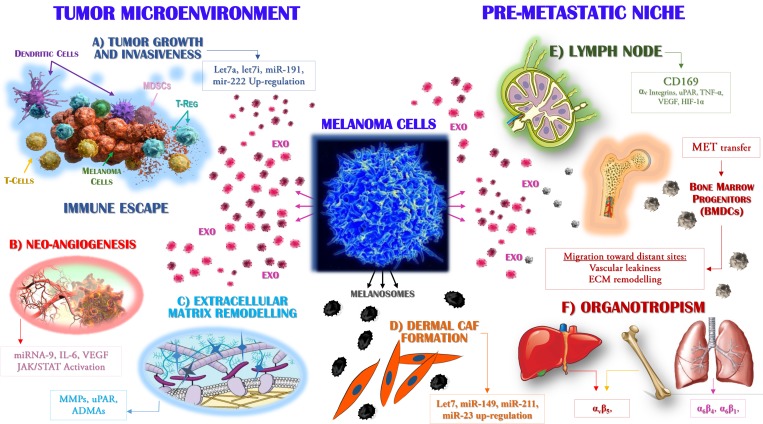
Figure 2. Exosomes drive the metastasis of melanoma cells.
Primary melanoma cells produce Exo that are able to target distinct populations, both within the tumor microenvironment (left) and at distant sites where they participate to the pre-metastatic niche formation (right). The molecular mechanisms by which Exo interact with target cells include the cytokines, miRNAs and receptors variably expressed by or accumulated in these vesicles. Exo enriched in Let7a, Let7i, miR-191 and miR-222 (A) promote tumor growth and invasiveness while are also able to impair immune system activity, leading to the defective maturation of dendritic cells, impaired cytotoxicity of T-cells and the expansion of suppressive populations, including regulatory T-cells (Treg) and myeloid-derived suppressor cells (MDSCs). (B) The expression by Exo of miR-9 and the high levels of both interleukin (IL)-6 and vascular endothelial growth factor (VEGF) drive the neo-angiogenesis, through the activation of the JAK/STAT pathway, whereas uPAR and ADAMs proteases (C) promote the remodelling and degradation of the extracellular matrix. Melanocytes produce melanosomes (D) as vesicles engulfed of melanin that are found accumulated in keratinocytes. Malignant melanoma cells stimulate the collagen-associated fibroblasts (CAFs) of the dermis leading to increased melanoma cell proliferation through the over-production of miR-149, -211, -23, -let7a and -let7b. In addition, the up-regulation of CD169 by Exo is a key step in the recruitment of melanoma cells to lymph nodes (E), whose colonisation by metastatic cells is also favoured by αv integrins, hypoxia-inducible factor (HIF)-1α and tumor necrosis factor (TNF)-α. In addition, specific organotropism (F) is driven by MET-expressing Exo, which in turn promotes the mobilisation of bone marrow progenitors (BMDCs) implicated in neo-vasculogenesis and pre-metastatic niche formation.