Abstract
Background
Hepatitis C virus (HCV) infection has been the leading indication for liver transplantation (LT) in the United States. Since 2013, interferon-free antiviral therapy has led to sustained virologic response in many LT candidates. We compared the waitlist mortality of HCV patients with that of patients with other chronic liver diseases.
Methods
Data for primary LT candidates were obtained from the Organ Procurement and Transplantation Network database. Adult waitlist registrants were divided into three cohorts: Cohort 1 included patients on the waitlist as of January 1, 2004; Cohort 2 as of January 1, 2009; and Cohort 3 as of January 1, 2014. The primary outcome was waitlist mortality, and the secondary outcome was the rate of change in Model for End-stage Liver Disease (MELD). Multivariable Cox proportional hazards analysis was performed to evaluate 12-month waitlist mortality.
Results
The cohorts included 7,627 LT candidates with HCV and 13,748 patients without HCV. Compared with Cohort 2, HCV patients in Cohort 3 had a 21% lower risk of death (hazard ratio [HR] 0.79, 95% CI 0.67–0.93). Among patients with non-HCV liver disease, no difference in mortality was seen between Cohorts 2 and 3 (HR 0.97, 95% CI 0.86–1.09). Among HCV patients, the mean rate of change in MELD decreased from 2.35 per year for Cohort 2 to 1.90 per year for Cohort 3, compared to 1.90 and 1.66 in Cohorts 2 and 3, respectively, among non-HCV patients.
Conclusion
In this population-based study, waitlist mortality and progression of disease severity decreased in recent HCV patients for whom direct-acting antiviral agents were available.
Keywords: Sustained virologic response, direct acting antiviral therapy, waitlist outcomes, waitlist mortality, delta-MELD
Introduction
According to the World Health Organization, approximately 3% of people worldwide (more than 170 million people) have been infected with hepatitis C virus (HCV).(1) In the United States, the prevalence of HCV infection is estimated to be 2.7 to 5.2 million, with up to 20% having developed cirrhosis. (2–4) As the HCV population ages, the number of patients with complications of liver cirrhosis including hepatocellular carcinoma (HCC) has increased, a trend projected to continue in the foreseeable future.(5) Advanced liver disease due to HCV infection has been the most common indication for liver transplantation (LT) in the United States.(6)
LT candidates with HCV may benefit from effective antiviral therapy. Sustained virologic response (SVR) in LT candidates eliminates the risk of post-LT HCV recurrence and can potentially prevent further clinical deterioration on the waitlist.(7, 8) In the interferon era, HCV treatment in patients with decompensated cirrhosis was not only ineffective, but also unsafe.(9) In late 2013, approval of sofosbuvir, a potent and safe direct-acting antiviral (DAA), heralded a revolutionary era in the treatment of chronic HCV. The efficacy and safety of sofosbuvir-based therapy in patients with decompensated cirrhosis have been demonstrated, with most patients exhibiting improved Model for End-Stage Liver Disease (MELD) scores after treatment.(10, 11) Given the high achievable rate of SVR and tolerability in patients with compensated and even decompensated liver cirrhosis, we hypothesize that patients on the LT waiting list would benefit from DAA-based treatment.
The aims of this study were (1) to examine whether waitlist mortality in the DAA era has changed for LT candidates with HCV, compared to other chronic liver diseases, and (2) to assess for alterations in the trajectory of disease severity in waitlist registrants with HCV. We hypothesized that HCV therapy in waitlist registrants for whom DAAs were available may have led to a greater number of patients achieving SVR, which would manifest at the aggregate level as a mitigation in the progression in MELD scores, accompanied by reduction in mortality.
Methods
Study Data
Data for all adult LT waitlist registrants in the United States were obtained from the Organ Procurement and Transplantation Network (OPTN) as of June 17, 2016. We determined the first registration date and the end date for each patient on the LT waiting list. Patients with multiple registration records were identified, and data were merged into single record per registrant. The end date was the date of transplant, date of death, or the date of last follow-up. Patients were excluded if their first transplant date was earlier than January 1, 2004. Patients with age less than 18 years or previous LT were excluded. Patients with HCV infection was identified by OPTN diagnosis codes 4104, 4106, 4204, 4206, or 4593. Patients with a diagnosis of combined HCV and alcoholic cirrhosis were set aside in the main analysis, to prevent potential misclassification of the exposure.
To determine trends in waitlist outcomes, prevalence cohorts of waitlist registrants over a 10-year period were created. Given our hypothesis, the most recent cohort was created to include HCV patients who would have had a chance to receive DAA and had a reasonable duration of follow-up. Thus, this cohort, which is designated as Cohort 3, was defined to include LT candidates on the LT waiting list as of Jan 1, 2014 who met the inclusion criteria. Prior prevalent cohorts were created in a similar fashion – Cohort 2 consisted of candidates waiting as of Jan 1, 2009 and Cohort 1 those as of Jan 1, 2004. The 5-year intervals were chosen to minimize the number of patients represented in more than one cohort, while they are separate enough to represent different eras of LT. Patients who existed in more than one cohort were sorted into the most recent cohort.
Demographic data (age, sex, and race/ethnicity), anthropometric variables such as body mass index, diagnostic codes for underlying liver disease, the presence of hepatocellular carcinoma, and laboratory data including components of the MELD score, were extracted from the OPTN database. The start date was determined by the date of the MELD score closest to January 1 of the relevant cohort — data available on the start date were designated as baseline data. Patients were excluded if the closest available MELD score was earlier than 6 months prior to January 1 of the relevant cohort.
Statistical Analysis
The primary outcome variable was death on the waiting list within 1 year of start of study period, removal from the waitlist with known subsequent death, or removal from the list due to deteriorated condition or too sick to transplant. Patients were censored when they underwent LT, were lost to follow-up, or after 1 year of follow-up was complete. Survival time was defined as the number of days from the date of the first recorded MELD score closest to January 1 of the relevant cohort, until the primary outcome event occurred. The multivariable Cox proportional hazards analysis was conducted to evaluate the differences in 1-year mortality between cohorts, adjusted for baseline age and MELD score. The Cox model assumes that censored patients had the same risk as patients with the same covariate values, such as the MELD score, who were not censored. Thus, once the model was adjusted for MELD score, concern about potential biases, such as informative censoring, could be minimized.
To determine whether the pattern of MELD score progression has changed in the DAA treatment era, the slope of MELD score change, referred to hereafter as delta-MELD, was calculated for all cohorts. For this analysis, only patients with at least 4 available MELD scores within 1 year of the cohort start date were included. Patients whose last available MELD score was recorded prior to the corresponding January 1 of each cohort were excluded. Delta-MELD scores for the eligible population were then calculated based on the average of the first 2 available MELD scores and the average of the last 2 available MELD scores available, divided by time. Because the time intervals between the two averages of the MELD scores were variable, this part of the analysis only included patients with at least 365 days between the two MELD score averages.
Finally, as one of the potential explanations for differences in waitlist outcome for HCV patients, data were extracted to assess the availability and utilization of HCV positive donors over time. Trends in median waitlist time, HCC exception points, and biochemical MELD for recipients with HCV and recipients of HCV positive grafts were described.
Results
Patient Characteristics
There were 11,823 patients on the waiting list on January 1, 2004, 12,381 on January 1, 2009 and 15,624 on January 1, 2014. When the eligibility criteria were applied, Cohort 1 included 6,000 patients, Cohort 2 6,566 patients and Cohort 3 8,809 patients (Supplementary Figure 1). Of these registrants, 7,627 (35.7%) belonged in the HCV group, and the remaining 13,748 in the non-HCV group.
Baseline characteristics of patients in the three cohorts are presented in Tables 1 (HCV) and 2 (non-HCV). HCV patients in the three cohorts had similar proportions of men, whereas the more recent cohorts were older. The proportion of white registrants decreased, while African American and Hispanic registrants increased in the more recent cohorts. The median MELD score at baseline increased over time, albeit modestly, from 13 in the 2004 and 2009 cohorts to 14 in the 2014 cohort.
Table 1.
Baseline characteristics of liver transplant waitlist registrants with hepatitis C, by cohort.
| Cohort 1 (2004) n=2,408 |
Cohort 2 (2009) n=2,402 |
Cohort 3 (2014) n=2,817 |
P value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 52 (48–57) | 55 (51–59) | 58 (54–62) | <0.001 |
| Men, n (%) | 1,548 (64.3) | 1,602 (66.7) | 1,832 (65.0) | 0.20 |
| Race/Ethnicity, n (%) | <0.001 | |||
| White | 1,742 (72.3) | 1,692 (70.4) | 1,910 (67.8) | |
| Black | 154 (6.4) | 196 (8.2) | 265 (9.4) | |
| Hispanic | 416 (17.3) | 416 (17.3) | 522 (18.5) | |
| Asian | 73 (3.0) | 70 (2.9) | 91 (3.2) | |
| Other | 23 (1.0) | 28 (1.2) | 29 (1.0) | |
| BMI(kg/m2), median (IQR) | 28.3 (25.2–31.9) | 28.5 (25.2–32.1) | 28.4 (25.0–32.9) | 0.65 |
| Diabetes mellitus, n (%) | 463 (19.8) | 508 (21.6) | 615 (22.0) | 0.13 |
| Hepatocellular carcinoma, n (%) | 390 (16.2) | 429 (17.9) | 499 (17.7) | 0.23 |
| MELD score, median (IQR) | 13.0 (11.0–16.0) | 13.0 (10.0–16.0) | 14.0 (11.0–17.0) | <0.001 |
| Serum creatinine, median (IQR) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.9 (0.7–1.2) | 0.75 |
| Serum bilirubin, median (IQR) | 1.9 (1.3–2.8) | 1.9 (1.2–2.9) | 2.0 (1.2–3.0) | 0.69 |
| INR, median (IQR) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | 0.08 |
| Serum albumin, median (IQR) | 3.0 (2.6–3.3) | 3.0 (2.6–3.4) | 3.0 (2.6–3.5) | <0.001 |
| Serum sodium, median (IQR) | 137 (135–140) | 137 (135–140) | 138 (135–140) | 0.71 |
Abbreviations: IQR, interquartile range; BMI, body mass index; MELD, Model for End-stage Liver Disease; INR, international normalized ratio
Table 2.
Baseline characteristics of liver transplant waitlist registrants with liver disease other than hepatitis C, by cohort.
| Cohort 1 (2004) n=3,592 |
Cohort 2 (2009) n=4,164 |
Cohort 3 (2014) n=5,992 |
p-value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 55 (48–61) | 57 (50–63) | 58 (50–64) | <0.001 |
| Men, n (%) | 1,988 (55.3) | 2,360 (56.7) | 3,633 (60.6) | <0.001 |
| Race/Ethnicity, n (%) | 0.10 | |||
| White | 2,701 (75.2) | 3,076 (73.9) | 4,397 (73.4) | |
| Black | 218 (6.1) | 232 (5.6) | 351 (5.9) | |
| Hispanic | 484 (13.5) | 616 (14.8) | 900 (15.0) | |
| Asian | 161 (4.5) | 200 (4.8) | 265 (4.4) | |
| Other | 28 (0.8) | 40 (1.0) | 79 (1.3) | |
| BMI(kg/m2), median (IQR) | 27.4 (24.0–31.9) | 27.5 (24.0–31.9) | 28.2 (24.4–32.4) | 0.047 |
| Diabetes mellitus, n (%) | 841 (24.3) | 1,157 (28.6) | 1,686 (28.3) | <0.001 |
| Hepatocellular carcinoma, n (%) | 350 (9.7) | 538 (12.9) | 755 (12.6) | <0.001 |
| Etiology, n (%) | <0.001 | |||
| Alcoholic liver disease | 976 (27.2) | 1,147 (27.5) | 1,889 (31.5) | |
| Nonalcoholic steatohepatitis | 11 (0.3) | 413 (9.9) | 1,039 (17.3) | |
| Cryptogenic cirrhosis | 714 (19.9) | 632 (15.2) | 578 (9.6) | |
| Primary sclerosing cholangitis | 317 (8.8) | 337 (8.1) | 479 (8.0) | |
| Primary biliary cirrhosis | 375 (10.4) | 278 (6.7) | 268 (4.5) | |
| Secondary biliary cirrhosis | 21 (0.6) | 12 (0.3) | 33 (0.6) | |
| Autoimmune hepatitis | 230 (6.4) | 284 (6.8) | 387 (6.5) | |
| Hepatitis B virus | 230 (6.4) | 170 (4.1) | 247 (4.1) | |
| Other | 718 (20.0) | 891 (21.4) | 1,072 (17.9) | |
| MELD score, median (IQR) | 13.0 (10.0–16.0) | 13.0 (10.0–16.0) | 14.0 (10.0–17.0) | <0.01 |
| Serum creatinine, median (IQR) | 0.9 (0.8–1.2) | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | 0.90 |
| Serum bilirubin, median (IQR) | 2.0 (1.3–3.2) | 1.9 (1.2–3.1) | 2.0 (1.2–3.3) | 0.02 |
| INR, median (IQR) | 1.3 (1.1–1.5) | 1.3 (1.1–1.4) | 1.3 (1.2–1.5) | <0.001 |
| Serum albumin, median (IQR) | 3.2 (2.7–3.6) | 3.2 (2.8–3.7) | 3.3 (2.8–3.7) | <0.001 |
| Serum sodium, median (IQR) | 138 (135–140) | 138 (136–140) | 138 (135–140) | 0.71 |
Abbreviations: IQR, interquartile range; BMI, body mass index; MELD, Model for End-stage Liver Disease; INR, international normalized ratio
Among the non-HCV registrants, members of Cohort 3 were older and more likely to be male, obese, and diabetic, compared with those in the first two cohorts (Table 2). As expected, the proportion with non-alcoholic steatohepatitis increased, whereas primary biliary cirrhosis and hepatitis B virus infection decreased. When compared to the HCV cohorts, the non-HCV cohorts had higher proportions with diabetes (p<0.01 for Cohorts 1, 2, and 3) and lower proportions with HCC (p≤0.01 for Cohorts 1, 2, and 3). Although waitlist registrants became progressively older over time, the trend was more pronounced among HCV patients. Laboratory data and MELD scores were statistically different between HCV and non-HCV cohorts, although numerical differences were small and unlikely to be clinically meaningful.
Waitlist Outcomes
Table 3 summarizes waitlist outcomes for the three cohorts. There was no apparent difference in 1-year fatality rates among them, with 63–65% of cohort members surviving and still waiting for a graft at the end of the year. The proportion that underwent liver transplantation decreased over time, to 18.9% for HCV patients and 17% for non-HCV patients in Cohort 3. The proportion of patients in the HCV cohorts withdrawn from the waitlist increased over time at a higher rate compared to the proportion in non-HCV cohorts. HCV patients withdrawn for improved conditions increased from 1.5% in Cohort 2 to 2.2% in Cohort 3, while non-HCV patients withdrawn for the same reason were 2.8% and 2.9% in Cohorts 2 and 3, respectively.
Table 3.
Waitlist outcomes of liver transplant waitlist registrants with and without hepatitis C (HCV), by cohort.
| HCV | Cohort 1 (2004) n=2,408 | Cohort 2 (2009) n=2,402 | Cohort 3 (2014) n=2,817 |
|
| |||
| Status at 1 year after start date, n (%) | |||
| Alive and waiting | 1,549 (64.3) | 1,511 (62.9) | 1,782 (63.3) |
| Underwent transplantation | 515 (21.4) | 512 (21.3) | 533 (18.9) |
| Died | 275 (11.4) | 277 (11.5) | 330 (11.7) |
| Withdrawn from waiting lista | 69 (2.9) | 102 (4.2) | 172 (6.1) |
| Follow-up, months, median (IQR) | 12.0 (7.9–12.0) | 12.0 (7.1–12.0) | 12.0 (7.3–12.0) |
|
| |||
| Non-HCV | Cohort 1 (2004) n=3,592 | Cohort 2 (2009) n=4,164 | Cohort 3 (2014) n=5,992 |
|
| |||
| Status at 1 year after start date, n (%) | |||
| Alive and waiting | 2,293 (63.8) | 2,626 (63.1) | 3,889 (64.9) |
| Underwent transplantation | 780 (21.7) | 834 (20.0) | 1,020 (17.0) |
| Died | 397 (11.1) | 446 (10.7) | 685 (11.4) |
| Withdrawn from waiting listb | 122 (3.4) | 258 (6.2) | 398 (6.6) |
| Follow-up, months, median (IQR) | 12.0 (8.0–12.0) | 12.0 (7.4–12.0) | 12.0 (7.9–12.0) |
Withdrawn for following reasons: condition improved-transplant not needed (0.3%, 1.5%, 2.2% of Cohort 1, Cohort 2 and Cohort 3 respectively); refused transplant, unable to contact candidate, or other.
Withdrawnfor following reasons: condition improved-transplant not needed (1.0%, 2.8%, 2.9% of Cohort 1, Cohort 2 and Cohort 3 respectively); refused transplant, unable to contact candidate, or other.
Abbreviation: MELD, Model for End-stage Liver Disease
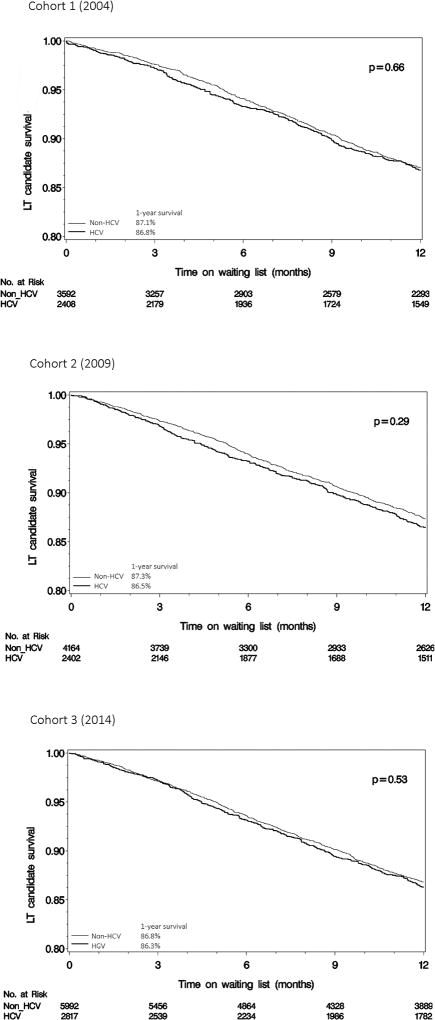
Similarly, without adjustment for baseline patient characteristics, there was no difference in the Kaplan-Meier estimates for waitlist survival between HCV and non-HCV cohorts of the same era, when the cohorts were assessed as a whole (Figure 1) or when they were divided into MELD groups of ≥ 20 and < 20 (Supplementary Figure 2).
Figure 1.
Survival Modeling
The results of the multivariable Cox regression analysis, adjusted for age and MELD score, are summarized in Table 4. Compared to patients in Cohort 2, those in Cohort 3 experienced a 21% reduction in waitlist mortality (hazard ratio [HR] 0.79, 95% confidence interval [CI] 0.67–0.93). There was no such improvement in the non-HCV cohorts of the same period (HR 0.97, 95% CI 0.86–1.09). There was no statistically significant improvement in survival from Cohort 1 compared to Cohort 2.
Table 4.
Multivariable Cox proportional hazards analysis for 12-month survival in liver transplant waitlist registrants with hepatitis C (HCV) or liver disease other than HCV.
| HCV | Non-HCV | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Total population | N=7,627 | N=13,748 |
| Cohort 1 | 1.05 (0.88–1.24) | 1.13 (0.99–1.30) |
| Cohort 2 | 1.00 | 1.00 |
| Cohort 3 | 0.79 (0.67–0.93) | 0.97 (0.86–1.09) |
| Age | 1.03 (1.02–1.04) | 1.04 (1.03–1.05) |
| Lab-MELD score | 1.18 (1.16–1.19) | 1.15 (1.14–1.16) |
|
| ||
| Baseline MELD below 20 | N=6,779 | N=12,070 |
| Cohort 1 | 1.09 (0.90–1.31) | 1.15 (0.99–1.35) |
| Cohort 2 | 1.00 | 1.00 |
| Cohort 3 | 0.88 (0.73–1.06) | 0.97 (0.84–1.12) |
| Age | 1.03 (1.02–1.05) | 1.04 (1.03–1.04) |
| Lab-MELD score | 1.21 (1.18–1.24) | 1.17 (1.15–1.19) |
|
| ||
| Baseline MELD above 20 | N=848 | N=1,678 |
| Cohort 1 | 0.94 (0.65–1.35) | 1.05 (0.79–1.39) |
| Cohort 2 | 1.00 | 1.00 |
| Cohort 3 | 0.59 (0.42–0.82) | 0.82 (0.65–1.02) |
| Age | 1.02 (1.00–1.04) | 1.05 (1.04–1.06) |
| Lab-MELD score | 1.15 (1.13–1.18) | 1.10 (1.09–1.12) |
Abbreviations: HR, hazard ratio; CI, confidence interval; MELD, Model for End-stage Liver Disease
When the analysis was stratified by baseline MELD score, the improvement in survival in the 2014 HCV cohort was predominantly driven by patients with MELD ≥ 20, with HR 0.59 (95% CI 0.42–0.82). In patients with lower MELD, there was a similar trend toward improved survival between Cohorts 2 and 3, but this was not statistically significant (HR 0.88, 95% CI 0.73–1.06). For non-HCV patients, no statistically significant change in survival was observed from Cohort 2 to Cohort 3 in either MELD stratum.
When patients with MELD ≥ 20 were further divided into MELD 20–25 and MELD ≥ 25, both HCV and non-HCV patients showed an improvement in survival over time; however, the change was more dramatic among HCV patients compared to non-HCV (Supplementary Table 1). When patients with MELD < 20 were further divided into MELD < 15 and MELD 15–20, no meaningful trend was found. A sensitivity analysis with 6-month mortality as the outcome variable yielded similar results (data not shown). A sensitivity analysis of patients with a combined diagnosis of HCV and alcohol showed no significant difference in survival across cohorts (Supplementary Table 6).
Change in MELD (delta-MELD)
The results of the delta-MELD analysis are shown in Table 5. In patients with HCV, the mean (± standard deviation) delta-MELD score increased from Cohort 1 (2.07 ± 4.5 per year) to Cohort 2 (2.35 ± 4.9 per year), but decreased in Cohort 3 (1.90 ± 5.0 per year). Among waitlist registrants without HCV, the trend was a more linear decrease in the mean delta-MELD score from Cohort 1 to Cohort 3.
Table 5.
Change in MELD(Delta-MELD) per year by cohort in liver transplant waitlist registrants with hepatitis C (HCV) or liver disease other than HCV.
| HCV | Non-HCV | |||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Total population | N=3,875 | N=6,584 | ||
| Cohort 1 | 2.07 (4.5) | 0.90 (−0.32–2.81) | 2.26 (4.9)a | 1.00 (−0.29–2.89) |
| Cohort 2 | 2.35 (4.9) | 0.91 (0.00–2.79) | 1.90 (4.4) | 0.84 (−0.38–2.58) |
| Cohort 3 | 1.90 (5.0)b | 0.68 (−0.43–2.36) | 1.66 (4.7)b | 0.61 (−0.60–2.41) |
|
| ||||
| Population excluding HCC | N=3,286 | N=6,000 | ||
| Cohort 1 | 2.16 (4.6) | 0.93 (−0.31–2.98) | 2.36 (5.0)b | 1.04 (0.00–2.98) |
| Cohort 2 | 2.45 (5.0) | 0.92 (0.00–2.84) | 1.89 (4.3) | 0.87 (−0.37–2.57) |
| Cohort 3 | 1.88 (5.0)b | 0.64 (−0.45–2.32) | 1.61 (4.7)b | 0.59 (−0.64–2.37) |
|
| ||||
| Baseline MELD above 20 | N=313 | N=577 | ||
| Cohort 1 | 4.39 (5.9) | 2.49 (0.56–6.26) | 3.68 (6.3) | 2.01 (0.31–4.99) |
| Cohort 2 | 3.70 (4.9) | 1.49 (0.33–7.21) | 3.38 (5.6) | 1.74 (−0.26–5.24) |
| Cohort 3 | 2.19 (5.9)b | 0.39 (−0.38–2.71) | 3.50 (5.6) | 1.64 (0.00–5.71) |
|
| ||||
| Baseline MELD below 20 | N=2,973 | N=5,423 | ||
| Cohort 1 | 2.00 (4.5) | 0.86 (−0.33–2.74) | 2.27 (4.9)b | 0.99 (0.00–2.89) |
| Cohort 2 | 2.36 (5.0) | 0.91 (0.00–2.73) | 1.76 (4.1) | 0.83 (−0.39–2.38) |
| Cohort 3 | 1.83 (4.9)b | 0.67 (−0.46–2.31) | 1.35 (4.5)b | 0.43 (−0.72–2.07) |
p-value <0.05 compared with Cohort 2
p-value <0.01 compared with Cohort 2
Abbreviations: SD, standard deviation; IQR, interquartile range; MELD, Model for End-stage Liver Disease; HCC, hepatocellular carcinoma
When patients were stratified by baseline MELD, the largest reduction in the delta-MELD was seen from Cohort 2 to Cohort 3 among HCV patients with baseline MELD ≥ 20 (3.70 per year to 2.19 per year, p<0.01). In contrast, the difference in delta-MELD for non-HCV patients with baseline MELD ≥ 20 was not statistically significant from Cohort 2 to Cohort 3. Sensitivity analysis excluding patients with MELD exception points for hepatocellular carcinoma and stratifying patients by baseline MELD ≥ 15 and < 15 showed similar results (Supplementary Table 2).
Delta-MELD may be made spuriously low, if a large number of patients with rising MELD were selectively transplanted and removed from the waiting list. In order to examine the possibility that survival and disease severity among patients in Cohort 3 may have improved due to changes in access to LT, transplantation rates were calculated for each cohort. Overall, the transplantation rate in our study population declined over time, from 26.6 (95% CI 25.1–28.0) transplants per 100 waitlist years in Cohort 1 to 21.6 (95% CI 20.6–22.7) in Cohort 3 (Supplementary Table 4). The transplantation rate for patients without tumor points showed a similar pattern of decline, regardless of HCV status. The transplantation rate for patients with HCC did increase more steeply for patients with HCV compared to those without HCV; however, HCC patients by and large have low biochemical MELD scores that tend not to change even over a relatively long period of time.
HCV Positive Donors
Out of the 21,375 patients who qualified for inclusion in our study cohorts, 4,194 underwent LT during the 1 year of follow-up. 166 (4.0%) were known to receive livers from hepatitis C positive donors, 146 of which were allocated to HCV-positive recipients (Supplementary Table 3A). Overall, 9.7% of HCV-positive recipients and 0.8% of HCV-negative recipients received HCV-positive donor organs. Although there was an increase in the proportion of HCV+ donors over time, the absolute number of HCV-positive organs transplanted over time was small. Out of the 166 HCV-positive organ recipients, 41 (24.7%) had HCC.
In our data, the median time from baseline to LT for HCV patients increased from 74 days (IQR 18–280) for Cohort 1 to 160 days (IQR 39–399) for Cohort 3, while the median MELD at LT did not change significantly for HCC and non-HCC patients (Supplementary Figure 3). These data suggest that increased availability of HCV-positive organs for Cohort 3 did not change the waitlist outcome appreciably. Finally, when the utilization of HCV-positive organs was analyzed by region, there was no clear correlation between the frequency of HCV-positive donor liver utilization and waitlist outcomes (Supplementary Table 5).
Discussion
Using national OPTN data, we demonstrate that there has been a decrease in waiting list mortality for patients with HCV in the DAA era. In addition, disease progression as measured by progression in MELD score appears attenuated for HCV patients in the DAA era. These two trends appear to be correlated, both seen predominantly in the most recent HCV cohort with higher MELD scores. The reductions in mortality risk and delta-MELD scores may be at least in part attributable to the use of DAA therapy after the approval of sofosbuvir in December 2013.
We also showed an increase in the proportion of waitlist registrations for black and Hispanic patients with HCV-related cirrhosis, and a concurrent decrease in the proportion of white registrants. This observation is consistent with recent studies that have demonstrated higher rates of cirrhosis among black and Hispanic patients and lower HCV treatment rates among racial minorities.(11–13) This trend also parallels the racial trends of LT overall, which may represent improved access to LT for minorities, who have been historically disadvantaged.(14–15)
Successful antiviral treatment of patients with HCV cirrhosis may reduce inflammation, halt or delay disease progression, and, in some cases, cause reversal of fibrosis, preventing further clinical deterioration and eventually death. Previous interferon-based regimens have been less effective and poorly tolerated; however, the availability of potent DAAs with minimal side effects has led to high rates of SVR with excellent safety profiles, even in decompensated cirrhosis.(16–20) In clinical trials of DAA-based HCV therapy for patients with decompensated cirrhosis, a majority of patients experience a decrease in MELD and Child-Pugh scores after antiviral treatment, suggesting that eradication of the virus improves hepatic function by alleviating the inflammation and injury resulting from active viral replication.(21) Similar effects are seen with the suppression of hepatitis B virus, with improvement in liver function and outcomes in patients with decompensated cirrhosis.(22)
In many patients with decompensated cirrhosis due to HCV, effective DAA-based antiviral therapy may result in reduction in portal hypertension and overall clinical improvement. (23–25) For LT candidates, this may lead to a delay in LT or even enable patients to avoid LT altogether.(7, 26) Over time, DAA-based therapy is also anticipated to reduce the overall burden of hepatitis C and need for liver transplantation. HCV has historically been the leading indication for LT in the United States, but waitlist registrations and rates of transplantation for HCV have already started to decrease as more patients are treated with antiviral therapy. (15, 27)
Our data suggest that in the recent DAA era, waitlist outcomes have improved for LT candidates with MELD ≥ 20, the sickest subgroup of HCV patients with the most advanced liver disease. There has been an emerging concern about treating these patients because attaining SVR may put them in a state referred to by some as “MELD purgatory,” which may reduce the opportunity for liver transplantation. In patients with mild hepatic decompensation, SVR would lead to disease stabilization and a decrease in the MELD score and measurable improvement in short-term outcomes, obviating the need for LT (26). This scenario may apply to some of the patients who were withdrawn from the waiting list for improvement in their condition. In other patients, however, SVR fails to resolve hepatic decompensation, and the patient still requires LT — now with little prospect of attaining a high enough MELD score to receive organ offers. These patients would lose priority on the LT waitlist while not achieving full hepatic recovery, leaving them with persistent complications and a decreased quality of life while awaiting LT. Our study may partly validate this concern, as it shows that while the outcomes of waitlist registrants with HCV in the current era improved and their delta-MELD was lower than before, they on average continue to experience an overall increase in MELD (i.e. delta-MELD >0). This suggests that LT candidates with HCV are not progressing to LT as rapidly, but that many do continue to progress over time.
Although our overarching hypothesis was that DAA therapy would improve waitlist outcomes in HCV patients with end stage liver disease, we are not able to directly link improvement in survival and lessened disease progression in LT candidates with HCV in the DAA era, because specific information regarding antiviral therapy was not available from the OPTN waitlist data. The improvement in survival and disease progression in this population could be attributed to improvement in the management of cirrhosis complications, advances in the quality of medical care in general, access to LT, or perhaps a difference in the selection of patients referred and listed for LT; however, the difference between HCV and non-HCV patients points to factors that are specific to HCV. In addition, patients in Cohort 3 were observed to have a lower incidence of transplantation compared to prior cohorts. The fact that we nonetheless detect improved survival among HCV patients in Cohort 3 despite a lower transplantation rate suggests that the true effect is even larger.
One possible alternate hypothesis for the improved waitlist outcomes among HCV positive LT candidates is the increased utilization of HCV-positive donors. In recent years, the transplant donor pool has been substantially expanded by the increased number of opioid drug users succumbing to overdose. A substantial proportion of these donor livers harbors HCV infection, leading to wider availability HCV-positive donors. Increased usage of those organs signals increasing confidence in the effectiveness of DAA therapy after LT. The addition of HCV positive organs to the donor pool may shorten waiting times and increase access to transplantation, especially for HCV patients. In our cohorts, HCV-positive LT recipients receiving HCV-positive grafts did increase from 7.2% to 13.0% over the study period. However, there was no appreciable reduction in waiting times or MELD at LT over time among those recipients. Along the same vein, with the ability to treat aggressive recurrent HCV in the graft, transplant programs may be using more organs that are thought to be high-risk, e.g. older donors, organs with macrosteatosis, and other extended criteria donors. These and other uncertainties, including the lack of HCV-specific information inherent in the registry data, prevent us from claiming a direct causality between HCV therapy and improved waitlist outcomes. Further studies are needed to characterize the response to HCV therapy in patients with HCV-related cirrhosis and its impact on the subsequent MELD trajectory in individual patients facing liver transplantation.
With those caveats, this is the first analysis, to our knowledge, to use a population-based database to show that survival and disease progression for patients with decompensated HCV cirrhosis are improved in the DAA era. The study takes advantage of the large sample size of the OPTN database, which provides outcome data as well as multiple data points to follow disease severity over time. The creation of time-based cohorts, inclusive only of patients on the waitlist on specific date, enables the detection of differences in clinical outcomes across time periods, and in particular, after the introduction of sofosbuvir and DAA-based therapy.
In conclusion, mortality for LT waiting list registrants with HCV has decreased in the DAA era, with concurrent attenuation in the progression of disease severity. This trend correlated with the availability of DAA-based antiviral therapy. These data, while ecological, represent the first evidence at a population level that DAA therapy has had an impact on hard end points such as survival and removal from the liver transplant waiting list, in addition to improvement in MELD score.
Supplementary Material
Acknowledgments
Financial support
This work was supported in part by a grant from the National Institute of Diabetes, Digestive and Kidney Disease (DK-34238 and T32 DK-007056). The funding organization played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
List of Abbreviations (in order of appearance)
- HCV
Hepatitis C
- HCC
hepatocellular carcinoma
- LT
liver transplantation
- SVR
sustained virologic response
- DAA
direct-acting antiviral
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- HR
hazard ratio
- CI
confidence interval
References
- 1.Saxena V, Terrault N. Current Management of Hepatitis C Virus: Regimens for Peri-Liver Transplant Patients. Clin Liver Dis. 2015;19:669–688. vi. doi: 10.1016/j.cld.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 4.Udompap P, Mannalithara A, Heo NY, Kim D, Kim WR. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J Hepatol. 2016;64:1027–32. doi: 10.1016/j.jhep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggins SW, Bambha KM, Terrault NA, Inadomi J, Shiboski S, Dodge JL, Gralla J, et al. Projected future increase in aging hepatitis C virus-infected liver transplant candidates: a potential effect of hepatocellular carcinoma. Liver Transpl. 2012;18:1471–1478. doi: 10.1002/lt.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15(Suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 7.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 8.Carrion JA, Navasa M, Garcia-Retortillo M, Garcia-Pagan JC, Crespo G, Bruguera M, Bosch J, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746–1756. doi: 10.1053/j.gastro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 9.Xirouchakis E, Triantos C, Manousou P, Sigalas A, Calvaruso V, Corbani A, Leandro G, et al. Pegylated-interferon and ribavirin in liver transplant candidates and recipients with HCV cirrhosis: systematic review and meta-analysis of prospective controlled studies. J Viral Hepat. 2008;15:699–709. doi: 10.1111/j.1365-2893.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 10.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, Fried MW, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Mathur AK, Ashby VB, Fuller DS, Zhang M, Merion RM, Leichtman A, et al. Variation in access to the liver transplant waiting list in the United States. Transplantation. 2014;98(1):94–9. doi: 10.1097/01.TP.0000443223.89831.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vutien P, Hoang J, Brooks L, Nguyen NH, Nguyen MH. Racial Disparities in Treatment Rates for Chronic Hepatitis C: Analysis of a Population-Based Cohort of 73,665 Patients in the United States. Medicine (Baltimore) 2016;95(22):e3719. doi: 10.1097/MD.0000000000003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu M, Li J, Rupp LB, Zhou Y, Holmberg SD, Moorman AC, et al. Changing trends in complications of chronic hepatitis C. Liver Int. 2017 doi: 10.1111/liv.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49(8):690–6. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 15.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17(Suppl 1):174–251. doi: 10.1111/ajt.14126. [DOI] [PubMed] [Google Scholar]
- 16.Bourliere M, Bronowicki JP, de Ledinghen V, Hezode C, Zoulim F, Mathurin P, Tran A, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15:397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 17.Reddy KR, Bourliere M, Sulkowski M, Omata M, Zeuzem S, Feld JJ, Lawitz E, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology. 2015;62:79–86. doi: 10.1002/hep.27826. [DOI] [PubMed] [Google Scholar]
- 18.Bunchorntavakul C, Reddy KR. Management of Hepatitis C Before and After Liver Transplantation in the Era of Rapidly Evolving Therapeutic Advances. J Clin Transl Hepatol. 2014;2:124–133. doi: 10.14218/JCTH.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunchorntavakul C, Tanwandee T. Treatment of Chronic Hepatitis C in Special Populations. Gastroenterol Clin North Am. 2015;44:883–900. doi: 10.1016/j.gtc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, Liapakis A, et al. Safety and Efficacy of Sofosbuvir-Containing Regimens in Hepatitis C Infected Patients with Impaired Renal Function. Liver Int. 2016;36:807–816. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 22.Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann HL, et al. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419–1427. doi: 10.1016/j.hep.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 23.Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692–699. doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017;152:142–156. e142. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 25.van der Meer AJ, Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol. 2016;65:S95–S108. doi: 10.1016/j.jhep.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, Morelli C, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65:524–531. doi: 10.1016/j.jhep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804–812. doi: 10.1002/hep.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.