Introduction
The prevalence of hypertension in the United States is estimated to have reached 85.7 million adults1 without taking into account new ACC/AHA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure2, which is certain to increase this estimate. Hypertension is a major risk factor for stroke, coronary heart disease and renal failure. Additionally, high blood pressure (BP) increases cardiovascular mortality rates, as well as progression of many other chronic diseases. Salt and water handling by the kidney directly affects BP, whereas renal ion channels and transporters maintain electrolyte homeostasis.3,4
Epidemiological and anthropological studies suggest that in isolated societies with diets composed primarily of fruits, vegetables, and nuts, hypertension affects only 1 % of the population. In contrast, countries consuming a modern Western diet high in processed foods and dietary sodium, the prevalence of hypertension is as high as 30%. This effect of the Western diet is not solely attributed to high sodium content, but rather the dramatically decreased dietary potassium to sodium ratio. In industrialized countries, the daily intakes of potassium and sodium are approximately 30 to 50 mmol and 80 to 250 mmol per day, respectively. This is in a sharp contrast with isolated or primitive societies, having the daily rates of 150 to 290 mmol for potassium and 20 to 40 mmol per day for sodium.5 Therefore, estimated potassium to sodium intake ratios range from 0.12 – 0.63 for industrialized societies and 3.8–14.5 for isolated societies. According to the National Health and Nutrition Examination Survey (NHANES), only about one-tenth of US adults have potassium to sodium intake ratios consistent with World Health Organization guidelines for reduced risk of mortality.6
Potassium is the most abundant intracellular ion, and its role in the regulation of BP is well established. Dietary supplementation with potassium can lower BP in normal and hypertensive patients. Potassium channels, along with Na+-K+-ATPase (also known as Na+-K+-pump), are central in determining the resting membrane potential and cell volume. Since the concentration of potassium is much higher in intracellular than extracellular medium, activation, and consecutive opening of potassium channels, results in hyperpolarization of the plasma membrane, thereby changing an electrogenic driving force for Na+ reabsorption in the distal nephron. The critical role of inward-rectifying potassium (Kir) channels in sodium and potassium homeostasis and the association of renal basolateral Kir channels with BP regulation is the focus of this brief review.
Role of Potassium in the Prevention and Treatment of Hypertension
It is well recognized that higher levels of sodium intake are associated with elevated BP.7 It was predicted more than a century ago that the effect of sodium on BP is dependent on diet composition, specifically potassium content8,9. Herbert Langford, who was among the first to suggest that differences in potassium consumption account for variations in the incidence of high BP among ethnic groups described10: “The interaction of sodium and potassium was the focus of von Bunge’s studies in Germany in the mid-1870s and remains a topic of interest. Von Bunge was concerned that the natriuresis produced by potassium would lead to serious disease.”11 Studies in the 20th century followed to provide further evidence of the critical role of potassium supplementation on BP. Addison evaluated the actions of potassium and sodium chloride in humans, including himself in the study. He reported in 1928 that potassium administration could lower the elevated BP and proposed that hypertension is due to a low potassium diet and excess sodium chloride consumption.12
The landmark Dietary Approaches to Stop Hypertension (DASH) diet13,14, a diet low in sodium and replete with potassium, calcium, and magnesium, is now being recommended as a standard lifestyle modification for patients with hypertension or other cardiovascular risk factors. The DASH diet recommends a daily intake of 4.7 g of potassium (Table 1 includes examples of foods rich in potassium). The standardized, worldwide epidemiological INTERSALT study also provides evidence that potassium intake is an essential determinant of BP, independent of sodium.15 A recent large-scale Prospective Urban Rural Epidemiology (PURE) study examined the association of urinary sodium and potassium excretion with BP in more than 100,000 participants and reported that higher potassium excretion is associated with a lower risk of death and major cardiovascular complications.16 The PURE investigators also noted an inverse relation between potassium excretion and systolic BP, with each gram increment in potassium excretion per day resulting in a 1.08 mmHg decrease in systolic BP (1 g sodium excretion produced an increment of 2.11 mmHg). The highest BP was observed in individuals with the maximum sodium excretion combined with the lowest potassium excretion.8 Similarly, a cluster-randomized, controlled trial, in which participants increased potassium consumption and reduced sodium consumption showed reduced cardiovascular mortality among those assigned to the higher-potassium group.17
Table 1.
Examples of common potassium-rich foods
| Name | Potassium content (per 100 g) | Citation |
|---|---|---|
| Apricots | 259 mg | USDA 09021, apricots, raw |
| Avocados | 485mg | USDA 09037, avocados, raw, all commercial varieties |
| Bananas | 358mg | USDA 09040, bananas, raw |
| Beans | 454mg | USDA 16051, beans, white, mature seeds, canned |
| Coconut Water | 250mg | USDA 12119, nuts, coconut water (liquid from coconut) |
| Dairy milk (especially fat-free or low-fat) | 156mg | USDA 01151, milk, nonfat, fluid, without added vitamin A or vitamin D |
| Fat-free yogurt | 255mg | USDA 01118, yogurt, plain, skim milk |
| Fish | 252mg | USDA 15117, fish, tuna, fresh, Bluefin, raw |
| Grapefruit | 135mg | USDA 09119, grapefruit, raw, pink and red, all areas |
| Lima beans | 220mg | USDA 16073, lima beans, large, mature seeds, canned |
| Melon | 267mg | USDA 09181, melons, cantaloupe, raw |
| Mushrooms | 318mg | USDA 11260, mushrooms, white, raw |
| Oranges | 181mg | USDA 09200, oranges, raw, all commercial varieties |
| Peas | 200mg | USDA 11300, peas, edible-podded, raw |
| Pomegranates | 236mg | USDA 09286, pomegranates, raw |
| Potatoes | 337mg | USDA 11507, sweet potato, raw, unprepared |
| Prunes | 732mg | USDA 09291, plums, dried (prunes), uncooked |
| Raisins and dates | 825mg | USDA 09299, raisins, seeded |
| Spinach | 558mg | USDA 11457, spinach, raw |
| Tomatoes | 237mg | USDA 11529, tomatoes, red, ripe, raw, year-round average |
| Watermelon | 112mg | USDA 09326, watermelon, raw |
Animal studies using established hypertensive models further provide critical evidence of potassium in the control of BP. In the nineteen-fifties, Meneely et al. found that potassium administration modulated BP and significantly enhanced survival of rats fed high NaCl diet.18,19 In another classical work, Dahl et al. found that the life expectancy of the rats, while shortened by high doses of sodium, increased back toward untreated values by concurrent supplementation with potassium.20 After establishing that the dietary Na/K ratio is crucial for long term survival, Dahl and colleagues went on to test the effect of different dietary Na/K ratios in hypertension-prone rats. All tested diets had high NaCl content (4.50%) but differed in KC1 concentration. It was concluded that the dietary Na/K molar ratio can be an important determinant for the severity, or even development, of salt-induced hypertension.20
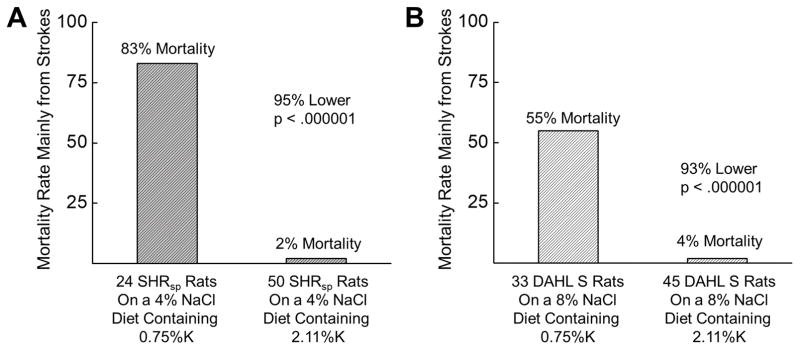
Tobian and colleagues further uncovered benefits of potassium supplementation during the development of hypertension and renal disease. Thus, the addition of 1.36% K+ to the diet reduced renal lesions (by 50% in the renal cortex, by 30% in the outer medulla, and by 44% in the inner medulla) in high salt-fed Dahl salt-sensitive (SS) rats.21 The added potassium also decreased BP moderately in Spontaneously Hypertensive (SHR) rats and modestly in Dahl SS rats. Importantly, the high K+ diet had a striking effect on mortality in both models. After 17 weeks on a 4% NaCl diet with no added K+, 20 of 24 SHR rats had died. On the contrary, 49 of 50 rats on the same diet plus 1.36% K+, were still alive. This resulted in a 98% reduction in mortality rate (Figure 1A).22 Similarly, after 9 weeks on the high salt (8% NaCl) diet with no K+ supplement, 18 of 33 Dahl SS had died, while only 2 out of 45 rats with 1.36% K+ supplementation had perished (overall, 93% reduction in mortality rate; Figure 1B).22 These changes in survival rate occurred independently from BP and it is likely that the majority of these deaths were due to stroke. Furthermore, it was reported that the level of dietary potassium has a marked influence on NaCl sensitivity in SHR rats, which are considered NaCl-resistant.23 Another chronic study of Dahl SS rats fed 1% NaCl with increasing dietary KCl revealed that after 8 months, Dahl SS rats fed 1% NaCl supplemented with 0.7% KCl had significantly increased mean arterial pressure, plasma volume, cardiac output and renal and cerebral vascular resistance compared with Dahl salt-resistant rats receiving the same diet. All these parameters were significantly reduced, when dietary K+ supplement was increased to 2.6%.24
Figure 1.
Mortality rate of SHR (A) and Dahl SS (B) rats challenged with the high salt diet supplemented with either normal or high dietary K+ intake. The actual final concentration of K+ in each diet is listed. For these experiments SHR rats were fed a 4% NaCl diet for 17 weeks, and Dahl SS rats were fed an 8% NaCl diet for 9 weeks. Redrawn and adapted from Tobian et al22 with permission from the publisher.
Providing further evidence supporting the importance of dietary potassium in renal disease and hypertension, it has been reported that potassium depletion and hypokalemia induce renal injury, salt sensitivity and hypertension in rats.25,26 For example, Sprague-Dawley rats have significant growth retardation, increased RAS activity, tubulointerstitial injury, macrophage infiltration, and early fibrosis when fed a potassium-deficient (<0.05% K+) diet. Furthermore, these rats had elevated BP and increased salt sensitivity.25
In summary, dietary supplementation of potassium can lower BP in human and animal models, especially if they prone to have salt-sensitivity. However, despite the clinical relevance and translational magnitude of these studies, the specific molecular mechanisms underlying the beneficial effects of a high potassium diet are still not fully understood.
Mechanisms of Regulation of Blood Pressure by Potassium
There are multiple mechanisms by which potassium may control BP. Here we will focus on mechanisms mediated by renal tubular K+ channels in the distal tubules. However, it should be taken into account that other tubular segments also contribute to potassium excretion. For example, recent studies by Wang et al. revealed that the activities of NKCC2 (Na+-K+-Cl− cotransporter) and Kir1.1 (also known as renal outer medullary K+ (ROMK) channel) are elevated in mice on a low Na+, high K+ diet.27,28
Furthermore, renal vascular potassium channels could be involved in regulation of BP.29 For instance, small changes in serum potassium can cause endothelium-dependent vasodilation by hyperpolarizing the endothelial and vascular smooth muscle cells. The high K+ diet might also improve the vascular integrity upon increased tension, as a result of hypertension. In studies of the kidneys of Dahl SS rats on normal and high K+ diets, K+ supplementation prevented the usual thickening of the arteriolar walls of the kidneys in hypertensive rats. Similarly, even during the development of severe hypertension, the high K+ diet promoted substantial reduction of wall thickening in either very large or small arteries of SHR rats.30
Potassium Homeostasis in the Kidney and its Transport in the Distal Tubules
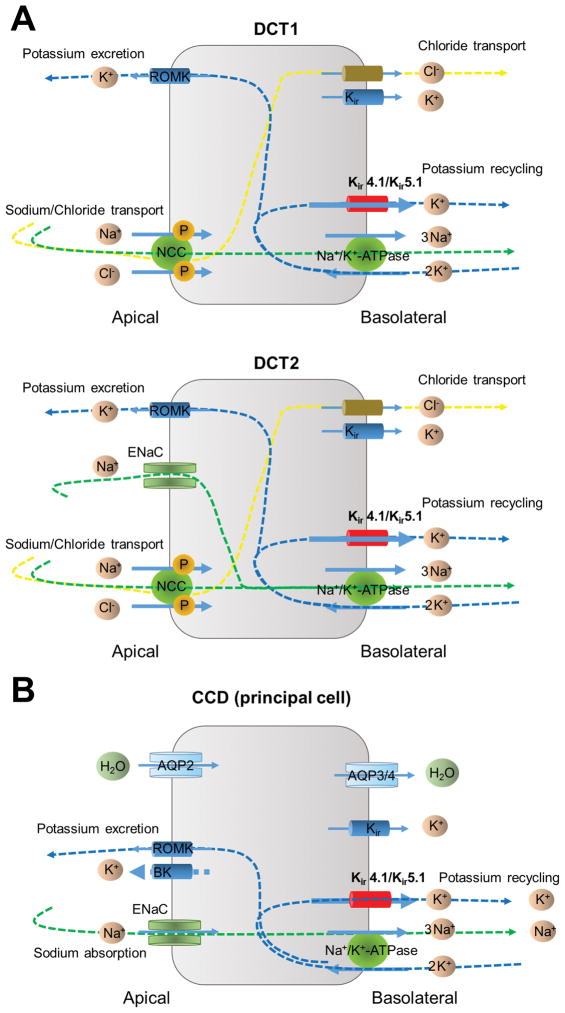
In the kidney, discretionary Na+ reabsorption and K+ secretion in the distal nephron and collecting duct is a critical determinant of the pressure-natriuresis relationship, which is of fundamental importance in the long-term control of arterial pressure. The distal convoluted tubule (DCT), connecting tubule (CNT), and cortical collecting duct (CCD) have been established as major targets for multiple hormones, also responding to sympathetic-nerve stimulation and changes in ion concentrations.31–33 Figure 2 shows simplified diagram illustrating water and electrolyte transport in the early (DCT1) and late (DCT2) DCT segments as well as in the CCD. While the Na+-Cl− transporter (NCC) contributes to sodium reabsorption in both DCT1 and DCT2, DCT2 differs in that the epithelial Na+ channel, ENaC, also involved in sodium absorption. Additionally, ENaC is the primary channel responsible for sodium transport in the CCD.
Figure 2.
Model of electrolyte transport in the early and late distal convoluted tubule (DCT1 and DCT2, respectively) (A) and the principal cells of cortical collecting duct (CCD) (B).
The ROMK and the large conductance calcium-activated K+ (BK) are the K+ channels in the apical membrane serving as the major pathways for controlled K+ secretion in the distal nephron (Figure 2). Mutations in the KCNJ1 gene, encoding Kir1.1 cause type II Barter’s syndrome.34 Most of these mutations are loss-of-function mutations. Interestingly, screening of subjects of the Framingham Heart Study also identified that variations in KCNJ1 produce clinically significant BP reductions and protect against development of hypertension.35 Zhou et al. addressed the role of ROMK by creating a Kcnj1 knock out in Dahl salt-sensitive rats (SSROMK−/−) 36,37. The authors demonstrated that the disruption of ROMK channels led to attenuation of salt-sensitive hypertension. It was reported that the survival rate of SSROMK−/− pups dramatically declined after postnatal day 14, body weight was significantly reduced, volume was severely depleted, whole blood electrolyte concentration (Na+, K+, and Cl−) was increased, metabolic acidosis was observed, and blood urea nitrogen level was elevated. The heterozygous SSROMK+/− rats, when challenged with a 4% salt diet, exhibited a reduced BP compared with their wild type littermates and when challenged with an 8% salt diet, the SSROMK+/− rats showed increased protection from salt-induced BP elevation and signs of protection from renal injury.38 Subsequent pharmacological studies revealed that chronic inhibition of ROMK not only prevented but also reversed development of hypertension and end-organ damage in Dahl SS rats.39
The role of BK channels in renal K+ excretion and blood pressure control has also been suggested.40–42 BK channels are essential for flow-induced K+ secretion triggered by loop diuretics and high K+ diet.43 BKα-deficient mice exhibited an impaired flow-dependent urinary K+ secretion and hyperaldosteronism and it was proposed that an upregulation of ROMK may compensate for the absence of functional BK channels.40 Similarly, deletions of the ancillary BKβ1 and BKβ4 subunits also result in deficient renal K+ excretion, hyperkalemia, primary hyperaldosteronism and hypertension.42,44 Further studies revealed that increased tubular flow in the distal nephron activate mechanosensitive Ca2+-permeable transient receptor potential vanilloid type 4 (TRPV4) channel to increase [Ca2+]i levels and activate BK channels. TRPV4 deletion results in hyperkalemia in response to dietary K+ load due to renal K+ retention.45 A number of recent excellent reviews have covered the role of ROMK and BK channels in potassium homeostasis in the kidney in great details.28,46,47
Basolateral Potassium Channels in the Distal Tubules
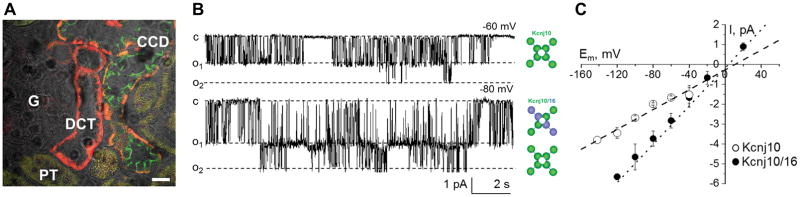
Inward-rectifier potassium channels, Kir4.1 and Kir5.1 (encoded by Kcnj10 and Kcnj16, respectively), play a dominant role in the control of the resting membrane potential of the basolateral membrane and transepithelial voltage, thereby modulating water and electrolyte transport in the distal nephron.48 Shown on Figure 3 are immunostaining images demonstrating Kir5.1 (encoded by Kcnj16) expression in DCT and CCD and representative single channel recordings as well as summarized current-voltage relationships for both homomeric Kir4.1 (Kcnj10) and heteromeric Kir4.1/Kir5.1 (Kcnj10/Kcnj16) channels. Kir4.1 channel subunits can form homomeric channels, or instead may polymerize with Kir5.1 subunits to form Kir4.1/Kir5.1 heteromers.49 It is established that the Kir4.1/Kir5.1 heteromer is the main basolateral K+ channel in the DCT and CDs.50–52 This heteromeric conformation has unique properties, including high sensitivity to pH changes within the physiologic range.53–55 Potassium recycling mediated by these channels is necessary to maintain a stable source of extracellular K+ in order to perform transcellular Na+ reabsorption driven by the Na+/K+-ATPase. Recently, it was also shown that basolateral Kir4.1/Kir5.1 channels in DCT are stimulated by low K+ intake and inhibited by high K+.56 In another study it was reported that Cl− intake also contribute to basolateral potassium and chloride conductance in principal and intercalated cells of CCD. Treatment of mice with high K+ diet without concomitant elevations in dietary Cl− elicited a comparable increase in basolateral K+-selective current and single channel Kir4.1/Kir5.1 activity in CCD principal cells. Furthermore, stimulation of aldosterone signaling by Deoxycorticosterone acetate recapitulated the stimulatory actions of high K+ intake on Kir4.1/Kir5.1 channels.57 This opposite regulation of Kir4.1/Kir5.1 by high K+ diet in DCT and CCD segments might suggest that they play discrete roles at corresponding segments.
Figure 3.
Expression and electrophysiological analysis of Kir4.1 homotetrameric and Kir4.1/Kir5.1 heterotetrameric channels. (A) Double immunostaining images show Kir5.1 expression (red) in the distal convoluted (DCT) and cortical collecting duct (CCD) cells. Aqp2 (green) was used as a marker of CD principal cells. Proximal tubules (PT) and glomerulus (G) are also shown. Scale bar is 20 μm. (B) Representative current traces and (C) current-voltage (I/V) relationships of the unitary current amplitude of Kir4.1 and Kir4.1/Kir5.1 channels measured in Dahl SS rats. Shown at −80 mV is activity of both heteromeric Kir4.1/Kir5.1 and homomeric Kir4.1 channels in the same patch. Adapted from Palygin al71 with permission from the publisher.
In humans, loss-of-function mutations in the Kcnj10 gene have been shown to cause SeSAME/EAST syndrome.58–60 The renal phenotype of these mutations includes salt wasting, hypomagnesemia, metabolic alkalosis and hypokalemia. It is thought that these mutations in Kcnj10 impair the function of heteromeric Kir4.1/Kir5.1 channels. Targeted disruption of the Kcnj16 gene in mice resulted in hypokalemic and hyperchloremic metabolic acidosis with hypercalciuria.61 Mutations in the Kcnj16 gene may also cause non-familial Brugada syndrome associated with sudden cardiac death.62 Furthermore, loss of transcriptional activation of Kir5.1 by hepatocyte nuclear factor 1 homeobox B (HNF1β) drives autosomal dominant tubulointerstitial kidney disease characterized by renal cysts and several hereditary forms of diabetes mellitus.63 Moreover, it was recently reported that the lack of Kcnj10 resulted in decreased expression of NCC in DCT64 and activation of ENaC.65
Kir channels are named “inward-rectifier” channels since they carry larger inward (at negative potentials) than outward currents. These channels are sensitive to extracellular K+ concentrations. According to the Nernst equation, reduction in plasma K+ concentration shifts the equilibrium potential for K+ (EK) to hyperpolarization, which alters channel gating and suppresses the overall conductance of Kir.66 Therefore, it was hypothesized that Kir4.1 (likely as a heteromer with Kir5.1) acts as the K+-sensor in the distal tubules.67 Special emphasis is paid to the key role NCC plays in mediating the effect of dietary K+ intake on K+ excretion by the kidney. Compelling evidence is provided that high K intake inhibits, whereas low K intake stimulates NCC activity.56,67–69
Homozygous Kcnj10−/− mice do not live more than 1–2 weeks59,64,70. Therefore, recent studies utilized techniques to conditionally delete Kir4.1 in the kidney after the completion of development using a Pa×8 promoter paired with the administration of doxycycline.67 Deletion of Kir4.1 in these mice led to moderate salt wasting, low BP, and profound potassium wasting.67 Notably, effects of dietary potassium on the basolateral potassium conductance and membrane potential in DCT were completely absent in these kidney-specific Kir4.1 KO mice. However, the same study also noted that dietary K+ intake affects Cl− conductance in DCT.56
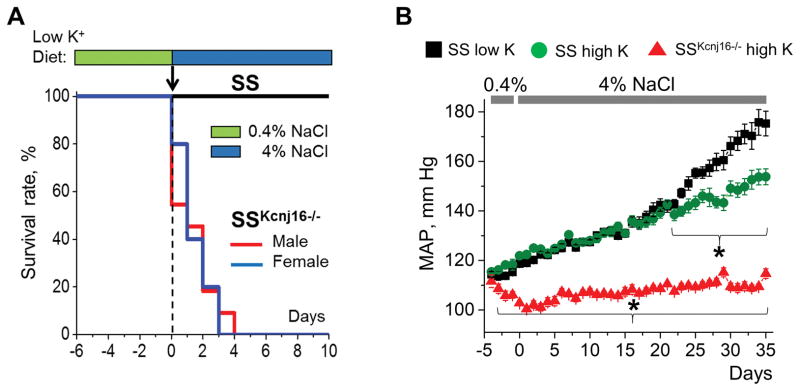
To define the importance of Kir4.1/Kir5.1 in BP control under conditions of salt-induced hypertension, we recently generated a Kcnj16 knockout in Dahl SS rats (SSKcnj16−/−).71 SSKcnj16−/− rats exhibited hypokalemia and reduced BP. Single-channel patch-clamp analysis of the basolateral K+ conductance in isolated CCDs of SS and SSKcnj16−/− rats revealed activity of only homomeric Kir4.1 channels. Expression of Kir4.1 was significantly increased in SSKcnj16−/− rats. However, an immunohistochemical analysis revealed that Kir4.1 channels were predominantly expressed in the cytosol of SSKcnj16−/− rats, in contrast to strong basolateral localization of this channel in SS rats. These data suggest that Kir5.1 is required for proper trafficking and localization of both Kir4.1 homomeric and Kir4.1/Kir5.1 heteromeric channels to the basolateral membrane of both DCT and CCD segments. The most striking phenotype was observed when SSKcnj16−/− rats were fed a high-salt diet. In contrast to wild type Dahl SS rats, SSKcnj16−/− rats experienced 100% mortality within a few days of switching to the high-salt diet, triggered by salt wasting and severe hypokalemia (Figure 4A). Importantly, administration of benzamil, an ENaC inhibitor, was able to rescue SSKcnj16−/− rats from mortality induced by a high-salt diet. In contrast, supplementation of drinking water with hydrochlorothiazide (an inhibitor of NCC in DCT) only slightly delayed the animals’ death, while furosemide (targeting NKCC2 in TAL) had no effect.71 Further studies revealed that SSKcnj16−/− rats survived when their high salt diet was supplemented with high K+ (Figure 4B). Figure 4B also shows that BP was reduced in SS rats fed a high salt diet when chow was supplemented with high potassium. Crucially, BP did not change in SSKcnj16−/− fed a high K+ diet, which demonstrates the critical role of this channel in the development of salt-induced hypertension.71
Figure 4.
High salt intake triggers rapid mortality of SSKcnj16−/− rats which is rescued by supplementation of high potassium. (A) Survival rate of SS and SSKcnj16−/− rats on a low potassium (0.36% K+) and 4% NaCl diet. (B) Mean arterial pressure in SS and SSKcnj16−/− rats. Animals were switched from a 0.4% to a 4% NaCl diet at day 0. Then, SS rats were fed either a standard 4% NaCl diet (black) or a 4% NaCl diet supplemented with high K+ (1.41% K+; green). SSKcnj16−/− rats were fed a 4% diet supplemented with high K+ (red). Adapted from Palygin et al71 with permission from the publisher.
Summary and Conclusions
It is well recognized that higher levels of sodium intake are associated with elevated BP. Importantly, the effect of high sodium on BP is dependent on diet composition, specifically on the potassium content. It is clear that high dietary potassium is associated with a decrease in BP, particularly in the presence of a high sodium diet. The studies summarized in this brief review emphasize the essential role of basolateral Kir channels, specifically Kir4.1/Kir5.1, in the control of potassium homeostasis and BP, respectively. Moreover, there are several other Kir channels identified in the renal tubular cells, such as Kir7.1 and Kir2.3 (encoded by Kcnj13 and Kcnj4, respectively)48,72–74, having largely unexplored roles. Further efforts are required to examine the relevance of these channels in BP and renal disease, especially under conditions of salt-induced hypertension and dietary supplementation with high potassium. Understanding of renal Kir channelopathies is essential for the development of new antihypertensive therapies. Some of these studies are underway39,75,76. Therefore, there is hope that novel small-molecule inhibitors selectively targeting Kir channels would be beneficial for the treatment of BP and kidney diseases.
Acknowledgments
I appreciate Dr. Oleg Palygin, Anna Manis (Medical College of Wisconsin) and Dr. Oleh Pochynyuk (UTHSC at Houston) for helpful discussion and critical reading of this review article. I am also grateful to Dr. Allen W. Cowley Jr. and Dr. Richard J. Roman for their nomination and the AHA Hypertension Council Awards Committee for selection me as a recepient of the 2017 Mid-Career Award for Research Excellence. I apologize to the investigators of K+ transport whose relevant publications were not directly discussed due to the space limit.
Sources of Funding
Work in the Staruschenko’s laboratory is supported by the American Heart Assositation (16EIA26720006) and the National Heart, Lung, and Blood Institute (R35 HL135749, R01 HL122662, and P01 HL116264).
Footnotes
Disclosures
None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018 doi: 10.1161/HYP.0000000000000065. [DOI] [Google Scholar]
- 3.Guyton AC. Blood pressure control-special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation. 2016;133:894–906. doi: 10.1161/CIRCULATIONAHA.115.018526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens. 2008;10:3–11. doi: 10.1111/j.1751-7176.2008.08575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey RL, Parker EA, Rhodes DG, Goldman JD, Clemens JC, Moshfegh AJ, Thuppal SV, Weaver CM. Estimating sodium and potassium intakes and their ratio in the American diet: data from the 2011–2012 NHANES. J Nutr. 2016;146:745–750. doi: 10.3945/jn.115.221184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease-a delicate balance. N Engl J Med. 2013;368:1229–1237. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 8.Mente A, O’Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–611. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 9.Weaver CM. Potassium and health. Adv Nutr. 2013;4:368S–377S. doi: 10.3945/an.112.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford HG. Sodium-potassium interaction in hypertension and hypertensive cardiovascular disease. Hypertension. 1991;17:I155–157. doi: 10.1161/01.hyp.17.1_suppl.i155. [DOI] [PubMed] [Google Scholar]
- 11.Von Bunge G. Ueber die Bedeutung des Kochsalzcs und das Verhalten der Kalisalze im menschlichen organismus. Z Biol. 1873:104–143. [Google Scholar]
- 12.Addison WL. The use of sodium chloride, potassium chloride, sodium bromide, and potassium bromide in cases of arterial hypertension which are amenable to potassium chloride. Can Med Assoc J. 1928;18:281–285. [PMC free article] [PubMed] [Google Scholar]
- 13.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 14.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 15.Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65:626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 17.Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, Tsai SY, Pan WH. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83:1289–1296. doi: 10.1093/ajcn/83.6.1289. [DOI] [PubMed] [Google Scholar]
- 18.Meneely GR, Ball CO. Experimental epidemiology of chronic sodium chloride toxicity and the protective effect of potassium chloride. Am J Med. 1958;25:713–725. doi: 10.1016/0002-9343(58)90009-3. [DOI] [PubMed] [Google Scholar]
- 19.Meneely GR, Ball CO, Youmans JB. Chronic sodium chloride toxicity: the protective effect of added potassium chloride. Ann Intern Med. 1957;47:263–273. doi: 10.7326/0003-4819-47-2-263. [DOI] [PubMed] [Google Scholar]
- 20.Dahl LK, Leitl G, Heine M. Influence of dietary potassium and sodium/potassium molar ratios on the development of salt hypertension. J Exp Med. 1972;136:318–330. doi: 10.1084/jem.136.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobian L, MacNeill D, Johnson MA, Ganguli MC, Iwai J. Potassium protection against lesions of the renal tubules, arteries, and glomeruli and nephron loss in salt-loaded hypertensive Dahl S rats. Hypertension. 1984;6:I170–176. doi: 10.1161/01.hyp.6.2_pt_2.i170. [DOI] [PubMed] [Google Scholar]
- 22.Tobian L, Lange J, Ulm K, Wold L, Iwai J. Potassium reduces cerebral hemorrhage and death rate in hypertensive rats, even when blood pressure is not lowered. Hypertension. 1985;7:I110. doi: 10.1161/01.hyp.7.3_pt_2.i110. [DOI] [PubMed] [Google Scholar]
- 23.Tobian L. Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension. 1991;17:I52–58. doi: 10.1161/01.hyp.17.1_suppl.i52. [DOI] [PubMed] [Google Scholar]
- 24.Manger WM, Simchon S, Stier CT, Jr, Loscalzo J, Jan KM, Jan R, Haddy F. Protective effects of dietary potassium chloride on hemodynamics of Dahl salt-sensitive rats in response to chronic administration of sodium chloride. J Hypertens. 2003;21:2305–2313. doi: 10.1097/00004872-200312000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Ray PE, Suga S, Liu XH, Huang X, Johnson RJ. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int. 2001;59:1850–1858. doi: 10.1046/j.1523-1755.2001.0590051850.x. [DOI] [PubMed] [Google Scholar]
- 26.Suga SI, Phillips MI, Ray PE, Raleigh JA, Vio CP, Kim YG, Mazzali M, Gordon KL, Hughes J, Johnson RJ. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol. 2001;281:F620–629. doi: 10.1152/ajprenal.2001.281.4.F620. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Wen D, Li H, Wang-France J, Sansom SC. Net K+ secretion in the thick ascending limb of mice on a low-Na, high-K diet. Kidney Int. 2017;92:864–875. doi: 10.1016/j.kint.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornelius RJ, Wang B, Wang-France J, Sansom SC. Maintaining K+ balance on the low-Na+, high-K+ diet. Am J Physiol Renal Physiol. 2016;310:F581–F595. doi: 10.1152/ajprenal.00330.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomonsson M, Brasen JC, Sorensen CM. Role of renal vascular potassium channels in physiology and pathophysiology. Acta Physiol. 2017;221:14–31. doi: 10.1111/apha.12882. [DOI] [PubMed] [Google Scholar]
- 30.Tobian L. The Jeremiah Metzger lecture. High potassium diets strongly protect against stroke deaths and renal disease: a possible legacy from prehistoric man. Trans Am Clin Climatol Assoc. 1986;97:123–140. [PMC free article] [PubMed] [Google Scholar]
- 31.Staruschenko A. Regulation of transport in the connecting tubule and cortical collecting duct. Compreh Physiol. 2012;2:1541–1584. doi: 10.1002/cphy.c110052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick JA, Ellison DH. Distal convoluted tubule. Compr Physiol. 2015;5:45–98. doi: 10.1002/cphy.c140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med. 2015;373:60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon DB, Karet FE, Rodriguez-Soriano J, et al. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 35.Ji W, Foo JN, O’Roak BJ, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40(5):592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW., Jr Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 2015;65:447–455. doi: 10.1161/HYPERTENSIONAHA.114.04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL. Salt sensitivity of blood pressure. A scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X, Zhang Z, Shin MK, et al. Heterozygous disruption of renal outer medullary potassium channel in rats is associated with reduced blood pressure. Hypertension. 2013;62:288–294. doi: 10.1161/HYPERTENSIONAHA.111.01051. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Forrest MJ, Sharif-Rodriguez W, Forrest G, Szeto D, Urosevic-Price O, Zhu Y, Stevenson AS, Zhou Y, Stribling S, Dajee M, Walsh SP, Pasternak A, Sullivan KA. Chronic inhibition of renal outer medullary potassium channel not only prevented but also reversed development of hypertension and end-organ damage in Dahl salt-sensitive rats. Hypertension. 2017;69:332–338. doi: 10.1161/HYPERTENSIONAHA.116.08358. [DOI] [PubMed] [Google Scholar]
- 40.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 41.Grimm PR, Sansom SC. BK channels and a new form of hypertension. Kidney Int. 2010;78:956–962. doi: 10.1038/ki.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci U S A. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 44.Wen D, Cornelius RJ, Rivero-Hernandez D, et al. Relation between BK-[alpha]/[beta]4-mediated potassium secretion and ENaC-mediated sodium reabsorption. Kidney Int. 2014;86:139–145. doi: 10.1038/ki.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mamenko MV, Boukelmoune N, Tomilin VN, Zaika OL, Jensen VB, O’Neil RG, Pochynyuk OM. The renal TRPV4 channel is essential for adaptation to increased dietary potassium. Kidney Int. 2017;91:1398–1409. doi: 10.1016/j.kint.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welling PA. Roles and Regulation of Renal K Channels. Annu Rev Physiol. 2016;78:415–435. doi: 10.1146/annurev-physiol-021115-105423. [DOI] [PubMed] [Google Scholar]
- 47.Penton D, Czogalla J, Loffing J. Dietary potassium and the renal control of salt balance and blood pressure. Pflügers Arch. 2015;467:513–530. doi: 10.1007/s00424-014-1673-1. [DOI] [PubMed] [Google Scholar]
- 48.Palygin O, Pochynyuk O, Staruschenko A. Role and mechanisms of regulation of the basolateral Kir4.1/Kir5.1 K+ channels in the distal tubules. Acta Physiol. 2017;219:260–273. doi: 10.1111/apha.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- 50.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol. 2008;294:F1398–F1407. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- 51.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol. 2002;538:391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sepúlveda FV, Pablo Cid L, Teulon J, Niemeyer MI. Molecular aspects of structure, gating, and physiology of pH-sensitive background K2P and Kir K+-transport channels. Physiol Rev. 2015;95:179–217. doi: 10.1152/physrev.00016.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tucker SJ, Imbrici P, Salvatore L, D’Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem. 2000;275:16404–16407. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- 54.Pessia M, Imbrici P, D’Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol. 2001;532:359–367. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Adamo MC, Shang L, Imbrici P, Brown SD, Pessia M, Tucker SJ. Genetic inactivation of Kcnj16 identifies Kir5.1 as an important determinant of neuronal PCO2/pH sensitivity. J Biol Chem. 2011;286:192–198. doi: 10.1074/jbc.M110.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang MX, Cuevas CA, Su XT, Wu P, Gao ZX, Lin DH, McCormick JA, Yang CL, Wang WH, Ellison DH. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int. 2018 doi: 10.1016/j.kint.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomilin VN, Zaika O, Subramanya AR, Pochynyuk O. Dietary K+ and Cl− independently regulate basolateral conductance in principal and intercalated cells of the collecting duct. Pflügers Arch. 2018;470:339–353. doi: 10.1007/s00424-017-2084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reichold M, Zdebik AA, Lieberer E, et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci U S A. 2010;107:14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bockenhauer D, Feather S, Stanescu HC, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paulais M, Bloch-Faure M, Picard N, et al. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci U S A. 2011;108:10361–10366. doi: 10.1073/pnas.1101400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juang JM, Lu TP, Lai LC, Ho CC, Liu YB, Tsai CT, Lin LY, Yu CC, Chen WJ, Chiang FT, Yeh SF, Lai LP, Chuang EY, Lin JL. Disease-targeted sequencing of ion channel genes identifies de novo mutations in patients with non-familial Brugada syndrome. Sci Rep. 2014;4:6733. doi: 10.1038/srep06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kompatscher A, de Baaij JHF, Aboudehen K, Hoefnagels APWM, Igarashi P, Bindels RJM, Veenstra GJC, Hoenderop JGJ. Loss of transcriptional activation of the potassium channel Kir5.1 by HNF1beta drives autosomal dominant tubulointerstitial kidney disease. Kidney Int. 2017;92:1145–1156. doi: 10.1016/j.kint.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1) Proc Natl Acad Sci U S A. 2014;111:11864–11869. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su XT, Zhang C, Wang L, Gu R, Lin DH, Wang WH. Disruption of KCNJ10 (Kir4.1) stimulates the expression of ENaC in the collecting duct. Am J Physiol Renal Physiol. 2016;310:F985–993. doi: 10.1152/ajprenal.00584.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng CJ, Kuo E, Huang CL. Extracellular potassium homeostasis: insights from hypokalemic periodic paralysis. Semin Nephrol. 2013;33:237–247. doi: 10.1016/j.semnephrol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang CL, Ellison DH, Wang WH. Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol. 2017;28:1814–1825. doi: 10.1681/ASN.2016090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 70.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight. 2017;2:e92331. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci U S A. 2017;114:E9989–E9998. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki Y, Yasuoka Y, Shimohama T, Nishikitani M, Nakamura N, Hirose S, Kawahara K. Expression of the K+ channel Kir7.1 in the developing rat kidney: role in K+ excretion. Kidney Int. 2003;63:969–975. doi: 10.1046/j.1523-1755.2003.00806.x. [DOI] [PubMed] [Google Scholar]
- 74.Hamilton KL, Devor DC. Basolateral membrane K+ channels in renal epithelial cells. Am J Physiol Renal Physiol. 2012;302:F1069–1081. doi: 10.1152/ajprenal.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swale DR, Kharade SV, Denton JS. Cardiac and renal inward rectifier potassium channel pharmacology: emerging tools for integrative physiology and therapeutics. Curr Opin Pharmacol. 2014;15:7–15. doi: 10.1016/j.coph.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kharade SV, Sheehan JH, Figueroa EE, Meiler J, Denton JS. Pore polarity and charge determine differential block of Kir1.1 and Kir7.1 potassium channels by small-molecule inhibitor VU590. Mol Pharmacol. 2017;92:338–346. doi: 10.1124/mol.117.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]