Abstract
The peroxisomal localization and characterization of NADP-dependent isocitrate dehydrogenase (perICDH) in young and senescent pea (Pisum sativum) leaves was studied by subcellular fractionation, kinetic analysis, immunoblotting, and immunoelectron microscopy. The subunit molecular mass for perICDH determined by immunoblotting was 46 kD. By isoelectric focusing (IEF) of the peroxisomal matrix fraction, the NADP-ICDH activity was resolved into four isoforms, perICDH-1 to perICDH-4, with isoelectric points (pIs) of 6.0, 5.6, 5.4, and 5.2, respectively. The kinetic properties of the NADP-ICDH in peroxisomes from young and senescent pea leaves were analyzed. The maximum initial velocity was the same in peroxisomes from young and senescent leaves, while the Michaelis constant value in senescent leaf peroxisomes was 11-fold lower than in young leaf peroxisomes. The protein levels of NADP-ICDH in peroxisomes were not altered during senescence. The kinetic behavior of this enzyme suggests a possible fine control of enzymatic activity by modulation of its Michaelis constant during the natural senescence of pea leaves. After embedding, electron microscopy immunogold labeling of NADP-ICDH confirmed that this enzyme was localized in the peroxisomal matrix. Peroxisomal NADP-ICDH represents an alternative dehydrogenase in these cell organelles and may be the main system for the reduction of NADP to NADPH for its re-utilization in the peroxisomal metabolism.
Peroxisomes are subcellular organelles that have an essentially oxidative type of metabolism, and catalase and H2O2-producing flavin oxidases as basic enzymatic constituents (Tolbert, 1981; Huang et al., 1983). Leaf peroxisomes are present in photosynthetic tissues and carry out the major reactions of the oxidative cycle of photorespiration (Huang et al., 1983). The presence of superoxide dismutases and the production of superoxide radicals (O2·−) in peroxisomes was first demonstrated in plant tissues (del Río et al., 1983, 1992; López-Huertas et al., 1997, 1999). Different lines of evidence found in recent years have shown that leaf peroxisomes can be responsible for a variety of induced oxidative stress situations (del Río et al., 1992, 1996). Very recently, the presence of the enzymes of the ascorbate-glutathione cycle in pea (Pisum sativum) leaf peroxisomes (Jiménez et al., 1997, 1998), as well as the two oxidative enzymes of the pentose-phosphate pathway, Glc-6-P dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH), have been reported (Corpas et al., 1998). All of these data point to the existence of a complex battery of oxidative and antioxidative enzymes in leaf peroxisomes.
During leaf senescence, numerous metabolic changes take place, such as protein degradation, nucleic acid and chlorophyll breakdown, and lipid and nitrogen remobilization (Buchanan-Wollaston, 1997). These changes also affect the metabolism of leaf peroxisomes, as in the case of the levels of the enzymes of the glyoxylate cycle, malate synthase and isocitrate lyase, which increase in peroxisomes of senescent leaves as a result of increased gene expression (Gut and Matile, 1988; De Bellis et al., 1990; Vicentini and Matile, 1993; Pastori and del Río, 1997). Moreover, the implication of the activated oxygen metabolism of leaf peroxisomes in the oxidative mechanism of leaf senescence has been proposed (Pastori and del Río, 1997; del Río et al., 1998).
NADP-dependent isocitrate dehydrogenase (NADP-ICDH; EC 1.1.1.42) catalyzes the oxidative decarboxylation of isocitrate to 2-oxoglutarate with the production of the reduced coenzyme NADPH (Gálvez and Gadal, 1995). This enzyme, together with the two dehydrogenases of the pentose-phosphate pathway and malic enzyme, is the main cellular source of NADPH, which is an essential electron donor in numerous biosynthetic and detoxification reactions. NADP-ICDH is widely distributed in living organisms, and there are numerous reports describing its characterization and subcellular distribution (Barroso, 1993; Gálvez and Gadal, 1995). In higher plant cells, NADP-ICDH activity has been detected and characterized in the cytosol (Chen et al., 1988, 1989; Fieuw et al., 1995; Canino et al., 1996; Palomo et al., 1998), mitochondria (Rasmusson and Møller, 1990; Attucci et al., 1994; Møller and Rasmusson, 1998), and chloroplasts (Randall and Givan, 1981; Gálvez et al., 1994). Breidenbach and Beevers (1967) and Donaldson (1982) reported the association of NADP-ICDH activity with glyoxysomes, and Yamazaki and Tolbert (1970) localized traces of this activity in leaf peroxisomes. However, the occurrence of NADP-ICDH in plant peroxisomes is still questioned by some authors and its physiological role in different cell compartments remains unknown (Gálvez and Gadal, 1995).
In the present study, NADP-ICDH was localized in peroxisomes purified from pea leaves using biochemical and immunocytochemical approaches, and the modulation of its activity during the natural senescence of pea leaves was studied. The function of NADP-ICDH as the main source of NADPH for its re-utilization in the leaf peroxisomal metabolism is proposed.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Pea (Pisum sativum L. cv Lincoln) seeds, obtained from Ramiro Arnedo SA (Calahorra, Spain), were surface-sterilized with 3% (v/v) commercial bleaching solution for 3 min, and then washed with distilled water and germinated in vermiculite for 15 d. Healthy and vigorous seedlings were selected and grown in the greenhouse in nutrient solutions under optimum conditions (del Río et al., 1985) for 15 d (young plants) and 50 d (senescent plants).
Purification of Peroxisomes
All operations were performed at 0°C to 4°C. Peroxisomes were purified from pea leaves by differential and Suc density-gradient centrifugation (35%–60%, w/w) (López-Huertas et al., 1995). Peroxisomes were detected in the gradient by measuring catalase activity as a marker enzyme. To assess possible contamination by mitochondria, fumarase activity was used as a marker for these organelles. The identifed peroxisomal fractions were pooled and diluted 5-fold with 100 mm potassium phosphate, pH 7.5, containing 1 mm EDTA, then incubated on ice for 60 min with gentle magnetic stirring. The suspensions were centrifuged at 120,000g for 30 min in a rotor (60 Ti, Beckman Instruments, Fullerton, CA), and supernatants containing the peroxisomal matrix fraction were recovered and concentrated by ultrafiltration using a PM-10 membrane (Amicon, Beverly, MA) (Distefano et al., 1997).
Enzyme Assays and Kinetic Analysis
Catalase activity was determined according to the method of Aebi (1984) and fumarase activity was measured by the method of Walk and Hock (1977). NADP-ICDH activity was determined spectrophotometrically by recording the reduction of NADP at 340 nm (Goldberg and Ellis, 1983). The assay was performed at 25°C in a reaction medium (1 mL) containing 50 mm HEPES, pH 7.6, 2 mm MgCl2, 0.8 mm NADP, and the reaction was initiated by the addition of 10 mm 2R,3S-isocitrate. One milliunit of activity was defined as the amount of enzyme required to reduce 1 nmol NADP min−1 at 25°C. For kinetic studies the range of substrate concentrations was 0.001 to 10 mm.
Kinetic data were analyzed by a nonlinear regression method based on the rectangular hyperbola described by the Michaelis-Menten equation (Dows and Riggs, 1965). This nonlinear plot was constructed with the aid of a computer program (GraFit software, Erithacus Software, Middlesex, UK). For illustrative and comparative analyses, data were also presented as linear, double-reciprocal plots. The catalytic efficiency (Vmax/Km) was defined as the ratio between the enzyme activity and its Km for each substrate. This parameter is an indication of the relationship between the total enzyme activity and the degree of interaction between the enzyme and its substrate.
Electrophoretic Methods and Immunoblot Analysis
IEF was carried out in a slab cell (Mini-Protein II, Bio-Rad Laboratories, Hercules, CA) using a pH gradient of 3.5 to 7.0, as described by Palma et al. (1997). Samples were prepared in a solution containing 15% (w/v) Suc, 2.3% (w/v) ampholytes, and 8 mm NADP+. The isoforms of NADP-ICDH were visualized by incubating the gels in a solution consisting of 50 mm Tris-HCl, pH 7.6, 0.8 mm NADP+, 5 mm EDTA, 2 mm MgCl2, 0.24 mm nitroblue tetrazolium, and 65 μm phenazine methosulfate containing 10 mm 2R,3S-isocitrate. When blue formazan bands appeared, the reaction was stopped by immersing the gels in 7% (v/v) acetic acid. The NADP-ICDH activity bands of the gels was quantified by measuring the relative absorbance of bands at 560 nm in a densitometer (model CS9000, Shimadzu, Columbia, MD). The pIs of ICDH isoenzymes were determined using pI markers (Bio-Rad Laboratories) that were isoelectric focused in parallel with the samples.
SDS-PAGE was carried out according to the method of Laemmeli (1970) in 12% acrylamide slab gels. Samples were prepared in 62.5 mm Tris-HCl, pH 6.8, containing 2% (w/v) SDS, 10% (v/v) glycerol, and 10 mm dithiothreitol, and were heated at 95°C for 5 min. For immunoblot analyses, the polypeptides were transferred onto polyvinylidene difluoride membranes (Immobilon P, Millipore, Bedford, MA) using a semi-dry transfer system (Bio-Rad Laboratories) with 10 mm 3-(cyclohexylamino)propanesulfonic acid (CAPS) buffer, 10% (v/v) methanol, pH 11.0, at 1.5 mA cm−2 for 2 h. For immunodetection of NADP-ICDH, a rabbit polyclonal antibody against cytosolic pea NADP-isocitrate dehydrogenase (Chen et al., 1989), diluted 1/4,000, was used. A goat anti-rabbit IgG-alkaline phosphatase conjugate (Promega, Madison, WI) diluted 1/10,000 was used as the secondary antibody, and the color was developed with the nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate reagent (Amresco, Solon, OH).
Electron Microscopy and Immunocytochemistry
Leaf segments of approximately 1 mm2 from young and senescent pea plants were fixed, dehydrated, and embedded in LR White resin as described by Corpas et al. (1994). Ultrathin sections were incubated for 3 h with IgG against pea NADP-ICDH (Chen et al., 1989) diluted 1/500 in TBS plus Tween 20 (TBST) buffer containing 2% (w/v) BSA and 1% (v/v) goat normal serum. The sections were then incubated for 1 h with goat anti-rabbit IgG conjugated to 15-nm gold particles (Bio Cell, Cardiff, UK) diluted 1/50 in TBST plus 2% (w/v) BSA. Sections were post-stained in 2% (v/v) uranyl acetate for 3 min and examined in a transmission electron microscope (EM 10C, Zeiss, Jena, Germany).
Other Assays
Protein levels were determined according to the method of Bradford (1976) using BSA as a standard. The density of the gradient fractions was calculated from the refractive index of the fractions, which was measured at room temperature using a refractometer (Atago, Japan).
RESULTS
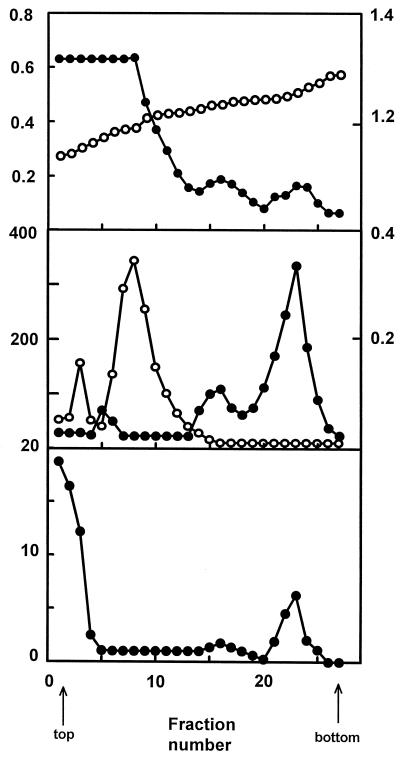
The purification of peroxisomes from 50-d-old pea leaves by Suc density-gradient centrifugation is shown in Figure 1. Peroxisomes (fractions 21–26) were identified by the peak of catalase activity, which was used as the peroxisomal marker enzyme. The peroxisomal fraction banded at an average equilibrium density of 1.24 g cm−3, characteristic for these intact organelles in Suc solutions (Huang et al., 1983; López-Huertas et al., 1995). The absence of fumarase activity in these fractions indicated that peroxisomes were essentially free of contamination by mitochondria. Likewise, no Cyt c reductase, acid phosphatase, or Fru-1,6-bisphosphatase activity was detected in the peroxisomal fraction, indicating that these organelles were not contaminated by endoplasmic reticulum, vacuoles, or chloroplasts, respectively (results not shown). A similar profile of organelle enzyme markers was obtained in 15-d-old plants. The NADP-ICDH activity was also measured throughout the gradient fractions. The activity was mainly found on the top of the Suc-density gradients (fractions 1–3), which corresponds to the broken organelles zone, but was also found in the peroxisomal fractions (tubes 21–25).
Figure 1.
Purification of peroxisomes from pea leaves. Cell organelles were purified from 50-d-old pea leaves by differential and Suc density-gradient centrifugations, as described by López-Huertas and co-workers (1995). Gradient fractions of 1.5 mL were eluted with a gradient fractionator and assayed for specific marker enzymes to localize cell organelles in the gradient: fumarase for mitochondria and catalase for peroxisomes. Catalase and fumarase activities are expressed in μmol min−1 mL−1 and NADP-ICDH activity in milliunits mL−1. Proteins were expressed as mg mL−1 and density as g cm−3. Top: ●, Proteins; ○, density. Middle: ●, Catalase; ○, fumarase. Bottom: NADP-IDH.
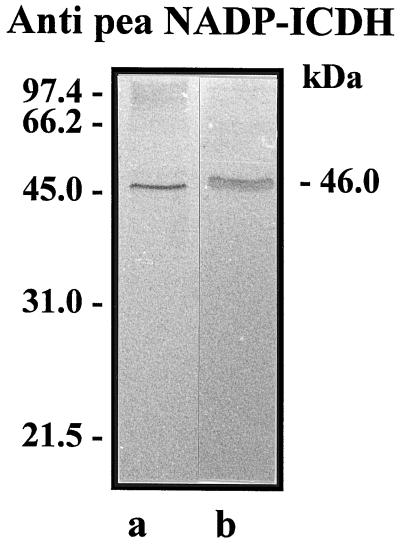
The analysis of cross-reactivity by western blot with a polyclonal antibody against cytosolic pea NADP-ICDH revealed an immunoreactive polypeptide of 46 kD in crude extracts and peroxisomal matrices of pea leaves (Fig. 2).
Figure 2.
Western-blot analysis of peroxisomes from senescent pea leaves. Samples were subjected to SDS-PAGE and then transferred to polyvinylidene difluoride membranes and incubated with a polyclonal antibody against pea NADP-ICDH (1/4,000 dilution). Lane a, Crude extract of pea leaves (50 μg of protein); lane b, matrices of pea leaf peroxisomes (45 μg of protein). Molecular mass standards are indicated on the left in kD.
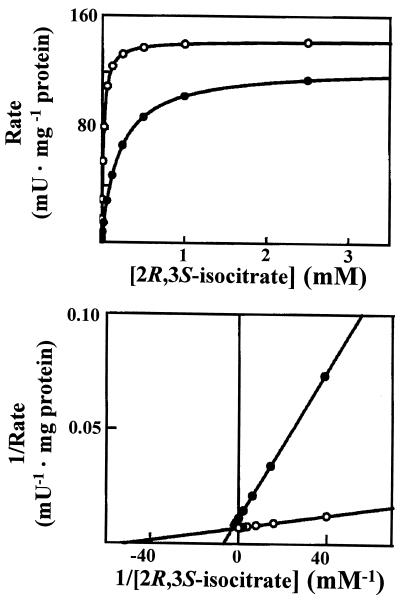
To establish the role of NADP-ICDH, the enzyme kinetic parameters in peroxisomes purified from young and senescent pea leaves were studied by measuring the formation of NADPH at 340 nm. The saturation curves and double-reciprocal plots are depicted in Figure 3. The initial rates of NADPH formation were measured as a function of 2R,3S-isocitrate (from 0.005–10 mm) in the presence of NADP (0.8 mm). In peroxisomes from young and senescent pea leaves, typical hyperbolic saturation curves were obtained for the activity of this NADPH-forming enzyme, without evidence of cooperativity. Double-reciprocal plots of the variations in initial enzyme rate as a function of substrate concentration showed a close linear relationship (Fig. 3), which also excluded the possibility of any significant cooperative effects. Hill coefficient (nH) values close to one were obtained, and this corroborated the absence of cooperativity in this enzyme activity in peroxisomes from young and senescent pea leaves. The kinetic parameters of the peroxisomal NADP-ICDH in senescent and young leaves are shown in Table I. No significant changes were observed in the specific activity or Vmax values of NADP-ICDH in the two experimental situations. However, in peroxisomes from senescent leaves, the Km of NADP-ICDH decreased almost 11-fold. This kinetic behavior resulted in a catalytic efficiency approximately 12 times higher for peroxisomal NADP-ICDH from senescent leaves.
Figure 3.
Effect of 2R,3S-isocitrate concentration on the NADP-ICDH activity of leaf peroxisomes from young (●) and senescent (○) pea leaves. The bottom panel shows the Lineweaver-Burk plot of the kinetic data. mU, Milliunits.
Table I.
Kinetic parameters of the NADP-ICDH in peroxisomes purified from young (15 d) and senescent (50 d) pea leaves
| Kinetic Parameter | Young Leaves | Senescent Leaves |
|---|---|---|
| Specific activity (milliunits mg−1 protein) | 124 ± 6 | 143 ± 7 NS |
| Vmax (microunits) | 4,150 ± 208 | 4,771 ± 239 NS |
| Km2R,3S-isocitrate (μm) | 202 ± 8 | 19 ± 1* |
| Catalytic efficiency (Vmax/Km) (106 microunits m−1) | 20 ± 1 | 239 ± 11* |
| nH | 1.1 | 1.1 |
Kinetic parameters were determined by using a non-linear-regression analysis program. Data are means ± se of five different experiments. Differences from young leaf values were significant at P < 0.05 (*). NS, Not significant.
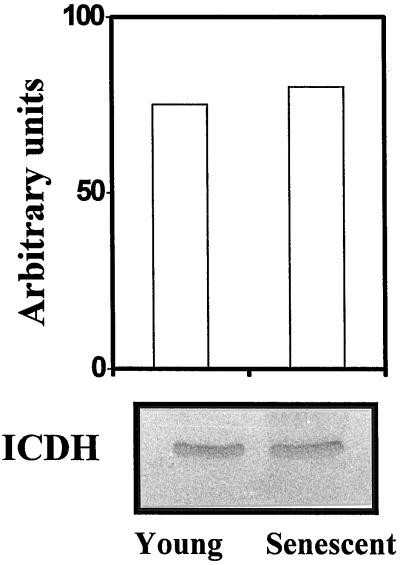
The protein levels of NADP-ICDH in peroxisomes from young and senescent leaves were estimated by western blot of total peroxisomal matrix proteins. The densitometric scan of specific bands did not reveal significant differences (Fig. 4).
Figure 4.
Western-blot analysis of NADP-ICDH protein in peroxisomes from young and senescent pea leaves. The western-blot conditions were identical to those described in Figure 2. The upper panel shows the quantification of NADP-ICDH levels by scanning densitometry at 560 nm expressed as arbitrary absorbance units. The lower panel shows an immunoblot indicating the NADP-ICDH protein levels.
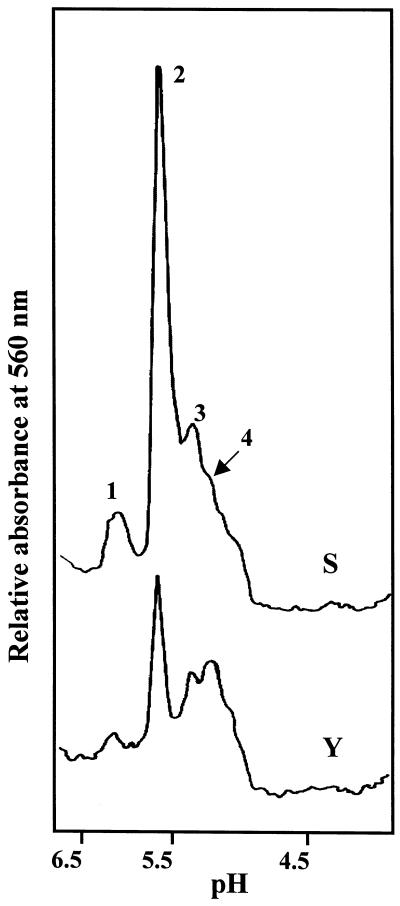
The activity of NADP-ICDH in peroxisomal matrices was also studied by IEF. Figure 5 shows the densitometric scan of NADP-ICDH activity-stained IEF gels. In the range of pH values used (pH 3.5–7.0), four NADP-ICDH isoforms were detected in peroxisomal matrices from young and senescent plants with pIs of 6.0, 5.6, 5.4, and 5.2, which were designated as ICDH-1 to ICDH-4, respectively. The ICDH-2 was the most prominent isoform and the total activity of peroxisomes was apparently higher in senescent than in young plants. However, this activity increment in the IEF gels could be due to the effect of small local changes of pH on the activity of NADP-ICDH isoforms. As indicated in Table I, the specific activity of NADP-ICDH was not significantly different in peroxisomes from young and senescent pea leaves.
Figure 5.
Densitograms of NADP-ICDH isoforms in peroxisomes from young (Y) and senescent (S) pea leaves. Samples of peroxisomal matrices (200 μg of protein) were subjected to IEF in a pH gradient of 3.5 to 7.0. NADP-ICDH isoforms were identified by activity staining, and gels were scanned at 560 nm. 1, ICDH-1; 2, ICDH-2; 3, ICDH-3; 4, ICDH-4.
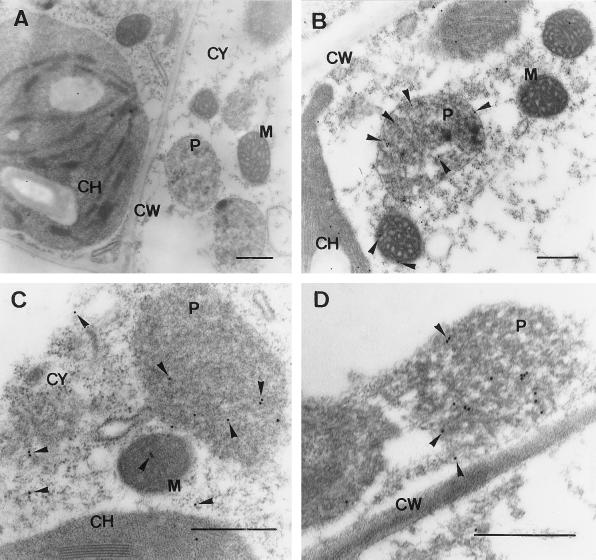
The cellular localization of NADP-ICDH in young and senescent pea leaves was also studied by EM immunocytochemistry (Fig. 6). Using a polyclonal antibody against cytosolic pea NADP-ICDH, immunogold particles appeared in cytosol, chloroplasts, mitochondria, and peroxisomes. The cell wall was used as a control for unspecific immunolabeling. Table II shows the average number of gold particles per square micrometer counted in the different cellular compartments of young and senescent pea leaves. In both pea plants, peroxisomes and mitochondria showed a density of immunogold particles higher than chloroplasts and cytoplasm. Although statistical analysis of immunogold particles per square micrometer showed significant increases in the labeling of peroxisomes and other cell compartments of senescent leaves (Table II), this result could be artifactual. In senescent pea leaves a reduction of 20% to 70% of the area of the different cell compartments and an enlargement of the vacuole size took place as a result of the senescence process. This reduction in size of the cellular compartments produces an apparent increase in the immunocytochemical labeling when expressed as the number of gold particles per square micrometer. Therefore, the apparent augmentation of peroxisome labeling in senescent leaves is not in contradiction with the specific activity and western-blot data of perICDH given in Table I and Figure 4, respectively.
Figure 6.
EM immunocytochemical localization of NADP-ICDH in pea leaves. The electron micrographs are representative of thin sections of pea leaves. Cell sections were probed with preimmune serum (dilution 1:500) (A). Immunogold labeling with anti-pea NADP-ICDH (dilution 1:500) was carried out in young (B) and senescent (C and D) pea leaves. Arrows indicate 15-nm gold particles. CH, Chloroplast; CW, cell wall; M, mitochondrion; P, peroxisome; CY, cytosol. Bars = 0.5 μm.
Table II.
Immunocytochemical labeling intensity of NADP-ICDH in young (15 d) and senescent (50 d) pea leaf cells
| Cellular Compartment | Young Leaves | Senescent Leaves |
|---|---|---|
| no. of gold particles μm−2 | ||
| Chloroplast | 4.5 ± 0.3 | 12.6 ± 0.6 (B) |
| Mitochondrion | 10.0 ± 0.5 | 24.0 ± 1.8 (B) |
| Peroxisome | 11.0 ± 0.7 | 22.2 ± 3.0 (A) |
| Cytoplasm | 1.1 ± 0.1 | 2.0 ± 0.2 (A) |
| Cell wall | 0.8 ± 0.2 | 0.9 ± 0.2 (NS) |
NADP-ICDH labeling density is given as the number of gold particles per square micrometer. Two separately embedded blocks were used to cut sections of each class of leaves, which were photographed from random fields. An average of 17 photographs were used for quantitative analysis for each type of leaves, and results are given as the means ± se. Differences from young leaf values were significant at: P ≤ 0.01 (A); P ≤ 0.001 (B). NS, Not significant.
DISCUSSION
The association of traces of NADP-ICDH activity with leaf peroxisomes was reported for the first time by Tolbert's group in 1970 (Yamazaki and Tolbert, 1970). Since that time, no more information was known on this NADP dehydrogenase in leaf peroxisomes, and the occurrence of this enzyme in these organelles is still questioned. Using biochemical and immunological approaches, we re-examined the presence of NADP-ICDH in leaf peroxisomes.
The procedure used to isolate leaf peroxisomes supplied highly purified peroxisomal fractions free of contamination by mitochondria, endoplasmic reticulum, vacuoles, or chloroplasts (López-Huertas et al., 1995; Distefano et al., 1997; Corpas et al., 1998).The data shown in Figure 1 demonstrate that peroxisomes were well separated from mitochondria, and the NADP-ICDH activity was detected in fractions corresponding to peroxisomes.
In addition to the enzyme activity, the presence of NADP-ICDH in the peroxisomal matrix fractions was corroborated using an immunological approach with a specific antibody against cytosolic NADP-ICDH from pea leaves. An immunoreactive polypeptide with a molecular mass of 46 kD was found on western-blot analysis, which was in the molecular mass range reported for subunits of other NADP-ICDHs (Chen et al., 1988; Fatania et al., 1993; Gálvez and Gadal, 1995; Yamamoto et al., 1995; Canino et al., 1996; Henke et al., 1998). The results obtained by EM immunocytochemistry clearly showed that NADP-ICDH has multiple subcellular localizations, including peroxisomes, and confirmed previous cell fractionation data reported by Yamazaki and Tolbert (1970) and Donaldson (1982). The amino acid sequence of different plant NADP-ICDHs was recently determined and it was found that at least two putative cytosolic NADP-ICDHs contain a C-terminal tripeptide (Ala-Lys-Ala) that is a type I peroxisomal targeting signal (PTS1) (Nekrutenko et al., 1998).
Previous experiments in pea plants showed that the activated oxygen metabolism of peroxisomes was involved in the mechanism of senescence (Pastori and del Río, 1997; del Río et al., 1998). NADPH is known to be essential for defense against oxidative stress, as it is the cofactor required for the reduction of oxidized glutathione by glutathione reductase, a component of the ascorbate-glutathione cycle that is present in leaf peroxisomes (Jiménez et al., 1997). In this sense, NADP-ICDH has been suggested to play a role in the provision of NADPH required to protect against oxidative damage in mitochondria (Møller and Rasmusson, 1998), and to provide the cytosol with reducing power, especially in biosynthetic processes and those metabolic situations in which the pentose-phosphate pathway is limited (Chen et al., 1988). Therefore, we decided to study the regulatory mechanisms of peroxisomal NADP-ICDH (perICDH) during natural plant senescence.
The main effects of senescence on the perICDH activity were found at sub-saturating substrate concentrations. The values obtained for the activity ratios in both physiological situations (young and senescent plants) could indicate the existence of a fine control of the enzymatic activity of perICDH during senescence. In leaf peroxisomes from 50-d-old pea plants, almost 40% of the maximum activity was reached at the sub-saturating isocitrate concentration, whereas in leaf peroxisomes from young pea plants, only 6% of the Vmax was found at the same substrate concentration. The significant increase in the enzyme activity at the cellular isocitrate concentration was caused by a drastic decline of the Km value of NADP-ICDH in leaf peroxisomes of senescent plants. This indicated a significant increase (more than 20-fold) in the affinity for isocitrate without any change in Vmax, and accounts for the similar increase in the enzymatic catalytic efficiency in leaf peroxisomes from senescent plants. However, the Km value determined for NADP-ICDH in leaf peroxisomes of senescent pea plants was similar to those reported for the cytosolic NADP-ICDH from pea leaves and cucumber cotyledons (Chen et al., 1988; Canino et al., 1996).
The changes found in the kinetic behavior of perICDH could be due to alterations in the activity of the preexistent enzyme (Lupiáñez et al., 1981; García-Salguero and Lupiáñez, 1989; Hortelano et al., 1991) without changes in its cellular concentration. This fact was corroborated by immunoblot analysis (Fig. 4) showing that the protein levels of perICDH did not change with senescence. In contrast, in pea plants grown with 50 μm cadmium chloride, a significant increase in the activity and protein level of perICDH was observed (Romero-Puertas et al., 1999). This indicates a different mechanism of regulation of leaf perICDH of pea plants during natural senescence and in response to abiotic stress.
The analysis of the perICDH activity by IEF revealed the presence of at least four isoforms. It is well known that NADP-ICDH has isoenzyme polymorphism (Gálvez and Gadal, 1995) and this characteristic was sometimes used for cultivar identification (Kiang and Gorman, 1985). Cucumber cotyledon crude extracts showed the presence of four isoforms by IEF in the range 4.55 to 5.85, and some of the isoforms disappeared when the cotyledons were exposed to light (Canino et al., 1996). However, the presence of four isoforms in the same cell organelle could appear somewhat unusual, but there are several enzymes that show similar characteristics. For example, glutathione reductase purified from chloroplasts and mitochondria was resolved into five and three isoforms, respectively (Edwards et al., 1990). Another closer example is the peroxisomal Glc-6-P dehydrogenase from pea leaves, which has three isoforms (Corpas et al., 1998). However, the physiological reason for the polymorphism of perICDH is still not clear.
The additional NADPH supplied by the activity of NADP-ICDH during senescence is required for its utilization in peroxisomal metabolism, probably in the ascorbate-glutathione cycle (Jiménez et al., 1997, 1998). This enzymatic cycle is an important antioxidative system, together with catalase, against the H2O2 generated in peroxisomes during the oxidative metabolism of these organelles (Jiménez et al., 1997, 1998; del Río et al., 1998). The physiological significance of the observed changes in the Km of peroxisomal NADP-ICDH during senescence is probably double: first to compete with isocitrate lyase, an enzyme of the glyoxylate cycle present in peroxisomes from senescent leaves (Gut and Matile, 1988; Pastori and del Río, 1997), for the intracellular pool of isocitrate; and second to provide a higher, constant supply of NADPH to eliminate the excess of H2O2 produced during senescence when catalase activity decreases dramatically (Pastori and del Río, 1997). An additional potential function for NADPH in peroxisomes could be related to the mechanism of protein import into these organelles. It was recently shown that the NADPH to NADP ratio is important in peroxisomal protein import (Pool et al., 1998). This ratio could also be involved in the senescence-induced transition of glyoxysomes into leaf-type peroxisomes.
In previous reports, we have shown the presence in pea leaf peroxisomes of the dehydrogenases of the pentose-phosphate pathway, G6PDH and 6PGDH, which could mediate the reduction of NADP in these cell organelles (Corpas et al., 1998). However, the production of NADPH by perICDH is 13-fold and 5-fold higher than that due to peroxisomal G6PDH and 6PGDH, respectively. This fact, together with the observed fine control of NADP-ICDH, strongly indicates the importance of this enzyme in the enzymatic systems of NADPH recycling, which are required for the operativity of the ascorbate-glutathione cycle in peroxisomes.
In conclusion, the presence of NADP-ICDH in pea leaf peroxisomes has been clearly demonstrated, and this enzyme exhibits a significant increase in its affinity for isocitrate during the senescence of leaves. The presence of NADP-ICDH in leaf peroxisomes, along with the NADP-dependent dehydrogenases G6PDH and 6PGDH, represents a very efficient system to recycle NADPH for its re-utilization in the peroxisomal metabolism and also to be used as a defense against oxidative stress in peroxisomes.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Pierre Gadal, Université de Paris-Sud, Orsay cedex, France, for his generous donation of antibody to pea NADP-ICDH, and to Drs. Manuel Gómez and Stefania Distefano for their valuable help. The Centre of Scientific Instrumentation, University of Granada, is acknowledged for the technical assistance in electron microscopic analyses.
Footnotes
This work was supported by the Dirección General de Enseñanza Superior e Investigación Científica (grant no. PB95–0004–01) and by the Junta de Andalucía (research groups nos. CVI 0157 and CVI 0192), Spain, and the European Union (contract no. CHRX–CT94–0605).
LITERATURE CITED
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Attucci S, Rivoal J, Brouquisse R, Carde JP, Pradet A, Raymond P. Characterization of a mitochondrial NADP-dependent isocitrate dehydrogenase in axes of germinating sunflower seeds. Plant Sci. 1994;102:49–59. [Google Scholar]
- Barroso JB. Influencias nutricionales y de la edad sobre el comportamiento cinético de los sistemas productores de NADPH en diferentes tejidos de la trucha arco-iris (Oncorhynchus mykiss). PhD thesis. Spain: University of Granada; 1993. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breidenbach RW, Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967;27:462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V. The molecular biology of leaf senescence. J Exp Bot. 1997;48:181–199. [Google Scholar]
- Canino S, Nieri B, Pistelli L, Alpi A, De Bellis L. NADP+-isocitrate dehydrogenase in germinating cucumber cotyledons: purification and characterization of a cytosolic isoenzyme. Physiol Plant. 1996;98:13–19. [Google Scholar]
- Chen R, Bismuth E, Champigny ML, Gadal P. Chromatographic and immunological evidence that chloroplastic and cytosolic pea (Pisum sativum L.) NADP-isocitrate dehydrogenases are distinct isoenzymes. Planta. 1989;178:157–163. doi: 10.1007/BF00393190. [DOI] [PubMed] [Google Scholar]
- Chen R, Le Maréchal P, Vidal J, Jacquot JP, Gadal P. Purification and comparative properties of the cytosolic isocitrate dehydrogenases (NADP) from pea (Pisum sativum) roots and green leaves. Eur J Biochem. 1988;175:565–572. doi: 10.1111/j.1432-1033.1988.tb14229.x. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Sandalio LM, Distefano S, Palma JM, Lupiáñez JA, del Río LA. A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem J. 1998;330:777–784. doi: 10.1042/bj3300777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Bunkelmann J, Trelease RT. Identification and immunological characterization of a family of peroxisome membrane proteins (PMPs) in oilseed glyoxysomes. Eur J Cell Biol. 1994;65:280–290. [PubMed] [Google Scholar]
- De Bellis L, Picciarelli L, Pistelli L, Alpi A. Localization of glyoxylate-cycle marker enzymes in peroxisomes of senescent leaves and green cotyledons. Planta. 1990;180:435–439. doi: 10.1007/BF00198797. [DOI] [PubMed] [Google Scholar]
- del Río LA, Lyon DS, Olah I, Glick B, Salin ML. Immunocytochemical evidence for a peroxisomal localization of manganese superoxide dismutase in leaf protoplasts from a higher plant. Planta. 1983;158:216–224. doi: 10.1007/BF01075257. [DOI] [PubMed] [Google Scholar]
- del Río LA, Palma JM, Sandalio LM, Corpas FJ, Pastori GM, Bueno P, López-Huertas E. Peroxisomes as a source of superoxide and hydrogen peroxide in stressed plants. Biochem Soc Trans. 1996;24:434–438. doi: 10.1042/bst0240434. [DOI] [PubMed] [Google Scholar]
- del Río LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jiménez A, López-Huertas E, Hernández JA. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998;116:1195–1200. doi: 10.1104/pp.116.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río LA, Sandalio LM, Palma JM, Bueno P, Corpas FJ. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Rad Biol Med. 1992;13:557–580. doi: 10.1016/0891-5849(92)90150-f. [DOI] [PubMed] [Google Scholar]
- del Río LA, Sandalio LM, Yáñez J, Gómez M. Induction of a manganese-containing superoxide dismutase in leaves of Pisum sativum L. by high nutrient levels of zinc and manganese. J Inorg Biochem. 1985;24:25–34. [Google Scholar]
- Distefano S, Palma JM, Gómez M, del Río LA. Characterization of endoproteases from plant peroxisomes. Biochem J. 1997;327:399–405. doi: 10.1042/bj3270399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson RP. Nicotinamide cofactors (NAD and NADP) in glyoxysomes, mitochondria, and plastids isolated from castor bean endosperm. Arch Biochem Biophys. 1982;215:274–279. doi: 10.1016/0003-9861(82)90305-8. [DOI] [PubMed] [Google Scholar]
- Dows JE, Riggs DS. A comparison of estimates of Michaelis-Menten kinetic constants from various linear transformations. J Biol Chem. 1965;240:863–869. [PubMed] [Google Scholar]
- Edwards EA, Rawsthorne S, Mullineaux P. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.) Planta. 1990;180:278–284. doi: 10.1007/BF00194008. [DOI] [PubMed] [Google Scholar]
- Fatania H, Al-Nassar KE, Sidhan V. Purification and partial characterization of NADP-linked isocitrate dehydrogenase from rat liver cytosol. FEBS Lett. 1993;320:57–60. doi: 10.1016/0014-5793(93)81657-l. [DOI] [PubMed] [Google Scholar]
- Fieuw S, Müller-Röber B, Gálvez S, Willmitzer L. Cloning and expression analysis of the cytosolic NADP+-dependent isocitrate dehydrogenase from potato. Plant Physiol. 1995;107:905–913. doi: 10.1104/pp.107.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez S, Bismuth E, Sarda C, Gadal P. Purification and characterization of chloroplastic NADP-isocitrate dehydrogenase from mixotrophic tobacco cells. Plant Physiol. 1994;105:593–600. doi: 10.1104/pp.105.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez S, Gadal P. On the function of the NADP-dependent isocitrate dehydrogenase isoenzymes in living organisms. Plant Sci. 1995;105:1–14. [Google Scholar]
- García-Salguero L, Lupiáñez JA. Metabolic adaptation of the renal carbohydrate metabolism. III. Effects of a high protein diet on the gluconeogenic and glycolytic fluxes in the proximal and distal rat-renal tubules. Mol Cell Biochem. 1989;90:99–110. doi: 10.1007/BF00221209. [DOI] [PubMed] [Google Scholar]
- Goldberg DM, Ellis G. Isocitrate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Ed 3. New York: Academic Press; 1983. pp. 183–190. [Google Scholar]
- Gut H, Matile P. Apparent induction of key enzymes of the glyoxylic acid in senescent barley. Planta. 1988;176:548–550. doi: 10.1007/BF00397663. [DOI] [PubMed] [Google Scholar]
- Henke B, Girzalsky W, Berteaux-Lecellier V, Erdmann R. IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the β-oxidation of unsaturated fatty acids. J Biol Chem. 1998;273:3702–3711. doi: 10.1074/jbc.273.6.3702. [DOI] [PubMed] [Google Scholar]
- Hortelano P, García-Salguero L, Alleyne GAO, Lupiáñez JA. Variations in the kinetic response of several different phosphate-dependent glutaminase isozymes during acute metabolic acidosis. Mol Cell Biochem. 1991;108:113–123. doi: 10.1007/BF00233115. [DOI] [PubMed] [Google Scholar]
- Huang AHC, Trelease RN, Moore TS., Jr . Plant Peroxisomes. New York: Academic Press; 1983. [Google Scholar]
- Jiménez A, Hernández JA, del Río LA, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, Pastori G, del Río LA, Sevilla F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang YT, Gorman MB. Inheritance of NADP-active isocitrate dehydrogenase isozymes in soybeans. J Hered. 1985;76:279–284. [Google Scholar]
- Laemmeli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- López-Huertas E, Corpas FJ, Sandalio LM, del Río LA. Characterization of membrane polypeptides from pea leaf peroxisomes involved in superoxide radical generation. Biochem J. 1999;337:531–536. [PMC free article] [PubMed] [Google Scholar]
- López-Huertas E, Sandalio LM, del Río LA. Integral membrane polypeptides of pea leaf peroxisomes: characterization and response to plant stress. Plant Physiol Biochem. 1995;33:295–302. [Google Scholar]
- López-Huertas E, Sandalio LM, Gómez M, del Río LA. Superoxide radical generation in peroxisomal membranes: evidence for the participation of the 18-kDa integral membrane polypeptide. Free Radic Res. 1997;26:497–506. doi: 10.3109/10715769709097820. [DOI] [PubMed] [Google Scholar]
- Lupiáñez JA, Hortelano P, Sánchez-Medina F, Sánchez-Pozo A, McFarlane-Anderson N, Barnswell J, Alleyne GAO. The mechanism of the increase in renal ammoniagenesis in the rat with acute metabolic acidosis. FEBS Lett. 1981;128:361–363. doi: 10.1016/0014-5793(81)80117-2. [DOI] [PubMed] [Google Scholar]
- Møller IM, Rasmusson G. The role of NADP in the mitochondrial matrix. Trends Plant Sci. 1998;3:21–27. [Google Scholar]
- Nekrutenko A, Hillis DM, Patton JC, Bradley RD, Baker RJ. Cytosolic isocitrate dehydrogenase in humans, mice, and voles and phylogenetic analysis of the enzyme family. Mol Biol Evol. 1998;15:1674–1684. doi: 10.1093/oxfordjournals.molbev.a025894. [DOI] [PubMed] [Google Scholar]
- Palma JM, Pastori GM, Bueno P, Distefano S, del Río LA. Purification and properties of cytosolic copper, zinc superoxide dismutase from watermelon (Citrullus vulgaris Schrad.) cotyledons. Free Radic Res. 1997;26:83–91. doi: 10.3109/10715769709097787. [DOI] [PubMed] [Google Scholar]
- Palomo J, Gallardo F, Suárez MF, Cánovas FM. Purification and characterization of NADP-linked isocitrate dehydrogenase from Scots pine. Plant Physiol. 1998;118:617–626. doi: 10.1104/pp.118.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, del Río LA. Natural senescence of pea leaves: an activated oxygen-mediated function for peroxisomes. Plant Physiol. 1997;113:411–418. doi: 10.1104/pp.113.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool MR, López-Huertas E, Horng JT, Baker A. NADPH is a specific inhibitor of protein import into glyoxysomes. Plant J. 1998;15:1–14. doi: 10.1046/j.1365-313x.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- Randall DD, Givan CV. Subcellular localization of NADP-isocitrate dehydrogenase in Pisum sativum leaves. Plant Physiol. 1981;68:70–73. doi: 10.1104/pp.68.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson A, Møller M. NADP-utilizing enzymes in the matrix of plant mitochondria. Plant Physiol. 1990;94:1012–1018. doi: 10.1104/pp.94.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas MC, McCarthy I, Sandalio LM, Palma JM, Corpas FJ, Gómez M, del Río LA (1999) Cadmium toxicity and oxidative metabolism in pea leaf peroxisomes. Free Radic Res (in press) [DOI] [PubMed]
- Tolbert NE. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Vicentini F, Matile P. Gerontosomes, a multifunctional type of peroxisomes in senescent leaves. J Plant Physiol. 1993;142:50–56. [Google Scholar]
- Walk R, Hock B. Glyoxysomal malate dehydrogenase of watermelon cotyledons: de novo synthesis of cytoplasmic ribosomes. Planta. 1977;134:277–285. doi: 10.1007/BF00384194. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Atomi H, Ueda M, Tanaka A. Novel NADP-linked isocitrate dehydrogenase present in peroxisomes of n-alkane yeast, Candida tropicalis: comparison with mitochondrial NAD-linked isocitrate dehydrogenase. Arch Microbiol. 1995;163:104–111. doi: 10.1007/BF00381783. [DOI] [PubMed] [Google Scholar]
- Yamazaki RK, Tolbert N. Enzymatic characterization of leaf peroxisomes. J Biol Chem. 1970;245:5137–5144. [PubMed] [Google Scholar]