Abstract
Natural Killer (NK) cells are involved in the host immune response against infections due to viral, bacterial and fungal pathogens, all of which are a significant cause of morbidity and mortality in immunocompromised patients. Since the recovery of the immune system has a major impact on the outcome of an infectious complication, there is major interest in strengthening the host response in immunocompromised patients, either by using cytokines or growth factors or by adoptive cellular therapies transfusing immune cells such as granulocytes or pathogen-specific T-cells. To date, relatively little is known about the potential of adoptively transferring NK cells in immunocompromised patients with infectious complications, although the anti-cancer property of NK cells is already being investigated in the clinical setting. This review will focus on the antimicrobial properties of NK cells and the current standing and future perspectives of generating and using NK cells as immunotherapy in patients with infectious complications, an approach which is promising and might have an important clinical impact in the future.
Keywords: natural killer cell, adoptive immunotherapy, virus, bacterium, fungus
INTRODUCTION
Immunocompromised patients, such as patients suffering from cancer or hematopoietic stem cell transplant (HSCT) recipients, are at a significantly increased risk for an infectious complication due to viral, bacterial or fungal pathogens [1–4]. In addition, in this patient population, infections often have a more severe clinical course and are an important cause of mortality [4]. Depending on the pathogen, different components of the host immune system play a role in the response to an infection. Lymphocytes are important in the combat against viruses, whereas granulocytes play a key role in bacterial and fungal infections, and proliferation and activation of these immune cells is regulated by cytokines and interferons. Over the last decades it became clear that also Natural Killer (NK) cells are involved in the host response against all of these pathogens.
In the immunocompromised patient, the recovery of the immune system has a major impact on the outcome of an infectious complication [4, 5]. Therefore, there is major interest in strengthening the host response in immunocompromised patients, either by using cytokines or growth factors or by adoptive cellular therapies transfusing immune cells such as granulocytes or pathogen-specific T-cells [6–8]. To date, relatively little is known about the potential of adoptively transferred NK cells in patients with infectious complications, although the anti-cancer property of NK cells is intensively being investigated in the clinical setting. Notably, in addition to strengthen the host response against a pathogen, potential adverse effects have to be considered, such as excessive inflammation by pro-inflammatory cytokines resulting in tissue damage [9–11]. This review will focus on the antimicrobial properties of NK cells and the current standing and future perspectives of generating and using NK cells as immunotherapy in patients with infectious complications, an approach which is promising and might have an important clinical impact in the future.
NK cell biology
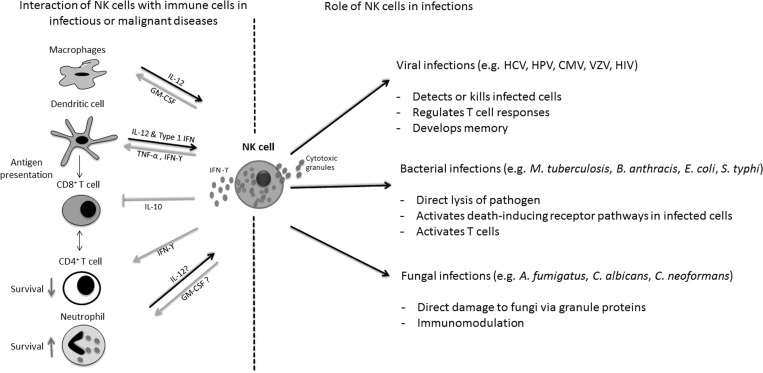
Human NK cells are cytolytic innate immune cells that are defined by the expression of CD56 and by the absence of the T cell marker CD3. NK cells originate from the bone marrow, home to secondary lymphoid tissues, and represent up to 15% of peripheral blood mononuclear cells. In respect to the surface expression density of CD56 and CD16, two main subpopulations of NK cells can be distinguished, namely the cytotoxic CD56dimCD16bright and the immune regulatory CD56brightCD16dim subsets [12]. The activity of NK cells depends on the surface expression of several activating and inhibitory receptors that recognize MHC class-1 molecules [13–17]. Activating receptors include the natural cytotoxicity receptors NKp46, NKp44, or NKp30 and NKG2D which recognize ligands and are upregulated during cellular stress such as tumor transformation and viral infections [16, 18]. Among the inhibitory receptors, the killer-immunoglobulin-like receptors (KIRs) play an important role in NK cell alloreactivity [19]. NK cells are able to kill their target directly by cytotoxic molecules such as perforin or granzyme B, and by death receptor mediated apoptosis [20, 21]. In addition to their cytotoxic function, NK cells are able to modify the immune response of the host by secreting different chemokines, like tumor-necrosis-factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) or CCL5 (RANTES) and interferon (IFN)-γ [22–24] (Figure 1). NK cells have recently been classified closely to group 1 innate lymphoid cells (ILCs), which are characterized by the ability to produce IFN-γ, but not type 2 cytokines [25].
Figure 1. Antimicrobial activities of Natural Killer (NK) cells.
NK cells not only detect and damage various viral, bacterial and fungal pathogens (right side), but also modulate proliferation and activation of a variety of cells of the innate and adaptive immune system (left side). IL interleukin; IFN interferon; TNF tumor-necrosis factor; GM-CSF granulocyte-macrophage colony-stimulating factor; HCV hepatitis C virus; HPV human papilloma virus; CMV cytomegalovirus; VZV Varicella-Zoster virus; HIV human immunodeficiency virus.
Current research has shown that NK cell education and differentiation plays an important role in both the direct and antibody-dependent functionality of NK cells [26, 27]. In this respect, various models have been developed to explain the process of NK cell “education” [23, 28]. In general, the expression of self-recognizing inhibitory receptors (SRIR) guides NK cell development towards fully functional mature NK cells and has been termed “licensing” process [29]. The “disarming” model describes NK cells lacking SRIR that become anergic due to chronic activation [30], and the more dynamic “rheostat model” has been introduced to describe that stronger inhibitory signaling through more SRIR interactions leads to greater functional responsiveness of NK cells [31, 32]. It has been reported that SRIR deficient NK cells can be primed to a functional state upon cytokine stimulation [29]. Of note, also uneducated NK cells may play important roles in the combat against viral infections, since SRIR-deficient NK cells strongly respond toward murine CMV [33]. In conclusion, all these results underline the value of a highly diverse NK cell repertoire with unlicensed and licensed cell subsets that interact in the defense of infectious diseases [34]. Importantly, NK cells also have the ability to shape adaptive immunity such as immunological memory [35–37], as both animal models and human studies indicate that NK cells can develop long-lasting antigen-specific memory cells [38]. These aspects of NK cell regulation are highly interesting as it demonstrates the high potential of NK cells for immuno-prophylactic and/or immunotherapeutic strategies, which may be important not only in the field of cancer therapy but also for infectious diseases [23, 39].
Anti-cancer activity of NK cells
It is well known that NK cells play a crucial role in the defense against cancer, and that low NK cell activity is associated with an increased risk for malignancy [40, 41]. The direct cytotoxicity of human expanded NK cells against several tumor cell lines could be demonstrated in various experimental settings, for example against human U87 glioblastoma cells [42], the neuroblastoma cell line UKF-NB-3 [43], and various Ewing sarcoma and rhabdomyosarcoma cell lines [44]. While cytotoxic NK cells directly eliminate tumor cells, regulatory NK cells also play an important role in the antitumor activity by orchestrating the complex interaction of other immune cells through secreted cytokines and chemokines [20, 45, 46].
Anti-cancer immunotherapy with NK cells – clinical aspects
A number of clinical trials investigated the immunotherapeutic anti-cancer property of NK cells in various patient populations. For example, regional arterial administration of autologous cytokine-stimulated NK cells (lymphokine-activated killer cells; LAK) in combination with daily administration of low IL-2 doses to patients with metastatic renal cell carcinoma resulted in the regression of five out of 15 treated metastases and pain relief in six out of ten patients [47]. A similar approach was used in patients with recurrent glioblastoma [48]. Although the response rate of immunotherapy was 33%, the survival of 18 months from diagnosis could not be improved. A phase I clinical trial using expanded autologous NK cells (approximately 4720-fold expansion) in patients with advanced digestive cancer demonstrated that the cells were well tolerated [49]. Despite the fact that the cells were highly lytic and a strong expression of functional markers such as NKG2D and CD16 was found, no clinical response could be observed. Safety and efficacy of multiple infusions of autologous activated and expanded NK cells in combination with anti-myeloma drugs were evaluated in a recent single-arm open-label phase I clinical trial which included five patients with relapsed or refractory multiple myeloma who had received two to seven prior lines of therapy [50]. Four of the five patients showed disease stabilization prior to the end of treatment, and two showed a 50% reduction in bone marrow infiltration and a long-term response of more than one year. The use of allogeneic NK cells for immunotherapy is currently being evaluated in different settings, such as acute lymphoblastic or myeloid leukemia (ALL and AML, respectively), but also in high-risk solid tumors (reviewed in [19]). Most of the patients receive a combined treatment which includes haploidentical HSCT and the adoptive transfer of NK cells from the same haploidentical donor followed by IL-2 application, and the overall results suggest a decrease of the relapse rate. For example, in one trial, the adoptive transfer of human haploidentical NK cells in AML patients induced complete hematologic remission in five out of 19 poor-prognosis patients [39]. In contrast to the haploidentical transplant setting, the use of allogeneic NK cells in unrelated HSCT is less clear and a matter of controversy [19]. In a phase I clinical study which evaluated allogeneic NK cells from random healthy donors in 17 patients with malignant lymphoma or advanced or recurrent solid tumors, no serious adverse event occurred [51]. This corroborates the data of other studies that immunotherapy with NK cells is usually safe and well tolerated, and only temporary side effects such as fever, weight gain, or neurotoxicity are observed [52]. Notably, in a phase II study which enrolled 20 patients with recurrent ovarian and breast cancer, one ovarian cancer patient developed tumor lysis syndrome within 6 h of the initial NK cell infusion. The timing of the event, together with the detection of NK cells in the necrotic liver specimen on autopsy, suggests that NK cells could have contributed to the tumor lysis [53].
To this end, the use of NK cells as immunotherapy against cancer is promising, but further studies are warranted in order to identify patient populations which will significantly benefit from this strategy, and to tailor pharmacological and immunological therapies to the individual patients´ characteristics.
NK cells in the host response against infectious pathogens
There is a growing body of evidence that NK cells play a major role in the host response against various pathogens. For example, a number of studies have demonstrated that genetic mutations may lead to reduced NK cell numbers or functional NK cell impairment such as mutations affecting genes IL2RG, JAK3, and ADA which cause severe combined immunodeficiency syndromes [54, 55] or a mutation in the ITGB2 gene associated with leukocyte adhesion deficiency [56]. These immunocompromised patients have an increased susceptibility to viral infections, such as infections with herpes simplex virus (HSV), Varicella Zoster virus (VZV), Cytomegalovirus (CMV), and with human papilloma virus [22, 41, 57]. However, as these patients display multiple defects of the immune system, the exact role of NK cells in the increases risk of viral infection remains unclear. An early report described a young girl who experienced a series of recurrent and severe viral infections during childhood and adolescence, including infections by multiple herpes viruses, which was thought to be the result of non-functional NK cells [58]. Other studies reported on children with altered forms of the Fc receptor for IgG type IIIA (CD16) on their NK cells, who suffered from recurrent viral infections such as infections due to HSV, Epstein-Barr virus (EBV) and VZV, respectively [59, 60]. The clinical condition of these children significantly improved with acyclovir prophylaxis. Recently, it has been shown that decidua NK cells inhibit human immunodeficiency virus (HIV)-1 infection in pregnancy [61]. Similar to the fight against cancer cells, NK cells limit viral burden not only by killing of infected cells [38], but also by modulating the cytokine milieu, which in turn influences other immune cells such as T cells. For example, NK cell derived IFN-γ is not only important for the direct non-cytopathic inhibition of the replication of the hepatitis C virus [62], but also regulates the immune responses of CD4+ and CD8+ T cells [63–65]. Importantly, recent data of animal and human studies indicate that NK can develop long-lasting antigen specific memory cells [38]. Much work has been performed on the evaluation of the importance of NK cells in the host response against influenza virus. It has become clear that the severity of influenza disease is not uniform, with a severe clinical course being associated with transient T and NK cell deficiency [66] and with specific haplotypes of killer-immunoglobulin-like receptors (KIRs) [67]. In a mouse model, infection with a high dose of influenza virus led to the impairment of cytotoxicity and IFN-γ production by spleen NK cells and to decreased virus-specific killing mediated by cytotoxic T lymphocytes. Importantly, the latter could be reversed by the adoptive transfer of spleen NK cells harvested from low-dose-infected mice [68]. During influenza infection, NK cells are activated by different mechanisms, such as by influenza nucleoprotein (NP) and matrix 1 (M1) antibodies [69], and CD16 seems to play an important role in the early activation of NK cells after vaccination against influenza [70]. A recent study demonstrated that shortly after infection with influenza virus, licensed (“functional”) NK cells serve as early innate effectors as they produce IFN-γ in inflamed parenchymal tissues and further mediate direct antiviral responses [34]. In contrast, NK cells which lack self-specific MHC-I receptors (“unlicensed” NK cells) are localized in the draining lymph nodes and help to promote activation and expansion of dendritic cells, which ultimately results in a sustained antigen-specific CD8+ response. In addition to the killing of virus-infected cells, NK cells provide vital cytokines for tissue regeneration, such as IL-22 [71]. However, it is important to note that in mouse models, NK cells might mediate pathology as the depletion of NK cells in vivo reduced mortality from influenza infection, whereas the adoptive transfer of NK cells from influenza-infected lung, but not from uninfected lung resulted in increased mortality in influenza-infected mice, probably due to a deleterious NK cell-dependent alteration of T cell responses [72].
Compared to the antiviral activity of NK cells, considerably less data are available for the interaction of NK cells with bacteria and fungi. NK cells exhibit direct activity against a variety of Gram-positive and Gram-negative bacteria such as Mycobacterium tuberculosis, Bacillus anthracis, Escherichia coli or Salmonella typhi by the secretion of the soluble molecules perforin and granulysin [73–76]. In addition, NK cells have an antibacterial effect against intracellular bacterial pathogens by using death inducing receptor pathways such as Fas-FasL and TNF-related apoptosis-inducing ligand (TRAIL) pathways [77, 78], which ultimately induce caspase-dependent apoptosis of the target cell [21, 77, 79]. The important role of the antibacterial activity of NK cells in vivo is demonstrated by animal models which showed higher survival rates and lower bacterial titers during infection with Shigella flexneri in mice lacking B and T cells but having NK cells as compared to mice which lack all three cell types [80].
Similar to the antibacterial activity, NK cells exhibit in vitro antifungal activity against a number of pathogenic fungi such as Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, or different species of mucormycetes [81–86]. Cytotoxic molecules including NK cell derived perforin seem to be important in the antifungal activity. In addition, upon stimulation by fungi, NK cells release a number of cytokines, which modulate both innate and adaptive immune responses [87]. The in vitro data of the antifungal activity of NK cells are supported by observations made in animal studies. For example, it has been shown that NK cells proliferate in mice experimentally infected with Aspergillus niger, and this proliferation was associated with an inhibition of the fungal growth [88]. Antibody mediated depletion of NK cells in mice inoculated with C. neoformans [89] resulted in a significant higher fungal burden in the lungs as compared to untreated controls, corroborating studies in NK-depleted mice which revealed the pivotal role of NK cells in the host response against A. fumigatus, C. albicans, and Histoplasma capsulatum [90–95].
Although these data clearly demonstrate that NK cells exhibit important activities in the host immune response to different viral, bacterial and fungal pathogens, many questions have to be resolved. For example, further studies have to evaluate how and to which extent a pathogen may exert an immunosuppressive effect on NK cells as well as on other cells of the immune system, which has been shown for bacteria such as Pseudomonas aeruginosa and for fungi such as A. fumigatus. This knowledge may be important in the clinical setting for specifically strengthening the host response during infection.
The strategy of adoptive immunotherapy in infectious complications
Both severity and duration of a defect in the immune system are associated with risk and outcome of an infectious complication [4]. Therefore, since the 1970s, there was great interest to improve the prognosis of persistently neutropenic patients suffering from severe infections with the administration of granulocytes, and the availability of recombinant hematopoietic growth factors such as granulocyte-colony stimulating factor (G-CSF) extended the use of this strategy even into the prophylactic setting (“adoptive immunotherapy”) [96]. A prospective, non-randomized study evaluated transfusions of granulocytes in order to control acute life-threatening infections or to prevent recurrence of severe fungal infections during HSCT or intensive chemotherapy [6]. Granulocyte transfusions achieved control in 82% of acute life-threatening infections, and no single reactivation of a previous infection occurred with prophylactically administered granulocytes, whereas a recent randomized study failed to demonstrate a significant benefit of this strategy [97]. In patients suffering from invasive fungal infections, a recent review of available data did not find strong evidence of a benefit of granulocyte transfusions, but it is to hope that ongoing randomized controlled studies such as the GRANITE study (German Clinical Trials Register number DRKS00000218) will provide helpful results [98]. Notably, granulocyte transfusions are often associated with febrile transfusion reactions and pulmonary complications, including transfusion-related acute lung injury (TRALI), and a monocenter retrospective analysis of 128 patients with a hematological malignancy, prolonged neutropenia and invasive aspergillosis suggested that patients receiving granulocyte transfusions had a worse outcome [99]. Another approach which aims to reconstitute the long-lasting impairment of cellular immunity of allogeneic HSCT recipients became possible with the development of techniques to isolate and to generate pathogen-specific T cells. Infusion of CMV-specific T cell clones largely prevented CMV reactivation and reduced CMV mortality [7, 100], and studies reported on a clinical benefit for adoptively transferred T cells specific against adenovirus [101] and Epstein-Barr-virus (EBV) [102], respectively. Similarly, a proof-of principle study showed that the adoptive transfer of pathogen-specific Aspergillus CD4+ T cells resulted in a rapid decline of Aspergillus antigen in the blood, and more importantly, 9 of 10 patients cleared invasive aspergillosis [7]. In contrast to most studies which use donor-derived pathogen specific T cells, a recent trial reported on the successful use of “off the shelf” T cells generated from eligible third-party donors against a variety of viruses such as BK virus, human herpesvirus 6, adenovirus or EBV [103].
Although immunotherapy with adoptively transferred pathogen specific T cells seems to be a promising strategy, the use of T cells may be associated with the risk of graft-versus-host disease (GvHD) [104].
NK cells as potential immunotherapeutic agent in infectious complications
As compared to studies investigating NK cells as immunotherapeutic tool in patients with an underlying malignancy, relatively little is known regarding the in vivo effect of adoptively transferred NK cells into a host suffering from an infectious complication. In Aspergillus infected mice, the depletion of NK cells resulted in a higher fungal load and lower survival, whereas the transfer of activated NK cells to these mice led to greater pathogen clearance from the lungs [93]. Similarly, cyclophosphamide pretreated mice suffering from cryptococcosis showed an enhanced clearance of the fungus when they had received an NK cell-enriched graft as compared to mice which had received an NK cell-depleted graft [105, 106]. Although these results suggest that adoptively transferred NK cells may be a potential tool in patients suffering from fungal infections, no study to date has proven this concept in the clinical setting. Importantly, there may be a major difference of the adoptive transfer of NK cells between immunocompromised and immunocompetent patients. Whereas safety data in immunocompromised patients with cancer are promising [107, 108], results on the adoptive transfer of NK cells into immunocompetent human individuals are lacking. However, it has been demonstrated that NK cells infused in mice with polymicrobial intra-abdominal bacterial infection contributed to an excessive induction of pro-inflammatory cytokines which ultimately resulted in a lethal septic shock [10, 11, 109–111]. Another study in the murine model demonstrated that infection by Listeria monocytogenes resulted in excessive IFN-γ production by CD27+ NK cells, which in turn, impaired innate anti-bacterial host defenses by inducing down-regulation of CXCR2 on granulocytes and thus inhibiting the recruitment of granulocytes at the site of infection [112]. However, the mice could be rescued by antibodies blocking CD27 signaling or by depleting IFN-γ. Therefore, further studies have to better define the preconditions in which the adoptive transfer of NK cells may be beneficial or harmful, such as the degree of the host´s immune impairment or the pathogen(s) causing an infection, as well as the optimal schedule and dosage of adoptively transferred NK cells in each setting. On the other hand, as compared to pathogen-specific T cells which have already been evaluated in the clinical setting, NK cells may be of advantage since they are active against a broad range of pathogens including viruses, bacteria, or fungi.
Generation of high numbers of functionally active NK cells for adoptive immunotherapy
When employing NK cells to fight against a malignancy or to combat infectious complications, only functional active NK cells administered at a sufficient effector-to-target (E:T) ratio might result in a beneficial effect. Studies addressing both mouse and human NK cell immunity have shown that NK cells are a heterogeneous population consisting of not only phenotypically but also functionally distinct subsets, and cytokine-stimulation induces significant changes not only in proliferation, maturation status and finally in the subset composition of the ex vivo expanded NK cell product [45, 113]. Therefore, knowledge on mouse NK cells cannot directly be transferred to the human system, and expansion and activation studies are mainly performed on human NK cell preparations from peripheral blood or umbilical cord blood leading to first preclinical data evaluating the cytotoxic capacity of the final NK cell product in vitro and in vivo in xenograft NSG (NOD scid gamma) mouse models against various human tumors [114].
Most clinical trials with NK cells in the autologous or allogeneic setting administer ex vivo expanded NK cells [115, 116]. Of note, the expansion of NK cells usually needs several days to weeks, depending on the protocols used [114, 117]. In order to improve the rapid expansion of isolated NK cells, cytokines like IL-2 have been investigated, which can be added either alone or in combination with an anti-CD3 antibody or additional cytokines such as IL-15 and IL-21 [117–120]. Although the exact role of anti-CD3 antibodies is unclear, it has been suggested that OKT-3 leads to a profound outgrowth of NK cells, which is probably due to the activation of T cells [114]. Other studies suggest that co-culture of NK cells with stimulatory cells such as EBV-transformed lymphoblastoid cells or a Wilms tumor-derived cell line also enhances the proliferation of NK cells [121, 122]. In an elegant approach, HLA-negative K562 cells were genetically modified to express membrane-bound IL-15 and 4-1 BB Ligand (4-1BBL), which specifically activate NK cells and promote their proliferation and survival, and this strategy resulted in a dramatic enhancement of NK cell expansion and activation [123, 124]. After further improvement, this method has been adapted to large-scale Good Manufacturing Practices (GMP) conditions [121, 125]. In addition to autologous or allogeneic NK cells, it may be possible to differentiate NK cells in vitro from umbilical cord blood CD34+ cells, which is currently being tested in two clinical trials (NCT01619761 and NCT01729091) [126].
In addition to proliferation, cytokine stimulation may also improve the functional activity of NK cells. For example, when NK cells are resting, lytic granules are distributed randomly or diffuse, whereas after exposure to IL-2, granules congregate to the microtubule-organizing center [127]. Thus, IL-2 stimulation not only activates NK cells, but also accelerates their transition into NK cells which are ready to exhibit their cytotoxic function. Another study demonstrated that exposure of murine NK cells to IL-12, IL-15 and IL-18 resulted in a sustained effector function of NK cells in vivo [128]. Only IL-12/15/18-preactivated NK cells, but not naïve, IL-15- nor IL-2-pretreated NK cells, respectively, reduced the growth of tumors when mice were concomitantly irradiated. Similarly, prestimulation with a cytokine cocktail including IL-12, IL-15, and IL-18 resulted in enhanced IFN-γ production of human NK cells after restimulation with K562 leukemia cells or with these cytokines [129]. Even if there are known differences in the biology of murine and human NK cells, this observation suggests that both murine and human NK cells receive functional memory-like properties after cytokine activation, which may provide a novel rationale for integrating cytokine preactivation into NK cell immunotherapeutic strategies. However, at the same time, the use of cytokines may alter the phenotype of the NK cell and result in a potential loss of responsiveness to some stimuli [130, 131]. For example, IL-2 stimulation of NK cells decreased CD16 [132], and NK cell activation by intramuscular influenza vaccination and HIV-positive plasma induced a matrix metalloproteinase-mediated cleavage of cell surface CD16, whereas inhibition of CD16 shedding potentiated NK cell cytotoxic function [70, 133].
In addition to the exposure to various cytokines, improvement of NK cell activity can be achieved by the manipulation of NK cell receptors. For example, NK cell cytotoxicity against tumor targets could be improved by a retroviral transduction of a receptor termed NKG2D-DAP10-CD3ζ that is composed of NKG2D plus two signaling molecules, DAP10 and CD3ζ [134]. These modified NK cells exhibit higher killing activity against a number of ALL cell lines such as CEM-C7 or MOLT-4, and against solid tumor-derived cell lines such as the human prostate adenocarcinoma cell line LNCaP or the hepatocellular carcinoma cell line HepG2 [134]. Importantly, the increased activity of the modified cells could also be demonstrated in vivo, since in immunodeficient mice, NKG2D-DAP10-CD3ζ-transduced NK cells killed osteosarcoma or hepatocellular carcinoma cells more effectively compared to mock-transduced NK cells [134, 135]. However, soluble NKG2D ligands (sNKG2DL) such as sMICA/B or sULBP2 are able to impair NK cell activity as it has been demonstrated in the setting of various tumors and of HIV [43, 136]. Naïve HIV-positive patients display increased plasma levels of sMICA and reduced NKD2D expression on NK cells, and sNKG2DLs impair NKG2D-mediated cytotoxicity of NK cells [136]. Interestingly, highly active antiretroviral therapy (HAART) resulted in the drop of sNKD2DL and recovery of NKD2D expression.
Another interesting approach is the transduction of chimeric antigen receptors (CARs) into immune cells in order to improve their activity. CARs are engineered receptors with the ability to bind to specific antigens which are expressed on the surface of tumor cells or on the surface of a pathogen. The strategy of CARs in patients’ T cells has recently been reported, which resulted in impressive regression of B cell malignancies, and the promising results of first clinical studies on CAR-T cells have recently been reported [137–140]. Of note, CAR constructs expressed in the NK cell line NK92 [141], primary NK cells [137, 142] or cord blood derived NK cells [143] have demonstrated efficient killing in preclinical settings [144]. Two clinical trials testing haploidentical donor-derived CAR NK cells for targeting of refractory CD19+ ALL with a second-generation anti-CD19 CAR that incorporates the 4-1BB costimulatory domain are currently recruiting patients (NCT00995137 from St. Jude and NCT01974479 from The National University Health System, Singapore) (for review see [145]).
In contrast, studies on NK cells with modified receptors or CAR-NK cells against infections are lacking, and challenges of this strategy include the relatively long time to generate these cells and the complex antigenic properties of many pathogens, which is seen in particular in fungi [146].
The functional activity of NK cells can also be enhanced by the forced expression of the high-affinity CD16-158V (HA-CD16) Fc receptor, for which the minority of patients is homozygous [147, 148]. Several groups demonstrated that engraftment of HA-CD16 on primary NK cells from donors which express a low-affinity CD16-158F/F (LA-CD16) Fc receptor [149] or on the NK-92 cell line [150] significantly increases the antibody-dependent cell-mediated cytotoxicity (ADCC) against target cells coated with rituximab, an antibody directed against CD20, of which the Fc portion mediates both antibody-dependent cell-mediated and complement-dependent cytotoxicity. Rituximab is used as therapeutic compound in many patients suffering from B cell non-Hodgkin lymphoma.
The genetic disruption of NK cell inhibitory receptors such as KIR or NKG2A via inhibiting antibodies or via shRNA silencing may also be used to overcome tumor evasion mechanisms related to MHC I expression. In this regard, in tumor bearing mice, NKG2A-silenced cells of the NKL cell line revealed enhanced killing activity of 721.221 HLA-E expressing EBV-LCL tumor cells [151–153]. Similarly, in the setting of an infection, blocking the interaction between NK cell inhibitory receptors [e.g., CD159a (NKG2A), CD158a (KIR2DL1), and 158b (KIR2DL2)] and MHC class I molecules (e.g., HLA-C and HLA-E) on HIV-infected autologous tumor cells resulted in a drastic increase in killing of anti-gp120-coated HIV-infected cells by NK cells [154].
Immunotherapy in infectious complications using NK cells from a cell bank
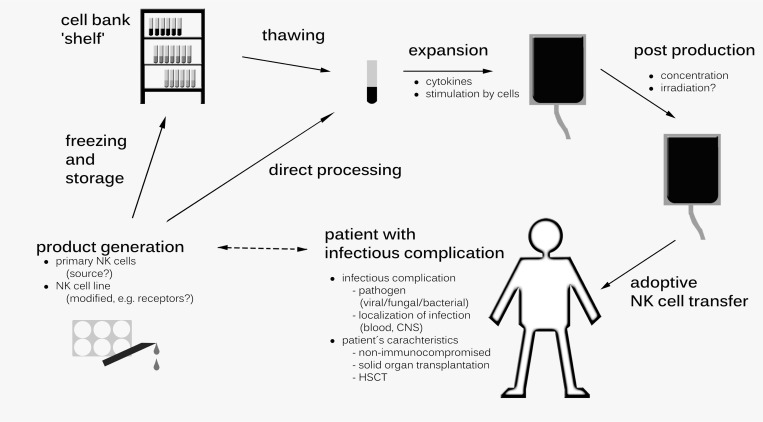
In particular in immunocompromised patients, infections often have a sudden onset with a rapid clinical course. Therefore, an immunotherapeutic tool in this setting has to be quickly available, which might be different to the setting of immunotherapy used against an underlying malignancy. In other words, the timely access to a suitable NK cell product is crucial when planning clinical studies evaluating NK cells in patients with viral, bacterial, or fungal infections (Figure 2). In this respect, NK cell products which are standardized, well characterized and cryopreserved would be ideal and open new perspectives in this emerging field. However, the long-term storage of NK cell products remains controversial. Whereas it has been demonstrated that NK cells maintain their cytotoxic activity against the leukemia cell line K562 after cryopreservation [126, 155], standard methods of cryopreservation seem to have a negative impact on cell expansion in vivo [156]. Interestingly, in one clinical trial NK cells were expanded upon medical need from aliquots of individual cryopreserved leukapharesis cryopreserved peripheral blood mononuclear cells [157].
Figure 2. Potential strategies of generating Natural Killer (NK) cells as an immunotherapeutic tool for patients suffering from infectious complications.
Both the infectious complication (e.g., pathogen, localization) and the patient´s characteristic influence the generation of the NK product (e.g., primary cells, NK cell line), which can be directly processed or be frozen and stored. CNS, central nervous system; HSCT, hematopoetic stem cell transplantation.
NK cell lines as the source for NK cell immunotherapy
Having identified the challenges in derivation, activation and expansion of NK cells directly from patients, NK cell lines may be considered as an ideal source for cell-based immunotherapy. Advantages using NK cell lines for immunotherapy would include 1) the possibility to establish a master cell bank, and 2) the fact that the cell source would be extremely well standardized and characterized. As one example, the permanent NK-92 cell line is cryopreserved in GMP-compliant master cell bank, from which it can be easily and reproducibly expanded [158]. The cell line exhibits cytotoxicity against a broad spectrum of tumor targets in vitro such as various leukemia, lymphoma and myeloma cell lines as well as against primary leukemic blasts [159–162]. When used as immunotherapy against cancer, preliminary data demonstrated the safety and tolerability of NK-92 cells in the clinical setting [163, 164]. A number of groups are exploring the use of CAR-modification to enhance the antitumor activity of these cells in preclinical and clinical studies [165, 166] (for review see: [145, 167]).
However, to date, relatively little is known about the activity of the NK-92 cell line against infectious pathogens. NK-92 cells reveal in vitro activity against EBV-infected B lymphocytes and the supernatant collected from NK-92 cells inhibits HIV replication in PBMCs from HIV-infected subjects in a dose-dependent manner [168]. In contrast, hepatitis B virus (HBV) antigens HBsAg and HBeAg have a direct negative impact on NK-92 cell activation, cytokine production and cytotoxic granule release [169]. In addition, the lack of toll-like receptor (TLR) 4 and CD16 (Fc receptors FcγRIIIa and FcγRIIIb) might be a relevant limitation of this cell line regarding the antimicrobial activity, since, for example, TLR4 is responsible for the detection of FimH, which is present of fimbria of some bacteria [170]. Other drawbacks in the clinical use of NK-92 cells include the fact that the NK-92 cell line is derived from an NK cell lymphoma and therefore has the potential risk for uncontrolled proliferation, in particular if cells are resistant or not fully hit by prior irradiation. In turn, irradiation, which is mandatory prior to infusion, severely limits the survival and function of the transferred cells. Since NK-92 cells are dependent on IL-2, the repeated IL-2 injections raise concerns regarding toxicity [171]. Whether this potential toxicity can be abrogated by genetic modification of the cells leading to constitutive expression of IL-2 and resulting in auto-activated and auto-proliferating cells is unclear to date [148, 149]. Of note, in a promising preclinical study, transduction of clinically applicable NK-92 cells with lentiviral vectors encoding human IL-15 resulted in predominantly intracellular expression of the cytokine, proliferation and cytotoxicity of the producer cells in the absence of IL-2 [172].
Based on these experiences, further studies evaluating the activity of the NK-92 cell line against viral, bacterial, and fungal pathogens are urgently needed.
It is important to mention that in addition to the NK-92 cell line, there are other cell lines which potentially could be used as adoptive NK cell-based immunotherapy, both in patients with cancer and in patients with infectious complications. For example, the KHYG-1 cell line derived from NK leukemia has superior cytotoxicity compared to NK-92 cells [173]. In addition, irradiation of these cells does not abrogate their cytotoxicity towards tumor targets. Similarly, the cell line NKL, which is biologically and functionally very similar to primary NK cells, exhibits enhanced cytotoxicity against certain tumor cells as compared to NK-92 [174]. Again, the antimicrobial activity of these cells lines is unclear and needs further evaluation.
CONCLUSIONS AND FUTURE PERSPECTIVES
Whereas the anticancer effect of NK cells is currently investigated in multiple clinical trials, little is known about the potential of adoptively transferred NK cells in patients suffering from infectious complications. In vitro data clearly demonstrate that NK cells are active against viral, bacterial, and fungal pathogens, and animal studies suggest that NK cells could be a promising tool in the antimicrobial immunotherapy. Current investigation focuses on the optimal and rapid generation of high numbers of functionally active NK cells and includes strategies such as genetic modification of the cell and its receptors and the use of NK cell lines for treatment of hemato-oncological diseases. However, before testing adoptively transferred NK cells in the clinical setting of infectious complications, a number of questions have to be resolved. For example, it is well known that activation of the host immune system (e.g., by the release of pro-inflammatory cytokines) is necessary in order to successfully fight the pathogen, but, at the same time, might cause severe complications, as it was documented in patients with pulmonary aspergillosis who received granulocyte transfusions [99]. Therefore, both the patient population (immunocompromised versus immunocompetent) and the optimal time point of the adoptive transfer of NK cells are unknown to date, or, in other words, it is unclear when and whom adoptively transferred NK cells will help to overcome an infectious complication or will ultimately harm. Based on the promising results in animal studies and due to the facts that HSCT recipients suffering from fungal infections lack a sufficient immune response and outcome of this patient population is extremely poor, first clinical trials might focus on invasive fungal disease in this patient population. Although it will be necessary to fully characterize the optimal patient population, the best time point of therapy as well as the best approach to generate NK cells for immunotherapy in infectious complication, this strategy might become important in this setting, in particular since antimicrobial compounds have limited activity and we witness emerging resistance of pathogens all over the world.
Footnotes
Author contributions
All authors were involved in designing and writing the manuscript, and all authors read and approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Editorial note
This paper has been accepted based in part on peer-review conducted by another journal and the authors’ response and revisions as well as expedited peer-review in Oncotarget.
REFERENCES
- 1.Atilla E, Atilla PA, Bozdağ SC, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection. 2017;45:403–11. doi: 10.1007/s15010-017-1016-1. https://doi.org/10.1007/s15010-017-1016-1. [DOI] [PubMed] [Google Scholar]
- 2.Hermann B, Lehners N, Brodhun M, Boden K, Hochhaus A, Kochanek M, Meckel K, Mayer K, Rachow T, Rieger C, Schalk E, Weber T, Schmeier-Jürchott A, et al. Influenza virus infections in patients with malignancies— characteristics and outcome of the season 2014/15. A survey conducted by the Infectious Diseases Working Party (AGIHO) of the German Society of Haematology and Medical Oncology (DGHO) Eur J Clin Microbiol Infect Dis. 2017;36:565–73. doi: 10.1007/s10096-016-2833-3. https://doi.org/10.1007/s10096-016-2833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532–39. doi: 10.1182/blood-2007-05-091942. https://doi.org/10.1182/blood-2007-05-091942. [DOI] [PubMed] [Google Scholar]
- 4.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–40. doi: 10.7326/0003-4819-64-2-328. https://doi.org/10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 5.Stuehler C, Kuenzli E, Jaeger VK, Baettig V, Ferracin F, Rajacic Z, Kaiser D, Bernardini C, Forrer P, Weisser M, Elzi L, Battegay M, Halter J, et al. Immune Reconstitution After Allogeneic Hematopoietic Stem Cell Transplantation and Association With Occurrence and Outcome of Invasive Aspergillosis. J Infect Dis. 2015;212:959–67. doi: 10.1093/infdis/jiv143. https://doi.org/10.1093/infdis/jiv143. [DOI] [PubMed] [Google Scholar]
- 6.Mousset S, Hermann S, Klein SA, Bialleck H, Duchscherer M, Bomke B, Wassmann B, Böhme A, Hoelzer D, Martin H. Prophylactic and interventional granulocyte transfusions in patients with haematological malignancies and life-threatening infections during neutropenia. Ann Hematol. 2005;84:734–41. doi: 10.1007/s00277-005-1055-z. https://doi.org/10.1007/s00277-005-1055-z. [DOI] [PubMed] [Google Scholar]
- 7.Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F, Martelli MF, Romani L, Velardi A. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106:4397–406. doi: 10.1182/blood-2005-05-1775. https://doi.org/10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safdar A, Rodriguez GH, Lichtiger B, Dickey BF, Kontoyiannis DP, Freireich EJ, Shpall EJ, Raad II, Kantarjian HM, Champlin RE. Recombinant interferon gamma1b immune enhancement in 20 patients with hematologic malignancies and systemic opportunistic infections treated with donor granulocyte transfusions. Cancer. 2006;106:2664–71. doi: 10.1002/cncr.21929. https://doi.org/10.1002/cncr.21929. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson HL, Estes MD, Thirumalapura NR, Walker DH, Ismail N. Natural killer cells promote tissue injury and systemic inflammatory responses during fatal Ehrlichia-induced toxic shock-like syndrome. Am J Pathol. 2010;177:766–76. doi: 10.2353/ajpath.2010.091110. https://doi.org/10.2353/ajpath.2010.091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza-Fonseca-Guimaraes F, Adib-Conquy M, Cavaillon JM. Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol Med. 2012;18:270–85. doi: 10.2119/molmed.2011.00201. https://doi.org/10.2119/molmed.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emoto M, Miyamoto M, Yoshizawa I, Emoto Y, Schaible UE, Kita E, Kaufmann SH. Critical role of NK cells rather than V alpha 14(+)NKT cells in lipopolysaccharide-induced lethal shock in mice. J Immunol. 2002;169:1426–32. doi: 10.4049/jimmunol.169.3.1426. https://doi.org/10.4049/jimmunol.169.3.1426. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. https://doi.org/10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 13.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. https://doi.org/10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 14.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–59. doi: 10.1038/sj.emboj.7600019. https://doi.org/10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bléry M, Olcese L, Vivier E. Early signaling via inhibitory and activating NK receptors. Hum Immunol. 2000;61:51–64. doi: 10.1016/s0198-8859(99)00157-3. https://doi.org/10.1016/S0198-8859(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 16.Biassoni R. Human natural killer receptors, co-receptors, and their ligands. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im1410s84. Chapter 14:Unit 14.10. https://doi.org/10.1002/0471142735.im1410s84. [DOI] [PubMed] [Google Scholar]
- 17.Del Zotto G, Marcenaro E, Vacca P, Sivori S, Pende D, Della Chiesa M, Moretta F, Ingegnere T, Mingari MC, Moretta A, Moretta L. Markers and function of human NK cells in normal and pathological conditions. Cytometry B Clin Cytom. 2017;92:100–14. doi: 10.1002/cyto.b.21508. https://doi.org/10.1002/cyto.b.21508. [DOI] [PubMed] [Google Scholar]
- 18.Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology. 2013;2:e26097. doi: 10.4161/onci.26097. https://doi.org/10.4161/onci.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggeri L, Parisi S, Urbani E, Curti A. Alloreactive Natural Killer Cells for the Treatment of Acute Myeloid Leukemia: From Stem Cell Transplantation to Adoptive Immunotherapy. Front Immunol. 2015;6:479. doi: 10.3389/fimmu.2015.00479. https://doi.org/10.3389/fimmu.2015.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. https://doi.org/10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 21.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–10. doi: 10.1016/j.molimm.2004.07.034. https://doi.org/10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. https://doi.org/10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132:536–44. doi: 10.1016/j.jaci.2013.07.006. https://doi.org/10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michel T, Hentges F, Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol. 2013;3:403. doi: 10.3389/fimmu.2012.00403. https://doi.org/10.3389/fimmu.2012.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–49. doi: 10.1038/nri3365. https://doi.org/10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 26.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagné F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. https://doi.org/10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Parsons MS, Zipperlen K, Gallant M, Grant M. Killer cell immunoglobulin-like receptor 3DL1 licenses CD16-mediated effector functions of natural killer cells. J Leukoc Biol. 2010;88:905–12. doi: 10.1189/jlb.1009687. https://doi.org/10.1189/jlb.1009687. [DOI] [PubMed] [Google Scholar]
- 28.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–72. doi: 10.1016/j.it.2011.06.001. https://doi.org/10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. https://doi.org/10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. https://doi.org/10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodin P, Lakshmikanth T, Johansson S, Kärre K, Höglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–41. doi: 10.1182/blood-2008-05-156836. https://doi.org/10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 32.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–80. doi: 10.4049/jimmunol.0803900. https://doi.org/10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11:321–27. doi: 10.1038/ni.1849. https://doi.org/10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamora AE, Aguilar EG, Sungur CM, Khuat LT, Dunai C, Lochhead GR, Du J, Pomeroy C, Blazar BR, Longo DL, Venstrom JM, Baumgarth N, Murphy WJ. Licensing delineates helper and effector NK cell subsets during viral infection. JCI Insight. 2017;2:415–22. doi: 10.1172/jci.insight.87032. https://doi.org/10.1172/jci.insight.87032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–97. doi: 10.4049/jimmunol.1003035. https://doi.org/10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narni-Mancinelli E, Vivier E, Kerdiles YM. The ‘T-cell-ness’ of NK cells: unexpected similarities between NK cells and T cells. Int Immunol. 2011;23:427–31. doi: 10.1093/intimm/dxr035. https://doi.org/10.1093/intimm/dxr035. [DOI] [PubMed] [Google Scholar]
- 37.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. 2014;155:213–19. doi: 10.1016/j.clim.2014.10.005. https://doi.org/10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol. 2017;44:43–51. doi: 10.1016/j.coi.2016.11.003. https://doi.org/10.1016/j.coi.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–57. doi: 10.1182/blood-2004-07-2974. https://doi.org/10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 40.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–58. doi: 10.1016/s1286-4579(02)00038-2. https://doi.org/10.1016/S1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 41.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. https://doi.org/10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 42.Oh SJ, Yang JI, Kim O, Ahn EJ, Kang WD, Lee JH, Moon KS, Lee KH, Cho D. Human U87 glioblastoma cells with stemness features display enhanced sensitivity to natural killer cell cytotoxicity through altered expression of NKG2D ligand. Cancer Cell Int. 2017;17:22. doi: 10.1186/s12935-017-0397-7. https://doi.org/10.1186/s12935-017-0397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader P, Passweg J, Klingebiel T, Schwabe D, Koehl U. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol. 2010;40:3255–67. doi: 10.1002/eji.201040568. https://doi.org/10.1002/eji.201040568. [DOI] [PubMed] [Google Scholar]
- 44.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010;16:3901–09. doi: 10.1158/1078-0432.CCR-10-0735. https://doi.org/10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. https://doi.org/10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 46.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–94. doi: 10.1038/ni1580. https://doi.org/10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 47.Hayakawa M, Hatano T, Ogawa Y, Gakiya M, Ogura H, Osawa A. Treatment of advanced renal cell carcinoma using regional arterial administration of lymphokine-activated killer cells in combination with low doses of rIL-2. Urol Int. 1994;53:117–24. doi: 10.1159/000282651. https://doi.org/10.1159/000282651. [DOI] [PubMed] [Google Scholar]
- 48.Boiardi A, Silvani A, Ruffini PA, Rivoltini L, Parmiani G, Broggi G, Salmaggi A. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother. 1994;39:193–97. doi: 10.1007/BF01533386. https://doi.org/10.1007/BF01533386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, Mineno J, Naito Y, Itoh Y, Yoshikawa T. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. doi: 10.1186/s12967-015-0632-8. https://doi.org/10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leivas A, Perez-Martinez A, Blanchard MJ, Martín-Clavero E, Fernández L, Lahuerta JJ, Martinez-Lopez J. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. Oncoimmunology. 2016;5:e1250051. doi: 10.1080/2162402X.2016.1250051. https://doi.org/10.1080/2162402X.2016.1250051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, Lim O, Kim TM, Ahn YO, Choi H, Chung H, Min B, Her JH, Cho SY, Keam B, Lee SH, Kim DW, Hwang YK, Heo DS. Phase I Study of Random Healthy Donor-Derived Allogeneic Natural Killer Cell Therapy in Patients with Malignant Lymphoma or Advanced Solid Tumors. Cancer Immunol Res. 2016;4:215–24. doi: 10.1158/2326-6066.CIR-15-0118. https://doi.org/10.1158/2326-6066.CIR-15-0118. [DOI] [PubMed] [Google Scholar]
- 52.Choi I, Yoon SR, Park SY, Kim H, Jung SJ, Kang YL, Lee JH, Lee JH, Kim DY, Lee JL, Park HS, Choi EJ, Lee YS, et al. Donor-Derived Natural Killer Cell Infusion after Human Leukocyte Antigen-Haploidentical Hematopoietic Cell Transplantation in Patients with Refractory Acute Leukemia. Biol Blood Marrow Transplant. 2016;22:2065–76. doi: 10.1016/j.bbmt.2016.08.008. https://doi.org/10.1016/j.bbmt.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K, Bliss R, Downs LS, Miller JS. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. https://doi.org/10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Notarangelo LD, Giliani S, Mazza C, Mella P, Savoldi G, Rodriguez-Pérez C, Mazzolari E, Fiorini M, Duse M, Plebani A, Ugazio AG, Vihinen M, Candotti F, Schumacher RF. Of genes and phenotypes: the immunological and molecular spectrum of combined immune deficiency. Defects of the gamma(c)-JAK3 signaling pathway as a model. Immunol Rev. 2000;178:39–48. doi: 10.1034/j.1600-065x.2000.17812.x. https://doi.org/10.1034/j.1600-065X.2000.17812.x. [DOI] [PubMed] [Google Scholar]
- 55.Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, Roberts JL, Puck JM. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–87. doi: 10.1016/s0022-3476(97)70199-9. https://doi.org/10.1016/S0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 56.Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, Nakauchi H, Shibuya A. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 1999;11:615–23. doi: 10.1016/s1074-7613(00)80136-3. https://doi.org/10.1016/S1074-7613(00)80136-3. [DOI] [PubMed] [Google Scholar]
- 57.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–94. doi: 10.1146/annurev-immunol-032712-100001. https://doi.org/10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 58.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–35. doi: 10.1056/NEJM198906293202605. https://doi.org/10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 59.Jawahar S, Moody C, Chan M, Finberg R, Geha R, Chatila T. Natural Killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II) Clin Exp Immunol. 1996;103:408–13. doi: 10.1111/j.1365-2249.1996.tb08295.x. https://doi.org/10.1111/j.1365-2249.1996.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Vries E, Koene HR, Vossen JM, Gratama JW, von dem Borne AE, Waaijer JL, Haraldsson A, de Haas M, van Tol MJ. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 1996;88:3022–27. [PubMed] [Google Scholar]
- 61.Quillay H, El Costa H, Duriez M, Marlin R, Cannou C, Madec Y, de Truchis C, Rahmati M, Barré-Sinoussi F, Nugeyre MT, Menu E. NK cells control HIV-1 infection of macrophages through soluble factors and cellular contacts in the human decidua. Retrovirology. 2016;13:39. doi: 10.1186/s12977-016-0271-z. https://doi.org/10.1186/s12977-016-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. https://doi.org/10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 63.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481:394–98. doi: 10.1038/nature10624. https://doi.org/10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waggoner SN, Daniels KA, Welsh RM. Therapeutic depletion of natural killer cells controls persistent infection. J Virol. 2014;88:1953–60. doi: 10.1128/JVI.03002-13. https://doi.org/10.1128/JVI.03002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol. 2015;98:153–62. doi: 10.1189/jlb.4HI1214-594R. https://doi.org/10.1189/jlb.4HI1214-594R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox A, Le NM, Horby P, van Doorn HR, Nguyen VT, Nguyen HH, Nguyen TC, Vu DP, Nguyen MH, Diep NT, Bich VT, Huong HT, Taylor WR, et al. Severe pandemic H1N1 2009 infection is associated with transient NK and T deficiency and aberrant CD8 responses. PLoS One. 2012;7:e31535. doi: 10.1371/journal.pone.0031535. https://doi.org/10.1371/journal.pone.0031535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aranda-Romo S, Garcia-Sepulveda CA, Comas-García A, Lovato-Salas F, Salgado-Bustamante M, Gómez-Gómez A, Noyola DE. Killer-cell immunoglobulin-like receptors (KIR) in severe A (H1N1) 2009 influenza infections. Immunogenetics. 2012;64:653–62. doi: 10.1007/s00251-012-0623-3. https://doi.org/10.1007/s00251-012-0623-3. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Zheng J, Liu Y, Wen L, Huang L, Xiang Z, Lam KT, Lv A, Mao H, Lau YL, Tu W. Uncompromised NK cell activation is essential for virus-specific CTL activity during acute influenza virus infection. Cell Mol Immunol. 2017 Apr 17 doi: 10.1038/cmi.2017.10. https://doi.org/10.1038/cmi.2017.10. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanderven HA, Ana-Sosa-Batiz F, Jegaskanda S, Rockman S, Laurie K, Barr I, Chen W, Wines B, Hogarth PM, Lambe T, Gilbert SC, Parsons MS, Kent SJ. What Lies Beneath: Antibody Dependent Natural Killer Cell Activation by Antibodies to Internal Influenza Virus Proteins. EBioMedicine. 2016;8:277–90. doi: 10.1016/j.ebiom.2016.04.029. https://doi.org/10.1016/j.ebiom.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodier MR, Lusa C, Sherratt S, Rodriguez-Galan A, Behrens R, Riley EM. Sustained immune complex-mediated reduction in CD16 expression after vaccination regulates NK cell function. Front Immunol. 2016;7:384. doi: 10.3389/fimmu.2016.00384. https://doi.org/10.3389/fimmu.2016.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013;6:69–82. doi: 10.1038/mi.2012.49. https://doi.org/10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou G, Juang SW, Kane KP. NK cells exacerbate the pathology of influenza virus infection in mice. Eur J Immunol. 2013;43:929–38. doi: 10.1002/eji.201242620. https://doi.org/10.1002/eji.201242620. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Peñarrubia P, Koster FT, Kelley RO, McDowell TD, Bankhurst AD. Antibacterial activity of human natural killer cells. J Exp Med. 1989;169:99–113. doi: 10.1084/jem.169.1.99. https://doi.org/10.1084/jem.169.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brill KJ, Li Q, Larkin R, Canaday DH, Kaplan DR, Boom WH, Silver RF. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect Immun. 2001;69:1755–65. doi: 10.1128/IAI.69.3.1755-1765.2001. https://doi.org/10.1128/IAI.69.3.1755-1765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzales CM, Williams CB, Calderon VE, Huante MB, Moen ST, Popov VL, Baze WB, Peterson JW, Endsley JJ. Antibacterial role for natural killer cells in host defense to Bacillus anthracis. Infect Immun. 2012;80:234–42. doi: 10.1128/IAI.05439-11. https://doi.org/10.1128/IAI.05439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu CC, Wu TS, Hsu YJ, Chang CJ, Lin CS, Chia JH, Wu TL, Huang TT, Martel J, Ojcius DM, Young JD, Lai HC. NK cells kill mycobacteria directly by releasing perforin and granulysin. J Leukoc Biol. 2014;96:1119–29. doi: 10.1189/jlb.4A0713-363RR. https://doi.org/10.1189/jlb.4A0713-363RR. [DOI] [PubMed] [Google Scholar]
- 77.Arase H, Arase N, Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–38. doi: 10.1084/jem.181.3.1235. https://doi.org/10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zucchini N, Crozat K, Baranek T, Robbins SH, Altfeld M, Dalod M. Natural killer cells in immunodefense against infective agents. Expert Rev Anti Infect Ther. 2008;6:867–85. doi: 10.1586/14787210.6.6.867. https://doi.org/10.1586/14787210.6.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clin Exp Immunol. 2005;139:287–96. doi: 10.1111/j.1365-2249.2004.02686.x. https://doi.org/10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP. Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol. 2005;175:1735–40. doi: 10.4049/jimmunol.175.3.1735. https://doi.org/10.4049/jimmunol.175.3.1735. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt S, Tramsen L, Hanisch M, Latgé JP, Huenecke S, Koehl U, Lehrnbecher T. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J Infect Dis. 2011;203:430–35. doi: 10.1093/infdis/jiq062. https://doi.org/10.1093/infdis/jiq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt S, Tramsen L, Perkhofer S, Lass-Flörl C, Hanisch M, Röger F, Klingebiel T, Koehl U, Lehrnbecher T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology. 2013;218:939–44. doi: 10.1016/j.imbio.2012.10.013. https://doi.org/10.1016/j.imbio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Voigt J, Hünniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, Löffler J, Kurzai O. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis. 2014;209:616–26. doi: 10.1093/infdis/jit574. https://doi.org/10.1093/infdis/jit574. [DOI] [PubMed] [Google Scholar]
- 84.Levitz SM, Dupont MP, Smail EH. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect Immun. 1994;62:194–202. doi: 10.1128/iai.62.1.194-202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hidore MR, Mislan TW, Murphy JW. Responses of murine natural killer cells to binding of the fungal target Cryptococcus neoformans. Infect Immun. 1991;59:1489–99. doi: 10.1128/iai.59.4.1489-1499.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt S, Schneider A, Demir A, Lass-Flörl C, Lehrnbecher T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses. 2016;59:34–38. doi: 10.1111/myc.12431. https://doi.org/10.1111/myc.12431. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt S, Zimmermann SY, Tramsen L, Koehl U, Lehrnbecher T. Natural killer cells and antifungal host response. Clin Vaccine Immunol. 2013;20:452–58. doi: 10.1128/CVI.00606-12. https://doi.org/10.1128/CVI.00606-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benedetto N, Sabatini P, Sellitto C, Romano Carratelli C. Interleukin-2 and increased natural killer activity in mice experimentally infected with Aspergillus niger. Microbiologica. 1988;11:339–45. [PubMed] [Google Scholar]
- 89.Lipscomb MF, Alvarellos T, Toews GB, Tompkins R, Evans Z, Koo G, Kumar V. Role of natural killer cells in resistance to Cryptococcus neoformans infections in mice. Am J Pathol. 1987;128:354–61. [PMC free article] [PubMed] [Google Scholar]
- 90.Algarra I, Ortega E, Serrano MJ, Alvarez de Cienfuegos G, Gaforio JJ. Suppression of splenic macrophage Candida albicans phagocytosis following in vivo depletion of natural killer cells in immunocompetent BALB/c mice and T-cell-deficient nude mice. FEMS Immunol Med Microbiol. 2002;33:159–63. doi: 10.1111/j.1574-695X.2002.tb00586.x. https://doi.org/10.1111/j.1574-695X.2002.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 91.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112:1862–70. doi: 10.1172/JCI18125. https://doi.org/10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tewari RP, Von Behren LA. Immune responses in histoplasmosis, a prototype of respiratory mycoses. Indian J Chest Dis Allied Sci. 2000;42:265–69. [PubMed] [Google Scholar]
- 93.Park SJ, Hughes MA, Burdick M, Strieter RM, Mehrad B. Early NK cell-derived IFN-{gamma} is essential to host defense in neutropenic invasive aspergillosis. J Immunol. 2009;182:4306–12. doi: 10.4049/jimmunol.0803462. https://doi.org/10.4049/jimmunol.0803462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balish E, Warner T, Pierson CJ, Bock DM, Wagner RD. Oroesophageal candidiasis is lethal for transgenic mice with combined natural killer and T-cell defects. Med Mycol. 2001;39:261–68. doi: 10.1080/mmy.39.3.261.268. https://doi.org/10.1080/mmy.39.3.261.268. [DOI] [PubMed] [Google Scholar]
- 95.Quintin J, Voigt J, van der Voort R, Jacobsen ID, Verschueren I, Hube B, Giamarellos-Bourboulis EJ, van der Meer JW, Joosten LA, Kurzai O, Netea MG. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Eur J Immunol. 2014;44:2405–14. doi: 10.1002/eji.201343828. https://doi.org/10.1002/eji.201343828. [DOI] [PubMed] [Google Scholar]
- 96.Lehrnbecher T, Chanock SJ. Controversies in the treatment of neutropenia in cancer patients. Curr Opin Hematol. 1998;5:26–32. doi: 10.1097/00062752-199801000-00005. https://doi.org/10.1097/00062752-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, Silling G, Grimminger W, Einsele H. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant. 2008;42:679–84. doi: 10.1038/bmt.2008.237. https://doi.org/10.1038/bmt.2008.237. [DOI] [PubMed] [Google Scholar]
- 98.West KA, Gea-Banacloche J, Stroncek D, Kadri SS. Granulocyte transfusions in the management of invasive fungal infections. Br J Haematol. 2017;177:357–74. doi: 10.1111/bjh.14597. https://doi.org/10.1111/bjh.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raad II, Chaftari AM, Al Shuaibi MM, Jiang Y, Shomali W, Cortes JE, Lichtiger B, Hachem RY. Granulocyte transfusions in hematologic malignancy patients with invasive pulmonary aspergillosis: outcomes and complications. Ann Oncol. 2013;24:1873–79. doi: 10.1093/annonc/mdt110. https://doi.org/10.1093/annonc/mdt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, Hamprecht K, Handgretinger R, Lang P, Einsele H. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–67. doi: 10.1182/blood-2010-01-262089. https://doi.org/10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 101.Qian C, Campidelli A, Wang Y, Cai H, Venard V, Jeulin H, Dalle JH, Pochon C, D'aveni M, Bruno B, Paillard C, Vigouroux S, Jubert C, et al. Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: a successful phase I/II multicenter clinical trial. J Hematol Oncol. 2017;10:102. doi: 10.1186/s13045-017-0469-0. https://doi.org/10.1186/s13045-017-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, Greil J, Albert MH, Schwinger W, Nathrath M, Schumm M, Stevanovic S, Handgretinger R, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31:39–48. doi: 10.1200/JCO.2011.39.8495. https://doi.org/10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 103.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, Carrum G, Sasa G, Lulla P, Watanabe A, Kuvalekar M, Gee AP, Wu MF, et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol. 2017;35:3547–57. doi: 10.1200/JCO.2017.73.0655. https://doi.org/10.1200/JCO.2017.73.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lehrnbecher T, Kalkum M, Champer J, Tramsen L, Schmidt S, Klingebiel T. Immunotherapy in invasive fungal infection—focus on invasive aspergillosis. Curr Pharm Des. 2013;19:3689–712. doi: 10.2174/1381612811319200010. https://doi.org/10.2174/1381612811319200010. [DOI] [PubMed] [Google Scholar]
- 105.Hidore MR, Murphy JW. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986;51:547–55. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hidore MR, Murphy JW. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986;137:3624–31. [PubMed] [Google Scholar]
- 107.Rubnitz JE, Inaba H, Kang G, Gan K, Hartford C, Triplett BM, Dallas M, Shook D, Gruber T, Pui CH, Leung W. Natural killer cell therapy in children with relapsed leukemia. Pediatr Blood Cancer. 2015;62:1468–72. doi: 10.1002/pbc.25555. https://doi.org/10.1002/pbc.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huenecke S, Zimmermann SY, Kloess S, Esser R, Brinkmann A, Tramsen L, Koenig M, Erben S, Seidl C, Tonn T, Eggert A, Schramm A, Bader P, et al. IL-2-driven regulation of NK cell receptors with regard to the distribution of CD16+ and CD16- subpopulations and in vivo influence after haploidentical NK cell infusion. J Immunother. 2010;33:200–10. doi: 10.1097/CJI.0b013e3181bb46f7. https://doi.org/10.1097/CJI.0b013e3181bb46f7. [DOI] [PubMed] [Google Scholar]
- 109.Etogo AO, Nunez J, Lin CY, Toliver-Kinsky TE, Sherwood ER. NK but not CD1-restricted NKT cells facilitate systemic inflammation during polymicrobial intra-abdominal sepsis. J Immunol. 2008;180:6334–45. doi: 10.4049/jimmunol.180.9.6334. https://doi.org/10.4049/jimmunol.180.9.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab Invest. 2004;84:1655–65. doi: 10.1038/labinvest.3700184. https://doi.org/10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 111.Adib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014;92:256–62. doi: 10.1038/icb.2013.99. https://doi.org/10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 112.Viegas N, Andzinski L, Wu CF, Komoll RM, Gekara N, Dittmar KE, Weiss S, Jablonska J. IFN-γ production by CD27+ NK cells exacerbates Listeria monocytogenes infection in mice by inhibiting granulocyte mobilization. Eur J Immunol. 2013;43:2626–37. doi: 10.1002/eji.201242937. https://doi.org/10.1002/eji.201242937. [DOI] [PubMed] [Google Scholar]
- 113.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96. doi: 10.1182/blood-2008-10-187179. https://doi.org/10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 114.Granzin M, Wagner J, Köhl U, Cerwenka A, Huppert V, Ullrich E. Shaping of natural killer cell antitumor activity by ex vivo cultivation. Front Immunol. 2017;8:458. doi: 10.3389/fimmu.2017.00458. https://doi.org/10.3389/fimmu.2017.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luhm J, Brand JM, Koritke P, Höppner M, Kirchner H, Frohn C. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res. 2002;11:651–57. doi: 10.1089/15258160260194794. https://doi.org/10.1089/15258160260194794. [DOI] [PubMed] [Google Scholar]
- 116.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–97. doi: 10.1158/1078-0432.CCR-11-1347. https://doi.org/10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Childs RW, Berg M. Bringing natural killer cells to the clinic: ex vivo manipulation. Hematology Am Soc Hematol Educ Program. 2013;2013:234–46. doi: 10.1182/asheducation-2013.1.234. https://doi.org/10.1182/asheducation-2013.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.London L, Perussia B, Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: IL 2 induces into cell cycle most peripheral blood NK cells, but only a minor subset of low density T cells. J Immunol. 1986;137:3845–54. [PubMed] [Google Scholar]
- 119.Alici E, Sutlu T, Björkstrand B, Gilljam M, Stellan B, Nahi H, Quezada HC, Gahrton G, Ljunggren HG, Dilber MS. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–62. doi: 10.1182/blood-2007-09-110312. https://doi.org/10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 120.Wagner J, Pfannenstiel V, Waldmann A, Bergs JW, Brill B, Huenecke S, Klingebiel T, Rödel F, Buchholz CJ, Wels WS, Bader P, Ullrich E. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front Immunol. 2017;8:676. doi: 10.3389/fimmu.2017.00676. https://doi.org/10.3389/fimmu.2017.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berg M, Lundqvist A, McCoy P, Jr, Samsel L, Fan Y, Tawab A, Childs R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11:341–55. doi: 10.1080/14653240902807034. https://doi.org/10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harada H, Watanabe S, Saijo K, Ishiwata I, Ohno T. A Wilms tumor cell line, HFWT, can greatly stimulate proliferation of CD56+ human natural killer cells and their novel precursors in blood mononuclear cells. Exp Hematol. 2004;32:614–21. doi: 10.1016/j.exphem.2004.03.011. https://doi.org/10.1016/j.exphem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 123.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–17. doi: 10.1158/0008-5472.CAN-08-3712. https://doi.org/10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. https://doi.org/10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tomchuck SL, Leung WH, Dallas MH. Enhanced cytotoxic function of natural killer and CD3+CD56+ cells in cord blood after culture. Biol Blood Marrow Transplant. 2015;21:39–49. doi: 10.1016/j.bbmt.2014.10.014. https://doi.org/10.1016/j.bbmt.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Domogala A, Madrigal JA, Saudemont A. Cryopreservation has no effect on function of natural killer cells differentiated in vitro from umbilical cord blood CD34(+) cells. Cytotherapy. 2016;18:754–59. doi: 10.1016/j.jcyt.2016.02.008. https://doi.org/10.1016/j.jcyt.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 127.James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, Orange JS. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood. 2013;121:2627–37. doi: 10.1182/blood-2012-06-437012. https://doi.org/10.1182/blood-2012-06-437012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–65. doi: 10.1084/jem.20120944. https://doi.org/10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–60. doi: 10.1182/blood-2012-04-419283. https://doi.org/10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, Luo X, Cooley S, Verneris M, Walcheck B, Miller J. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121:3599–608. doi: 10.1182/blood-2012-04-425397. https://doi.org/10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Felices M, Lenvik AJ, McElmurry R, Chu S, Hinderlie P, Bendzick L, Geller MA, Tolar J, Blazar BR, Miller JS. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 2018;3:96219. doi: 10.1172/jci.insight.96219. Epub ahead of print. https://doi.org/10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peruzzi G, Femnou L, Gil-Krzewska A, Borrego F, Weck J, Krzewski K, Coligan JE. Membrane-type 6 matrix metalloproteinase regulates the activation-induced downmodulation of CD16 in human primary NK cells. J Immunol. 2013;191:1883–94. doi: 10.4049/jimmunol.1300313. https://doi.org/10.4049/jimmunol.1300313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang CC, Isitman G, Bruneau J, Tremblay C, Bernard NF, Kent SJ, Parsons MS. Phenotypical and functional profiles of natural killer cells exhibiting matrix metalloproteinase-mediated CD16 cleavage after anti-HIV antibody-dependent activation. Clin Exp Immunol. 2015;181:275–85. doi: 10.1111/cei.12593. https://doi.org/10.1111/cei.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–86. doi: 10.1158/0008-5472.CAN-12-3558. https://doi.org/10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 135.Kamiya T, Chang YH, Campana D. Expanded and Activated Natural Killer Cells for Immunotherapy of Hepatocellular Carcinoma. Cancer Immunol Res. 2016;4:574–81. doi: 10.1158/2326-6066.CIR-15-0229. https://doi.org/10.1158/2326-6066.CIR-15-0229. [DOI] [PubMed] [Google Scholar]
- 136.Matusali G, Tchidjou HK, Pontrelli G, Bernardi S, D’Ettorre G, Vullo V, Buonomini AR, Andreoni M, Santoni A, Cerboni C, Doria M. Soluble ligands for the NKG2D receptor are released during HIV-1 infection and impair NKG2D expression and cytotoxicity of NK cells. FASEB J. 2013;27:2440–50. doi: 10.1096/fj.12-223057. https://doi.org/10.1096/fj.12-223057. [DOI] [PubMed] [Google Scholar]
- 137.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. https://doi.org/10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. https://doi.org/10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–48. doi: 10.1056/NEJMoa1709866. https://doi.org/10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378:449–59. doi: 10.1056/NEJMoa1709919. https://doi.org/10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, Ullrich E, Ottmann OG, Klingemann H, Tonn T, Wels WS. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy. 2017;19:235–49. doi: 10.1016/j.jcyt.2016.10.009. https://doi.org/10.1016/j.jcyt.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 142.Klöß S, Oberschmidt O, Morgan M, Dahlke J, Arseniev L, Huppert V, Granzin M, Gardlowski T, Matthies N, Soltenborn S, Schambach A, Koehl U. Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells. Hum Gene Ther. 2017;28:897–913. doi: 10.1089/hum.2017.157. https://doi.org/10.1089/hum.2017.157. [DOI] [PubMed] [Google Scholar]
- 143.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, Orange J, Wan X, Lu X, Reynolds A, Gagea M, Banerjee P, Cai R, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–31. doi: 10.1038/leu.2017.226. https://doi.org/10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li L, Liu LN, Feller S, Allen C, Shivakumar R, Fratantoni J, Wolfraim LA, Fujisaki H, Campana D, Chopas N, Dzekunov S, Peshwa M. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010;17:147–54. doi: 10.1038/cgt.2009.61. https://doi.org/10.1038/cgt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther. 2017;25:1769–81. doi: 10.1016/j.ymthe.2017.06.012. https://doi.org/10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Latgé JP. Tasting the fungal cell wall. Cell Microbiol. 2010;12:863–72. doi: 10.1111/j.1462-5822.2010.01474.x. https://doi.org/10.1111/j.1462-5822.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 147.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–70. doi: 10.1172/JCI119616. https://doi.org/10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lehrnbecher TL, Foster CB, Zhu S, Venzon D, Steinberg SM, Wyvill K, Metcalf JA, Cohen SS, Kovacs J, Yarchoan R, Blauvelt A, Chanock SJ. Variant genotypes of FcgammaRIIIA influence the development of Kaposi’s sarcoma in HIV-infected men. Blood. 2000;95:2386–90. [PubMed] [Google Scholar]
- 149.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–47. doi: 10.1200/JCO.2003.05.013. https://doi.org/10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 150.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180:6392–401. doi: 10.4049/jimmunol.180.9.6392. https://doi.org/10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Furutani E, Su S, Smith A, Berg M, Childs R. siRNA Inactivation of the Inhibitory Receptor NKG2A Augments the Anti-Tumor Effects of Adoptively Transferred NK Cells In Tumor-Bearing Hosts. Blood. 2010;116:1015. [Google Scholar]
- 152.Figueiredo C, Seltsam A, Blasczyk R. Permanent silencing of NKG2A expression for cell-based therapeutics. J Mol Med (Berl) 2009;87:199–210. doi: 10.1007/s00109-008-0417-0. https://doi.org/10.1007/s00109-008-0417-0. [DOI] [PubMed] [Google Scholar]
- 153.Godal R, Bachanova V, Gleason M, McCullar V, Yun GH, Cooley S, Verneris MR, McGlave PB, Miller JS. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16:612–21. doi: 10.1016/j.bbmt.2010.01.019. https://doi.org/10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ward JP, Bonaparte MI, Barker E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS. 2004;18:1769–79. doi: 10.1097/00002030-200409030-00005. https://doi.org/10.1097/00002030-200409030-00005. [DOI] [PubMed] [Google Scholar]
- 155.Holubova M, Miklikova M, Leba M, Georgiev D, Jindra P, Caprnda M, Ciccocioppo R, Kruzliak P, Lysak D. Cryopreserved NK cells in the treatment of haematological malignancies: preclinical study. J Cancer Res Clin Oncol. 2016;142:2561–67. doi: 10.1007/s00432-016-2247-8. https://doi.org/10.1007/s00432-016-2247-8. [DOI] [PubMed] [Google Scholar]
- 156.Szmania S, Lapteva N, Garg T, Greenway A, Lingo J, Nair B, Stone K, Woods E, Khan J, Stivers J, Panozzo S, Campana D, Bellamy WT, et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother. 2015;38:24–36. doi: 10.1097/CJI.0000000000000059. https://doi.org/10.1097/CJI.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shimasaki N, Coustan-Smith E, Kamiya T, Campana D. Expanded and armed natural killer cells for cancer treatment. Cytotherapy. 2016;18:1422–34. doi: 10.1016/j.jcyt.2016.06.013. https://doi.org/10.1016/j.jcyt.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 158.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–44. doi: 10.1089/15258160152509145. https://doi.org/10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 159.Tam YK, Miyagawa B, Ho VC, Klingemann HG. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J Hematother. 1999;8:281–90. doi: 10.1089/106161299320316. https://doi.org/10.1089/106161299320316. [DOI] [PubMed] [Google Scholar]
- 160.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–58. [PubMed] [Google Scholar]
- 161.Klingemann HG, Wong E, Maki G. A cytotoxic NK-cell line (NK-92) for ex vivo purging of leukemia from blood. Biol Blood Marrow Transplant. 1996;2:68–75. [PubMed] [Google Scholar]
- 162.Yan Y, Steinherz P, Klingemann HG, Dennig D, Childs BH, McGuirk J, O’Reilly RJ. Antileukemia activity of a natural killer cell line against human leukemias. Clin Cancer Res. 1998;4:2859–68. [PubMed] [Google Scholar]
- 163.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–70. doi: 10.1016/j.jcyt.2013.06.017. https://doi.org/10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 164.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32. doi: 10.1080/14653240802301872. https://doi.org/10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 165.Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, Zeng T, Huang H, Zhang X, Sun W, Man-Yuen Sze D, Yi Q, Hou J. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8:297–310. doi: 10.1016/j.molonc.2013.12.001. https://doi.org/10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schönfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, Nowakowska P, Bönig H, Köhl U, Kloess S, Köhler S, Holtgreve-Grez H, Jauch A, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23:330–38. doi: 10.1038/mt.2014.219. https://doi.org/10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mehta RS, Rezvani K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front Immunol. 2018;9:283. doi: 10.3389/fimmu.2018.00283. https://doi.org/10.3389/fimmu.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang T, Li Y, Wang YJ, Wang X, Young M, Douglas SD, Ho WZ. Natural killer cell inhibits human immunodeficiency virus replication in chronically infected immune cells. Antiviral Res. 2007;73:132–39. doi: 10.1016/j.antiviral.2006.08.006. https://doi.org/10.1016/j.antiviral.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 169.Yang Y, Han Q, Zhang C, Xiao M, Zhang J. Hepatitis B virus antigens impair NK cell function. Int Immunopharmacol. 2016;38:291–97. doi: 10.1016/j.intimp.2016.06.015. https://doi.org/10.1016/j.intimp.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 170.Mian MF, Lauzon NM, Andrews DW, Lichty BD, Ashkar AA. FimH can directly activate human and murine natural killer cells via TLR4. Mol Ther. 2010;18:1379–88. doi: 10.1038/mt.2010.75. https://doi.org/10.1038/mt.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tam YK, Maki G, Miyagawa B, Hennemann B, Tonn T, Klingemann HG. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum Gene Ther. 1999;10:1359–73. doi: 10.1089/10430349950018030. https://doi.org/10.1089/10430349950018030. [DOI] [PubMed] [Google Scholar]
- 172.Sahm C, Schönfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother. 2012;61:1451–61. doi: 10.1007/s00262-012-1212-x. https://doi.org/10.1007/s00262-012-1212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Suck G, Branch DR, Smyth MJ, Miller RG, Vergidis J, Fahim S, Keating A. KHYG-1, a model for the study of enhanced natural killer cell cytotoxicity. Exp Hematol. 2005;33:1160–71. doi: 10.1016/j.exphem.2005.06.024. https://doi.org/10.1016/j.exphem.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 174.Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 2015;6:605. doi: 10.3389/fimmu.2015.00605. https://doi.org/10.3389/fimmu.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]