Abstract
O-GlcNAcylation is emerging as a critical regulatory post-translational modification, impacting proteins that regulate cell division, apoptosis, metabolism, cell signaling, and transcription. O-GlcNAc also affects biological homeostasis by integrating information coming from the environment, such as nutrient conditions and extracellular stimuli, with cellular response. Aberrant O-GlcNAc modulation has been linked to metabolic and neurodegenerative diseases, as well as cancers. While many studies have highlighted the significance of O-GlcNAc in cancer, a specific function for O-GlcNAc during tumorigenesis remains unclear and seems to differ according to cancer type. Herein, we review the impact of altered O-GlcNAcylation in breast, ovarian and uterine cancers.
Introduction
O-GlcNAcylation is a highly dynamic and reversible posttranslational modification of nuclear and cytoplasmic proteins. The two enzymes responsible for O-GlcNAc cycling are O-GlcNAc transferase (OGT), which adds a single N-acetylglucosamine sugar to serine and threonine residues, and O-GlcNAcase (OGA), which removes the modification. The widespread distribution of O-GlcNAc on tumor suppressor proteins and oncoproteins suggests that this modification may play an important role in the development of many diseases, including cancer. O-GlcNAcylation in diverse cancers has been reviewed elsewhere; this review will focus on O-GlcNAcylation in women’s cancers of the breast, ovary and uterus.
Breast Cancer
Breast cancer is a disease that will affect 1 in 8 women during their lifetime, and represents the second most common cancer diagnosed in women [1]. Therefore, understanding how breast cancer develops, and what drives the progression of breast cancer, is of great clinical interest. Levels of O-GlcNAcylation have been determined in many different epithelial tumors (reviewed in [2]), including lung [3], liver [4], colon [5], prostate [6], and ovarian [7] and breast (reviewed herein). For the majority of tumor types, O-GlcNAcylation is elevated in cancer, however it is apparent that the correlation between O-GlcNAcylation and tumorigenesis is cell-type dependent [2].
A growing body of evidence is accumulating that implicate O-GlcNAcylation in the development and progression of breast cancer. The earliest worked aimed at measuring O-GlcNAcylation in breast cancer was performed using breast cancer cell lines, such as SKBR3 and MDA-MB-453, as compared to a non-transformed mammary gland cell line, MCF10A. This work showed that global O-GlcNAc levels were elevated in the breast cancer cell lines, along with elevated expression of OGT [8]. Further analysis in breast cancer patient samples have confirmed this phenotype of elevated O-GlcNAcylation in breast cancer. Multiple studies using powerful tissue microarrays (TMA) constructed using breast tumor tissue, along with matched/adjacent benign tissue, revealed that O-GlcNAc levels were elevated in tumors [9, 10]. This was also observed via Western blotting from digested, pooled tumor samples (representing multiple grades) as compared to benign tissue [11]. Moreover, TMA data comparing primary breast tumors to breast metastases isolated from lymph nodes (n=50) revealed that O-GlcNAc levels were even further elevated in the metastatic breast cancer tissue [9]. Finally, increased levels of O-GlcNAcylation also correlated with poor prognosis, as a high extent of nuclear and cytoplasmic O-GlcNAc staining was observed in patients with increased relapse rates, increased sites of distant metastases and poor outcome [12]. Cumulatively, these data imply that O-GlcNAc levels are correlated with breast cancer progression, covering the spectrum from benign breast tissue to distant metastatic sites.
Although the data correlating high O-GlcNAc levels to breast cancer are compelling, they are correlative studies and don’t provide evidence that O-GlcNAc and associated proteins, such as OGT or OGA, are tumor-promoting vs tumor-associated. Further, many studies correlate high OGT, and low OGA, expression with breast cancer, using both tumor cell line models and patient samples (primarily TMA) [8, 11, 13–15]. The addition of numerous studies where OGT, and subsequently O-GlcNAc, levels were experimentally decreased have further implicated O-GlcNAc-modulating enzymes, such as OGT, in the process of tumorigenesis. Caldwell and colleagues used shRNA to decrease OGT levels in breast cancer cell lines, and found that lack of OGT decreased cell line growth in vitro, breast tumor xenograft formation in mice and cell cycle progression [8]. Similar results were seen when OGT knockdown lead to inhibition of anchorage-independent growth [11]. Gu et al employed a syngeneic mouse model in which OGT levels were decreased using shRNA [9]. In contrast to what was observed using the human xenograft models, no differences in primary tumor growth were observed when OGT was knocked-down using this model. However, they did observe a dramatic inhibition in breast cancer lung metastases when OGT levels were decreased [9]. Despite the observed inconsistencies in OGT’s effect on primary tumor growth, which are likely attributable to the differences in mouse tumor model systems, cumulatively these data suggest that OGT promotes the tumorigenic potential of breast cancer and is directly involved in promoting breast cancer.
Many diverse models have been put forth that explain how increased O-GlcNAc levels, potentiated OGT activity, and decreased OGA activity promote breast tumorigenesis; those models are presented in brief herein. Multiple papers from the Reginato lab have detailed a link between O-GlcNAc and stability of the oncogenic transcription factor, FoxM1 [8, 15]. O-GlcNAc regulates SIRT1 levels and activity, which in turn is linked to proteosomal degradation of FOXM1. Reducing cellular O-GlcNAc levels leads to instability of FOXM1, and SIRT1 activity is required for OGT’s tumor promoting potential in breast cancer. O-GlcNAc-dependent promotion of HIF1α stability has been shown to regulate pro-growth metabolic reprogramming and survival-associated stress signaling in breast cancer cells [15]. Breast cancer TMA data have correlated elevated O-GlcNAc levels with proteins that regulate glycosaminoglycan hyaluronan (HA) such as hyaluronan synthases 1-3 (HAS1-3; [12]), proteins known to be regulated by O-GlcNAcylation. As HA enhances tumor progression [16, 17], this suggests that high HAS levels seen in breast cancer are correlated to their increased O-GlcNAcylation [12]. Finally, Sodi et al investigated how OGT levels, themselves, are upregulated in breast cancer. They detail regulation of OGT protein expression in breast cancer through a c-MYC/mTOR-dependent post-transcriptional mechanism [18].
Multiple studies have been done modeling how O-GlcNAc promotes breast cancer invasion and metastasis. O-GlcNAcylation of cofilin, an actin-binding protein involved in cancer invasion through the regulation of cell motility, is required for its ability to promote breast cancer cell invasion [19]. Moreover, O-GlcNAcylation has been shown to decrease cell surface E-cadherins which enhances breast cancer cell migration and metastasis [9]. Recent studies by Liu and colleagues have shown that OGT expression is regulated by miR24; decreased OGT expression reduced breast cancer cell invasion. The authors also found that decreased OGT activity lead to destabilization of FOXA1, a key transcriptional regulator of proteins promoting the epithelial to mesenchymal transition (EMT) [20]. Finally, work by Tao et al in triple negative breast cancer cell lines has shown that O-GlcNAcylation of TAK1-binding protein 3 (TAB3), a novel regulator of the NF-κB pathway, promotes metastasis in these tumor models [21].
Other models for how O-GlcNAcylation promotes breast cancer involve regulation of two key transcription factors involved in breast cancer, the estrogen receptor (ER) and progesterone receptor (PR). These steroid-activated nuclear receptors are present in 70% of breast cancers upon diagnosis [1]. ER/PR expression is a positive predictive marker for therapeutic response, as targeted-therapies exist to decrease primarily ER/estrogen function in breast cancer (reviewed in [22, 23]). Of interest to this review, ER and PR have both been shown to be O-GlcNAcylated, and this modification leads to a decrease in RNA and protein levels of both receptors [10, 24–26]. This O-GlcNAc-dependent turnover of ER has been linked to resistance to ER-based targeted therapies [27]. Interestingly, the converse may be true for PR action in breast cancer. Although O-GlcNAcylation leads to a decrease in PR expression [10, 26], the remaining PR protein was shown to be more active at activating PR-dependent gene transcription [10]. Finally, in breast cancer TMA data, PR-positive tumors exhibited higher levels of OGT expression when compared to PR-negative tumors [10]. The development of ER- and PR-O-GlcNAc-specific antibodies will help define how ER/PR O-GlcNAcylation is relevant in human tumors.
Endometrial cancer
Endometrial cancer is the most common cancer of the female reproductive organs [28]. This particular condition is common among women suffering from hyperglycemia, however little is known about the underlying relationship between glucose and endometrial cancer [29–31]. Mounting data show that O-GlcNAcylation can be a sensor of the cell’s glucose status [32]. Numerous studies confirm that cancer cells increase their consumption of glucose, which is used in the hexosamine biosynthesis pathway (HBP) to generate UDP-GlcNAc, the metabolic substrate for O-GlcNAcylation. Therefore, unbalanced glucose metabolism can affect O-GlcNAcylation of proteins, leading to cellular dysfunctions and, possibly, cancer. Zhou et al [33] revealed that endometrial cancer cell lines treated with high levels of glucose showed increased cellular O-GlcNAcylation levels and expression of β-catenin, a key effector of the WNT/β-catenin pathway which plays an essential role in oncogenic gene transcription. Glucose-induced activation of the HBP enhanced β-catenin stability through its O-GlcNAcylation and increased the protein’s activity as a transcriptional coactivator. These data suggest that modulation of O-GlcNAcylation may be a potential target for the therapy of hyperglycemic endometrial cancer patients.
mRNA expression levels of O-GlcNAc cycling enzymes OGT and OGA have been found to be significantly higher in undifferentiated endometrial tumors compared to well-differentiated ones [34]. However, Sgantzos et al [35] showed that immunohistochemical expression of O-GlcNAc fluctuates in normal and neoplastic uterine tissues, which may reflect differences in the expression of O-GlcNAcylated cellular proteins. Their work implies that the role of O-GlcNAc in oncogenesis may be mediated by addition or removal of this modification on tumor-related proteins and sheds light on the relevance of this modification in normal histophysiology and in the pathogenesis of uterine muscle cell tumors.
Ovarian Cancer
Ovarian cancer is a malignancy that frequently remains clinically silent and is usually diagnosed at an advanced stage, which renders it the fifth most common cause of cancer-associated mortality in females due to a high rate of metastasis [36, 37]. Accumulating evidence suggests that O-GlcNAcylation may play a significant role in tumor metastasis, and multiple studies have investigated the significance of this modification in cell migration [38, 39]. When comparing highly-metastatic (HO-9810PM) to low-metastatic (OVCAR3) ovarian cancer cell lines, Jin et al [38] found that expression of OGT mRNA levels and O-GlcNAcylation levels of total proteins were higher in the cell lines with greater metastatic potential. To investigate whether O-GlcNAcylation affected cell migration, total O-GlcNAc levels were decreased using OGT shRNA in HO-9810PM cells and increased using OGA inhibitors in OVCAR3 cells. Migration capacity was significantly inhibited in HO-9810PM cells and increased in OVCAR3 cells, suggesting that O-GlcNAcylation is linked to this cellular feature relevant to metastasis. The group investigated these cells for levels of E-cadherin, a protein previously shown to be O-GlcNAcylated in ovarian cancer [40] and a key regulator of epithelial cell-cell adhesion, and they found that its expression was decreased in the context of high O-GlcNAcylation. This finding suggests that O-GlcNAcylation may promote loss of cell adhesion, a crucial requirement for tumor invasion, by compromising the formation of the E-cadherin/catenin/p120 complex. Another model put forth by Niu et al [39] showed that O-GlcNAc upregulation may promote the migration and invasion of ovarian cancer cells by activating the RhoA/ROCK/MLC pathway, a signaling cascade which has been found to be upregulated in several types of cancer, including bladder, gastric, testicular, breast, and ovarian.
Ovarian cancer has one of the highest p53 mutation rates and many studies have shown that defects in the p53 pathway are intrinsically related to cell malignancy and tumor development [41, 42]. p53 was found to be O-GlcNAcylated and stabilized by this modification [43]. Muniz de Queiroz and colleagues aimed at identifying a link between O-GlcNAc homeostasis (the balance between addition and removal of O-GlcNAc) and wild type p53 in ovarian cancer [7]. They observed that any change in O-GlcNAc cycling, either through inhibition or silencing of OGA, or overexpression of OGT (O-GlcNAc levels increase) or overexpression of OGA (O-GlcNAc levels decrease), resulted in stabilization of wild type p53 and increased activation of the p53 pathway. These results suggest that changes in O-GlcNAc homeostasis may trigger stress-response activation of the p53 pathway, which in turn can induce cell cycle arrest or apoptosis. Furthermore, they found that OGA inhibition (TMG treatment) improved the efficiency of cisplatin, a chemotherapeutic agent used in the treatment of ovarian cancer: ovarian cancer cells treated with a combination of TMG and cisplatin showed decreased growth, as compared to cisplatin treated only, and this effect was partially dependent on p53 activation. Taken together, their findings suggest that modulation of O-GlcNAc homeostasis in association with traditional therapy may be beneficial for ovarian cancer treatment.
Future direction of O-GlcNAc in women’s cancers
The field of protein O-GlcNAcylation in cancers, in particular women’s cancers, is young and much work remains to be done. These following major unanswered questions highlight the areas of research focus needed in breast, ovarian and uterine cancers, but can also be applied to the broader question of general cancer biology:
Is altered O-GlcNAcylation a cause or effect of oncogenesis? As with many alternations in post-translational modifications, O-GlcNAcylation of proteins in cancer may occur simply because global glycosylation-regulatory systems are impaired in cancer. Mounting data, however, suggest that changes in O-GlcNAcylation may be a driving event in oncogenesis. Further studies in mouse models with regulatable levels of O-GlcNAc are warranted to best answer this question.
How do hormonal influences affect O-GlcNAcylation in cancer? The women’s cancers reviewed herein are all highly regulated by hormonal influences, particularly those of the ovarian hormones estrogen and progesterone. Perhaps hormonal influences can affect protein O-GlcNAcylation in these cancers, as well as other hormonally regulated/influenced cancers such as prostate cancer. Studies of O-GlcNAcylation in pre- and post-menopausal women would begin to address how hormones influence cellular and protein-specific O-GlcNAcylation.
Does alternation of O-GlcNAc differ based upon molecular mechanism of oncogenesis in each tumor type? While most data suggest that O-GlcNAc (cellular, and on specific proteins) increases as cancer progresses, there are data to suggest the opposite. Are their different molecular mechanisms in these tumors that determine if O-GlcNAcylation is a tumor promoting or tumor preventing phenotype?
As cancer biologist understand more about altered glucose metabolism in cancer cells, understanding protein O-GlcNAcylation as a readout for this process is becoming increasingly relevant to understanding the pathology of human cancer.
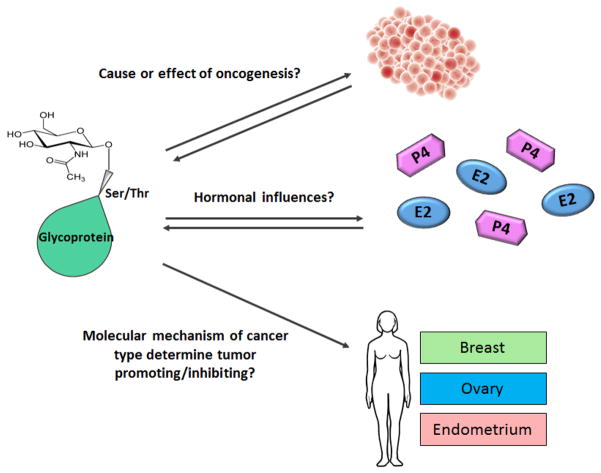
Figure 1.
Future directions to determine role of O-GlcNAcylation in women’s cancers.
Top: It remains to be shown if altered O-GlcNAcylation is a cause (does it promote tumorigenesis?) or an effect (is it a result of tumorigenesis?) of oncogenesis and tumor formation. Middle: How do hormones that are known to play a role in the development of women’s cancers, such as progesterone (P4; purple) and estrogen (E2; blue), affect O-GlcNAcylation of target proteins? And, alternatively, does O-GlcNAcylation affect how these hormones transduce signals in their respective ligand receptor-positive cells? Bottom: Does the impact of O-GlcNAcylation on the development/progression of a tumor vary by the molecular mechanism that promotes oncogenesis in that particular tumor or tumor/type?
Acknowledgments
This work was supported by R00CA166643 (CRH), DOD BCRP W81XWH-16-1-0320 (CRH), Susan G Komen Foundation CCR16376147 (CRH), V Foundation V2015-025 (CRH), and the NCI Cancer Center Support Grant P30 CA168524 (CRH).
Footnotes
The authors declare no conflict of interest.
References
- 1.Breast Cancer Facts & Figures 2015–2016. 2016 Available from: www.cancer.org/statistics.
- 2.Lynch TP, Reginato MJ. O-GlcNAc transferase: a sweet new cancer target. Cell Cycle. 2011;10(11):1712–3. doi: 10.4161/cc.10.11.15561. [DOI] [PubMed] [Google Scholar]
- 3.Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011;1812(4):514–9. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, Xing C, Zhang F, Zheng S. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol. 2012;29(2):985–93. doi: 10.1007/s12032-011-9912-1. [DOI] [PubMed] [Google Scholar]
- 5.Yang YR, Kim DH, Seo YK, Park D, Jang HJ, Choi SY, Lee YH, Lee GH, Nakajima K, Taniguchi N, Kim JM, Choi EJ, Moon HY, Kim IS, Choi JH, Lee H, Ryu SH, Cocco L, Suh PG. Elevated O-GlcNAcylation promotes colonic inflammation and tumorigenesis by modulating NF-kappaB signaling. Oncotarget. 2015;6(14):12529–42. doi: 10.18632/oncotarget.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287(14):11070–81. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Queiroz RM, Madan R, Chien J, Dias WB, Slawson C. Changes in O-Linked N-Acetylglucosamine (O-GlcNAc) Homeostasis Activate the p53 Pathway in Ovarian Cancer Cells. J Biol Chem. 2016;291(36):18897–914. doi: 10.1074/jbc.M116.734533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29(19):2831–42. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 9.Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010;70(15):6344–51. doi: 10.1158/0008-5472.CAN-09-1887. [DOI] [PubMed] [Google Scholar]
- 10.Trinca GM, Goodman ML, Papachristou EK, D’Santos CS, Chalise P, Madan R, Slawson C, Hagan CR. O-GlcNAc-Dependent Regulation of Progesterone Receptor Function in Breast Cancer. Horm Cancer. 2017 doi: 10.1007/s12672-017-0310-9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J. Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics. 2013;13(14):2088–99. doi: 10.1002/pmic.201200126. [DOI] [PubMed] [Google Scholar]
- 12.Tiainen S, Oikari S, Tammi M, Rilla K, Hamalainen K, Tammi R, Kosma VM, Auvinen P. High extent of O-GlcNAcylation in breast cancer cells correlates with the levels of HAS enzymes, accumulation of hyaluronan, and poor outcome. Breast Cancer Res Treat. 2016;160(2):237–247. doi: 10.1007/s10549-016-3996-4. [DOI] [PubMed] [Google Scholar]
- 13.Krzeslak A, Forma E, Bernaciak M, Romanowicz H, Brys M. Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med. 2012;12(1):61–5. doi: 10.1007/s10238-011-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netsirisawan P, Chokchaichamnankit D, Srisomsap C, Svasti J, Champattanachai V. Proteomic Analysis Reveals Aberrant O-GlcNAcylation of Extracellular Proteins from Breast Cancer Cell Secretion. Cancer Genomics Proteomics. 2015;12(4):201–9. [PubMed] [Google Scholar]
- 15.Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54(5):820–31. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auvinen P, Tammi R, Kosma VM, Sironen R, Soini Y, Mannermaa A, Tumelius R, Uljas E, Tammi M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int J Cancer. 2013;132(3):531–9. doi: 10.1002/ijc.27707. [DOI] [PubMed] [Google Scholar]
- 17.Auvinen P, Rilla K, Tumelius R, Tammi M, Sironen R, Soini Y, Kosma VM, Mannermaa A, Viikari J, Tammi R. Hyaluronan synthases (HAS1-3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat. 2014;143(2):277–86. doi: 10.1007/s10549-013-2804-7. [DOI] [PubMed] [Google Scholar]
- 18.Sodi VL, Khaku S, Krutilina R, Schwab LP, Vocadlo DJ, Seagroves TN, Reginato MJ. mTOR/MYC Axis Regulates O-GlcNAc Transferase Expression and O-GlcNAcylation in Breast Cancer. Mol Cancer Res. 2015;13(5):923–33. doi: 10.1158/1541-7786.MCR-14-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Pan Q, Sun D, Chen W, Shen A, Huang M, Ding J, Geng M. O-GlcNAcylation of cofilin promotes breast cancer cell invasion. J Biol Chem. 2013;288(51):36418–25. doi: 10.1074/jbc.M113.495713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Huang H, Cao Y, Wu Q, Li W, Zhang J. Suppression of OGT by microRNA24 reduces FOXA1 stability and prevents breast cancer cells invasion. Biochem Biophys Res Commun. 2017;487(3):755–762. doi: 10.1016/j.bbrc.2017.04.135. [DOI] [PubMed] [Google Scholar]
- 21.Tao T, He Z, Shao Z, Lu H. TAB3 O-GlcNAcylation promotes metastasis of triple negative breast cancer. Oncotarget. 2016;7(16):22807–18. doi: 10.18632/oncotarget.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med. 2014;12:32. doi: 10.1186/1741-7015-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll JS, Hickey TE, Tarulli GA, Williams M, Tilley WD. Deciphering the divergent roles of progestogens in breast cancer. Nat Rev Cancer. 2017;17(1):54–64. doi: 10.1038/nrc.2016.116. [DOI] [PubMed] [Google Scholar]
- 24.Cheng X, Cole RN, Zaia J, Hart GW. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry. 2000;39(38):11609–20. doi: 10.1021/bi000755i. [DOI] [PubMed] [Google Scholar]
- 25.Cheng X, Hart GW. Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: post-translational regulation of turnover and transactivation activity. J Biol Chem. 2001;276(13):10570–5. doi: 10.1074/jbc.M010411200. [DOI] [PubMed] [Google Scholar]
- 26.Bowe DB, Sadlonova A, Toleman CA, Novak Z, Hu Y, Huang P, Mukherjee S, Whitsett T, Frost AR, Paterson AJ, Kudlow JE. O-GlcNAc integrates the proteasome and transcriptome to regulate nuclear hormone receptors. Mol Cell Biol. 2006;26(22):8539–50. doi: 10.1128/MCB.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanwal S, Fardini Y, Pagesy P, N’Tumba-Byn T, Pierre-Eugene C, Masson E, Hampe C, Issad T. O-GlcNAcylation-inducing treatments inhibit estrogen receptor alpha expression and confer resistance to 4-OH-tamoxifen in human breast cancer-derived MCF-7 cells. PLoS One. 2013;8(7):e69150. doi: 10.1371/journal.pone.0069150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 29.Trabert B, Wentzensen N, Felix AS, Yang HP, Sherman ME, Brinton LA. Metabolic syndrome and risk of endometrial cancer in the united states: a study in the SEER-medicare linked database. Cancer Epidemiol Biomarkers Prev. 2015;24(1):261–7. doi: 10.1158/1055-9965.EPI-14-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, Huang Y. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57(11):2261–9. doi: 10.1007/s00125-014-3361-2. [DOI] [PubMed] [Google Scholar]
- 31.Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Tjonneland A, Olsen A, Overvad K, Jakobsen MU, Chajes V, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Lukanova A, Boeing H, Pischon T, Trichopoulou A, Christina B, Trichopoulos D, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Gram IT, Lund E, Quiros JR, Travier N, Martinez-Garcia C, Larranaga N, Chirlaque MD, Ardanaz E, Berglund G, Lundin E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Bingham S, Khaw KT, Allen N, Key T, Ferrari P, Rinaldi S, Slimani N, Riboli E. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2007;14(3):755–67. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- 32.Hardiville S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20(2):208–13. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Huo J, Liu Y, Liu H, Liu G, Chen Y, Chen B. Elevated glucose levels impair the WNT/beta-catenin pathway via the activation of the hexosamine biosynthesis pathway in endometrial cancer. J Steroid Biochem Mol Biol. 2016;159:19–25. doi: 10.1016/j.jsbmb.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Krzeslak A, Wojcik-Krowiranda K, Forma E, Bienkiewicz A, Brys M. Expression of genes encoding for enzymes associated with O-GlcNAcylation in endometrial carcinomas: clinicopathologic correlations. Ginekol Pol. 2012;83(1):22–6. [PubMed] [Google Scholar]
- 35.Sgantzos MN, Galani V, Arvanitis LD, Charchanti A, Psathas P, Nakou M, Havaki S, Kallioras V, Marinos E, Vamvakopoulos NC, Kittas C. Expression of the O-linked N-acetylglucosamine containing epitope H in normal myometrium and uterine smooth muscle cell tumors. Pathol Res Pract. 2007;203(1):31–7. doi: 10.1016/j.prp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Ferlay J, SH, Bray F, Forman D, Mathers C, Parkin DM. IARC Cancer Base No 10 [Internet] Lyon, France: IARC Press; 2010. GLOBOCAN 2008v1.2. Cancer incidence, mortality and prevalence worldwide. and 18. [Google Scholar]
- 37.Statistics, C.C.
- 38.Jin FZ, Yu C, Zhao DZ, Wu MJ, Yang Z. A correlation between altered O-GlcNAcylation, migration and with changes in E-cadherin levels in ovarian cancer cells. Exp Cell Res. 2013;319(10):1482–90. doi: 10.1016/j.yexcr.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Niu Y, Xia Y, Wang J, Shi X. O-GlcNAcylation promotes migration and invasion in human ovarian cancer cells via the RhoA/ROCK/MLC pathway. Mol Med Rep. 2017;15(4):2083–2089. doi: 10.3892/mmr.2017.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Leber B, Andrews DW. Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J. 2001;20(21):5999–6007. doi: 10.1093/emboj/20.21.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19(6):607–14. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 42.Malaguarnera R, Vella V, Vigneri R, Frasca F. p53 family proteins in thyroid cancer. Endocr Relat Cancer. 2007;14(1):43–60. doi: 10.1677/erc.1.01223. [DOI] [PubMed] [Google Scholar]
- 43.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8(10):1074–83. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]