Abstract
Background
The high levels of eicosanoid production and clinical efficacy of leukotriene-modifying pharmacotherapies for patients with aspirin-exacerbated respiratory disease suggests that other interventions targeting arachidonic acid dysregulation may also improve disease control.
Objective
To assess the utility of a high omega-3/low omega-6 diet for the treatment of AERD.
Methods
Prospective, non-blinded dietary intervention in 10 adult patients with AERD at Brigham and Women’s Hospital in Boston, MA. The primary objective was for subjects to reduce dietary omega-6 fatty acid consumption to less than 4 grams per day and increase omega-3 intake to more than 3 grams per day. The primary outcome was change in urinary LTE4, with changes in other eicosanoids, platelet activation, lung function, and changes in patient-reported questionnaires also assessed.
Results
Of the 10 subjects that screened for the study, all 10 completed the dietary intervention. Urinary LTE4 decreased 0.17 ng/mg (95% CI, −0.29 to −0.04; P = 0.02) and tetranor PGD-M decreased 0.66 ng/mg creatinine (95% CI, −1.21 to −0.11; P = 0.02). There was a 15.1-point reduction in SNOT-22 score (95% CI, −24.3 to −6.0; P = 0.01), a 0.27-point reduction in ACQ-7 score (95% CI, −0.52 to −0.03; P = 0.03), and no change in FEV1 % predicted (P = 0.92) or FVC % predicted (P = 0.74). All patients lost some weight over the two-week intervention period, and there were no diet-associated adverse events.
Conclusions
A high omega-3/low omega-6 diet may be an appropriate adjunct treatment option for patients with AERD.
Keywords: Aspirin-exacerbated respiratory disease, AERD, Samter’s triad, diet, asthma, nasal polyps, aspirin, NSAIDs, fatty acids, omega-3, omega-6, LTE4
INTRODUCTION
Aspirin-exacerbated respiratory disease (AERD) is characterized by adult-onset asthma, chronic rhinosinusitis with nasal polyposis, and clinical reactions to aspirin and other cyclooxygenase-1 (COX-1) inhibitors. Dysregulation of arachidonic acid metabolism plays an integral role in the pathogenesis of AERD; excessive production of cysteinyl leukotrienes and other inflammatory lipid mediators, including prostaglandin D2 (PGD2), are responsible for the hallmark respiratory and sino-nasal symptoms at baseline and during aspirin-induced reactions.1 Dietary fatty acids (FAs) are requisite precursors in the generation of arachidonic acid, and thus dietary modifications may provide therapeutic relief for patients with AERD. Leukotrienes and prostaglandins are generated by the metabolism of arachidonic acid (20:4n-6), a polyunsaturated omega-6 FA, through the enzymatic activity of 5-lipoxygenase and cyclooxygenase, respectively. The amount of arachidonic acid in inflammatory cells is directly correlated to the dietary intake of arachidonic acid and of its precursor linoleic acid (18:2n-6), also an omega-6 FA,2,3 and increased consumption of polyunsaturated long-chain omega-3 FAs, such as eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) present largely in fish oil, increases the proportions of omega-3 FAs in inflammatory cells. As this reduces the amount of arachidonic acid substrate available for synthesis of the leukotrienes, supplementation of the diet with omega-3 FAs has been shown to result in decreased generation of inflammatory leukotrienes.4,5
In the United States, a thousand-fold increase in the prevalence of corn and soybean oil over the past century has led to a sizeable shift in the ratio of omega-6 to omega-3 FA consumption. Although in the early 20th century it is estimated that humans generally consumed food sources with roughly equivalent omega-6 and omega-3 FA content, the ratio in modern Western diets is now estimated to be as high as 30:1. This change has been mirrored by a reduction in the proportion of omega-3 FAs present in tissue phospholipids.6 A robust body of evidence suggests that omega-3 FAs aid in the prevention and management of several diseases, and the disequilibrium of dietary FA consumption may be detrimental to human health.7 Although many studies have examined the effect of omega-3 FA supplementation on various disease states, the role of reducing dietary omega-6 FA consumption remains poorly understood and is worthy of further consideration.
In this trial, we sought to decrease the availability of arachidonic acid, and therefore the generation of downstream lipid mediators, through a two-week dietary intervention aimed at restoring a more balanced omega-6 to omega-3 FA ratio. The clinical efficacy of zileuton, a medication that blocks 5-lipoxygenase enzymatic activity and inhibits leukotriene biosynthesis, supports this approach.8,9 Zileuton has been shown to decrease urinary leukotriene E4 (LTE4) by up to 36%, and in one study of aspirin-intolerant asthmatics, a two-week course of zileuton increased lung function by an average of 9% compared to placebo and provided improvements in sense of smell and rhinorrhea.9 These data suggest that other leukotriene-modifying interventions may also provide clinical benefit to patients with AERD.
We hypothesized that strict adherence to a diet rich in omega-3 FAs and deficient in omega-6 FAs could diminish the downstream production of both cysteinyl leukotrienes and PGD2, and thereby improve upper and lower airway symptoms in patients with AERD. To test this hypothesis, we designed a prospective pilot trial and assessed the feasibility of a dietary intervention aimed at markedly decreasing the ratio of omega-6 to omega-3 FA consumption.
METHODS
Study Design
In this prospective trial, ten subjects were recruited to complete three in-person visits over a four-week study period. Following a screening visit to confirm study eligibility and obtain written informed consent, subjects began Phase I, a two-week period during which they were instructed to maintain their ‘normal’ diet without alteration. The inclusion of Phase I allowed for standardized collection of baseline nutrition data. In Phase II, the two-week treatment period, subjects were asked to (a) increase their daily omega-3 FA intake above three grams per day (either through the frequent addition of wild-caught fish to their diet, or through fish oil supplementation) and (b) decrease their omega-6 FA consumption below four grams per day. At the start of Phase II, subjects were counseled by the study team and provided with educational materials on how to achieve these objectives. A summary of dietary recommendations appears in Table 1. Subjects maintained a daily food and drink diary for the duration of the trial, and entries were reviewed in detail during weekly telephone consultations with a Registered Dietitian at the Center for Clinical Investigation Nutrition Core at Brigham and Women’s Hospital. Each subject received a total of $150 in compensation to help offset study-related costs, including fish oil supplements and other food items. This trial was approved the Partners Human Research Committee (Protocol 2013P002683) and is registered with ClinicalTrials.gov (Identifier NCT02064738).
Table 1.
Summary of dietary recommendations
| Recommended |
| Wild-caught, cold-water, oily fish (salmon, sardines, mackerel, herring, anchovies, tuna) |
| Fat-free dairy products |
| Egg whites |
| Dark leafy green vegetables (Brussels sprouts, kale, spinach, broccoli, salad greens) |
| Other vegetables (cabbage, turnips, green beans, carrots, sweet potatoes, squash) |
| Raw fruits |
| Ground flaxseeds or flaxseed oil |
| Wild-caught fish oils |
| Suggested in limited quantities |
| Kidney beans, mungo beans, black beans, pinto beans |
| Other beans (cowpeas, navy beans, lentils, lima beans, split peas) |
| Potatoes |
| White rice and grains (barley) |
| Olive oil |
| Butter from grass-fed cows |
| Discouraged |
| Meat |
| Poultry |
| Fat-containing dairy products (regular milk, cheese, and yogurt) |
| Egg yolks |
| Peanuts and peanut butter |
| Tree nuts (almond, cashew, pistachio) |
| Avocados |
| Fried food |
| High-fat sweets and desserts |
| Margarine and vegetable oils, including corn oil, grape seed oil, soybean oil, safflower oil, sunflower, and cottonseed oil |
Participant Selection and Eligibility
This study was conducted between April 2014 and April 2017 at the Brigham and Women’s Hospital in Boston, MA. Inclusion criteria specified that eligible participants were between the ages of 18 and 70 with a physician diagnosis of AERD, defined as a current history of asthma, nasal polyposis, and at least one physician-observed reaction to oral aspirin or other non-selective cyclooxygenase inhibitor with features of lower and/or upper airway involvement. Subjects were excluded if they were current tobacco smokers, were pregnant or breastfeeding, had a body mass index (BMI) < 18.5, had fish allergy or were unwilling to eat fish during the treatment period, had an implanted defibrillator, used zileuton or oral steroids within the two weeks prior to screening, or anticipated the use of any cyclooxygenase inhibitors during the study.
Interventions and Measurements
The Asthma Control Questionnaire (ACQ-7) and the Sino-Nasal Outcome Test (SNOT-22) are validated questionnaires that were administered at each study visit to assess the severity of respiratory and sino-nasal symptoms, respectively. The published minimally important difference for ACQ-7 is 0.5 points10 and for SNOT-22 is 8.9 points;11 these thresholds were used to provide clinical context to our findings. Vital signs, including height and weight, were collected at each visit. Pulmonary function tests (PFTs) were performed at all three visits with a CareFusion Micro I spirometer to measure forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). Blood samples were collected at Visit 2 (baseline) and Visit 3 (post-treatment) to quantify plasma lipid levels, blood eosinophil counts, and platelet activation. Similarly, urine samples were collected pre- and post-treatment to measure urinary LTE4, tetranor PGD-M, an endogenous metabolite of PGD2 and PGE-M, an endogenous metabolite of PGE2. Participants were encouraged to maintain their usual medications throughout the trial, and they were interrogated at each visit about the occurrence of any potential adverse events.
Plasma samples were analyzed at the University of California, San Diego Lipidomics Core by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) as previously described.12 Urinary samples for LTE4, PGD-M, and PGE-M were analyzed by mass spectrometric assay at the Vanderbilt University Eicosanoid Core Laboratory as previously described.13 Complete blood counts with differentials were performed by LabCorp (Raritan, NJ).
To monitor platelet activation (surface expression of CD62P on free platelets), peripheral blood was drawn into heparinized tubes, kept at room temperature, and assayed within 1 hour of collection. Platelet-rich plasma (PRP) was obtained from the top layer of blood samples after a 20-minute centrifuge at 200g. We incubated 10 μL of PRP with directly-conjugated antibodies specific for CD61 and CD62P, or appropriate isotype controls (BD Biosciences) for 20 minutes, then fixed the cells in 1% paraformaldehyde. At least 50,000 platelets were recorded for each sample in a FACSAria flow cytometer (BD Biosciences).
End Points
The primary endpoints were changes in urinary LTE4 and plasma LTB4. Secondary endpoints were changes in patient-reported questionnaires (ACQ-7 and SNOT-22) and pulmonary function (FEV1 and FVC). Exploratory endpoints included changes in urinary prostaglandin metabolites, blood eosinophil count, and platelet activation. All end points were pre-specified and calculated as the change between Visit 2 (baseline) and Visit 3 (post-treatment).
Statistical Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at Brigham and Women’s Hospital. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies.14 Quantitative differences between treatment phases were analyzed using the paired Student’s t-test (alpha = 0.05), and all data were analyzed in GraphPad Prism version 7.03 for Windows, GraphPad Software, La Jolla California USA. Data are represented as the mean and 95% confidence interval unless stated otherwise. Treatment assignments and secondary clinical outcomes were not blinded to the subject or study team; however, biomarkers for the primary and exploratory end points including urinary and plasma lipid levels and platelet activation were analyzed without knowledge of the corresponding subject ID or collection time point.
RESULTS
Baseline Characteristics of the Study Population
Baseline characteristics are summarized in Table 2. All ten study subjects lost body weight during the two-week intervention phase, with an average loss of 3.25 pounds (95% CI, −4.94 to −1.57; P = 0.002). Likewise, average BMI also significantly decreased by 0.58 (95 % CI, −0.99 to −0.06, P = 0.03). Nine of ten subjects were female. Although female participants were over-represented in our pilot trial, AERD is known to be more prevalent in women compared to men. In our AERD patient registry with 546 patients, 344 (63.0%) are female.
Table 2.
Baseline demographics and clinical characteristics of the study population
| Subject | Age (Yr) | Sex | Race | BMI | Montelukast (Y/N) | Lifetime polypectomies (No.) | ICS Use (mcg/day)* |
|---|---|---|---|---|---|---|---|
| 01 | 31 | F | White | 19.46 | N | 3 | 0 |
| 02 | 33 | F | White | 19.79 | N | 1 | 0 |
| 03 | 44 | F | White | 29.42 | Y | 7 | 1760 |
| 04 | 57 | M | White | 26.23 | Y | 2 | 0 |
| 05 | 55 | F | Black | 33.47 | Y | 1 | 940 |
| 06 | 65 | F | Black | 22.36 | Y | 2 | 1240 |
| 07 | 46 | F | White | 20.48 | N | 1 | |
| 08 | 59 | F | White | 23.93 | Y | 1 | 220 |
| 09 | 63 | F | White | 30.95 | N | 200 | |
| 10 | 54 | F | White | 23.68 | Y | 2 | 320 |
| Median | 54.5 | – | – | 23.81 | – |
ICS use is calculated as fluticasone equivalent dose.
Compliance with the Dietary Intervention
All ten subjects that were screened for the study met eligibility criteria and completed enrollment. Participants demonstrated greater than 95% compliance with recording their daily food and drink diary, defined as percent of days with complete records. Nutrient intake data was calculated from the subject diaries by a registered dietitian and is summarized in Table 3. Overall, participants achieved their goal of increasing omega-3 FA intake to greater than 3 grams per day while decreasing omega-6 FA intake below 4 grams per day. Dietary omega-3 was increased by an average of 3.5 g/day (95% CI, 0.9 to 6.1; P = 0.01) from Phase I to Phase II, while dietary omega-6 was simultaneously reduced by 10.7 g/day (95% CI, −12.9 to −8.6, P = <0.001) over the same time interval (Figure 1). Accordingly, the omega-6:omega-3 ratio decreased 10-fold (from 7.3 to 0.7) in Phase II compared to Phase I. Daily total energy consumed decreased by an average of 385.7 kcal/day (95% CI, −526.8 to −244.5; P = 0.001) during the study intervention. Although study subjects consumed similar quantities of protein (P = 0.08) and carbohydrates (P = 0.97) in both phases, overall fat intake was substantially reduced in Phase II (P <0.001).
Table 3.
Nutrient intake pre- vs. post-treatment
| Nutrients | Phase I (Normal Diet) | Phase II (Treatment Diet) | P Value |
|---|---|---|---|
| Carbohydrates, g/day | 200.0 ± 54.4 | 199.0 ± 70.9 | 0.97 |
| % of energy | 45.2 ± 7.1 | 57.1 ± 10.6 | – |
| Protein, g/day | 77.6 ± 12.3 | 71.1 ± 15.0 | 0.08 |
| % of energy | 17.4 ± 2.2 | 20.4 ± 4.3 | – |
| Total fat, g/day | 73.4 ± 16.1 | 32.9 ± 18.5 | <0.001 |
| % of energy | 37.3 ± 6.6 | 21.2 ± 8.1 | – |
| Saturated fat, g/day | 23.4 ± 8.1 | 9.4 ± 7.0 | 0.001 |
| MUFA, g/day | 27.3 ± 6.0 | 9.1 ± 6.2 | <0.001 |
| PUFA, g/day | 16.7 ± 4.5 | 8.9 ± 6.1 | <0.001 |
| Total Omega-3, g/day | 2.0 ± 0.6 | 5.5 ± 4.0 | 0.01 |
| from food sources, g/day | 2.0 | 3.8 | – |
| from supplements, g/day | 0.0 | 1.7 | – |
| Total Omega-6, g/day | 14.5 ± 4.2 | 3.8 ± 2.6 | <0.001 |
| from food sources, g/day | 14.5 | 3.8 | – |
| from supplements, g/day | 0 | 0 | – |
| Total energy, kcal/day | 1787.8 ± 333.3 | 1393.5 ± 463.8 | <0.001 |
Data are presented as mean ±STDEV.
Figure 1. Omega-3 and omega-6 FA intake during two-week dietary intervention.
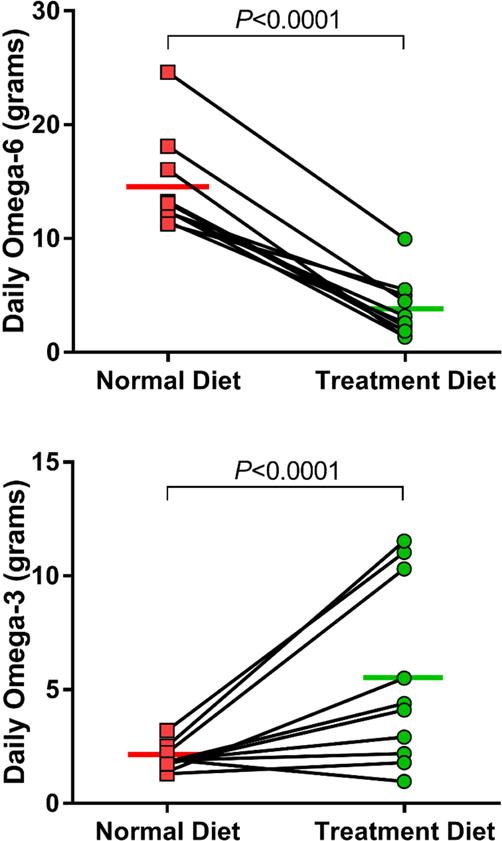
Change in average daily nutritional intake of omega-6 (TOP) and omega-3 (BOTTOM) fatty acids are shown. Individual data points are shown with means for each phase.
Laboratory End Points
Urinary LTE4 decreased by 0.17 ng/mg creatinine after the two-week dietary intervention period compared to baseline (95% CI, −0.29 to −0.04; P = 0.02), and there was a notable decrease in the production of tetranor PGD-M of 0.66 ng/mg creatinine (95% CI, −1.21 to −0.11; P = 0.02) (Figure 2); there was no change in the production of PGE-M (data not shown). Plasma LTB4 was undetectable in 75% of the samples tested, and therefore could not be analyzed. Plasma levels of the free omega-3 FAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) increased significantly by 4-fold (P = 0.016) and 2-fold (P = 0.015), respectively. Additionally, the combined plasma levels of detectable EPA-derived lipid metabolites (5-HEPE, 15-HEPE, 12-HEPE, 14(15)-EpETE) increased by 2-fold (P = 0.041), as did the plasma levels of DHA-derived metabolites (Resolvin D1, 7,17-dHDPA, 19(20)-EpDPE, 19,20-DiHDPA) (P < 0.001).
Figure 2. Urinary LTE4 and PGD-M levels during two-week dietary intervention.
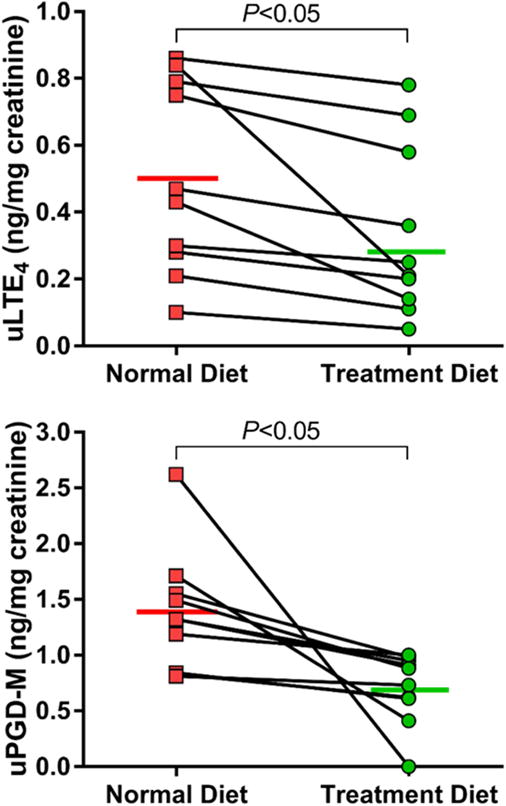
Change in uLTE4 (TOP) and uPGD-M (BOTTOM) levels are shown. Individual data points are shown with means for each phase.
Absolute blood eosinophil count remained unchanged after the treatment diet, with a baseline average of 540 eosinophils/μL and a post-treatment diet average of 510/μL (mean of differences = −0.03; 95% CI, −0.16 to 0.10; P = 0.60). Platelet activation levels of free plasma platelets were also unchanged on the treatment diet, with a baseline average of 29.9% CD62P+ platelets and a post-treatment diet average of 31.8% CD62P+ platelets (mean of differences = 1.9; 95% CI, −5.0 to 8.8; P = 0.55).
Clinical End Points
For patient-reported outcomes, SNOT-22 score decreased by 15.1 points (95% CI, −24.3 to −6.0; P = 0.01), and ACQ-7 score decreased by 0.27 points (95% CI, −0.52 to −0.03; P = 0.03) (Figure 3). The decrease in SNOT-22 score is clinically significant; the improvement in ACQ-7 score, although statistically significant, does not meet the published threshold for clinical significance.10,11 There were no significant correlations between clinical symptom improvement and total change in uLTE4, u-PGD-M, or quantified fatty acid consumption. No differences were observed with respect to the pulmonary function tests (FEV1 % predicted: mean of differences = −0.1 %; 95% CI, −3.3 to 3.0; P = 0.92; FVC % predicted: mean of differences = 0.5 %; 95% CI, −2.9 to 3.9; P = 0.74). No adverse events were reported in either the pre- or post-treatment phases.
Figure 3. SNOT-22 and ACQ scores during two-week dietary intervention.
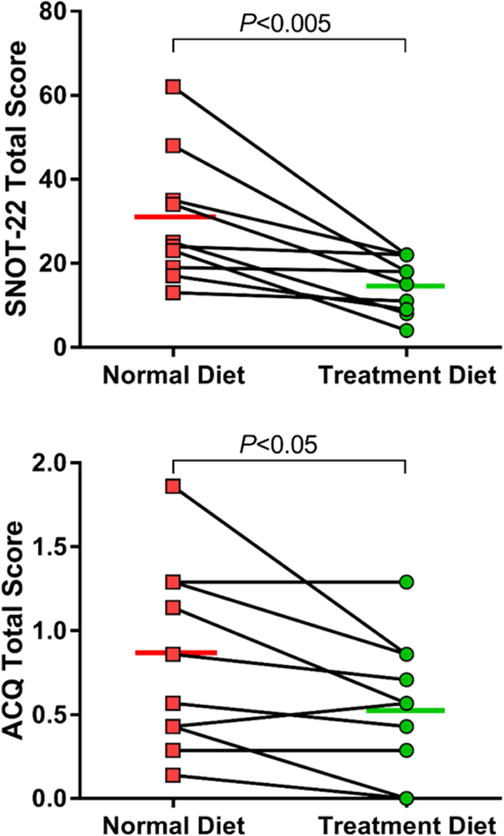
Change in patient-reported Sino-Nasal Outcome Test-22 (TOP) and Asthma Control Questionnaire (BOTTOM) scores are shown. Individual data points are shown with means for each phase.
DISCUSSION
In the two-week intervention period, we found that a diet high in omega-3 fatty acids and low in omega-6 fatty acids was generally safe and well-tolerated, improved AERD-associated symptoms, and decreased levels of pro-inflammatory biomarkers. We were able to demonstrate that the dietary intervention changed cellular fatty acid composition sufficiently to provide for a measurable reduction in both LTE4 and PGD-M, two arachidonic acid-derived inflammatory lipids relevant in AERD,13,15 while maintaining unchanged levels of the metabolite of PGE2, which may be protective in the disease.16,17 Furthermore, we found evidence that the dietary intervention also increased production of omega-3 fatty acid-derived lipid metabolites, including resolving D1, which may promote the resolution of chronic airway inflammation.18 This combination of biochemical changes allowed for the improved symptomatic control reported by the patients in this study.
Previous literature has remained inconclusive about the protective role of omega-3 supplementation on asthma control and pulmonary function. Our subjects failed to show improvement in their pulmonary function tests after the two-week treatment period. This result is consistent with a study by Brannan et al. that showed that fish oil supplementation in a population of asthmatics did not produce a change in FEV1 percent predicted compared to placebo after three weeks of supplementation.19 However, two studies by Mickleborough et al. demonstrated a protective effect of fish oil supplementation on exercise-induced bronchoconstriction in adult asthmatics20 and in a cohort of elite athletes.21 The discrepancy in our results may be attributed to the differences in subject phenotypes as well as the heterogeneic nature of asthma. Also, our patients had generally normal FEV1 values, and reversibility of bronchoconstriction was not an inclusion criterion, so large changes in FEV1 were not necessarily expected.
To date, only two AERD-specific diet trials have been published, both by Sommer et al. for evaluation of a low-salicylate diet.22,23 In the larger of their two studies, a total of 30 patients were randomly assigned to either maintain their regular diet or start on a low-salicylate diet for six weeks, after which they crossed over into the opposite treatment arm an additional six weeks. Compared to the regular diet arm, patients randomized to the low-salicylate diet did demonstrate improvement with respect to several subjective and objective measurements.23 The biological rationale for a low-salicylate diet in the treatment of AERD, however, is unclear. Aspirin (acetylsalicylic acid)-induced respiratory reactions are triggered by irreversible inhibition of COX-1 activity; acetylation of serine 530 creates a steric hindrance that blocks entry of arachidonic acid into the enzyme’s active site.24 The generation of salicylic acid occurs downstream of COX-1 inhibition and is therefore unlikely to contribute to AERD-related symptoms. There is no known mechanism by which dietary salicylates would perturb the biological pathways implicated in the pathogenesis of AERD.
The current study does have several limitations. First, the intervention period was just two weeks in length, in part because we were uncertain about the long-term feasibility of adhering to the study diet. The observed weight loss would likely be unsustainable if the diet were followed for an indefinite period. Future studies should introduce additional measures to ensure that participants consume enough protein and carbohydrates to balance the net reduction in dietary fats and thus maintain adequate total caloric intake. Second, because the study diet required substantial dietary modifications, it was not practical to blind the participants or the study staff to the intervention. Third, the small sample size and predominantly female study population may limit the generalizability of the results. Fourth, disparities in access to healthy food options may hinder widespread implementation of this diet, and the cost of fish oil supplements and other recommended foods such as wild-caught fish or animal products from grass-fed sources may be prohibitive for some patients.
Strengths of this study include meticulous documentation and accounting of nutrient intake as well as excellent subject adherence to the target omega-3 and omega-6 FA objectives. Although there exists a large body of research examining the health effects of omega-3 supplementation, information about the role of dietary omega-6 reduction remains scarce. Our study is novel because it emphasized the importance of reducing omega-6 consumption in conjunction with omega-3 supplementation to maintain a more historically natural balance between the two.
The treatment options for AERD remain limited. Current management includes nasal polypectomy, aspirin desensitization, nasal and oral corticosteroids, leukotriene-modifying agents, monoclonal asthma medications such as omalizumab and mepolizumab, and inhaled corticosteroids.25 Our study takes an important step toward expanding the arsenal of available treatment options available to patients and clinicians. Specifically, with the decrease of both urinary LTE4 and PGD-M, combined with the increase in pro-resolving lipid metabolites, this dietary approach has several biochemical advantages over the existing anti-leukotriene medications. Our results generally support the hypothesis that a diet high in omega-3 FAs and low in omega-6 FAs may be a viable non-pharmaceutical option for patients looking to augment their standard treatment plan. Additional studies are warranted to more precisely determine the effect size of our findings and to assess the long-term feasibility of a low omega-6/high omega-3 diet.
Table 4.
Study outcomes
| End Point | Visit 2 (Baseline) | Visit 3 (Post-Treatment) | P Value |
|---|---|---|---|
| Laboratory end points | |||
| Urinary LTE4, ng/mg creatinine | 0.50 ± 0.39 | 0.34 ± 0.26 | 0.02 |
| Urinary PGD2, ng/mg creatinine | 1.37 ± 0.54 | 0.71 ± 0.32 | 0.03 |
| Blood EOS, absolute | 0.54 ± 0.29 | 0.51 ± 0.27 | 0.60 |
| Platelet activation (%CD62P+) | 29.9 ± 9.6 | 31.8 ± 13.8 | 0.55 |
| Clinical end points | |||
| ACQ | 0.83 ± 0.55 | 0.56 ± 0.40 | 0.03 |
| SNOT-22 | 30.0 ± 15.2 | 14.9 ± 6.6 | 0.02 |
| FEV1, L | 2.36 ± 0.42 | 2.37 ± 0.45 | 0.70 |
| FEV1, % predicted | 85.0 ± 11.6 | 84.9 ± 11.4 | 0.92 |
| FVC, L | 3.11 ± 0.45 | 3.16 ± 0.51 | 0.37 |
| FVC, % predicted | 90.7 ± 12.7 | 91.2 ± 11.6 | 0.74 |
Data are presented as mean ±STDEV.
HIGHLIGHTS BOX.
- 1. What is already known about this topic?
- It is known that patients with AERD over-produce the pro-inflammatory lipids, LTE4 and PGD2, and that these lipids are derived from metabolism of dietary sources of omega-6 fatty acids.
- 2. What does this article add to our knowledge?
- This study demonstrates that in patients with AERD, dietary modifications to increase omega-3 and decrease omega-6 fatty acid consumption can decrease their systemic production of LTE4 and PGD2, and can lead to improved respiratory symptom control.
- 3. How does this study impact current management guidelines?
- Our results suggest that a diet high in omega-3 and low in omega-6 fatty acids may be a viable non-pharmaceutical adjunct for patients with AERD.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (Grants K23HL111113, K23AI118804, R01HL128241, and T32AI007306-32, and U19AI095219-07)
Abbreviations
- AERD
(aspirin-exacerbated respiratory disease)
- COX-1
(cyclooxygenase-1)
- FA
(fatty acid)
- LTE4
(leukotriene E4)
- BMI
(body mass index)
- ACQ-7
(Asthma Control Questionnaire)
- SNOT-22
(Sino-Nasal Outcome Test)
- PFT
(pulmonary function test)
- FEV1
(forced expiratory volume in one second)
- FVC
(forced vital capacity)
- LTB4
(leukotriene B4)
- PGDM
(tetranor prostaglandin D-M)
- PGD2
(prostaglandin D2)
- PRP
(platelet-rich plasma)
- UPLC-MS/MS
(ultra-performance liquid chromatography-tandem mass spectrometry)
- REDCap
(Research Electronic Data Capture)
- EPA
(eicosapentaenoic acid)
- DHA
(docosahexaenoic acid)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laidlaw TM, Boyce JA. Aspirin-Exacerbated Respiratory Disease–New Prime Suspects. The New England journal of medicine. 2016;374(5):484–488. doi: 10.1056/NEJMcibr1514013. [DOI] [PubMed] [Google Scholar]
- 2.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001;131(7):1918–1927. doi: 10.1093/jn/131.7.1918. [DOI] [PubMed] [Google Scholar]
- 3.Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Arachidonic acid supplementation enhances synthesis of eicosanoids without suppressing immune functions in young healthy men. Lipids. 1998;33(2):125–130. doi: 10.1007/s11745-998-0187-9. [DOI] [PubMed] [Google Scholar]
- 4.Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese J, Spur BW, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. The New England journal of medicine. 1985;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- 5.von Schacky C, Kiefl R, Jendraschak E, Kaminski WE. n-3 fatty acids and cysteinyl-leukotriene formation in humans in vitro, ex vivo, and in vivo. J Lab Clin Med. 1993;121(2):302–309. [PubMed] [Google Scholar]
- 6.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70(3 Suppl):560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 8.Laidlaw TM, Fuentes DJ, Wang Y. Efficacy of zileuton in patients with asthma and history of aspirin sensitivity: A retrospective analysis of data from two phase 3 studies. J Allergy Clin Immunol. 2017;139(2):1. [Google Scholar]
- 9.Dahlen B, Nizankowska E, Szczeklik A, Zetterstrom O, Bochenek G, Kumlin M, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. American journal of respiratory and critical care medicine. 1998;157(4 Pt 1):1187–1194. doi: 10.1164/ajrccm.157.4.9707089. [DOI] [PubMed] [Google Scholar]
- 10.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respiratory medicine. 2005;99(5):553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical otolaryngology: official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 12.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51(11):3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119(16):3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology. 2015;135(1):245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(42):16987–16992. doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahill KN, Raby BA, Zhou X, Guo F, Thibault D, Baccarelli A, et al. Impaired E Prostanoid2 Expression and Resistance to Prostaglandin E2 in Nasal Polyp Fibroblasts from Subjects with Aspirin-Exacerbated Respiratory Disease. Am J Respir Cell Mol Biol. 2016;54(1):34–40. doi: 10.1165/rcmb.2014-0486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy BD. Resolvin D1 and Resolvin E1 Promote the Resolution of Allergic Airway Inflammation via Shared and Distinct Molecular Counter-Regulatory Pathways. Front Immunol. 2012;3:390. doi: 10.3389/fimmu.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brannan JD, Bood J, Alkhabaz A, Balgoma D, Otis J, Delin I, et al. The effect of omega-3 fatty acids on bronchial hyperresponsiveness, sputum eosinophilia, and mast cell mediators in asthma. Chest. 2015;147(2):397–405. doi: 10.1378/chest.14-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129(1):39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]
- 21.Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003;168(10):1181–1189. doi: 10.1164/rccm.200303-373OC. [DOI] [PubMed] [Google Scholar]
- 22.Sommer DD, Hoffbauer S, Au M, Sowerby LJ, Gupta MK, Nayan S. Treatment of aspirin exacerbated respiratory disease with a low salicylate diet: a pilot crossover study. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;152(1):42–47. doi: 10.1177/0194599814555836. [DOI] [PubMed] [Google Scholar]
- 23.Sommer DD, Rotenberg BW, Sowerby LJ, Lee JM, Janjua A, Witterick IJ, et al. A novel treatment adjunct for aspirin exacerbated respiratory disease: the low-salicylate diet: a multicenter randomized control crossover trial. International forum of allergy & rhinology. 2016;6(4):385–391. doi: 10.1002/alr.21678. [DOI] [PubMed] [Google Scholar]
- 24.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. The Journal of clinical investigation. 1975;56(3):624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchheit KM, Laidlaw TM. Update on the Management of Aspirin-Exacerbated Respiratory Disease. Allergy Asthma Immunol Res. 2016;8(4):298–304. doi: 10.4168/aair.2016.8.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]


