Abstract
How distinct are visual memory representations from visual perception? Although evidence suggests that briefly remembered stimuli are represented within early visual cortices, the degree to which these memory traces resemble true visual representations remains something of a mystery. Here, we tested whether both visual memory and perception succumb to a seemingly ubiquitous neural computation: normalization. Observers were asked to remember the contrast of visual stimuli, which were pitted against each other to promote normalization either in perception or in visual memory. Our results revealed robust normalization between visual representations in perception, yet no signature of normalization occurring between working memory stores—neither between representations in memory nor between memory representations and visual inputs. These results provide unique insight into the nature of visual memory representations, illustrating that visual memory representations follow a different set of computational rules, bypassing normalization, a canonical visual computation.
Keywords: visual memory, normalization, visual perception, psychophysics
Visual memory allows us to briefly retain information we have just seen, despite the fact that we constantly experience rapid, moment-to-moment changes in visual inputs. What are the qualitative properties of representations stored within visual memory? A prevailing theory, the sensory recruitment hypothesis, posits that the retention of visual memories involves maintenance of visual information within visual cortices in the absence of visual input (Christophel, Klink, Spitzer, Roelfsema, & Haynes, 2017; Harrison & Tong, 2009; Offen, Schluppeck, & Heeger, 2009; Pasternak & Greenlee, 2005; Serences, Ester, Vogel, & Awh, 2009). Indeed, the contents of visual memory appear to share some properties in common with true visual representations (Harrison & Tong, 2009; Pasternak & Greenlee, 2005; Serences et al., 2009; Sneve, Alnæs, Endestad, Greenlee, & Magnussen, 2011; Supèr, Spekreijse, & Lamme, 2001; Tanaka & Sagi, 1998; Xing, Ledgeway, McGraw, & Schluppeck, 2013). For instance, neuroimaging studies have demonstrated that information regarding the remembered stimulus is still evident in the ensemble pattern of activity residing within striate cortex—so much so that training a classifier on true visual stimuli allows for reasonable generalization of classification to patterns of voxel activity corresponding to the remembered orientation (Harrison & Tong, 2009) or contrast (Xing et al., 2013), suggesting that visual memory and visual perception share a representational structure. However, it remains unknown whether representations stored within visual memory function like visual representations.
To address this, we tested whether visual memory representations abide by the same rules as visual perception, examining the degree to which representations in visual memory undergo one of the most essential computations that supports perception: divisive normalization. Under divisive normalization, the neural response to a stimulus is attenuated by the presence of neighboring responses (Carandini & Heeger, 2012; Heeger, 1992). Models of normalization have long served as cornerstone principles for computational accounts of early vision (Carandini & Heeger, 2012; Heeger, 1992; Ling & Blake, 2012) and have been shown to generalize to a variety of other sensory modalities and cognitive processes (Rabinowitz, Willmore, Schnupp, & King, 2011; Rangel & Clithero, 2012), suggesting that normalization may serve as a canonical neural computation (Carandini & Heeger, 2012). Interestingly, apparently unrelated modulatory processes, such as attention, have been theorized to act by co-opting the same neural machinery to alter the relative gain of responses to selected information (Herrmann, Montaser-Kouhsari, Carrasco, & Heeger, 2010; Reynolds & Heeger, 2009). Does normalization act on visual memories?
To examine whether the contents of visual memory undergo contrast normalization, we leveraged a classic demonstration of this computation in action within primary visual cortex: center-surround suppression. With center-surround suppression, the response to a stimulus is dampened by adding additional stimulation in its surrounding region, which has been shown to be linked to decreases in perceived contrast (Shushruth et al., 2013; Xing & Heeger, 2001; Zenger-Landolt & Heeger, 2003)—an interaction that emerges naturally from divisive normalization. Another trademark of divisive normalization is its feature-tuned nature, whereby stimuli with similar features suppress each other’s response more so than those with dissimilar features, implying that surround suppression is mediated by orientation-specific inhibitory interactions within early visual areas (Shushruth et al., 2013). If visual perception and visual memory truly succumb to the same neural computations, presenting stimulation in the surrounding region of an item retained in memory should also attenuate its remembered contrast. Evidence for center-surround suppression in memory would indicate that visual memory representations are pooled by normalization, much like visual representations are.
In Experiment 1, we investigated the degree to which surrounding visual stimulation can influence an actively maintained visual memory representation of a center contrast stimulus. To test for normalization within visual perception, we presented the surround stimulus simultaneously with the center stimulus (simultaneous condition), while to test normalization within visual memory, this surrounding stimulus was instead presented sequentially, during the maintenance interval (sequential condition). We observed surround suppression only when center and surround were presented simultaneously during visual encoding; visual memory representations were left unaffected by the potentially normalizing influence of a surrounding stimulus presented during retention. In Experiment 2, we tested whether normalization operates between multiple representations stored within visual memory. To do so, we tested the degree to which representations stored in visual memory compete with each other by asking observers to retain a visual memory of both the center and surround stimulus, which were presented either simultaneously or sequentially. We again found suppression when center and surround were presented simultaneously but no signature of contrast normalization between representations of sequentially presented stimuli stored in visual memory, suggesting that visual memory representations do not interact like true visual representations. Taken together, these results suggest that visual memory fails to take advantage of a neural computation that could potentially mediate between competing neural representations—results that are striking considering the limited capacity of visual memory (Alvarez & Cavanagh, 2004; Todd & Marois, 2004).
Experiment 1: Normalization Between Visual Memory and Vision
Method
Observers
Twelve healthy adult volunteers between the ages of 20 and 31 years (6 female; mean age = 24.1), with normal or corrected-to-normal vision, participated in Experiment 1. A minimum sample size of 12 was chosen a priori on the basis of sample sizes of comparable studies (Kiyonaga & Egner, 2016; Xing & Heeger, 2001), and a power calculation illustrated that the current sample size yielded a statistical power greater than 90%. All observers provided written informed consent and were reimbursed for their time. The Boston University Institutional Review Board approved the study.
Stimuli
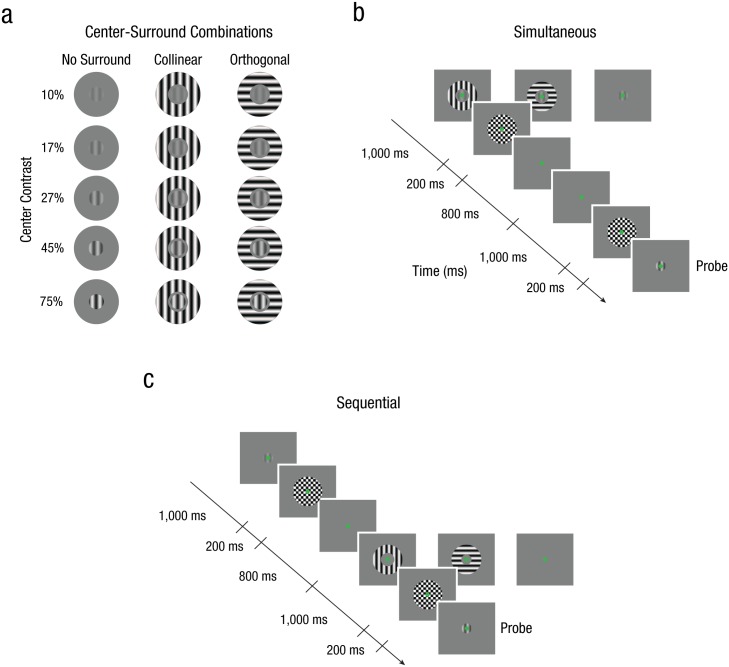
Stimuli were generated using MATLAB (Release 2013b; The MathWorks, Natick, MA) in conjunction with the Psychophysics Toolbox (Pelli, 1997), rendered on a PC running Ubuntu 14.04 LTS, and presented on a gamma-corrected CRT monitor (1,400- × 1,050-pixel resolution; 60 Hz refresh rate). Observers were placed comfortably with their heads in a chin rest at a viewing distance of 68 cm from the screen and were instructed to maintain steady fixation throughout all experimental trials. Stimuli consisted of foveally presented oriented gratings (spatial frequency = 3 cycles/°; randomized spatial phase) on a uniform gray background (mean luminance = 52.05 cd/m2). In each trial, the center stimulus (subtending 1° of visual angle) had a random orientation (between 1° and 180°) and varied from trial to trial in its contrast (five contrast levels, linearly spaced on a log scale between 10% and 75% Michelson contrast; Fig. 1a).
Fig. 1.
Stimuli and example trial sequences from Experiment 1. Each stimulus (a) was composed of one of three different surround configurations at five different center contrast levels (10%–75% contrast). Example trial sequences are shown for the simultaneous (b) and the sequential (c) conditions. Observers viewed a center stimulus for 1,000 ms, which varied from trial to trial in contrast and orientation. In both conditions, observers were required to match the contrast of the probe to the remembered center stimulus after a 2,200-ms retention interval. During the simultaneous condition, the center stimulus was enveloped by a full-contrast surround stimulus, which had orientation content that was either collinearly or orthogonally oriented to the center. In the sequential condition, this surround stimulus was moved into the retention interval. After every interval in which a stimulus could appear, a counterphase flickering, full-contrast checkerboard masking stimulus was presented to reduce any lingering afterimages. Stimuli are modified for illustrative purposes.
Depending on the experimental condition, an oriented surround stimulus (spatial frequency = 3 cycles/°; inner diameter = 1.08°; outer diameter = 3°; randomized spatial phase; 100% Michelson contrast) was presented either simultaneously or sequentially with the center stimulus. The sequential condition was constructed to ensure that any normalization-driven suppression we may observe was not simply due to suppression during perceptual encoding but instead due to normalization during visual memory retention. To ensure that the stimulus presentation would not cause any lingering afterimages, we always directly followed the presentation of both center and surround stimuli with a brief, counterphase flickering, full-contrast checkerboard masking stimulus (diameter = 3°; presented for 200 ms at 40 Hz).
Procedure
Behavioral performance was measured by means of a method-of-adjustment contrast replication task. Throughout both the simultaneous and sequential conditions, the general outline of the task was the same (Figs. 1b and 1c). First, a randomly oriented center grating target was presented for 1,000 ms, and observers were asked to remember the contrast of this grating. After a retention interval (2,200 ms), we presented a probe grating that matched the orientation of the center grating but differed in spatial phase and contrast intensity. Note that in both conditions, the maintenance duration of the center contrast was identical. Presentation of each stimulus was followed by a brief, full-contrast checkerboard stimulus (for 200 ms at 40 Hz) to ensure that the center stimulus did not evoke a negative afterimage. Observers were asked to manually operate a knob (PowerMate; Griffin Technology, Nashville, TN) to match the contrast of the probe to the contrast of the center stimulus held in memory. Once satisfied with the replicated contrast, observers proceeded to the next trial.
There were no time constraints for responses (mean duration = 3.08 s, SD = 1.13 s); instead, the precision of replication performance was stressed throughout the experiment. Observers were required to practice the task before the start of the experiment to get acquainted with the knob. For each of the two experimental conditions (simultaneous and sequential), observers performed a total of six runs of 120 trials (~15 min) each, resulting in 48 repetitions for each contrast-surround configuration. Observers participated in four sessions of data collection, with each session occurring on separate days. Within a session, only one of the two experimental conditions was tested, and the order of the experimental conditions over sessions was counterbalanced across observers.
Simultaneous condition
In the simultaneous condition, we examined the influence of divisive normalization on perceived contrast by introducing a full-contrast surround stimulus (100% Michelson contrast), which was presented simultaneously with the center grating. This surrounding stimulus could have the same orientation as the center (collinear condition) or could be oriented 90° relative to the center grating’s orientation (orthogonal condition; Fig. 1a). Observers were instructed that the surrounding stimuli were irrelevant and that they should attend to and remember only the center stimulus’s contrast. Trials without the presentation of a surrounding stimulus were interleaved throughout the experiment in order to obtain a baseline measure (no-surround condition) for contrast-replication precision, matching the total duration of a trial sequence (Fig. 1b).
Sequential condition
In the sequential condition, we examined whether a visual memory representation can undergo normalization similarly to perception by moving the full-contrast surround stimulus into the retention interval. As in the simultaneous condition, the surround could be collinearly or orthogonally oriented relative to the center grating but was presented 1,000 ms after the offset of the center stimulus and was displayed for 1,000 ms. Observers were told that the surrounding stimuli were irrelevant and were instructed to focus only on retaining the center stimulus’s contrast. To obtain a baseline measure (no-surround condition) for contrast-replication precision, we also measured perceived contrast of the center stimulus in the absence of the surrounding stimulus during the retention interval (Fig. 1c).
Model-fitting procedure
Perceived contrast of the center stimulus in both the simultaneous and sequential conditions was formalized within the normalization framework. The normalization model proposes that the neural response to a stimulus is comprised of an excitatory component that is divided by an inhibitory component (Carandini & Heeger, 2012; Heeger, 1992). We assumed that perceived contrast scales proportionally to the signal-to-noise ratio of the underlying contrast response function (Herrmann et al., 2010; Ling & Blake, 2012). Specifically, changes in the neural contrast response function under this framework directly impact an observer’s perceived contrast for a stimulus. The neural response to an isolated center stimulus, Ra, can be formally expressed as
where ca corresponds to the center-stimulus contrast in the absence of a surround stimulus, C50 is the inflection point of the response function, and n represents the nonlinear transducer, determining the steepness of the function.
We extended Equation 1 to include surround suppression, as described in previous work (Xing & Heeger, 2001). The neural response to the test center stimulus when enveloped by a surround stimulus, Rt, can be formally expressed as
where ct corresponds to the center stimulus contrast, cs is the contrast of the surround stimulus (here fixed to 100% contrast), and γ is a parameter that represents the degree of normalization induced by the surround.
In order to fit our data, we assumed that the underlying contrast response for the center stimulus in the no-surround and surround conditions was equal, with only γ free to describe the influence of the surround on perceived center contrast. We used MATLAB’s fminsearch function to optimize the parameter estimates for C50, n, and γ, using nonlinear regression, for each individual observer in the simultaneous and sequential conditions independently. The fitting procedure was performed concurrently for all surround conditions using the no-surround condition to estimate C50 and n and two independent γ parameters to capture the differences in normalization evoked by either the collinear or orthogonal surround conditions.
Results
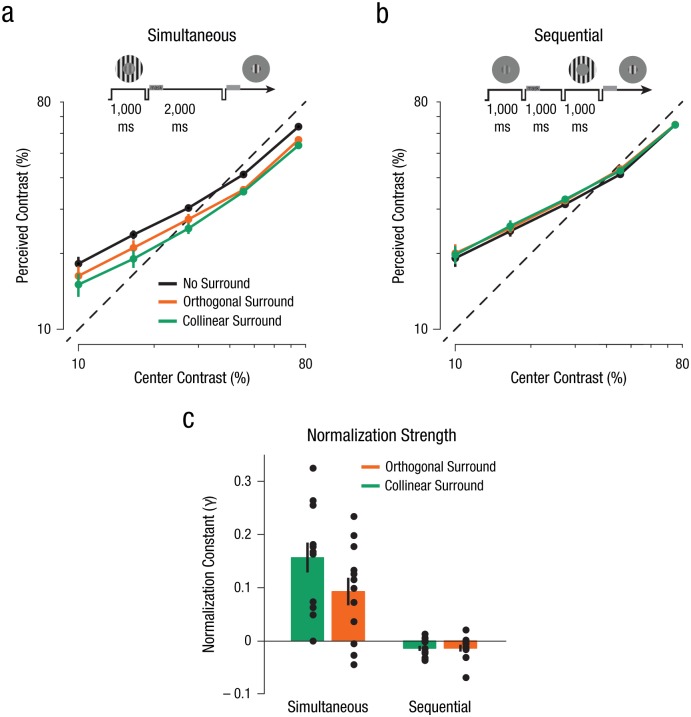
We first confirmed that the contrast of a stimulus could be reliably retained within visual memory by analyzing contrast estimates in the no-surround condition. Observers’ subjective reports of the center contrast retained in visual memory in the absence of a surround stimulus were near veridical: Measures of apparent contrast closely matched the objective contrast of the stimulus, albeit with a slight bias; specifically, lower contrasts were remembered as slightly higher than reality, and higher contrasts were remembered as slightly lower (Figs. 2a and 2b).
Fig. 2.
Results from Experiment 1. Perceived contrast of the center stimuli is shown separately for the (a) simultaneous and (b) sequential conditions. Observers’ estimates of the center stimulus contrast were near veridical (indicated by the dashed line). Data points reflect the apparent contrast estimates across all contrast levels, averaged over observers (N = 12), for the three different surround conditions (collinear, orthogonal, and no surround). Error bars denote ±1 SEM (note that in some cases the error bars are smaller than the data points). Schematics above the graphs illustrate the general experimental design. Normalization strength estimates (c) were derived from the normalization model. Parameter estimates illustrate the influence of the surround (collinear and orthogonal) on perceived contrast of the center stimulus for both the simultaneous and sequential conditions (see the Supplemental Material available online for additional parameter estimates). Error bars denote ±1 SEM.
When the center grating was simultaneously enveloped by a surrounding stimulus, we found a substantial suppression of the center’s remembered contrast across all contrast levels (Fig. 2a)—the signature of normalization-driven surround suppression within early visual areas. This attenuation in apparent contrast was evident both when the orientation content of the surrounding stimulus matched that of the center (collinear condition), as well as when the surround orientation content did not match (orthogonal condition). We found that the magnitude of perceptual suppression depended on the match between the center and surround stimuli; the collinear condition engendered stronger suppression than the orthogonal condition (Fig. 2a; see also Figs. S1 and S3a in the Supplemental Material available online).
The previous results established that our stimuli configurations gave rise to multiple signatures of divisive normalization when presented simultaneously. However, does a visual memory representation of the actively maintained center contrast also succumb to contrast normalization when a surround stimulus is instead presented during the retention interval? Our results revealed that the presence of the surround stimulus during the retention interval did not have an effect on the remembered contrast of the center stimulus, either for the collinear or orthogonal configurations (Fig. 2b; see also Figs. S2 and S3a in the Supplemental Material); however, the precision of responses was highly comparable between the simultaneous and sequential conditions (Fig. S5 in the Supplemental Material). In a separate experiment, we confirmed that differences in the timing of the onset of the surround stimulus between the simultaneous and sequential conditions did not influence the differences in suppression between these conditions (Fig. S3b in the Supplemental Material).
Fig. 3.
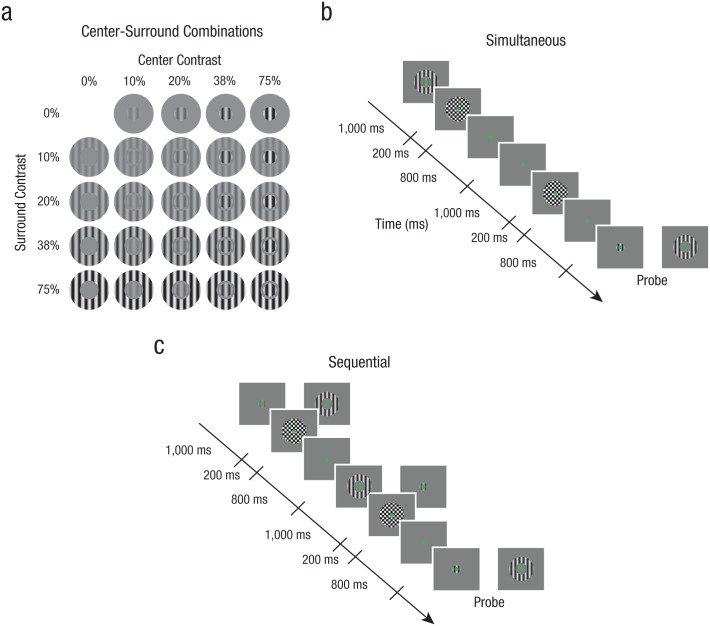
Stimuli and example trial sequences from Experiment 2. Stimuli (a) were composed of a center and a surround stimulus that both varied in contrast. Each component could be one of four contrast levels (10%–75% contrast). Example trial sequences are shown for the simultaneous (b) and sequential (c) conditions. The contrast of both center and surround stimuli had to be remembered, and after a retention period, observers were asked to match the contrast of the probe to either the center or surround that had been held in memory. Counterphase flickering, full-contrast masks were presented to reduce any lingering afterimages. Stimuli are modified for illustrative purposes.
To quantify the degree of normalization brought about by the surround in both the simultaneous and sequential conditions, we fitted the perceived contrast estimates with a variant of the normalization model (Carandini & Heeger, 2012; Heeger, 1992; Xing & Heeger, 2001; see Equation 2). The model fitted well to all our individual observers’ data (mean R2 = .92, SD = .03; Fig. S4 in the Supplemental Material), capturing the slight compression of perceived contrast for visual stimuli, as well as the suppression in the presence of the surround. Normalization strength, as indexed by the normalization constant, γ, differed substantially when the surround was presented simultaneously or sequentially. These results were confirmed by utilizing a paired-samples t test for both the collinear surround, t(11) = 6.37, p < .001 (95% confidence interval, CI = [0.11, 0.23], d = 1.84), and the orthogonal surround, t(11) = 4.57, p = .001 (95% CI = [0.06, 0.16], d = 1.32).
Specifically, the model fits revealed that with competing visual stimulation in the simultaneous condition, normalization strength, γ, was substantially greater than zero across our observers (Fig. 2c; see also Fig. S4). Right-tailed one-sided t tests and Jeffreys-Zellner-Siow Bayes factors (JZS BFs; BayesFactor package for R; see Morey & Rouder, 2011) confirmed these results for both the collinear surround, t(11) = 5.63, p < .001 (95% CI = [0.11, ∞], d = 1.63, estimated JZS BF10 = 399.46), and the orthogonal surround, t(11) = 3.61, p = .002 (95% CI = [0.05, ∞], d = 1.04, estimated JZS BF10 = 24.42). Moreover, normalization strength, γ, was greater in the collinear surround condition compared with the orthogonal surround condition, confirming orientation-tuned divisive normalization for visual representations, t(11) = 5.32, p < .001 (paired-samples t test, 95% CI = [0.04, 0.09], d = 1.53).
However, when fitting the normalization model to the sequential conditions, we found no evidence for suppression, indicated by the normalization constant, γ, between memory stores and visual inputs (Fig. 2c; see also Fig. S4). Right-tailed one-sided t tests confirmed these results for both the collinear surround, t(11) = −2.80, p = .99 (95% CI = [−0.02, ∞], d = −0.81, estimated JZS BF10 = 0.10, JZS BF20 = 7.49), and the orthogonal surround, t(11) = −2.07, p = .97 (95% CI = [−0.03, ∞], d = −0.60, estimated JZS BF10 = 0.11, JZS BF20 = 2.67). The right-tailed one-sided t test was motivated by our a priori hypothesis that normalization should suppress perceived contrast of the center. While the visual memory condition hints toward a subtle increase in perceived contrast, this is not in agreement with divisive normalization and might reflect an attractor bias toward the irrelevant surround stimulus presented during the maintenance period, a memory bias that has been observed for other visual features (Rademaker, Bloem, De Weerd, & Sack, 2015). Furthermore, there was no signature of orientation-tuned normalization, t(11) = −0.03, p = .979 (paired-samples t test, 95% CI = [−0.01, 0.01], d = −0.01).
Fig. 4.
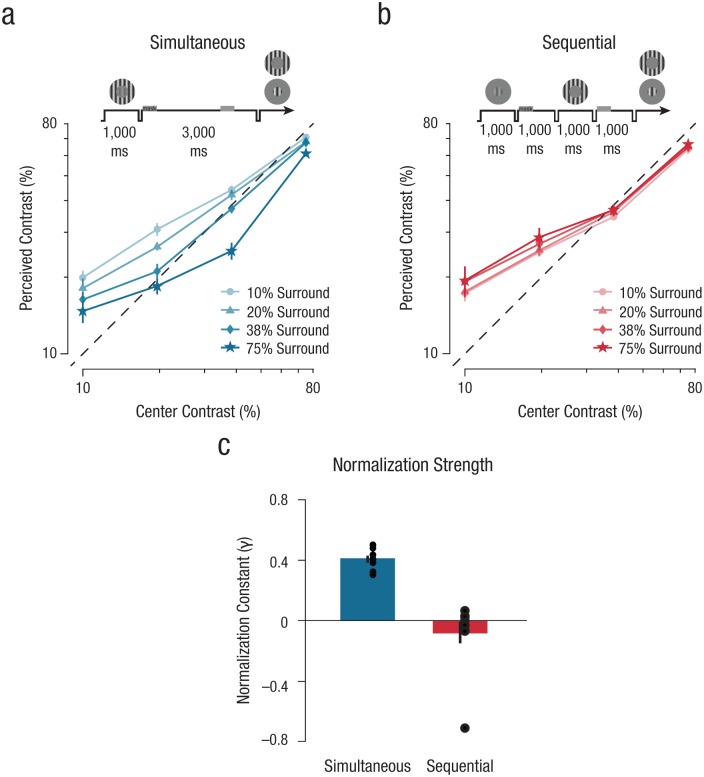
Results from Experiment 2. Perceived contrast of the center stimuli is shown separately for the (a) simultaneous and (b) sequential conditions. Data points reflect the apparent center contrast estimates across all contrast levels, averaged over observers (N = 10), for each surround contrast condition (10%–75% surround). Dashed black lines indicate veridical contrast estimation. Error bars denote ±1 SEM (note that in some cases the error bars are smaller than the data points). Schematics above the graphs illustrate the general experimental design. Normalization strength estimates (c) were derived from the normalization model. Parameter estimates illustrate the influence of the surround on perceived contrast of the center stimulus for both the simultaneous and sequential conditions (perception = blue; visual memory = red; see the Supplemental Material for additional parameter estimates). Error bars denote ±1 SEM.
Discussion
Experiment 1 suggests that visual memory representations appear immune to divisive normalization induced by visual stimulation during retention. However, it is possible that our visual memory condition did not elicit normalization because observers could ignore the sequentially presented stimulus. Previous work has shown that only attended memory representations elicit a decodable neural representation, suggesting that different attentional states might have different mechanisms supporting the memory representations (LaRocque, Riggall, Emrich, & Postle, 2016). While Experiment 1 showed that normalization may not occur between visual memory representations and visual inputs, it is possible that normalization operates between two attended visual memories stored within early visual areas. In our second experiment, we set out to test this hypothesis, asking whether multiple memory representations that are stored within early visual areas undergo normalization-driven competition.
Experiment 2: Normalization Between Visual Memory Representations
Method
Observers
Ten observers between the ages of 20 and 31 years (5 female; mean age = 26.1), including 7 who had previously participated in Experiment 1, participated in Experiment 2. All had normal or corrected-to-normal vision. A minimum sample size of 10 was chosen a priori on the basis of sample sizes of comparable studies (Kiyonaga & Egner, 2016; Xing & Heeger, 2001); furthermore, a power calculation illustrated that the current sample size yielded a statistical power greater than 90%. All observers provided written informed consent and were reimbursed for their time. The Boston University Institutional Review Board approved the study.
Stimuli
Stimuli were similar to those used in Experiment 1, except that now both center and surround stimuli varied from trial to trial in contrast (four contrast levels linearly spaced on a log scale between 10% and 75% Michelson contrast, randomized orientation and spatial phase), and the surround was always collinearly oriented to the center stimulus. As in Experiment 1, the presentation of both center and surround stimuli was always directly followed by a brief, counterphase flickering, full-contrast checkerboard masking stimulus (diameter = 3°; presented for 200 ms at 40 Hz).
Procedure
Throughout Experiment 2, the general outline of the task was similar to that of Experiment 1 (Figs. 3b and 3c). Center and surround components could be presented simultaneously or sequentially, but here both components varied in contrast from trial to trial (Fig. 3a) and had to be remembered. After a retention interval (3,000 ms), a probe grating was presented at a random initial contrast and spatial phase, which cued observers to match the contrast of the probe to either the center or surround held in memory. As in Experiment 1, there were no time constraints for responses (mean duration = 2.92 s, SD = 0.76 s), and the precision of replication performance was stressed throughout the experiment. For each of the two experimental conditions (simultaneous and sequential), observers performed a total of nine runs of 80 trials (~10 min) each, resulting in 18 repetitions for each contrast configuration. The data were collected over 4 testing days, two separate sessions for each experi-mental condition, the order of which was counterbalanced across observers.
Simultaneous condition
In the simultaneous condition, we examined the influence of surround contrast on the perceived contrast of the center stimulus, as well as the influence of center contrast on the perceived contrast of the surround stimulus, by asking observers to maintain representations of both the center and surround contrast in visual memory. Center and surround were presented simultaneously at the start of a trial, and observers did not know until the appearance of the subsequent probe (retention interval = 3,000 ms) which of the two they would be asked to replicate (Fig. 3b). Additionally, we measured perceived contrast for the presentation of each component individually in order to compare any differences in observers’ ability to replicate the center or surround contrast in isolation while maintaining identical retention-interval durations.
Sequential condition
In the sequential condition, we examined whether normalization governs competition within visual memory by presenting the center and surround components sequentially, carefully counterbalancing the order of appearance (Fig. 3c). One of the two components appeared at the trial onset for 1,000 ms, while the second component was moved 1,000 ms into the retention interval (identical to Experiment 1’s sequential condition). The probe appeared after an additional 1,000 ms, allowing observers to match the probe contrast to either the center or surround stimulus. Trials with only the presentation of an individual component at the start of the trial were interleaved throughout the experiment in order to compare any differences in observers’ ability to replicate the center or surround contrast.
Model-fitting procedure
We modeled the interaction between center and surround contrast when both were held in visual memory by using the same model as in Experiment 1 (Equation 2). For each individual observer, we used MATLAB’s fminsearch function to optimize the parameter estimates for C50, n, and γ for the simultaneous and sequential conditions independently. The fitting procedure was performed concurrently for all surround contrast conditions when the center stimulus was probed, and likewise, a separate fitting procedure was performed for all center contrast conditions when the surround stimulus was probed (however, note that in this experiment ct represented the surround contrast and cs the center contrast).
Results
Consistent with Experiment 1, results showed that observers’ subjective reports of the center contrast were near veridical in the absence of a surround stimulus. The normalization model parameters estimated in Experiment 1 were able to explain more than 90% of the variance of the perceived contrast estimates in Experiment 2, confirming that the increase in retention duration did not influence memory fidelity (Fig. S6 in the Supplemental Material).
Turning next toward the visually competing perception condition, we found evidence for divisive normalization when center and surround were presented simultaneously. The perceived contrast of the center stimulus gradually decreased as a function of the surround contrast (Fig. 4a; see also Fig. S7 in the Supplemental Material). Similarly, as the center increased in contrast, the perceived contrast of the surround decreased as well (Fig. S8 in the Supplemental Material). This modulatory effect was greatest for mid-to-low contrasts, which is consistent with previous findings that have been obtained using similar configurations to test perceptual normalization (Xing & Heeger, 2001). We once again found reliable fits of this data with the normalization model (Equation 2; mean R2 = .91, SD = .10, Fig. S9 in the Supplemental Material): Normalization strength, as indexed by the normalization constant, γ, differed substantially depending on whether the stimuli were presented simultaneously or sequentially, t(9) = 5.87, p < .001 (paired-samples t test, 95% CI = [0.30, 0.68], d = 1.86). Specifically, the normalization constant, γ, was greater than zero across observers when center and surround were presented simultaneously (Fig. 4c), t(9) = 17.45, p < .001 (right-tailed one-sample t test, 95% CI = [0.37, ∞], d = 5.52, estimated JZS BF10 = 666961.7).
We then turned to the sequential condition to examine whether normalization governs competition between representations stored in visual memory. Observers produced near-veridical reports of remembered contrasts for both the center and surround in this condition (Fig. 4b; see also Figs. S7 and S8). To quantify these results, we fitted the normalization model to the data in the sequential condition and found that the normalization constant, γ, was near zero across all observers in this condition (Fig. 4c), t(9) = −1.08, p = .846 (right-tailed one- sample t test, 95% CI = [−0.21, ∞], d = −0.34, estimated JZS BF10 = 0.171, JZS BF20 = 0.822). When the two stimuli were both attended, there was no indication of an increase in perceived contrast (see Experiment 1), indicating no normalization between memory stores. The model was also able to capture the mutual inhibitory effects that the center stimulus had on the perceived contrast of the surround, demonstrating multiple signatures of normalization-driven suppression in the simultaneous condition and the lack of normalization in the sequential condition (Figs. S8 and S10 in the Supplemental Material).
General Discussion
Visual memory is essential for human behavior, allowing us to actively retain representations of our visual environment after this information can no longer be sensed directly. Here, we tested whether visual memory representations abide by the same rules as perception, examining whether they succumb to divisive normalization. Experiment 1 illustrated that while divisive normalization exercises potent suppression among visual information, visual memory stores may be exempt from this normalization-driven suppression. While observers’ memory for contrast was of reasonably high fidelity (Pasternak & Greenlee, 2005; Tanaka & Sagi, 1998), visual memories appear to have been computationally segregated from visual representations, curtailing contrast normalization between visual information and remembered information. Experiment 2 demonstrated no signature of normalization between visual representations stored in memory, suggesting that visual memories do not compete with each other within early visual areas in the same manner as true visual representations. Taken together, our results point toward a key distinction between visual representations and memory representations—a matter of active debate (e.g., Serences, 2016; Xu, 2017). While visual memory representations modulate activity within early visual cortices (Harrison & Tong, 2009; Serences et al., 2009; Xing et al., 2013), they follow a different set of computational rules, bypassing contrast normalization.
While a growing body of work on visual memory suggests that memory representations have some characteristics that are akin to visual representations, there is reason to believe that these memories are distinct from true visual representations. For instance, although information regarding the remembered stimulus is evident in the ensemble pattern of activity residing within striate cortex (Harrison & Tong, 2009; Xing et al., 2013), the mean functional MRI blood-oxygen-level-dependent response for remembered stimuli exhibits very weak signals of the remembered stimulus (Harrison & Tong, 2009; Xing et al., 2013). Furthermore, while some work has shown that visual memory representations for features are bound to spatial location, exhibiting a retinotopic organization in striate cortex (Pratte & Tong, 2014; Sneve et al., 2011), other work has suggested that visual memories may not remain at the remembered-stimulus location, instead spreading its patterning across retinotopic space, much like feature-based attention (Ester, Serences, & Awh, 2009; Treue & Maunsell, 1999). Indeed, while early visual areas may support visual memory representations, these representations may be distributed across the cortex, rendering them somewhat quarantined from incoming visual information (Christophel et al., 2017).
Recently, normalization has been incorporated as a key operating component into prominent population-encoding models to account for the decreasing neural activity per item as set size increases (e.g., Bays, 2015). Specifically, working memory is assumed to be a fixed limited resource, which must be normalized over all stimuli maintained in memory. Here, we demonstrated in our second experiment that no contrast normalization occurred when two memory representations were pitted against each other. While, at first glance, this seems at odds with the previously described population-encoding models (Bays, 2015), we cannot exclude the possibility of normalization occurring between higher-order memory representations further along the visual hierarchy. Here, we tested a canonical computation within early visual areas, to which visual memories should succumb if they truly share a representational structure with perceptual inputs, as proposed by the sensory recruitment hypothesis. Our results therefore suggest that visual memory representations are distinct from perceptual representations—results that square with recent theories proposing that memory traces within sensory regions do not rely on persistent spiking activity but are instead based on discrete dynamics (Mongillo, Barak, & Tsodyks, 2008; Stokes, 2015).
Visual memory and attention have long been intertwined, with theories of visual memory often positing that attention is necessary in order to retain items in memory (Awh & Jonides, 2001; Desimone & Duncan, 1995; LaRocque et al., 2016). Attention has been strongly linked to divisive normalization—models propose that the gain of visual responses with attention arise through a release from normalization (Reynolds & Heeger, 2009). Our results do not necessarily indicate mutual exclusivity between visual attention and visual memory, where attention selectively enhances representations by leveraging normalization, and visual memory appears to be incapable of doing so. While recent work has suggested that attention and visual memory may operate together to alter center-surround inhibition of memory representations in color space (Kiyonaga & Egner, 2016), there are a number of methodological and theoretical limitations that prevent one from interpreting those results as evidence for divisive normalization within working memory. For instance, there is little evidence for cortical or subcortical processes with color representations that correspond to the implemented color space (hue, saturation, and value [HSV] color space), and therefore, the predicted perceptual signature of normalization-driven inhibition across color space is difficult to pin down (Bae, Olkkonen, Allred, & Flombaum, 2015; Brouwer & Heeger, 2013). In our study, we probed stimulus orientation and contrast, two of the most well-understood features in the context of divisive normalization, and found qualitative differences in the representations supporting vision and visual memory.
Another recent study examined whether visual memory has the same spatial resolution as found in perception (Tamber-Rosenau, Fintzi, & Marois, 2015) by utilizing crowding to induce visual competition. While this study hints at distinctions between perceptual and memory representations, crowding was always induced during encoding, making it difficult to disentangle the effects of perceptual versus memory processes. Here, we presented our stimuli simultaneously or sequentially with a fixed retention interval in both conditions to test whether visual memories bypass normalization.
Visual memory’s immunity from normalization may be adaptive in some cases and potentially maladaptive in others. Consider the results of our first experiment. If visual memory representations were truly prone to the influence of normalization by ongoing visual stimulation, the incessant barrage of visual information would induce constant distortions of memory representations, rendering them less useful. It is, however, surprising that multiple representations stored within visual memory do not leverage normalization more readily to regulate each other’s representations, as we discovered in our second experiment. One of the putative functional utilities of normalization is to carry out “redundancy reduction” (Schwartz & Simoncelli, 2001), compressing the amount of information needed for encoding via suppression of representations that share common features. Given visual memory’s infamously feeble storage capacity (Alvarez & Cavanagh, 2004; Todd & Marois, 2004), it is somewhat surprising that visual memory fails to co-opt contrast normalization to efficiently regulate among visual memory representations.
Supplemental Material
Supplemental material, BloemSupplementalMaterial for Visual Memories Bypass Normalization by Ilona M. Bloem, Yurika L. Watanabe, Melissa M. Kibbe, and Sam Ling in Psychological Science
Acknowledgments
We thank members of the Ling Lab (ClownCar), Megan Mariani, and Alejandra Lopez for their valuable comments and suggestions.
Footnotes
Action Editor: Edward S. Awh served as action editor for this article.
Author Contributions: I. M. Bloem, M. M. Kibbe, and S. Ling conceived and designed the experiments. I. M. Bloem and Y. L. Watanabe collected and analyzed the data. I. M. Bloem, Y. L. Watanabe, M. M. Kibbe, and S. Ling wrote the manuscript and approved the final version of the manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This research was funded by National Institutes of Health Grant EY028163 to S. Ling.
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617747091
References
- Alvarez G. A., Cavanagh P. (2004). The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science, 15, 106–111. [DOI] [PubMed] [Google Scholar]
- Awh E., Jonides J. (2001). Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences, 5, 119–126. [DOI] [PubMed] [Google Scholar]
- Bae G. Y., Olkkonen M., Allred S. R., Flombaum J. I. (2015). Why some colors appear more memorable than others: A model combining categories and particulars in color working memory. Journal of Experimental Psychology: General, 144, 744–763. [DOI] [PubMed] [Google Scholar]
- Bays P. M. (2015). Spikes not slots: Noise in neural populations limits working memory. Trends in Cognitive Sciences, 19, 431–438. [DOI] [PubMed] [Google Scholar]
- Brouwer G. J., Heeger D. J. (2013). Categorical clustering of the neural representation of color. The Journal of Neuroscience, 33, 15454–15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M., Heeger D. J. (2012). Normalization as a canonical neural computation. Nature Reviews Neuroscience, 13, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophel T. B., Klink P. C., Spitzer B., Roelfsema P. R., Haynes J. D. (2017). The distributed nature of working memory. Trends in Cognitive Sciences, 21, 111–124. [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Ester E. F., Serences J. T., Awh E. (2009). Spatially global representations in human primary visual cortex during working memory maintenance. The Journal of Neuroscience, 29, 15258–15265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. A., Tong F. (2009). Decoding reveals the contents of visual working memory in early visual areas. Nature, 458, 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger D. J. (1992). Normalization of cell responses in cat striate cortex. Visual Neuroscience, 9, 181–197. [DOI] [PubMed] [Google Scholar]
- Herrmann K., Montaser-Kouhsari L., Carrasco M., Heeger D. J. (2010). When size matters: Attention affects performance by contrast or response gain. Nature Neuroscience, 13, 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonaga A., Egner T. (2016). Center-surround inhibition in working memory. Current Biology, 26, 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocque J. J., Riggall A. C., Emrich S. M., Postle B. R. (2016). Within-category decoding of information in different attentional states in short-term memory. Cerebral Cortex, 27, 4881–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S., Blake R. (2012). Normalization regulates competition for visual awareness. Neuron, 75, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo G., Barak O., Tsodyks M. (2008). Synaptic theory of working memory. Science, 319, 1543–1546. [DOI] [PubMed] [Google Scholar]
- Morey R. D., Rouder J. N. (2011). Bayes factor approaches for testing interval null hypotheses. Psychological Methods, 16, 406–419. [DOI] [PubMed] [Google Scholar]
- Offen S., Schluppeck D., Heeger D. J. (2009). The role of early visual cortex in visual short-term memory and visual attention. Vision Research, 49, 1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T., Greenlee M. W. (2005). Working memory in primate sensory systems. Nature Reviews Neuroscience, 6, 97–107. [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [PubMed] [Google Scholar]
- Pratte M. S., Tong F. (2014). Spatial specificity of working memory representations in the early visual cortex. Journal of Vision, 14(3), Article 22. doi: 10.1167/14.3.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz N. C., Willmore B. D. B., Schnupp J. W. H., King A. J. (2011). Contrast gain control in auditory cortex. Neuron, 70, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker R. L., Bloem I. M., De Weerd P., Sack A. T. (2015). The impact of interference on short-term memory for visual orientation. Journal of Experimental Psychology: Human Perception and Performance, 41, 1650–1665. [DOI] [PubMed] [Google Scholar]
- Rangel A., Clithero J. A. (2012). Value normalization in decision making: Theory and evidence. Current Opinion in Neurobiology, 22, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. H., Heeger D. J. (2009). The normalization model of attention. Neuron, 61, 168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Simoncelli E. P. (2001). Natural signal statistics and sensory gain control. Nature Neuroscience, 4, 819–825. [DOI] [PubMed] [Google Scholar]
- Serences J. T. (2016). Neural mechanisms of information storage in visual short-term memory. Vision Research, 128, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences J. T., Ester E. F., Vogel E. K., Awh E. (2009). Stimulus-specific delay activity in human primary visual cortex. Psychological Science, 20, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shushruth S., Nurminen L., Bijanzadeh M., Ichida J. M., Vanni S., Angelucci A. (2013). Different orientation tuning of near- and far-surround suppression in macaque primary visual cortex mirrors their tuning in human perception. The Journal of Neuroscience, 33, 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneve M. H., Alnæs D., Endestad T., Greenlee M. W., Magnussen S. (2011). Modulation of activity in human visual area V1 during memory masking. PLOS ONE, 6(4), Article e18651. doi: 10.1371/journal.pone.0018651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes M. G. (2015). “Activity-silent” working memory in prefrontal cortex: A dynamic coding framework. Trends in Cognitive Sciences, 19(7), 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H., Spekreijse H., Lamme V. A. (2001). A neural correlate of working memory in the monkey primary visual cortex. Science, 293, 120–124. [DOI] [PubMed] [Google Scholar]
- Tamber-Rosenau B. J., Fintzi A. R., Marois R. (2015). Crowding in visual working memory reveals its spatial resolution and the nature of its representations. Psychological Science, 26, 1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Sagi D. (1998). A perceptual memory for low-contrast visual signals. Proceedings of the National Academy of Sciences, USA, 95, 12729–12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. J., Marois R. (2004). Capacity limit of visual short-term memory in human posterior parietal cortex. Nature, 428, 751–754. [DOI] [PubMed] [Google Scholar]
- Treue S., Maunsell J. H. R. (1999). Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. The Journal of Neuroscience, 19, 7591–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J., Heeger D. J. (2001). Measurement and modeling of center-surround suppression and enhancement. Vision Research, 41, 571–583. [DOI] [PubMed] [Google Scholar]
- Xing Y., Ledgeway T., McGraw P. V., Schluppeck D. (2013). Decoding working memory of stimulus contrast in early visual cortex. The Journal of Neuroscience, 33, 10301–10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. (2017). Reevaluating the sensory account of visual working memory storage. Trends in Cognitive Sciences, 21, 794–815. doi: 10.1016/j.tics.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Zenger-Landolt B., Heeger D. J. (2003). Response suppression in V1 agrees with psychophysics of surround masking. The Journal of Neuroscience, 23, 6884–6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, BloemSupplementalMaterial for Visual Memories Bypass Normalization by Ilona M. Bloem, Yurika L. Watanabe, Melissa M. Kibbe, and Sam Ling in Psychological Science