Abstract
Objective
Chronic inflammation in HIV-infected individuals drives disease progression and the development of co-morbidities, despite viral suppression with combined anti-retroviral therapy (CART). Here, we sought evidence that vagal dysfunction (VD), which occurs commonly as part of HIV-associated autonomic neuropathy (HIV-AN), could exacerbate inflammation through gastrointestinal (GI) dysmotility, small intestinal bacterial overgrowth (SIBO), and alterations in patterns of soluble immune mediators.
Design
This is a cross-sectional observational study.
Methods
Forty participants on stable CART with GI symptoms, and no causes for vagal or GI dysfunction other than HIV, underwent autonomic testing, hydrogen/methane breath testing (HMBT) for SIBO, and gastric emptying scintigraphy. A panel of 41 cytokines, high mobility group box 1 (HMGB-1), and markers of bacterial translocation (lipopolysaccharide, LPS) and monocyte/macrophage activation (sCD14 and sCD163) were tested in plasma.
Results
We found that participants with VD had delayed gastric emptying and higher prevalence of SIBO. SIBO was associated with IL-6, but not sCD14; LPS could not be detected in any participant. We also found alteration of cytokine networks in participants with VD, with stronger and more numerous positive correlations between cytokines. In the VD group, HMGB-1 was the only soluble mediator displaying strong negative correlations with other cytokines, especially those cytokines which had numerous other strong positive correlations.
Conclusions
This study provides evidence that the vagal component of HIV-AN is associated with changes in immune and GI function in individuals with well-treated HIV. Further study will be needed to understand whether therapies targeted at enhancing vagal function could be of benefit in HIV.
Keywords: HIV, vagal, autonomic, gastric emptying, small intestinal bacterial overgrowth (SIBO), inflammation
INTRODUCTION
Chronic HIV infection produces pathologic immune activation which drives disease progression and contributes to the development of serious co-morbid medical conditions, even in the setting of effective combination antiretroviral therapy (CART).1 Bacterial translocation across the gastrointestinal (GI) tract is a potent antigenic stimulus, and is an important mechanism in the generation of HIV-associated chronic immune activation.2 There are three mechanisms by which HIV-infection has thus far been shown to be permissive for GI bacterial translocation: disruption of the physical integrity of the mucosal barrier, depletion of CD4+ T cells resulting in altered mucosal immunity, and changes in the luminal microbiome.3
Autonomic neuropathy (AN) is a common but underappreciated neurologic complication of HIV, typically occurring in the setting of the more familiar distal symmetric polyneuropathy (DSP).4 The clinical manifestations of HIV-associated AN (HIV-AN) are not yet well described. One important function of the autonomic nervous system is promotion of GI motility via cholinergic fibers of the vagus nerve. A potential consequence of vagal nerve fiber loss is slowed motility, particularly in the stomach and proximal small intestine, where vagal contact with enteric neurons is especially rich,5 and where the migrating motor complex depends on vagal integrity.6 With slowed motility, there is a propensity for small intestinal bacterial overgrowth (SIBO); this in turn can promote bacterial translocation and drive inflammation.7–9 In addition, cholinergic stimulation, such as is provided by an intact vagus nerve, has been shown to modulate GI mucosal inflammation,10–13 and increase mucosal populations of CD4+ T cell in animal models.13 However, to our knowledge, these mechanisms have not been studied in CART-treated HIV.
Independent of its effects on GI motility, vagal dysfunction (VD) could also contribute to chronic inflammation in HIV via direct effects on the immune system. In vitro, cholinergic stimulation reduces the release of inflammatory cytokines from macrophages via stimulation of the α7 nicotinic acetyl choline receptor (α7nAChR), and subsequent prevention of NF-κB pathway activation.14 An important effect of this pathway, referred to as the cholinergic anti-inflammatory reflex (CAR), is reduced release of high mobility group box 1 (HMGB-1). HMGB-1 is a potent inflammatory mediator produced in the later stages of sepsis,15 and is also elevated in HIV infection, although this latter literature is more limited.16 It is currently unknown how deficits in cholinergic signaling due to VD would affect cytokine networks in HIV-infected humans, however outside the context of HIV it has been proposed that aberrant NF-κB pathway activation results in hyper-responsiveness of immune cells to antigenic stimuli.17
In this cross-sectional, observational study, we sought evidence to support these relationships (as depicted in figure 1) in a group of 40 participants (18 with VD, and 22 controls) with stable, well-treated HIV. Specifically, we tested the main hypothesis that in HIV, VD is associated with SIBO via slowed GI motility, and SIBO in turn is associated with systemic inflammation as reflected by increased levels of IL-6. In exploratory analyses, we also measured a panel of 41 additional cytokines (including HMGB-1), to compare cytokine networks in participants with and without VD, focusing on the number and strength of correlations between individual cytokines, the increase of which has been proposed as an indicator of immune hyper-responsiveness in HIV.18–20
Figure 1.
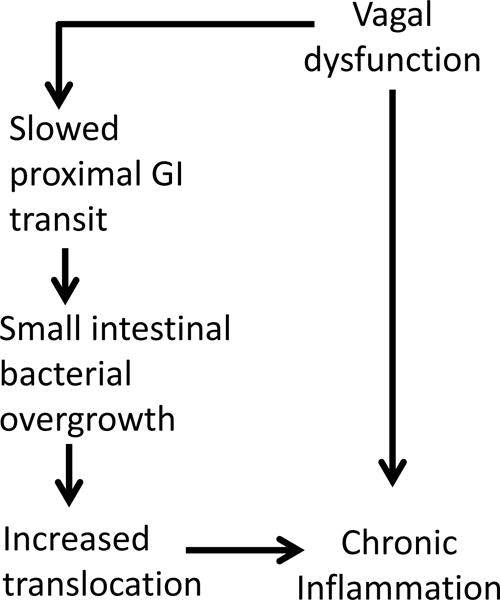
Hypothesized mechanisms by which vagal dysfunction could lead to chronic inflammation in HIV
METHODS
Participants
All procedures were performed in accordance with a protocol approved by the Institutional Review Board of the Icahn school of Medicine at Mount Sinai (ISMMS). All participants provided written informed consent. Included participants were HIV-infected adults (≥18 years), who were CART-treated for a minimum of 3 months, with HIV-1 plasma RNA load of ≤100 copies/ml, and at least one symptom potentially indicative of HIV-AN affecting the GI tract (as assessed using the Survey of Autonomic Symptoms, SAS).21 Exclusion criteria were: another diagnosis known to cause autonomic and/or GI dysfunction (e.g. diabetes); use of medications with significant autonomic or GI effects, or the ability to interfere with testing (e.g. prokinetics, antibiotics); contraindication to the testing; and urine drug testing positive for stimulants (sympathomimetic), or opiates/opioids (slowed GI motility). Given the potential for interference with gastric emptying studies (GES), participants who were prescribed benzodiazepines were included only if the use was on an as needed basis and urine toxicology was negative. In addition to these specific criteria, we sought to enrich the sample for participants with VD with a goal of approximately 50% of participants with VD. Accordingly, we specifically sought out participants likely to have VD, such as older patients, and those with prior autonomic testing or known DSP.4
Study procedures
Participants were seen for three visits. Visit one consisted of the informed consent process and assessment of eligibility including urine toxicology screening. Nicotine use was prohibited for 24 hours prior to each subsequent visit.
Visit two was the autonomic function tests (AFTs), which have been described previously.4,22 An individualized plan to delay any potentially interfering medications was developed for each participant. Participants refrain from caffeine use on the morning of testing (confirmed by self-report). Briefly, AFTs are a standard battery of non-invasive tests,23,24 which require about 90 minutes and include: quantitative sudomotor axon reflex testing (QSART), heart rate response to deep breathing (HRDB), Valsalva maneuver, and tilt table testing. The resultant data are compared to age and gender adjusted norms and summarized as the Composite Autonomic Severity Score (CASS) which includes sudomotor, vagal, and adrenergic sub-scores.23
Visit three took place over two days, one day for assessment of proximal GI motility using gastric emptying scintigraphy (GES), and one for SIBO using glucose breath testing (GBT). Participants were fasting for 12 hours prior to each test. In addition, a special diet (no slowly-digesting foods) was prescribed on the day prior to GBT. The GES involves ingestion of a standardized radiolabeled meal followed by acquisition of images of the stomach at 0.5, 1, 2, 3 and 4 hours, with results expressed as a percentage of retained gastric contents at each time point.25 The diagnosis of SIBO by GBT has been validated based on correlation with aspiration and culture techniques,26 and is based on the principle that hydrogen and methane are not produced by the human body, but are produced during fermentation of glucose by GI bacteria. Participants exhaled into a standardized apparatus at baseline, and then ingested a standardized glucose solution. Repeat breath samples were then collected every 20 minutes for 180 minutes, with attention paid to minimization of variability. The samples were analyzed by an external CLIA-certified laboratory (Aerodiagnostics, Lexington, MA), using gas chromatography (QuinTron Micro Analyzer, QuinTron Instrument Company, Inc.), with results expressed as parts per million (ppm). SIBO was diagnosed according to consensus guidelines of an increase in methane of ≥10 ppm, or in hydrogen ≥20ppm.27 The Composite Autonomic Symptom Scale (COMPASS) was also administered.28
Cytokines were quantified in the ISMMS Human Immune Monitoring Core, using a bead-based ELISA method by Milliplex xMAP technology (Millipore, Billerica, MA) with a Luminex 200 system (Luminex Corporation, Austin, TX). In addition to HMGB-1, we used the premixed 41 plex human cytokine/chemokine panel which includes: EGF, Eotaxin, G-CSF, GM-CSF, IFNα2, IFNγ, IL-10, IL-12P40, IL-12P70, IL-13, IL-15, IL-17A, IL-1RA, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, TNFα, TNFβ, VEGF,FGF-2, TGF-α, FLT-3L, Fractalkine, GRO, MCP-3, MDC, PDGF-AA, PDGF-AB/BB, sCD40L, and IL-9. Quantikine ELISA kits from R and D systems were utilized for quantification of plasma sCD14 and sCD163. Pyrosate Limulus Amebocyte Lysate assay (Associates of Cape Cod, East Falmouth, MA) was used to detect LPS, as per the manufacturer’s protocol. The limits of sensitivity for this assay are 0.03 EU/ml (3 pg/ml); plasma samples were run in a 1:10 dilution in the assay (threshold for detection was 30 pg/ml in the original sample). Additional technical information regarding cytokine analysis is included as a supplemental file.
Statistical procedures
Participants were classified as having abnormal vagal function if their CASS vagal sub-score was ≥1. The primary outcome for the GES was percent gastric retention at 4 hours.25 For SIBO, we considered both the dichotomous outcome as defined above, as well as the total increase in summated methane and hydrogen content of the breath (ppm). Participants with and without VD were compared using Chi-square of Fisher’s Exact tests for categorical variables, as well as Mann-Whitney U or independent samples t-test for continuous variables, as appropriate. Due to deviations from the assumption of bivariate normality, Spearman’s correlations (ρ) were used to examine the monotonic relationships between cytokines. All analyses were conducted in SAS 9.4 (Cary, NC) and with a type I error rate of 0.05. R 3.4.1 was used for generation of the heat maps accompanied by dendrograms.51,52
RESULTS
Participant characteristics
We contacted all eligible participants from prior studies,4,29,30 yielding 22 successful recruitments. Fourteen participants were recruited from our institution’s primary care HIV clinic, and four were self-referred. By design the majority of participants (90%) had a diagnosis of HIV-DSP, and 73% met criteria for HIV-AN (CASS≥3). Participants with and without VD were similar in terms of demographic and clinical factors (table 1).
Table 1.
Sample characteristics (n=40)
| Sample overall (n=40) |
Vagal dysfunction (n=18) |
Normal vagal function (n=22) |
p-value | |
|---|---|---|---|---|
| Age, years** | 58.0 ± 6.4 | 56.7 ± 6.6 | 59.1 ± 6.1 | 0.227 |
|
| ||||
| Sex | 0.455 | |||
| Male | 73% | 77% | 67% | |
| Female | 28% | 23% | 33% | |
|
| ||||
| Race/ethnicity | 0.623 | |||
| African-American | 48% | 50% | 45% | |
| Hispanic/Latino | 35% | 28% | 41% | |
| White | 18% | 22% | 14% | |
|
| ||||
| Current CD4+ count (cells/mm3)* | 624 (504 - 835) | 585 (490-754.5) | 685 (495.5-1060.75) | 0.147 |
|
| ||||
| Nadir CD4+ count (cells/mm3)* | 278 (110 - 423) | 279 (53-371.75) | 209 (70-459.5) | 0.668 |
|
| ||||
| Duration of known HIV infection, years* | 21 (16 - 25) | 21 (12-24) | 19.5 (16-28.25) | 0.806 |
|
| ||||
| Antiretroviral regimen | 0.918 | |||
| Single pill regimens | 43% | 40% | 46% | |
| Integrase based | 18% | 22% | 14% | |
| Protease inhibitor based | 15% | 17% | 14% | |
| NNRTI based | 10% | 11% | 9% | |
| Complex (multiple classes) | 15% | 11% | 18% | |
|
| ||||
| GI symptoms*** | ||||
| Early satiety | 51% | 44% | 58% | 0.641 |
| Post-prandial bloating | 70% | 72% | 67% | 0.884 |
| Nausea | 70% | 72% | 67% | 0.927 |
| Post-prandial vomiting | 18% | 22% | 14% | 0.520 |
| Moderate to severe diarrhea | 38% | 50% | 28% | 0.182 |
| Moderate to severe constipation | 34% | 39% | 30% | 0.622 |
|
| ||||
| GI symptom score*** | 8.0 (4.25-13.0) | 10.5 (4.75-14.00) | 6.5 (4.0-9.0) | 0.058 |
|
| ||||
| Abnormal CASS | NA | |||
| Vagal sub-score ≥1 | 45% | 100% | 0% | |
| Adrenergic sub-score ≥1 | 80% | 78% | 82% | |
| Sudomotor sub-score ≥1 | 85% | 83% | 86% | |
| Total CASS ≥3 | 73% | 83% | 64% | |
|
| ||||
| Meets diagnostic criteria for SIBO | 50% | 72% | 32% | 0.01 |
|
| ||||
| Increase in methane and hydrogen content of breath, ppm* | 12.5 (3.25, 31.5) | 29 (11.5, 47.5) | 7 (2, 24.25) | 0.004 |
|
| ||||
| Percent gastric retention at 4 hours* | 2 (1,3) | 3 (2, 4) | 2 (1, 3) | 0.043 |
Values are median (interquartile range).
Values are mean ± standard deviation.
Assessed using the Composite Autonomic Symptom Score (COMPASS)
NNRTI=non-nucleoside reverse transcriptase inhibitor; NA= not applicable; SIBO = small intestinal bacterial overgrowth; ppm=parts per million
VD is associated with slowed gastric emptying and SIBO
Participants with VD had a higher median percent retention of gastric contents at 4 hours (p=0.043). However, the difference was numerically small (3% vs. 2%) and in the normal range (<10%) for both groups. Only two participants (figure 2) met criteria for delayed gastric emptying at 4 hours, both of whom had VD. Participants with VD were more than twice as likely to have SIBO (72% vs 32%; χ2=6.5; p=.01). The median increase in summated methane and hydrogen breath content (indicative of SIBO severity) was >4 times higher in the group with VD: 29 vs. 7 ppm (p=.004). Both methane and hydrogen breath content tended to be higher in participants with VD (figure 3). Interestingly, although VD was associated with both GES results and SIBO, there were no significant associations between the results of GES and SIBO (p>0.15). Of note, all of these findings were specific to the vagal aspect of autonomic dysfunction; the total CASS and the sudomotor and adrenergic sub-scores were not associated with SIBO or gastric emptying (p>0.25 for all).
Figure 2.
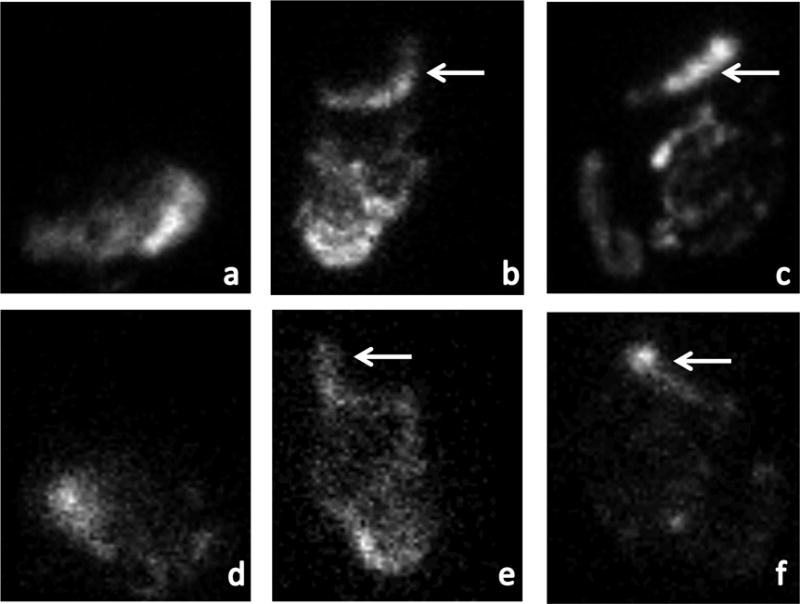
Gastric emptying abnormalities in participants with vagal dysfunction
shows gastric emptying scintigraphy images at 4 hours after ingestion of a radiolabeled meal. (a) is a normal anterior image in which all radiolabeled material has exited the stomach and moved distally. (b) and (c) are anterior images from the participants with vagal dysfunction and gastroparesis demonstrating retained radiolabeled material in the stomach (arrows). (d)-(f) are the analogous posterior images.
Figure 3.
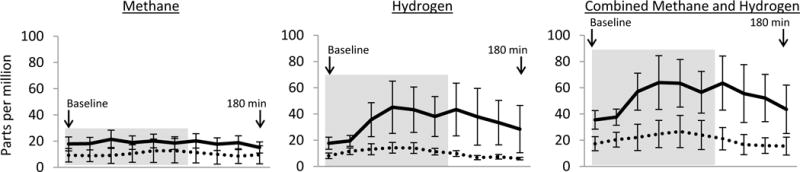
Gas content in breath following glucose ingestion in participants with and without vagal dysfunction
depicts mean hydrogen and methane breath content in parts per million (± standard error of the mean). The solid line represents participants with vagal dysfunction and the dotted line represents participants with normal vagal function. The baseline measurement is taken after an overnight fast. The participant then immediately drinks a standardized glucose solution and repeat samples are taken every 20 minutes for a total of 180 minutes. The grey box (minutes 0-100) represents the time during which gas production is likely to represent fermentation of glucose by small intestinal bacteria, whereas later times represent the large intestine.
SIBO is associated with elevated IL-6 levels in peripheral blood
Participants with SIBO had 24% higher levels of IL-6 (51.0 vs. 41.1 pg/ml, p=0.004), and more modestly elevated levels of IFNα and IL-2 (16% and 13% respectively, p<0.05 for both). However, we could not document direct evidence of bacterial translocation as the driver of the IL-6 elevation. Consistent with some prior reports in patients with well-controlled HIV, LPS was not detected in any of our participants regardless of SIBO status.31,32 In addition, there was no evidence of an association between SIBO and sCD14, a marker of macrophage activation commonly used as an indirect measure of translocation.32,33 Median sCD14 levels were slightly higher in the SIBO group (2235 vs. 2054 pg/ml) but the difference was not statistically significant (p=0.565).
Cytokine networks are more tightly correlated in participants with VD; HMGB-1 is an exception
There were a total of 946 possible bivariate correlations (Spearman’s ρ) between the inflammatory biomarkers we measured. Due to this high number, and the associated high potential for false discovery (type 1 error) none of these bivariate correlations can be considered statistically significant individually. However overall, a shift to more numerous and stronger positive correlations between cytokines was observed in the group with VD. In this group, 874 (92%) of the bivariate correlations were positive in sign, with 31 correlations being >0.80 in magnitude; whereas in the group with normal vagal function, 719 (76%) of the bivariate correlations were positive in sign, with only 4 correlations being >0.80 in magnitude. These findings are demonstrated visually in the heat maps (figure 4), in which each box represents a bivariate correlation between two biomarkers, with darker blue-green colors indicating stronger positive correlations.
Figure 4.
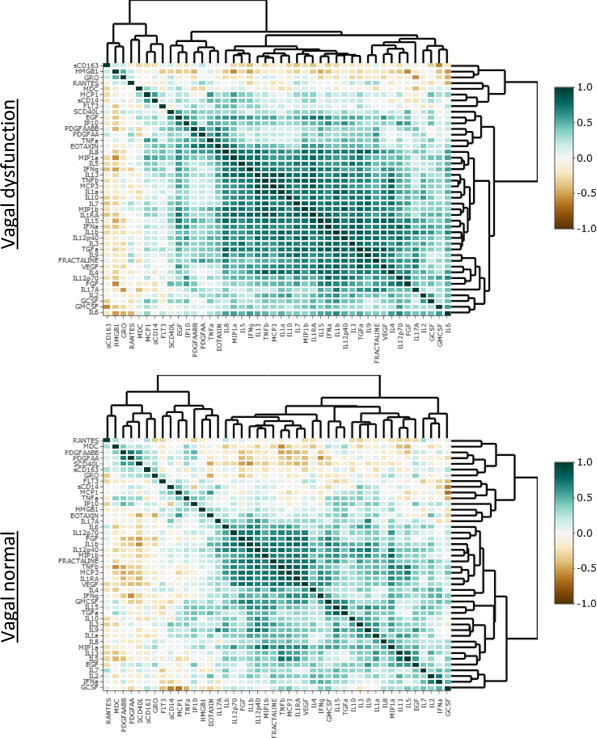
Correlations and hierarchical clustering of cytokine expression in participants with and without vagal dysfunction
depicts correlations between individual cytokines for participants with and without vagal dysfunction. Each small colored square represents the degree of correlation (i.e. value of Spearman’s ρ for bivariate correlation) between two individual cytokines. Darker shades of blue-green indicate stronger positive correlations. Cytokines are ordered according to the hierarchical clustering for each group as depicted by the dendrograms.
HMGB-1 was the exception to this pattern, being the only inflammatory mediator that was considerably less correlated with other cytokines in participants with VD. In the group with normal vagal function, HMGB-1 had 10 (24%) bivariate correlations with other cytokines which were negative in sign, compared to 37 (88%) in the group with VD. No other biomarker displayed such a large difference; the closest was sCD163 which had 21 (49%) negative correlations in the vagal normal group and 35 (81%) in the VD group.
DISCUSSION
Consideration of AN as an important neurologic complication of HIV dates back to the earliest days of the epidemic,34–36 although the topic has received sparse attention subsequently, with a few studies documenting mostly mild and sub-clinical cardiovascular AN,37–39 and more recent work demonstrating associations between HIV-AN and medical co-morbidites.22,40,41 Study of GI manifestations of HIV-AN has been limited to pre-CART anatomical studies demonstrating loss of autonomic fibers in jejunal biopsies from individuals with HIV,42,43 and a few studies suggesting GI dysmotility in HIV.44–47 We undertook the present work to provide evidence for the pathways illustrated in figure 1, specifically that VD, which occurs commonly as part of HIV-AN, is associated with abnormal states of inflammation in HIV, either through GI mechanisms or via impairment of the CAR.48
With regard to the GI mechanisms which could link VD with chronic inflammation, we found that that VD was associated with SIBO, and SIBO with elevations in IL-6. We had originally hypothesized that the association between VD and SIBO would be mediated by decreased GI motility,7–9 however this was not the case. A potential explanation is that subtle changes in motility may promote SIBO, but be insufficiently abnormal to be captured by GES, especially given the ability of unmeasured variables such as poor patient preparation to confound results.49 Alternatively, the main problem may be dysmotility of the small intestine, which we did not measure. Mechanisms other than dysmotility may also be important, such as exacerbation of GI mucosal immune abnormalities. Depletion of CD4+ T cells from the GI mucosa is well documented in HIV,50,51 and might be exacerbated by VD given the ability of cholinergic stimulation to increase the number of GI mucosal CD+ cells in animal models.13
Given our desire to study relationships between vagal and GI dysfunction we recruited only patients with GI symptoms, nonetheless the high prevalence of SIBO in our participants (50% overall) was surprising, considering that prior studies using similar diagnostic techniques have found SIBO in only 1-4% of healthy controls.52 The GI microbiome has received significant attention in HIV, but most studies focus on colonic flora.53–55 Little is known about the microbiome of the proximal GI tract in CART-era HIV, which is nearly sterile in healthy populations. To our knowledge there are only two relevant studies, both of which used aspiration techniques,56,57 and none using breath tests. The first studied only two HIV-infected patients.56 The second reported colonization of the duodenum with Proteobacteria in HIV-infected patients as compared to healthy controls.57 While such aspiration techniques are considered the gold standard, they have technical limitations and are invasive and not commonly performed in clinical practice.27 Larger studies, with broader inclusion criteria will be needed to understand the true prevalence of SIBO in the general HIV-infected patient population. Such studies would be particularly important given our observation that SIBO is associated with IL-6 which has been found to predict mortality during treated HIV infection, even among those with high CD4+ counts, and is also linked to a broad array of medical co-morbidities.58,59
In addition to these GI mechanisms, we also posited that VD might be linked to immune dysfunction in HIV via disruption of the CAR. Prior evidence for the CAR arises mostly from animal models of sepsis60,61 and in vitro studies of immune cells.14,62,63 Such studies demonstrate that vagal activity/cholinergic stimulation reduces the inflammatory response to LPS by reducing NF-κB activation and consequently the production of cytokines under its regulation.64,65 NF-κB regulates a broad array of cytokines including the majority of those in our panel,17,63,66–69 although cholinergic effects have only been demonstrated on a smaller subset (TNF-α, IL-1b, IL-6, IL-12 p40).64,65 Evidence for the CAR in humans is mostly from observational studies in sepsis, which have demonstrated correlations between lower heart rate variability (a reflection of VD), and elevation of individual cytokines such as IL-6.70–72 To our knowledge a role for the CAR in HIV-associated chronic inflammation has not been documented, although a significant body of literature has examined abnormalities of soluble immune mediators in HIV, most of which focuses on increases in absolute levels of individual molecules and pathways.2,59,73,74 A much smaller literature describes findings akin to ours with alteration in cytokine networks characterized by increased numbers and strength of correlations between cytokines of various classes. When detected, this latter phenomenon has been interpreted as maladaptive rigidity in cytokine responses evoked by the antigenic stimulus of chronic viral infection.19,75,76 Although our findings are preliminary, in need of future replication, and do not demonstrate causality, there is a biologically plausible mechanism by which VD could lead to more tightly correlated cytokine networks. Based on the pre-clinical studies described above, decreased cholinergic stimulation of immune cells (e.g. macrophages) is expected to lower the threshold for NF-κB activation, creating a state of hyper-responsiveness which could lead to the production of a broad array of inflammatory cytokines in response to antigenic stimulus (e.g. LPS).
HMGB-1 displayed a distinctive pattern in our study, in effect decoupling from other cytokines in our HIV-infected participants with VD. Secreted HMGB-1 acts as a pro-inflammatory cytokine. It is elevated in patients with HIV,16 and is also a critical mediator in the CAR.14 Cholinergic stimulation of primary human macrophages reduces extracellular HMGB-1 release in a dose dependent manner in vitro, and this close regulatory relationship is the primary reason we chose to focus on HMGB-1 in this study.14 Additionally, animal models of sepsis have shown that vagal nerve stimulation reduces HMGB-1 levels and improves outcomes.77 Thus it is not surprising that vagal function and HMGB-1 might have a unique relationship not shared by other cytokines, although the complexity of the relationship we observed was unexpected. Future studies are needed to confirm this finding, and understand its mechanism and clinical significance.
This study has limitations. First, the sample size is relatively small, the work was conducted at a single center, and all participants were residents of the same geographic area (New York City). Also our exclusionary criteria, while necessary for the study design, greatly narrowed the pool of potential participants since items like diabetes and opioid use are very common in our patient population. Since this was an initial study we did not use invasive GI diagnostic techniques, thus we cannot exclude subtle changes in motility or provide any specificity with regard to the bacterial species comprising the SIBO. We also did not test for the presence of Helicobacter pylori infection, which may cause symptoms and also impact inflammation. In addition, given the unexpectedly high rate of SIBO we had only three participants with VD who did not have SIBO, and so we cannot exclude the possibility that the changes in cytokine patterns in the VD group are due to SIBO or some other unmeasured variable. Also with regard to cytokines, although a comprehensive assay of soluble mediators was used, we did not directly measure monocyte-macrophage cell populations to assess activation of cholinergic pathways, and so can only speculate about underlying mechanisms. Finally and perhaps most importantly, we did not include HIV-negative controls, and therefore cannot comment on whether the relationships demonstrated are specific to HIV.
In summary, this study has several important and novel findings, which to our knowledge have not previously been demonstrated. We found that in the context of HIV, VD was associated with slower gastric emptying and SIBO; and SIBO (which was common even in participants without VD albeit to a lesser extent) was associated with an important marker of inflammation, IL-6. Also participants with VD displayed tighter correlation between cytokines overall, with the exception of HMGB-1 which reversed the pattern, becoming more negatively correlated with other cytokines in the setting of VD, particularly the most tightly correlated cytokines. These findings provide evidence that VD has demonstrable clinical impact on chronic inflammation, which is key to HIV pathogenesis. Given this first evidence of a neurological mechanism with the potential to contribute to disease progression in HIV, future work will turn to understanding the mechanisms underlying these phenomena, and whether therapies targeted at restoring diminished vagal function could be of benefit in HIV.
Supplementary Material
Acknowledgments
All authors were responsible for manuscript editing and providing final approval of the manuscript. Additional contributions follow. Dr. Robinson-Papp was responsible for conception and design; acquisition, analysis, and interpretation of data; and drafting. Dr. Morgello was responsible for conception and design, data interpretation. Dr. George was responsible for conception and design, and data acquisition. Drs. Nmashie, Sharma, Kim-Schulze, Machac, Heiba, Navis and Elicer and Ms. Murray were responsible for aspects of data acquisition and interpretation. Drs. Pedowitz, Mehandru, and Benn were responsible for aspects of data analysis and interpretation.
This study was funded by the National Institutes of Health (PI: Robinson-Papp, R21DK105917:“Autonomic neuropathy, gastrointestinal motility, and inflammation in HIV”). Additional laboratory support was provided by U24MH100931:“The Manhattan HIV Brain Bank.”
Footnotes
Conflicts of interest and source of funding: The authors report no conflicts of interest.
References
- 1.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 2.Vassallo M, Mercié P, Cottalorda J, Ticchioni M, Dellamonica P. The role of lipopolysaccharide as a marker of immune activation in HIV-1 infected patients: a systematic literature review. Virol J. 2012;9:174. doi: 10.1186/1743-422X-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13:19. doi: 10.1186/s12981-016-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson-Papp J, Sharma S, Simpson DM, Morgello S. Autonomic dysfunction is common in HIV and associated with distal symmetric polyneuropathy. J Neurovirol. 2013;19:172–180. doi: 10.1007/s13365-013-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powley T. Vagal input to the enteric nervous system. Gut. 2000;47:iv30–iv32. doi: 10.1136/gut.47.suppl_4.iv30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. 2012;9:271–285. doi: 10.1038/nrgastro.2012.57. [DOI] [PubMed] [Google Scholar]
- 7.Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158–1166. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stotzer PO, Bjornsson ES, Abrahamsson H. Interdigestive and postprandial motility in small-intestinal bacterial overgrowth. Scand J Gastroenterol. 1996;31:875–880. doi: 10.3109/00365529609051995. [DOI] [PubMed] [Google Scholar]
- 9.Husebye E, Skar V, Hoverstad T, Iversen T, Melby K. Abnormal intestinal motor patterns explain enteric colonization with gram-negative bacilli in late radiation enteropathy. Gastroenterology. 1995;109:1078–1089. doi: 10.1016/0016-5085(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 10.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr Protoc Immunol Ed John E Coligan Al. 2014;104 doi: 10.1002/0471142735.im1525s104. Unit-15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jonge WJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 13.Galitovskiy V, et al. Cytokine-induced alterations of α7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17- versus Th2-mediated colitis. J Immunol Baltim Md 1950. 2011;187:2677–2687. doi: 10.4049/jimmunol.1002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon ML. Alarmins and central nervous system inflammation in HIV-associated neurological disorders. J Intern Med. 2017;281:433–447. doi: 10.1111/joim.12570. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LISCO A, et al. HIV-1 imposes rigidity on blood and semen cytokine networks. Am J Reprod Immunol N Y N 1989. 2012;68:515–521. doi: 10.1111/aji.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, et al. Cytokine cascade and networks among MSM HIV seroconverters: implications for early immunotherapy. Sci Rep. 2016;6:36234. doi: 10.1038/srep36234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shebl FM, Yu K, Landgren O, Goedert JJ, Rabkin CS. Increased Levels of Circulating Cytokines with HIV-Related Immunosuppression. AIDS Res Hum Retroviruses. 2012;28:809–815. doi: 10.1089/aid.2011.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zilliox L, et al. Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology. 2011;76:1099–1105. doi: 10.1212/WNL.0b013e3182120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson-Papp J, Sharma SK. Autonomic neuropathy in HIV is unrecognized and associated with medical morbidity. AIDS Patient Care STDs. 2013;27:539–543. doi: 10.1089/apc.2013.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin ProceedingsMayo Clin. 1993;68:748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 24.Novak P. Quantitative Autonomic Testing. J Vis Exp JoVE. 2011 doi: 10.3791/2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abell TL, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 26.Erdogan A, et al. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil. 2015;27:481–489. doi: 10.1111/nmo.12516. [DOI] [PubMed] [Google Scholar]
- 27.Rezaie A, et al. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87:1196–1201. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George MC, Wongmek A, Kaku M, Nmashie A, Robinson-Papp J. A Mixed-Methods Pilot Study of Mindfulness Based Stress Reduction for HIV-Associated Chronic Pain. Behav Med Wash DC. 2015 Dec;11:1–12. doi: 10.1080/08964289.2015.1107525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson-Papp J, et al. Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch Neurol. 2008;65:1096–1101. doi: 10.1001/archneur.65.8.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balagopal A, et al. Detection of Microbial Translocation in HIV and SIV Infection Using the Limulus Amebocyte Lysate Assay is Masked by Serum and Plasma. PLOS ONE. 2012;7:e41258. doi: 10.1371/journal.pone.0041258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Sánchez M, et al. Different biological significance of sCD14 and LPS in HIV-infection: importance of the immunovirology stage and association with HIV-disease progression markers. J Infect. 2012;65:431–438. doi: 10.1016/j.jinf.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 33.De Voeght A, Maes N, Moutschen M. sCD14 is not a bona-fide biomarker of microbial translocation in HIV-1-infected Africans living in Belgium. AIDS Lond Engl. 2016;30:921–924. doi: 10.1097/QAD.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 34.Freeman R, Roberts MS, Friedman LS, Broadbridge C. Autonomic function and human immunodeficiency virus infection. Neurology. 1990;40:575–580. doi: 10.1212/wnl.40.4.575. [DOI] [PubMed] [Google Scholar]
- 35.Craddock C, Bull R, Pasvol G, Protheroe A, Hopkin J. CARDIORESPIRATORY ARREST AND AUTONOMIC NEUROPATHY IN AIDS. The Lancet. 1987;330:16–18. doi: 10.1016/s0140-6736(87)93054-6. [DOI] [PubMed] [Google Scholar]
- 36.Villa A, Foresti V, Confalonieri F. Autonomic nervous system dysfunction associated with HIV infection in intravenous heroin users. AIDS Lond Engl. 1992;6:85–89. doi: 10.1097/00002030-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Mittal CM, Wig N, Mishra S, Deepak KK. Heart rate variability in human immunodeficiency virus-positive individuals. Int J Cardiol. 2004;94:1–6. doi: 10.1016/j.ijcard.2003.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Sakhuja A, et al. Heart rate variability and autonomic function tests in HIV positive individuals in India. Clin Auton Res Off J Clin Auton Res Soc. 2007;17:193–196. doi: 10.1007/s10286-007-0412-5. [DOI] [PubMed] [Google Scholar]
- 39.Lebech AM, et al. Autonomic dysfunction in HIV patients on antiretroviral therapy: studies of heart rate variability. Clin Physiol Funct Imaging. 2007;27:363–367. doi: 10.1111/j.1475-097X.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 40.Askgaard G, et al. Decreased heart rate variability in HIV positive patients receiving antiretroviral therapy: importance of blood glucose and cholesterol. PloS One. 2011;6:e20196. doi: 10.1371/journal.pone.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow D, et al. Rates of autonomic dysfunction in HIV patients receiving antiretroviral therapy. J Neurovirol. 2013;19:511–512. doi: 10.1007/s13365-013-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batman PA, Miller AR, Sedgwick PM, Griffin GE. Autonomic denervation in jejunal mucosa of homosexual men infected with HIV. AIDS Lond Engl. 1991;5:1247–1252. doi: 10.1097/00002030-199110000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Batman PA, et al. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. J Clin Pathol. 1989;42:275–281. doi: 10.1136/jcp.42.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpstone D, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45:70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neild PJ, et al. Delayed gastric emptying in human immunodeficiency virus infection: correlation with symptoms, autonomic function, and intestinal motility. Dig Dis Sci. 2000;45:1491–1499. doi: 10.1023/a:1005587922517. [DOI] [PubMed] [Google Scholar]
- 46.Konturek JW, Fischer H, van der Voort IR, Domschke W. Disturbed gastric motor activity in patients with human immunodeficiency virus infection. Scand J Gastroenterol. 1997;32:221–225. doi: 10.3109/00365529709000198. [DOI] [PubMed] [Google Scholar]
- 47.Barnert J, Dumitrascu DL, Wienbeck M. Dyspepsia in AIDS is correlated to ultrasonographic changes of antral distension. Eur J Ultrasound Off J Eur Fed Soc Ultrasound Med Biol. 2000;11:189–197. doi: 10.1016/s0929-8266(00)00083-5. [DOI] [PubMed] [Google Scholar]
- 48.Tracey KJ. Reflex control of immunity. Nat Rev. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrell MB, Costello M, McKee JLD, Gordon LL, Fig LM. Compliance with Gastric-Emptying Scintigraphy Guidelines: An Analysis of the Intersocietal Accreditation Commission Database. J Nucl Med Technol. 2017;45:6–13. doi: 10.2967/jnmt.116.184473. [DOI] [PubMed] [Google Scholar]
- 50.Mehandru S, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah ED, Basseri RJ, Chong K, Pimentel M. Abnormal Breath Testing in IBS: A Meta-Analysis. Dig Dis Sci. 2010;55:2441–2449. doi: 10.1007/s10620-010-1276-4. [DOI] [PubMed] [Google Scholar]
- 53.Dillon SM, Frank DN, Wilson CC. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS Lond Engl. 2016;30:2737–2751. doi: 10.1097/QAD.0000000000001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vázquez-Castellanos JF, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015;8:760–772. doi: 10.1038/mi.2014.107. [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Santiago J, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS Lond Engl. 2013;27:1921–1931. doi: 10.1097/qad.0b013e3283611816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Rosenvinge EC, et al. Immune status, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. ISME J. 2013;7:1354–1366. doi: 10.1038/ismej.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, et al. HIV-induced immunosuppression is associated with colonization of the proximal gut by environmental bacteria. AIDS Lond Engl. 2016;30:19–29. doi: 10.1097/QAD.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deeks SG, Tracy R, Douek DC. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis. 2016;214:S44–S50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watkins LR, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 61.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 62.Rosas-Ballina M, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med Camb Mass. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nizri E, et al. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol Baltim Md 1950. 2009;183:6681–6688. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- 64.Rehani K, et al. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim Biophys Acta. 2008;1783:375–382. doi: 10.1016/j.bbamcr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 67.Nakayama T, et al. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein-Barr virus. J Virol. 2004;78:1665–1674. doi: 10.1128/JVI.78.4.1665-1674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hein H, et al. Genomic organization, sequence, and transcriptional regulation of the human eotaxin gene. Biochem Biophys Res Commun. 1997;237:537–542. doi: 10.1006/bbrc.1997.7169. [DOI] [PubMed] [Google Scholar]
- 69.Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF. Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NF-kappaB. FASEB J Off Publ Fed Am Soc Exp Biol. 2008;22:1807–1816. doi: 10.1096/fj.07-094235. [DOI] [PubMed] [Google Scholar]
- 70.Peterson CY, Krzyzaniak M, Coimbra R, Chang DC. Vagus nerve and postinjury inflammatory response. Arch Surg Chic Ill 1960. 2012;147:76–80. doi: 10.1001/archsurg.2011.237. [DOI] [PubMed] [Google Scholar]
- 71.Papaioannou VE, Dragoumanis C, Theodorou V, Gargaretas C, Pneumatikos I. Relation of heart rate variability to serum levels of C-reactive protein, interleukin 6, and 10 in patients with sepsis and septic shock. J Crit Care. 2009;24:625.e1–625.e7. doi: 10.1016/j.jcrc.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Tateishi Y, et al. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock Augusta Ga. 2007;28:549–553. doi: 10.1097/shk.0b013e3180638d1. [DOI] [PubMed] [Google Scholar]
- 73.Tasca KI, Calvi SA, de Souza L do R. Immunovirological parameters and cytokines in HIV infection. Rev Soc Bras Med Trop. 2012;45:663–669. doi: 10.1590/s0037-86822012000600002. [DOI] [PubMed] [Google Scholar]
- 74.Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olivier AJ, et al. Distinct cytokine patterns in semen influence local HIV shedding and HIV target cell activation. J Infect Dis. 2014;209:1174–1184. doi: 10.1093/infdis/jit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lisco A, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huston JM, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 51.R Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2016. R: A language and environment for statistical computing. Available at: https://www.r-project.org/. (Accessed: 20th October 2017) [Google Scholar]
- 52.Galili T, Sidi J, O’Callaghan A, Benjamini Y, heatmaply: Interactive Cluster Heat Maps Using ‘plotly’ R package version 0.11.1. 2017 https://CRAN.R-project.org/package=heatmaply.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


