Abstract
Pulmonary hypertension (PH) is a devastating disease and its successful treatment remains to be accomplished despite recent advances in pharmacotherapy. It has been proposed that PH be considered as a systemic disease, rather than primarily a disease of the pulmonary vasculature. Consequently, an investigation of intricate interplay between multiple organs such as brain, vasculature and lung in PH could lead to identification of new targets for its therapy. However, little is known about this interplay. This study was undertaken to examine the concept that altered autonomic-pulmonary communication is important in PH pathophysiology. Therefore, we hypothesize that activation of microglial cells in the paraventricular nucleus (PVN) of hypothalamus and neuroinflammation is associated with increased sympathetic drive and pulmonary pathophysiology contributing to PH. We utilized the monocrotaline (MCT) rat model for PH and intracerebroventricular (ICV) administration of minocycline for inhibition of microglial cells activation to investigate this hypothesis. Hemodynamic, echocardiographic, histological, immunohistochemical and confocal microscopic techniques assessed cardiac and pulmonary function and microglial cells. MCT treatment caused cardiac and pulmonary pathophysiology associated with PH. There were also increased activated microglial cells and mRNA for proinflammatory cytokines (IL-1β, IL-6 and TNF-α) in the PVN. Furthermore, increased sympathetic drive and plasma NE were observed in rats with PH. ICV infusion of minocycline inhibited all these parameters and significantly attenuated PH. These observations implicate a dysfunctional autonomic-lung communication in the development and progression of PH providing new therapeutic targets, such as neuroinflammation, for PH therapy.
Keywords: Pulmonary hypertension, Autonomic nervous system, Neuroinflammation, Minocycline, Echocardiography
Introduction
Pulmonary hypertension (PH) is a devastating disease that leads to progressive right heart failure (RHF), disability and death. Despite persistent efforts over the last several decades to develop effective therapies, limited progress has been made in improving quality of life, preventing or arresting progression of PH. The limited success of therapeutics could be due to the long-held dogma that PH is primarily a disease of the pulmonary vasculature. However, it has recently been suggested that PH should be approached as a systemic disease for the development of the next generation of therapy1. Our study was designed to evaluate this ‘paradigm shifting’ concept by investigating the involvement of brain-lung communication in PH.
It has long been established from both animal and human studies that the autonomic nervous system (ANS) has important influences on cardiopulmonary function2, 3. In fact, sympathetic nerve activity (SNA) has been shown to be markedly increased in PH patients4, 5 and SNA is an independent predictor of clinical deterioration that correlates with severity of disease6. Moreover, enhanced SNA is associated with RHF7. Consistent with these findings are observations of increased plasma NE and beneficial effects of beta-adrenergic receptor antagonists in animal models of PH8,9. Although little is known about the role of SNA and microglial activation in PH, considerable evidence exists linking them in systemic hypertension10. Collectively, these observations raise the potential involvement of microglial cells and neuroinflammation in PH. Consistent with this view is evidence of the involvement of SNA in other pulmonary diseases11, 12.
The rationales for focusing on the paraventricular nucleus (PVN) of the hypothalamus are: a) PVN is a key autonomic control center in the brain implicated in integrating SNA13,14 and a site implicated in the neurogenic origin of hypertension10. b) Our studies in systemic hypertension have shown association of activated microglial cells and neuroinflammation in the PVN with increased SNA. Additionally, intracerebroventricular (ICV) infusion of minocycline prevents microglial activation, increased SNA and hypertension induced by angiotensin II10. Collectively, these findings, led us to propose the following hypothesis: Increased activated microglial cells and neuroinflammation in the autonomic regions of the brain are important in PH. They participate in a sequence of physiological events including increased sympathetic activity, plasma NE and systemic inflammation to culminate in development of pulmonary pathophysiology associated with PH. The present study investigated this concept.
Methods
Animals
Authors declare that all supporting data in the article and supplement are available upon reasonable request. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Eight-week-old male SD rats (n=6–8/group) were used and PH was induced by a single subcutaneous injection of 50mg/kg body weight of monocrotaline (MCT). A subset of MCT-injected animals was infused ICV with minocycline. Four weeks after MCT administration, animals were sacrificed for the measurement of physiological and pathological parameters.
Stereotaxic surgery for intracerebroventricular (ICV) minocycline infusion
ICV minocycline infusion was carried out on the same day as MCT injection. Briefly, rats were anesthetized with Isoflurane/O2 mixture (4%), and the head was fixed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Body temperature was maintained at 37 ± 1°C using heating pads. A brain infusion cannula was implanted into the left cerebroventricle at coordinates 1.0 caudal to bregma, 1.9 mm lateral to midline and 4.5 mm ventral to the skull surface and fixed to the skull with stainless steel screws and dental cement. The outer end of the cannula was connected to an osmotic mini-pump (model 2004, Durect Corporation, Cupertino, CA) which was implanted in the neck subcutaneously. Using the cannulae and pumps, either minocycline (5 μg/h; Sigma-Aldrich, St. Louis, MO) or artificial cerebrospinal fluid (aCSF; Tocris Bioscience, UK) was infused for four weeks into the left cerebroventricle at a flow rate of 0.25 μl/h. Rats received a single dose of buprenorphine (0.05 mg/kg b.w.; Buprenex, Pfizer, NY) subcutaneously during surgery, and were monitored daily throughout the experimental period.
Statistical Analysis
For all the parameters, comparisons among the groups were made using one-way analysis of variance (ANOVA), p-Value <0.05 was accepted as significant and data were presented as mean ± SEM. 2-Way ANOVA was also used for key parameters to confirm interactions. Full details of all experimental protocols are presented in Methods of the ‘Online Supplement’.
Results
ICV administration of minocycline attenuates MCT-induced PH pathophysiology
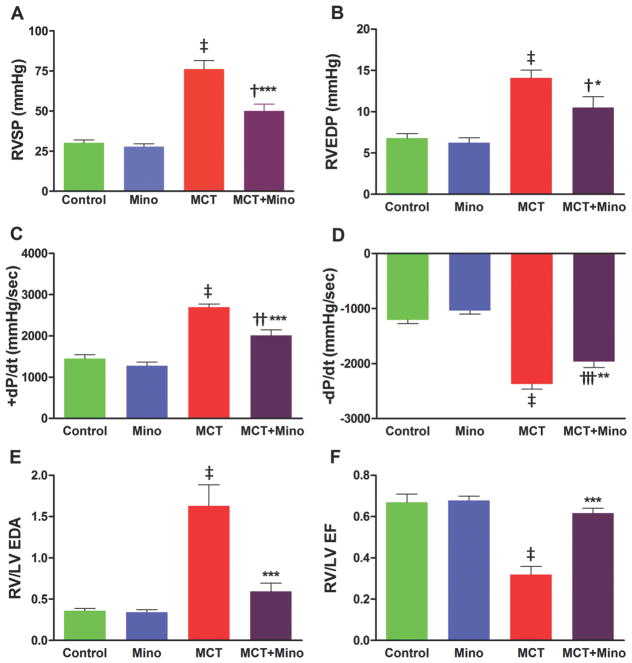
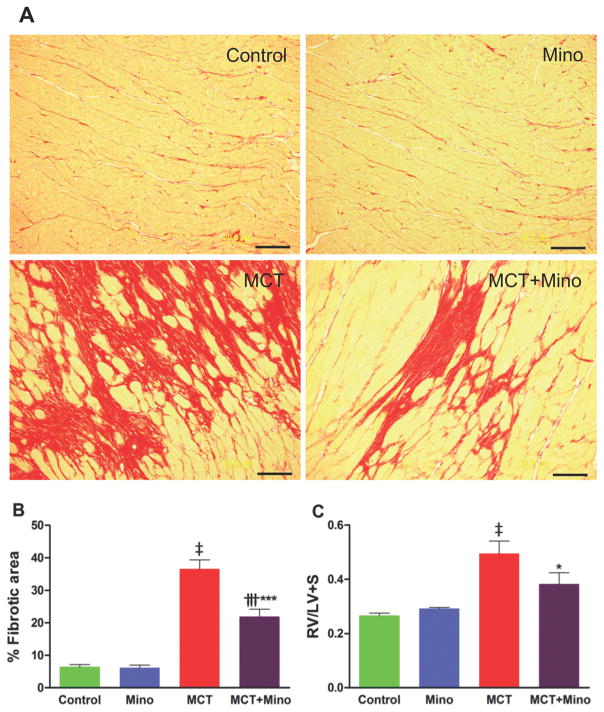
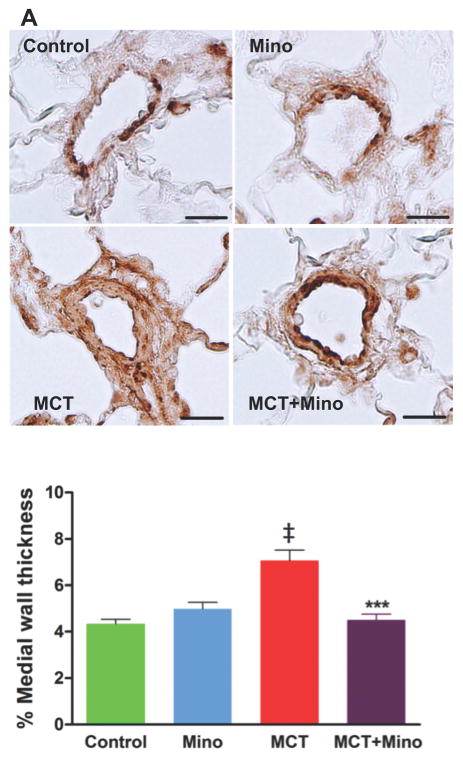
Our first objective was to determine if a neuroinflammatory process is involved in MCT-induced PH and if ICV minocycline would influence this process. The rationale for selecting minocycline is its well-documented effects on inhibition of microglial cells and neuroinflammation10. As expected, MCT-treated rats had a 153% increase in right ventricular systolic pressure (RVSP, 30 ± 1.8 mmHg control rats vs. 76 ± 5.3 mmHg MCT, p<0.001) (Figure 1A) indicative of PH development. Similarly, MCT causes alterations in multiple other hemodynamic parameters such as right ventricular end diastolic pressure (RVEDP) increased 107%, p<0.001; RV +dP/dt increased 86%, p<0.001; and RV -dP/dt decreased 97%, p<0.001 (Figure 1B–D). ICV minocycline infusion into control rats had no significant effect on any of these parameters. However, ICV minocycline infusion into MCT-treated rats reduced RVSP 34% (50 ± 4.2 mmHg, p<0.001) vs. MCT-treated rats (Figure 1A). Furthermore, minocycline beneficially attenuated other hemodynamic parameters (Figure 1B–D). Echocardiographic analysis documented attenuation of ventricular dilation and maladaptive structural remodeling of the right heart with 63% and 93% improvements in right ventricle/left ventricle (RV/LV) end-diastolic area (EDA) and RV/LV ejection fraction (EF) when minocycline was administered to MCT-treated rats (Figure 1E–F). Video echocardiographic clips documented an increase in RV/LV in MCT-treated rats vs. control rats and rats treated with minocycline alone, implying dilation of the right heart in PH animals (Online Videos S1A–C). However, ICV minocycline treatment prevented MCT-induced dilation of the right heart (Online Video S1D). MCT caused a significant right heart remodeling as evidenced by ~6-fold increase in RV collagen accumulation (p<0.001, Figure 2A–B) and ~2-fold increase in RV hypertrophy (RVH) (p<0.001, Figure 2C). Both the parameters were significantly attenuated by ICV minocycline treatment (Figure 2A–C). Furthermore, MCT treatment increased pulmonary artery medial wall thickness by ~2-fold that was significantly attenuated by concomitant treatment with ICV minocycline (p<0.001, Figure 3A–B). Finally, treatment with minocycline two-weeks after initiation of PH by MCT showed a significant arrest in the progression of PH-linked hemodynamics in four of six rats (RVSP, 70 ± 5.9 mmHg MCT vs. 50 ± 1.5 mmHg MCT + minocycline (p<0.01, n=4); RVH, 0.43 ± 0.04 MCT vs. 0.32 ± 0.01 MCT + minocycline (p<0.05, n=4). Collectively, these observations are the first demonstration of involvement of brain mechanisms in development of PH.
Figure 1. Minocycline attenuates monocrotaline (MCT)-induced pulmonary pathophysiology.
A, Right ventricular systolic pressure (RVSP) shown in controls and MCT-treated rats that were either untreated (vehicle) or treated with intracerebroventricular (ICV) minocycline. B, Measurement of right ventricular enddiastolic pressure (RVEDP). C, Ventricular contractility as shown by +dP/dt and D, -dP/dt. E, Ratio of right ventricle (RV) to left ventricle (LV) end-diastolic area (RV/LV EDA) as visualized by echocardiography and F, Ratio of RV to LV ejection fraction (EF) examined by echocardiography. ‡p<0.001 vs. control and minocycline, ***p<0.001, **p<0.01, *p<0.05 vs. MCT and †††p<0.001, ††p<0.01, †p<0.05 vs. control and minocycline (n= 6–7 rats/group); Mino = Minocycline. Data are represented as mean ± SEM, analyzed using one-way analysis of variance (ANOVA) with Newman-Keuls post hoc test. 2-way ANOVA also showed significant interactions between MCT and minocycline on RVSP (p=0.0043).
Figure 2. ICV minocycline treatment reduces the RV fibrosis (RVF) and hypertrophy (RVH) induced by MCT.
A, Representative micrographs of collagen stained with Picro Sirius Red revealing increased fibrosis in MCT-treated rats. B, Cumulative analysis of MCT-treated rats shows significant collagen accumulation in the RV indicating the development of fibrosis. C, RVH determined as the ratio of RV to LV and intraventricular septum (S) weight [RV/(LV+S)]. Minocycline treatment exerts anti-fibrotic and anti-hypertrophic effects in the RV of MCT-treated rats. ‡p<0.001 vs. control and minocycline; ***p<0.001, *p<0.05 vs. MCT; †††p<0.001 vs. control and minocycline (n= 5–6 rats/group) (scale bar =20 μm). Data are represented as mean ± SEM, analyzed using one-way ANOVA with Newman-Keuls post hoc test. 2-way ANOVA showed significant interaction between MCT and minocycline on RVF (p=0.0016).
Figure 3. Anti-remodeling effects of minocycline in MCT-treated rats.
A, Representative micrographs show remodeling in pulmonary arteries of external diameter 25 to 50 μM stained with α-smooth muscle actin antibody and quantified for medial wall thickness (scale bar=100μm). B, Cumulative data of α-smooth muscle actin staining showing MCT caused significant increases in pulmonary medial wall thickness and this increase was significantly prevented by minocycline treatment. ‡p<0.001 vs. control and minocycline; ***p<0.001 vs. MCT (n= 6–7 rats/group). Data are represented as mean ± SEM, analyzed using one-way ANOVA with Newman-Keuls post hoc test. 2-way ANOVA showed significant interaction between MCT and minocycline on % medial wall thickness (p=0.0001).
MCT-induction of microglial cells is inhibited by minocycline
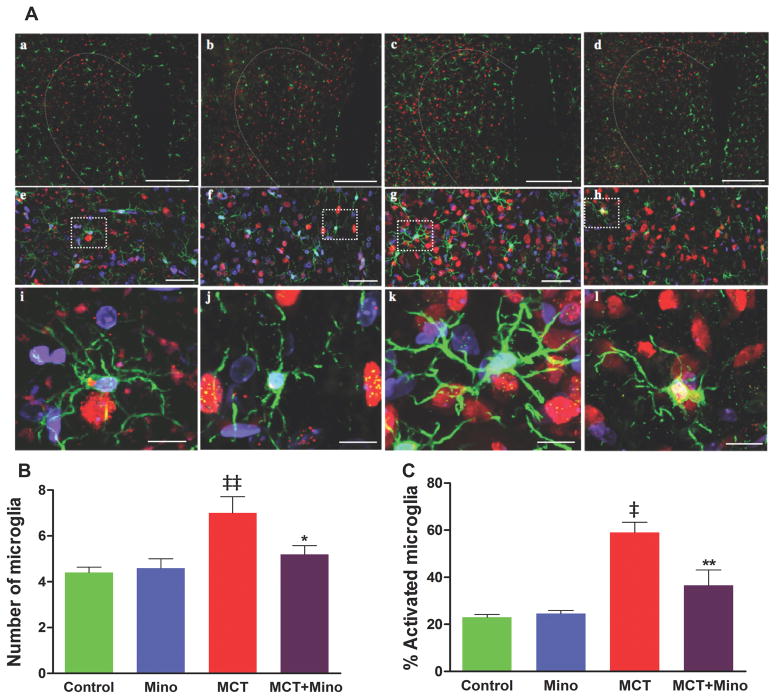
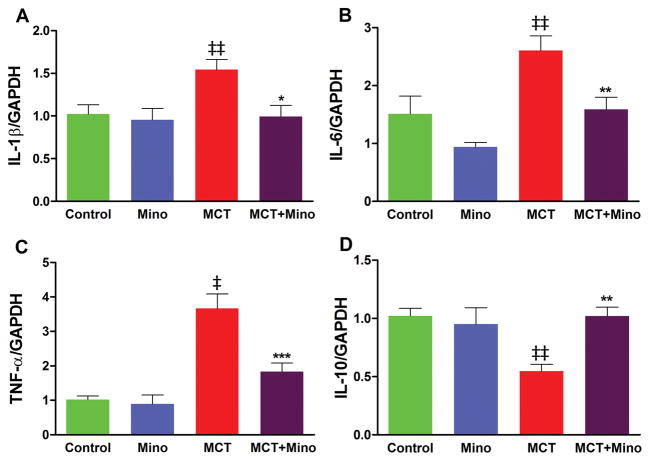
Our objective was to answer two questions: (i) Is MCT-induced PH associated with increases in activated microglia and if so, what autonomic brain region(s) is (are) predominantly involved, and (ii) Does minocycline inhibit this process? We observed that MCT-treated rats had a significant increase in total numbers of ionized calcium binding adaptor molecule 1 (Iba1) positive cells in the PVN of the hypothalamus (Figure 4). Iba1 is a marker of mature microglia in the brain. Increases in both the total number (~2-fold) and number of activated (~1.5-fold) microglial cells were observed (Figure 4A–C) in the PVN of MCT-treated rats. Concomitant minocycline and MCT treatments resulted in significant decreases in total number (25%) and number of activated microglial cells (42%) in the PVN (Figure 4B–C) versus the MCT-treated rats. Increases in the total number of microglial cells were also observed in other autonomic areas such as rostral ventrolateral medulla and nucleus tractus solitarii (Online Figure S1A–B). However, little change in activated microglial cells was seen in these areas. Furthermore, minocycline treatment had modest effects on total microglial cells but did not influence their activation in these regions in MCT-treated animals (Online Figure S1 A–B). Online Videos S2A–D are representative examples of 3D video clips of the same region of PVN from control (S2A), minocycline alone (S2B), MCT-treated (S2C), and MCT with minocycline (S2D) treated rats in order to fully appreciate the preponderance of microglial cells and their interactions with neurons in MCT-treated rats. The mRNA levels of certain cytokines measured by qPCR confirm the proinflammatory response in the PVN. IL-1β, IL-6, and TNF-α mRNAs, were increased 54% (p<0.01), 66% (p<0.001), and 260% (p<0.001), respectively, in the PVN of MCT-treated rats (Figure 5A–C). In contrast, anti-inflammatory IL-10 mRNA levels were decreased by 34% (p<0.05, Figure 5D). Minocycline treatment of MCT rats significantly attenuated transcription of proinflammatory cytokine genes and increased IL-10. These observations document that MCT treatment is associated with microglial cell activation and neuroinflammation, while ICV minocycline attenuates these processes.
Figure 4. MCT-induced microglial cells are inhibited by ICV minocycline treatment.
A, Representative micrographs show the paraventricular nucleus (PVN) sections stained with anti-ionized calcium binding adaptor molecule1 (Iba1) antibody indicative of microglia (green), anti-NeuN indicative of neurons (red) and DAPI showing DNA (blue). (a–d) (20x; scale bar= 200 μM). (e–h) Higher magnification (60x; scale bar=50 μM), and enlarged view of microglia showing resting microglia in control (i), minocycline (j) and MCT+ minocycline (l) compared with activated microglia in MCT-treated group (k). B, Total number of microglia (resting/activated) and C, % of activated microglia within the 200×200 μm2 area of PVN under each treatment condition. ‡p<0.001, ‡‡ p<0.01 vs. control and minocycline; **p<0.01, *p<0.05 vs. MCT (n= 6 rats/group). Data are represented as mean ± SEM, analyzed using one-way ANOVA with Newman-Keuls post hoc test. 2-way ANOVA showed significant interactions between MCT and minocycline on number of microglia (p=0.047) and % activated microglia (p=0.0086).
Figure 5. MCT-induced proinflammatory cytokines are attenuated by ICV minocycline.
PVN mRNA levels of IL-1β, IL-6 and TNF-α are increased while IL-10 is decreased by MCT treatment (A–D). Minocycline treatment restored IL-1β (A), IL-6 (B), TNF-α (C), and IL-10 (D) to the control levels. ‡p<0.001, ‡‡ p<0.01 vs. control and minocycline; ***p<0.001, **p<0.01, *p<0.05 vs. MCT (n= 6 rats/group). Data are represented as mean ± SEM, analyzed using one-way ANOVA with Newman-Keuls post hoc test. 2-way ANOVA showed significant interactions between MCT and minocycline on TNF-α (p=0.0079) and IL-10 (0.0094). However, no significant interaction was observed on IL-6 (p=0.066) and IL-1β (p=0.34).
Restoration of altered brain-ANS-lung communication in PH by minocycline
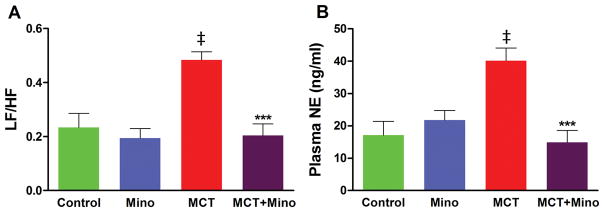
Power spectral analysis of heart rate variability data supported involvement of sympathetic activation in PH. The ratio of low-frequency (LF) and high-frequency (HF) is an index for the relationship between sympathetic and parasympathetic nerve activities15,16. MCT-treated rats had a 108% increase in LF/HF ratio (p<0.001, Figure 6A) indicating an increase in SNA in PH rats. However, minocycline co-treatment resulted in a complete attenuation of this LF/HF ratio increase (p<0.001, Figure 6A). These observations are supported by effects of MCT and minocycline on plasma norepinephrine (NE). MCT treatment increased plasma NE (135%) while minocycline significantly attenuated this increase (Figure 6B). Taken together, these observations document that ICV minocycline treatment attenuated the enhanced sympathetic activity of MCT-treated rats and suggest an altered PVN regulation of ANS in PH.
Figure 6. Minocycline improves autonomic imbalance in MCT-rats.
A, Spectral components analyzed using ECG signals, the ratio of low-frequency (LF) and high-frequency (HF); (LF/HF) represents the balance between sympathetic and parasympathetic system. Minocycline treatment restored the MCT-perturbed autonomic balance. B, Plasma norepinephrine (NE), indicative of sympathetic activation, was greatly increased in MCT-treated rats and minocycline treatment significantly reduced the NE to control. ‡p<0.001 vs. control and minocycline, ***p<0.001 vs. MCT (n= 6 rats/group). Data are represented as mean ± SEM, analyzed using one-way ANOVA with Newman-Keuls post hoc test. 2-way ANOVA showed significant interactions between MCT and minocycline on LF/HF (p=0.0097) and plasma NE (p=0.0007).
Discussion
Our results provide three novel observations: (i) microglial cell activation and neuroinflammation are associated with PH; (ii) increased SNA is associated with vascular remodeling of lung resulting in PH; and (iii) ICV minocycline infusion decreases microglial cells and microglial activation in the PVN, attenuates SNA, and prevents development of PH. Together, these observations support the suggestion that impaired neuronal-microglial interactions may play a previously unrecognized role in the initiation of dysfunctional brain-lung communication in PH.
Data for the involvement of neuroinflammation, predominantly in the PVN, are: a) increased microglial cells and proinflammatory cytokines in MCT-treated rats, and b) concomitant ICV infusion of minocycline decreased activation of microglial cells, reduced SNA and improved cardiopulmonary functional parameters to attenuate PH. Minocycline is a broad-spectrum anti-inflammatory antibiotic with rather selective effects to inhibit microglial cells in brain10,17. Minocycline easily crosses the blood-brain barrier after oral administration and exerts neuroprotective effects in several diseases involving activated microglial cells18,19. However, the mechanism of its action remains controversial. Direct roles of minocycline on microglial cells include (i) attenuation of NMDA toxicity by blocking the NMDA-stimulated activation of p38 mitogen-activated protein kinase (MAPK), an inflammatory mediator expressed on the microglial cells19. (ii) Inhibition of a specific p38 MAPK inhibitor and prevention of translocation of 5-lipoxygenase into the nucleus consequently blocking the release of cytokines and neuroinflammation19. Minocycline’s anti-inflammatory effects include inhibition of diverse enzymes (MMPs, phospholipase A2, protein kinase C, cyclooxygenase-2, nitric oxide synthase) and pathways (proinflammatory cytokines, anti-apoptotic effects, cell migration, and chemotaxis)20,21. Indirect effects on astrocytes, oligodendrocytes, and even on neurons have been suggested to contribute to its protective actions22. Thus, anti-PH effects of minocycline on microglial cells likely involve more than one of these signaling pathways. Nonetheless, our observations remain relevant because they are the first demonstration of the involvement of microglial cells in an autonomic brain region in the pathophysiology of PH.
Minocycline exerts strong anti-inflammatory effects, thus the possibility of its direct protective effect on lung inflammation cannot be excluded. It is conceivable that some ICV-administered minocycline diffuses to the peripheral circulation to influence lung vasculature. This view is supported by the observations that minocycline attenuates pulmonary fibrosis in mice23. However, we believe that this is a less likely possibility in our experimental paradigm as we used a significantly lower concentration of minocycline. Furthermore, effective concentrations of systemic minocycline in pulmonary disorders such as pulmonary fibrosis were significantly higher23. In the present study, to ensure that the observed effects were due to ICV infusion of minocycline, we verified cannula placement by microscopic visualization of the tract. Animals whose cannulae were misplaced did not respond to minocycline treatment; their data were not included in the results. Thus, their non-responsiveness argues against a systemic effect of this dose of minocycline. Even so, the possibility of indirect effects of minocycline, such as influence on astrocytes, neurons or peripheral-generated cytokines, can also not be ruled out from this study.
Peripheral inflammation impacts PH pathophysiology since circulating inflammatory markers and infiltration of inflammatory cells have been observed within lung tissue during PH24. However, a role for inflammation within the central nervous system (CNS) in PH has never been investigated. How neuroinflammation is triggered in CNS and modulates SNA to perpetuate PH is quite unclear and under active investigation. Evidence indicate that activated microglia release several proinflammatory cytokines10, and reactive oxygen species25 within the CNS. These inflammatory processes alone or in combination, increase the activity of PVN neurons converging on RVLM and resulted in increased sympathetic outflow to the periphery. Importantly, in a separate study we observed increased activated microglial cells in the PVN of mice with hypoxia-induced PH, unpublished, further supporting the existence of interplay between the CNS and PH.
These observations suggest altered autonomic-lung communication in PH. Evidence for this includes the following: (i) Increased ratio of low and high-frequency components (LF/HF ratio; represents a measure of sympatho-vagal balance) observed in MCT-induced PH rats and (ii) increased plasma NE commonly attributed to increased sympathetic drive. Moreover, these elevated autonomic indices were attenuated by ICV infusion of minocycline.
Our observations, coupled with existing evidence, leads us to propose the following hypothesis: we suggest that diverse pro-pulmonary hypertensive signals (environmental, genetics, etc.) are perceived by PVN neurons whose chemical and physical interactions with resident microglia cells cause their activation. This activated neuronal-microglial unit, with increased cytokines, neuronal activity, etc., increases SNA to the lung, increases inflammatory status, and imbalances plasma metabolites and cytokines. Together, these products impact the lung vasculature, leading to pulmonary pathophysiology associated with PH. Of course, this is a working hypothesis, but our study presents key data to support the concept.
However, many more questions remain for further investigation. They are: (i) Is the activity of pulmonary sympathetic nerves altered in PH? (ii) Do different autonomic brain regions integrate to mediate effects on pulmonary pathophysiology, and by what mechanism? (iii) Which actions of minocycline protect in PH, antibiotic, anti-inflammatory, or both? (iv) Is activation of microglial cells in the PVN due to direct effect of MCT or indirectly via MCT-stimulated inflammatory mediators? This seems rather unlikely since there is no clear evidence of blood brain barrier permeability of MCT. (v) The MCT rat model used in this study was selected because it represents a complex multi-organ disease process that culminates in PH and RHF, a concept that we have attempted to test here. However, we acknowledge its limitations and the brain-ANS-lung hypothesis must be investigated with other models of PH and in human PH. Nonetheless, lacking answers to these questions now should not minimize the importance of our novel observations, which provide the prerequisites to evaluate translational implications of disordered brain-ANS-lung communication in PH patients.
Perspectives
Significant evidence exist explaining lung pathophysiology in PH, such as endothelial cell dysfunction, smooth muscle cell proliferation, vascular inflammation, immune dysregulation and certain genetic determinants. Most current therapies are centered on these mechanisms. Despite considerable advances, the survival of PH patients remains poor. We considered whether PH is a disease with complex interactions of many organ systems ultimately affecting lungs. The present study demonstrated that central administration of minocycline, an anti-inflammatory antibiotic and inhibitor of microglial cells, inhibited microglial activation, reduced neuroinflammation, restored autonomic imbalance and prevented PH-associated pathologies in the MCT-induced rat model of PH. Thus, these results may present novel therapeutic targets for PH treatment.
Supplementary Material
Novelty and Significance.
What Is New?
Activation of microglial cells and neuroinflammation in the PVN region of the hypothalamus is involved in the pathophysiology of PH.
Inhibition of microglial cells by ICV administration of minocycline produces beneficial outcomes on pathophysiology of the cardiopulmonary system to attenuate PH by reducing sympathetic outflow.
Suggests dysfunctional brain-ANS-lung communication in PH.
What is relevant?
The first evidence that dysfunctional neuronal-microglial communication in higher nervous centers may be responsible for the autonomic imbalance that perpetuates PH.
Summary
PH is associated with neuroinflammation accompanied by autonomic imbalance. This suggests dysfunctional brain-ANS-lung interactions during PH. Minocycline treatment prevents these anomalies and provides favorable outcomes by inhibiting microglial activation and neuroinflammation in the PVN of the hypothalamus. Therapies targeting neuroinflammation may present a promising strategy to treat PH.
Acknowledgments
Source of funding: This study was supported by National Institute of Health grant HL102033 to M.K. Raizada.
Footnotes
Disclosure: None
References
- 1.Gurtu V, Michelakis ED. A Paradigm Shift Is Needed in the Field of Pulmonary Arterial Hypertension for Its Entrance Into the Precision Medicine Era. Circ Res. 2016;119:1276–1279. doi: 10.1161/CIRCRESAHA.116.309689. [DOI] [PubMed] [Google Scholar]
- 2.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–57. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 3.Kishi T. Heart failure as an autonomic nervous system. J Cardiol. 2012;59:117–22. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Velez-Roa S, Ciarka A, Najem B, Vachiery JL, Naeije R, van de Borne P. Increased sympathetic nerve activity in pulmonary artery. Circulation. 2004;110:1308–12. doi: 10.1161/01.CIR.0000140724.90898.D3. [DOI] [PubMed] [Google Scholar]
- 5.Mak S, Witte KK, Al-Hesayen A, Granton JJ, Parker JD. Cardiac sympathetic activation in patients with pulmonary arterial hypertension. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1153–7. doi: 10.1152/ajpregu.00652.2011. [DOI] [PubMed] [Google Scholar]
- 6.Ciarka A, Doan V, Velez-Roa S, Naeije R, van de Borne P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181:1269–75. doi: 10.1164/rccm.200912-1856OC. [DOI] [PubMed] [Google Scholar]
- 7.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa M, Sato N, Asai K, Takano T, Mizuno K. Effects of a pure alpha/beta-adrenergic receptor blocker on monocrotaline-induced pulmonary arterial hypertension with right ventricular hypertrophy in rats. Circ J. 2009;73:2337–41. doi: 10.1253/circj.cj-09-0213. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki R, Maehara R, Kobuchi S, Tanaka R, Ohkita M, Matsumura Y. Beneficial effects of γ-aminobutyric acid on right ventricular pressure and pulmonary vascular remodeling in experimental pulmonary hypertension. Life Sci. 2012;91:693–8. doi: 10.1016/j.lfs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreas S, Haarmann H, Klarner S, Hasenfuss G, Raupach T. Increased sympathetic nerve activity in COPD is associated with morbidity and mortality. Lung. 2014;192:235–41. doi: 10.1007/s00408-013-9544-7. [DOI] [PubMed] [Google Scholar]
- 12.Heindl S, Lehnert M, Criée CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med. 2001;164:597–601. doi: 10.1164/ajrccm.164.4.2007085. [DOI] [PubMed] [Google Scholar]
- 13.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 14.Choudhary RC, Sharma RK, Gulati K, Ravi K. Role of the paraventricular the reflex diuresis to pulmonary lymphatic obstruction in rabbits. Can J Physiol Pharmacol. 2016;94:18–27. doi: 10.1139/cjpp-2015-0109. [DOI] [PubMed] [Google Scholar]
- 15.Rigatto K, Casali KR, Shenoy V, Katovich J, Raizada MK. Diminazene aceturate improves autonomic modulation in hypertension. Eur J Pharmacol. 2013;713:89–93. doi: 10.1016/j.ejphar.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilzendeger AM, Shenoy V, Raizada MK, Katovich MJ. Neuroinflammation in pulmonary hypertension: concept, facts, and relevance. Curr Hypertens Rep. 2014;16:469. doi: 10.1007/s11906-014-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–52. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilakaratne A, Soory M. Anti-inflammatory Actions of Adjunctive Tetracyclines and Other Agents in Periodontitis and Associated Comorbidities. Open Dent J. 2014;8:109–24. doi: 10.2174/1874210601408010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orsucci D, Calsolaro V, Mancuso M, Siciliano G. Neuroprotective effects of tetracyclines: molecular targets, animal models and human disease. CNS Neurol Disord Drug Targets. 2009;8:222–31. doi: 10.2174/187152709788680689. [DOI] [PubMed] [Google Scholar]
- 22.Möller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, Eder C, Gan L, Garden GA, Hughes ZA, Pearse DD, Staal RG, Sayed FA, Wes PD, Boddeke HW. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia. 2016;64:1788–94. doi: 10.1002/glia.23007. [DOI] [PubMed] [Google Scholar]
- 23.Kalemci S, Dirican N, Cetin S, Sözen H, Uner AG, Yaylali A, Aksun S, Karacam V, Ulger E, Sütcü R, Dirican A. The efficacy of minocycline against methotrexate-induced pulmonary fibrosis in mice. Eur Rev Med Pharmacol Sci. 2013;17:3334–40. [PubMed] [Google Scholar]
- 24.Groth A, Vrugt B, Brock M, Speich R, Ulrich S, Huber LC. Inflammatory cytokines in pulmonary hypertension. Respir Res. 2014;15:47. doi: 10.1186/1465-9921-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao HL, Yu XJ, Liu KL, Shi XL, Qi J, Chen YM, Zhang Y, Bai J, Yi QY, Feng ZP, Chen WS, Cui W, Liu JJ, Zhu GQ, Kang YM. PVN Blockade of p44/42 MAPK Pathway Attenuates Salt-induced Hypertension through Modulating Neurotransmitters and Attenuating Oxidative Stress. Sci Rep. 2017;7:43038. doi: 10.1038/srep43038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.