Abstract
Aim: The associations between dietary saturated fatty acids and the risks of stroke subtypes in cohort studies were examined by a meta-analysis of separate ethnic Japanese and non-Japanese cohorts, and causes of their difference were elucidated.
Method: Log hazard ratio (HR) with 95% confidence interval (CI) of the highest versus the lowest saturated fat intake from cohort studies were weighed by an inverse variance method to combine HRs.
Results: Five studies of intracerebral hemorrhage and 11 studies/comparisons of ischemic stroke were selected. A meta-analysis of intracerebral hemorrhage excluding subarachnoid hemorrhage showed a strong inverse association in Japanese (n = 3, HR = 0.55, 95% CI 0.32–0.94) but not in non-Japanese (n = 2, HR = 0.98, 95% CI 0.62–1.53). A meta-analysis of ischemic stroke showed a mild inverse association in Japanese (n = 4, HR = 0.82, 95% CI 0.71–0.93) but not in non-Japanese (n = 7, HR = 0.93, 95% CI 0.84–1.03). The effect size of saturated fat in reducing the risk of stroke in Japanese was stronger for intracerebral hemorrhage (45% reduction) than for ischemic stroke (18% reduction).
Conclusions: In Japanese but not in non-Japanese, a diet high in saturated fat is associated with a low risk of intracerebral hemorrhage and ischemic stroke. This may be due to differences in the range of intake of saturated fat, genetic susceptibility, incidence of lacunar infarction, and/or confounding factors such as dietary proteins. An intervention study targeting Japanese will be required to verify the causality.
Keywords: Saturated fatty acid, Hemorrhagic stroke, Brain infarction, Relative risk
Introduction
The association of the intake of saturated fat (saturated fatty acids) with the risk of stroke has been a major concern in Japan1) because most cohort studies conducted in Japan showed an inverse association between the intake of saturated fat and the incidence of stroke or its mortality2–7), which is opposite to the guidelines that recommend a reduction in saturated fat intake to prevent cardiovascular disease8). Three previous meta-analyses of cohort studies were conducted to examine the association between the intake of saturated fat and the risk of stroke9–11). The first meta-analysis conducted in 2010, in which ischemic and hemorrhagic strokes were pooled, showed no significant association between the intake of saturated fat and the risk of stroke (n [studies] = 8, relative risk [RR] = 0.81, 95% confidence interval [CI] 0.62–1.05)9). The second meta-analysis conducted in 2015, in which only ischemic strokes were pooled, also showed no significant association between the intake of saturated fat and the risk of ischemic stroke (n [studies/comparisons] = 12/15, RR = 1.02: 95% CI 0.90–1.15)10). However, the third meta-analysis conducted in 2016, in which ischemic and hemorrhagic strokes were pooled, showed that a high intake of saturated fat was significantly associated with a reduction in stroke morbidity and mortality (n [studies/comparisons] = 15/16, RR = 0.89, 95% CI 0.82–0.96)11). Furthermore, many confounding factors in these studies, such as stroke subtypes, ethnicities, sex, amount of fat intake, duration of observations, and body mass index (BMI), affected this association11). In a subgroup analysis of stroke type, the intake of saturated fat showed a stronger inverse association with the risk of hemorrhagic stroke (n [studies] = 6, RR = 0.76, 95% CI 0.63–0.93) than with ischemic stroke (n [studies] = 10, RR = 0.90, 95% CI 0.82–0.99).
Most strokes were classified as subarachnoid hemorrhage (ICD-10 code I60), intracerebral hemorrhage (ICD-10 code I61), thrombotic infarction (ICD-10 code I63.0 or I63.3), embolic infarction (ICD-10 code I63.1 or I63.4), or lacunar infarction (ICD-10 code G46)12, 13). Each subtype of stroke may have a different etiology. Thus, lifestyle—including dietary habits—may affect the incidence of each stroke subtype differently. Indeed, in the previous studies in which subarachnoid hemorrhage and intracerebral hemorrhage were analyzed separately, the intake of saturated fat was inversely associated with mortality in patients with intraparenchymal hemorrhage (n [patients] = 224, hazard ratio [HR] = 0.48, 95% CI 0.27–0.85) but not with that of subarachnoid hemorrhage (n [patients] = 153, HR = 0.91, 95% CI 0.46–1.80)6). Another study showed that the intake of saturated fat was inversely associated with the incidence of intraparenchymal hemorrhage (n [patients] = 894, HR = 0.61, 95% CI 0.43–0.86) but not with that of subarachnoid hemorrhage (n [patients] = 348, HR = 0.87, 95% CI 0.50–1.52)7). However, in the previous meta-analyses9, 11), hemorrhagic stroke included cases of subarachnoid hemorrhage and intracerebral hemorrhage, and ischemic stroke (or cerebral infarction) included cases of thrombosis and embolism, by which the associations between each stroke subtypes with saturated fat may become unclear.
In the present meta-analysis, we analyzed the effects of dietary saturated fats on the incidence of intracerebral (intraparenchymal) haemorrhage excluding subarachnoid hemorrhage, and their confounding factors were discussed. However, there was only one cohort study of ischemic stroke in which cases of thrombosis and embolism were separately examined7) because it might be difficult to differentiate thrombosis from embolism by computed tomography (CT) or magnetic resonance imaging (MRI). Therefore, as a reference for intracerebral hemorrhage, the association of the intake of saturated fat with the risk of ischemic stroke including cases of thrombosis and embolism was also compared.
Methods
Data Sources and Searches
The PubMed database was searched for studies published before March 3, 2016. The computer-based searches were conducted by combining search terms related to saturated fatty acids (“fat” OR “dietary fat” OR “saturated fatty acids”), diet (“diet” OR “intake”), stroke (“stroke”), and cohort study (“cohort” OR “prospective study”), and 229 articles were identified (Fig. 1). The reference lists of the retrieved studies and review articles were manually searched to identify any eligible resources. We included studies that met the following criteria: (1) a prospective cohort design was used to examine the association between the intake of saturated fats and the incidence or death of intracerebral hemorrhage or ischemic stroke (or cerebral infarction); and (2) CT, MRI, or autopsy findings were used for diagnosis. Studies on mortality in which stroke was classified by death certificate were also included if they were conducted since the 1980s when CT or MRI became available14). Both authors (M.M. and O.E.) independently performed the selection of papers.
Fig. 1.
Flow chart of the selection of the studies.
Data Synthesis and Analysis
To calculate the risk of intracerebral hemorrhagic or ischemic stroke morbidity and mortality (the primary outcome), the log HRs of the highest versus the lowest values for cohort studies were combined by an inverse variance method. Most studies were conducted by Cox proportional hazards regression analysis, and thus the HR was used instead of RR. The odds ratios in one study15) were assumed to approximate the same measure of HR. When effect size was expressed by a per-1-SD change, the log risk estimates were transformed assuming a normal distribution, with the comparison between the top and bottom thirds being equal to 2.18 times the log HR for a 1-SD increase16). SEs of the log HRs were also calculated using published confidence limits, and we transformed the SEs in the same way. These log conversions were conducted on data from the Framingham Heart Study17). Studies that reported HRs with different degrees of adjustment for other risk factors required the most adjustment in their estimates.
A sensitivity analysis (secondary outcome) was conducted to detect any potential bias including that for ethnicity (Japanese, non-Japanese), sex, BMI, amount of saturated fat intake, and follow-up duration. Publication bias was assessed using funnel plots and Egger's test. A quantitative measure of inconsistency across individual studies was assessed by the I-square statistic18). A small I-square value is interpreted as meaning that the effect size is comparable across studies, whereas a large I-square value is interpreted as meaning that the effect size varies substantively across studies19).
Because our goal was to report on the dispersion of effects as function of a covariate (we assumed that the true effect size might be different from study to study by the effect of confounders), the random-effects model was used. However, when the number of samples was two, a fixed-effect model was used because we were not concerned with dispersion in the observed effects and a summary based on two studies would yield a more precise estimate of the true effect than either study alone20). The data were analyzed using Comprehensive Meta-Analysis Software Version 2.0 (Biostat, Eaglewood, NJ, USA).
Results
Study Selection
In total, 11 studies were selected3–7, 15, 17, 21–24) (Fig. 1). The Honolulu Heart Program study, the Caerphilly Prospective Study, and the European Prospective Investigation into Cancer and Nutrition were excluded because they only investigated total stroke (hemorrhagic and ischemic strokes were not separately analyzed)25–27). The Ni-Hon-San Study was excluded because the diagnoses of intracranial hemorrhage and thromboembolic stroke were based on the clinical image and not on CT or MRI2). Summaries of the included studies are shown in Table 1 (basic characteristics) and Table 2 (detailed characteristics).
Table 1. Basic characteristics of included studies.
| Source | Country, region/cohort | Followup, yrs | No. (women, %) | Age, yrs | Mean or median BMI | Mean or median saturated fat intake (g/d) | |
|---|---|---|---|---|---|---|---|
| 1 | Seino, 1997 | Japan, Shibata Study | 15.5 | 2283 (58%) | Over 40 | 21.5 | 11 |
| 2 | Gillman, 1997 | USA, Framingham Heart Study | 20 | 832 (0%) | 45–65 | 26.9 | 43.8 |
| 3 | Iso, 2001 | USA, Nurses' Health Study (NHS) | 14 | 85764 (100%) | 34–59 | 24.0 | 28 |
| 4 | He, 2003 | USA, Health Professional Follow Up Study (HPFS) | 14 | 43732 (0%) | 40–75 | 24.9 | 24 |
| 5 | Iso, 2003 | Japan, Circulatory Risk in Communities Study (CIRCS) | 14 | 4775 (52%) | 40–69 | 23.8 | 10.9 |
| 6 | Sauvaget, 2004 | Japan, Hiroshima and Nagasaki/Life Span Study (LSS) | 14 | 3731 (63%) | 35–89 | 22.3 | 12 |
| 7 | Yamagishi, 2010 | Japan, JACC study | 14 | 58453 (61%) | 40–79 | 22.8 | 14.4 |
| 8 | Wallstrom, 2012 | Sweden, Malmo Diet and Cancer Cohort (MDC) | 13.5 | 8139 (0%) 12535 (100%) |
44–73 | 26.1 (men) 25.4 (women) |
16.8 (men) 16.7 (women) |
| 9 | Larsson, 2012 | Sweden, Swedish Mammography Cohort (SMC) | 10.4 | 34670 (100%) | 49–83 | 25.0 | 26.9 |
| 10 | Yaemsiri, 2012 | USA, Women's Health Initiative Observational Study (WHI-OS) | 7.6 | 87025 (100%) | 50–79 | 27.2 | 14.5 |
| 11 | Yamagishi, 2013 | Japan, JPHC study | 11.1 | 81931 (54%) | 40–69 | 23.6 | 16.3 |
| Source | No. of patients, outcome | Methods of diagnosis | |
|---|---|---|---|
| 1 | Seino, 1997 | 75, Incidence of cerebral infarction (exclude TIA) | Clinical images and CT |
| 2 | Gillman, 1997 | 61, Incidence of ischemic stroke (including atherothrombotic brain infarction and embolus; excluding TIA) 14, Incidence of hemorrhagic stroke (including subarachnoid and intracerebral) | Clinical images and CT |
| 3 | Iso, 2001 | 385, Incidence of ischemic stroke (including thrombotic and embolic; excluding TIA) 74, Incidence of intraparenchymal hemorrhage | Clinical images, CT, MRI, and autopsy |
| 4 | He, 2003 | 455, Incidence of ischemic stroke (including embolism and thrombosis) 125, Incidence of hemorrhagic stroke (including subarachnoid and intracerebral) | Criteria of the National Survey of Stroke |
| 5 | Iso, 2003 | 68, Incidence of intraparenchymal hemorrhage | 85% were confirmed by CT and MRI |
| 6 | Sauvaget, 2004 | 60, Death from cerebral infarction (ICD-10 I63, I69.3) | Death certificates |
| 7 | Yamagishi, 2010 | 224, Death from intraparenchymal hemorrhage (ICD-10 I61) 321, Death from ischemic stroke (ICD-10 I63) | Death certificates |
| 8 | Wallstrom, 2012 | 401 (men), 354 (women), Incidence of ischemic stroke (ICD-10 I63 or I64) | CT, MRI, and autopsy |
| 9 | Larsson, 2012 | 1310, Incidence of cerebral infarction (ICD-10 I63) 233, Incidence of hemorrhagic stroke (ICD-10 I61) | Swedish hospital discharge registry and Swedish death registry |
| 10 | Yaemsiri, 2012 | 1049, Incidence of ischemic stroke (excluding TIA) | Criteria of the Trial of ORG 10172 Acute Stroke Trial (TOAST). Over 95% of cases were classified by brain imaging. |
| 11 | Yamagishi, 2013 | 894, Incidence of ischemic stroke (including large-artery thrombotic, lacunar, and embolic; excluding TIA) 1939, Incidence of intraparenchymal hemorrhage (excluding subarachnoid) | Criteria of the National Survey of Stroke. CT and MRI were available for 98% of registered stroke events. |
BMI, body mass index; CT, computed tomography; MRI, magnetic resonance imaging; TIA, transient ischemic attack
Table 2. Detailed characteristics of the included studies.
| Source | Method | Outcome measures | Adjustment factors | |
|---|---|---|---|---|
| 1 | Seino, 1997 | FFQ | Hazard ratio by Cox proportional hazard regression model | Sex, age, diastolic blood pressure, atrial fibrillation |
| 2 | Gillman, 1997 | 24-hour recall | Cumulative incidence rate (per 1000) by Mantel-Haenszel methods and relative risk by Cox proportional hazard regression model | Age, systolic blood pressure, cigarette smoking, glucose intolerance, BMI, physical activity index, left ventricular hypertrophy, and intake of energy, alcohol, and fruits and vegetables |
| 3 | Iso, 2001 | FFQ | Relative risk by logistic regression model | Age, smoking status, time interval, BMI, alcohol intake, menopausal status and postmenopausal hormone use, vigorous exercise, usual aspirin use, multivitamin use, vitamin E use, n-3 fatty acid intake, calcium intake, and histories of hypertension, diabetes, and high cholesterol levels, and total energy intake |
| 4 | He, 2003 | FFQ | Relative risk by Mantel-Haenszel methods and Cox proportional hazard regression model | BMI, physical activity, history of hypertension, smoking status, aspirin use, multivitamin use, consumption of alcohol, potassium, fiber, vitamin E, fruit and vegetables, total energy intake, hypercholesterolemia, polyunsaturated fatty acids, mono-unsaturated fatty acids, and trans fatty acids |
| 5 | Iso, 2003 | 24-hour recall | Relative risk by Cox proportional hazard regression model | Age, sex, quartiles of total energy intake, BMI, hypertension category, diabetes, serum total cholesterol, smoking status, ethanol intake, and menopausal status |
| 6 | Sauvaget, 2004 | 24-hour recall | Relative hazard by Cox proportional hazard regression model | Sex, age, radiation dose, city, BMI, smoking status, alcohol habits, and medical history of hypertension and diabetes |
| 7 | Yamagishi, 2010 | FFQ | Hazard ratio by Cox proportional hazard regression model | Age, sex, history of hypertension and diabetes, smoking status, alcohol consumption, BMI, mental stress, walking, sports, educational level, and dietary intakes of total energy, cholesterol, n-3 and n-6 polyunsaturated fatty acids, vegetables, and fruit |
| 8 | Wallstrom, 2012 | FFQ | Hazard ratio by Cox proportional hazard regression model | Age, method version, total energy intake, season, BMI class, smoking category, education, alcohol category, systolic blood pressure, antihypertensive treatment, antihyperlipidemic treatment, leisure time physical activity (quartiles), and quintiles of energy-adjusted dietary fiber |
| 9 | Larsson, 2012 | FFQ | Relative risk by Cox proportional hazard regression model | Age, smoking status and pack-years of smoking, education, BMI, total physical activity, history of hypertension, history of diabetes, aspirin use, family history of myocardial infarction, intakes of alcohol, protein, and dietary fiber, intakes of total fat, and quintiles of cholesterol |
| 10 | Yaemsiri, 2012 | FFQ | Hazard ratio by Cox proportional hazard regression model | Age, race, education, family income, years as a regular smoker, hormone replacement therapy use, total MET-hours per week, alcohol intake, history of coronary disease, history of atrial fibrillation, history of diabetes, aspirin use, use of antihypertensive medication, use of cholesterol-lowering medication, BMI, systolic blood pressure, and total energy intake |
| 11 | Yamagishi, 2013 | FFQ | Hazard ratio by Cox proportional hazard regression model | Age, sex, energy intake, cohort, cigarette smoking status, alcohol intake, BMI, sports during leisure time, walking and standing times, perceived mental stress, energy-adjusted dietary intakes of carbohydrate, protein, cholesterol, vegetables, fruit, and calcium |
BMI, body mass index; FFQ, food-frequency questionnaire
Intracerebral hemorrhage was analyzed in 5 studies4, 6, 7, 15, 24). Because subarachnoid hemorrhage was included as hemorrhagic stroke in the Framingham Heart Study and the Health Professionals Follow-Up Study17, 21), these two studies were excluded from our analysis, despite the fact that they were included in the previous meta-analyses9–11).
Ten studies were included in the analysis of ischemic stroke, of which 7 studies were described as evaluating ischemic stroke6, 7, 15, 17, 21–23), whereas that in 3 studies was described as evaluating cerebral infarction3, 5, 24). The definition of ischemic stroke (but not cerebral infarction) may include both cerebral infarction and transient ischemic attack (TIA), which is an acute episode of temporary neurologic dysfunction that results from focal ischemia and is not associated with acute tissue infarction (episode disappears within 24 hours after onset). The inclusion of TIA in ischemic stroke may lead to misclassification and biased results in epidemiological research28). However, all 7 studies, in which the outcomes of ischemic stroke were reported, excluded TIAs6, 7, 15, 17, 21–23). Thus, we considered ischemic stroke and cerebral infarction to be the same, and 10 studies were included in the present analysis. The Swedish Malmo Diet and Cancer Cohort study, in which ischemic stroke was separately analyzed in men and women, was counted as two comparisons23).
Primary Outcome
A high intake of saturated fat was significantly associated with a reduction in the risk of both intracerebral hemorrhage (n [studies] = 5, HR = 0.69, 95% CI 0.48–1.00) and ischemic stroke (n [studies] = 11, HR = 0.89, 95% CI 0.82–0.96) (Table 3 and Fig. 2A for intracerebral hemorrhage, Table 4 and Fig. 3A for ischemic stroke). The effect size in intracerebral hemorrhage (HR = 0.69) was larger than that in ischemic stroke (HR = 0.89).
Table 3. Hazard ratio and 95% CI of the risk of intracerebral hemorrhage by intake of saturated fat for race, sex, BMI, mean saturated fat intake, and followup duration.
| Outcome | Studies (n) | HR | 95% CI | P value | I-squared | |
|---|---|---|---|---|---|---|
| Hemorrhagic stroke | 5 | 0.69 | 0.48 | 1.00 | 0.048 | 58.1 |
| Race | ||||||
| Japanese | 3 | 0.55 | 0.32 | 0.94 | 0.03 | 68.6 |
| Non-Japanese | 2 | 0.98 | 0.62 | 1.53 | 0.91 | 37.9 |
| Sex | ||||||
| Women | 2 | 0.98 | 0.62 | 1.53 | 0.91 | 37.9 |
| Men | 0 | |||||
| Mixed | 3 (all Japanese) | 0.55 | 0.32 | 0.94 | 0.03 | 68.6 |
| BMI | ||||||
| < 24 | 3 (all Japanese) | 0.55 | 0.32 | 0.94 | 0.03 | 68.6 |
| ≥ 24 | 2 | 0.98 | 0.63 | 1.53 | 0.91 | 37.9 |
| Mean saturated fat intake | ||||||
| < 25 g/d | 3 (all Japanese) | 0.55 | 0.32 | 0.94 | 0.03 | 68.6 |
| ≥ 25 g/d | 2 | 0.98 | 0.63 | 1.53 | 0.91 | 37.9 |
| Follow-up duration | ||||||
| < 14 years | 2 (1 Japanese) | 0.93 | 0.58 | 1.45 | 0.74 | 53.5 |
| ≥ 14 years | 3 (2 Japanese) | 0.51 | 0.35 | 0.75 | 0.001 | 24.1 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio
Fig. 2.
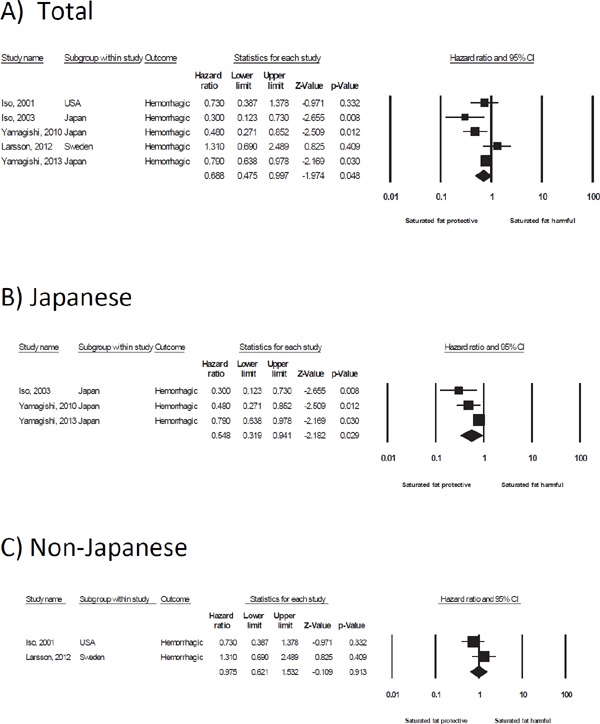
Forest plot of the hazard ratio for saturated fat and the risk of intracerebral hemorrhage in total (A), Japanese (B), and non-Japanese (C) populations. A summary of the hazard ratios is shown in the last row of the forest plot data (◆). CI, confidence interval
Table 4. Hazard ratio and 95% CI of risk of ischemic stroke by intake of saturated fat for race, sex, BMI, mean saturated fat intake, and follow-up duration.
| Outcome | Studies (n) | HR | 95% CI | P value | I-squared | |
|---|---|---|---|---|---|---|
| Ischemic stroke | 11 | 0.89 | 0.82 | 0.96 | 0.004 | 38.9 |
| Race | ||||||
| Japanese | 4 | 0.82 | 0.71 | 0.93 | 0.003 | 19.0 |
| Non-Japanese | 7 | 0.93 | 0.84 | 1.03 | 0.172 | 41.7 |
| Sex | ||||||
| Women | 4 | 1.04 | 0.90 | 1.21 | 0.586 | 5.0 |
| Men | 3 | 0.85 | 0.74 | 0.97 | 0.018 | 35.9 |
| Mixed | 4 (all Japanese) | 0.82 | 0.71 | 0.93 | 0.003 | 19.0 |
| BMI | ||||||
| < 24 | 4 (all Japanese) | 0.82 | 0.71 | 0.93 | 0.003 | 19.0 |
| ≥ 24 | 7 | 0.93 | 0.84 | 1.03 | 0.172 | 41.7 |
| Mean saturated fat intake | ||||||
| < 25 g/d | 8 (4 Japanese) | 0.92 | 0.83 | 1.02 | 0.111 | 43.3 |
| ≥ 25 g/d | 3 | 0.85 | 0.75 | 0.96 | 0.010 | 36.2 |
| Follow-up duration | ||||||
| < 14 years | 5 (1 Japanese) | 0.96 | 0.86 | 1.07 | 0.441 | 27.2 |
| ≥ 14 years | 6 (3 Japanese) | 0.79 | 0.70 | 0.90 | 0.000 | 11.2 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio
Fig. 3.
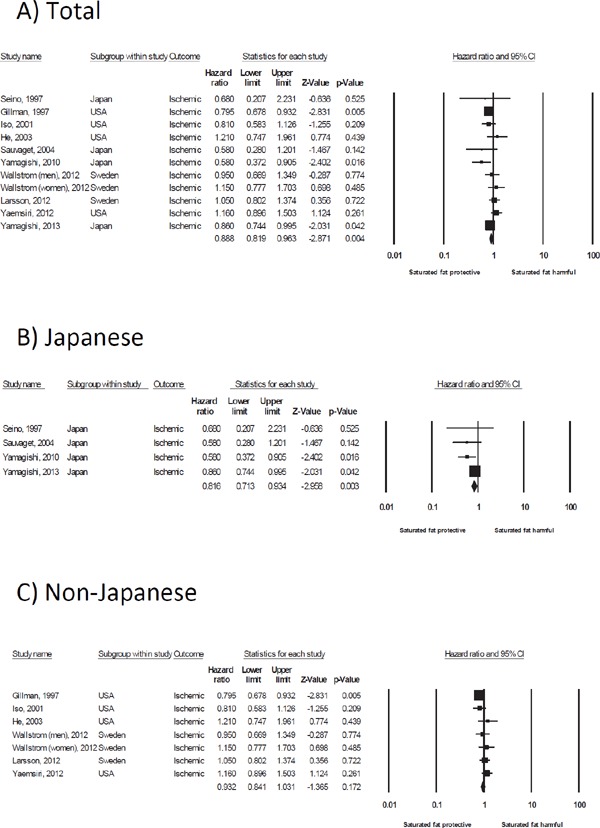
Forest plot of the hazard ratio for saturated fat and the risk of ischemic stroke in total (A), Japanese (B), and non-Japanese (C) populations. A summary of the hazard ratios is shown in the last row of the forest plot data (◆). CI, confidence interval
Secondary Outcome of Intracerebral Hemorrhage
In the 5 studies examined, all 3 Japanese studies showed a significant inverse association4, 6, 7), whereas the 2 non-Japanese studies showed no association15, 24). Therefore, in the subgroup meta-analysis of intracerebral hemorrhage, a high intake of saturated fat in Japanese was strongly associated with a reduction in the risk of intracerebral hemorrhage (n [studies] = 3, HR = 0.55, 95% CI 0.32–0.94)4, 6, 7), whereas this inverse association was not observed in non-Japanese (n [studies] = 2, HR = 0.98, 95% CI 0.62–1.53)15, 24) (Table 3, Fig. 2B, C).
The characteristics of all of the Japanese studies included mixed sex (women and men), the BMI values were < 24 kg/m2, and the mean intake of saturated fat was < 25 g/day. In contrast, the non-Japanese studies were of women only, the BMI values were ≥ 24 kg/m2, and the mean intake of saturated fat was ≥ 25 g/day. Thus, when a subgroup analysis was conducted according to sex, BMI, and the mean intake of saturated fat, the same studies used in a subgroup analysis of the Japanese and non-Japanese were selected. Therefore, in the studies of mixed sex (women and men), with BMI values of < 24 kg/m2, and mean intake of saturated fat of < 25 g/day, a strong inverse association was observed between the intake of saturated fat and the risk of intracerebral hemorrhage (Table 3). Studies over a follow-up period of ≥ 14 years, in which 2 Japanese studies were included, showed an inverse association between the intake of saturated fat and the risk of intracerebral hemorrhage (n [studies] = 3, HR = 0.51, 95% CI 0.35–0.75) (Table 3). The longer follow-up period may detect a higher incidence of stroke, which most often occurs in elderly adults.
Secondary Outcomes of Ischemic Stroke
In 4 Japanese studies, the 2 more recent studies showed that a high intake of saturated fat was associated with a reduction in the risk of ischemic stroke6, 7), whereas the other 2 older studies did not show a significant association3, 5). In 7 non-Japanese studies, one study of men showed that a high intake of saturated fat was associated with a reduction in the risk of ischemic stroke17), but the other 6 studies/comparisons did not show a significant association15, 21–24). In a subgroup meta-analysis of ischemic stroke, a high intake of saturated fat by Japanese was associated with a reduction in the risk of ischemic stroke (n [studies] = 4, HR = 0.82, 95% CI 0.71–0.93), whereas this association was not observed in non-Japanese (n [studies] = 7, HR = 0.93, 95% CI 0.84–1.03) (Table 4, Fig. 3B, C).
The characteristics of all of the Japanese studies included mixed sex (women and men) and BMI values of < 24 kg/m2, whereas those of the non-Japanese studies analyzed women or men separately and included patients with BMI values of ≥ 24 kg/m2. Thus, when subgroup analyses were conducted according to sex, BMI, and the mean intake of saturated fat, the analysis of mixed sex and patients with BMI values of < 24 kg/m2 revealed an inverse association between the intake of saturated fat and the risk of ischemic stroke (Table 4).
In an analysis in 7 non-Japanese studies, a subgroup meta-analysis in 3 studies of men showed that a high intake of saturated fat was associated with a reduction in the risk of ischemic stroke (n [studies] = 3, HR = 0.85, 95% CI 0.74 -0.97)17, 21, 23), whereas in the 4 studies of women, this association was not observed (n [studies] = 4, HR = 1.04, 95% CI 0.90–1.21)15, 22–24) (Table 4).
The studies in which the mean intake of saturated fat was ≥ 25 g/day were all in non-Japanese. However, a subgroup meta-analysis of studies in which the mean intake of saturated fat was ≥ 25 g/day showed that a high intake of saturated fat was associated with a reduction in the risk of ischemic stroke (n [studies] = 3, HR = 0.85, 95% CI 0.75–0.96)15, 17, 24) (Table 4).
Three of the 6 studies in which the follow-up period was ≥ 14 years were Japanese. A meta-analysis of these 6 studies showed that a high intake of saturated fat was associated with a reduction in the risk of ischemic stroke (n [studies] = 6, HR = 0.79, 95% CI 0.70–0.90)3, 5, 6, 15, 17, 21) (Table 4).
Publication Bias
A statistical analysis (Egger's test) indicated that there was no publication bias regarding studies that investigated the association between the intake of saturated fat and the risks of hemorrhagic and ischemic stroke (P = 0.517, 0.866, respectively), and funnel plots had a symmetrical appearance (by visual inspection) (Fig. 4A, B).
Fig. 4.
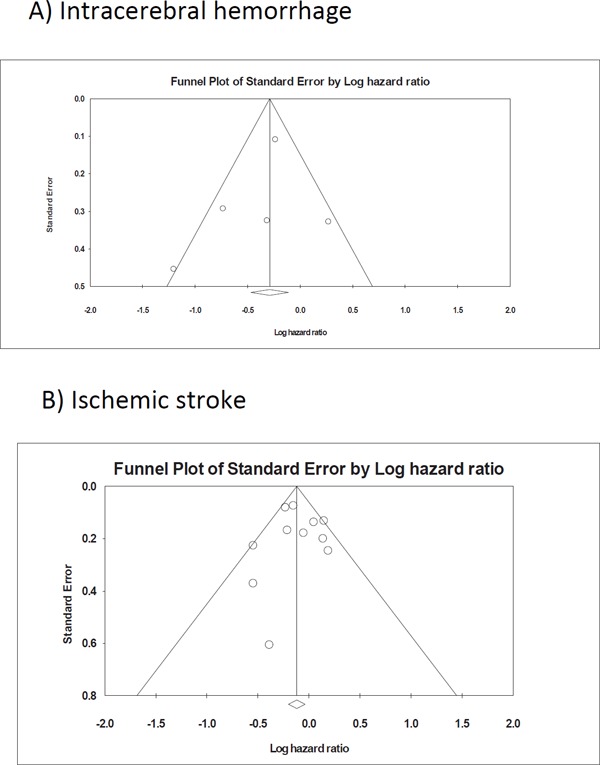
Funnel plot of the standard error by the log hazard ratio for intracerebral hemorrhage (A) and ischemic stroke (B)
Discussion
Association between Dietary Saturated fat Intake and Risk of Stroke Differs between Ethnic Japanese and Non-Japanese
In this meta-analysis, we focused on the risk of intracerebral hemorrhage, for which dietary saturated fat may have a substantial effect. For intracerebral hemorrhage, our meta-analysis of 3 studies targeting ethnic Japanese showed a strong inverse association (HR = 0.55, 95% CI 0.32 -0.94), whereas that of 2 studies targeting non-Japanese did not show any such association (HR = 0.98, 95% CI 0.62–1.53). Similarly to intracerebral hemorrhage, for ischemic stroke, our metaanalysis of 4 studies targeting Japanese showed a mild inverse association (HR = 0.82, 95% CI 0.71–0.93), whereas that of 7 studies targeting non-Japanese did not show any association (HR = 0.93, 95% CI 0.84–1.03). The effect size of saturated fat to reduce the incidence of stroke in Japanese was stronger for intracerebral hemorrhage (45% reduction) than for ischemic stroke (18% reduction). However, because the genetic and environmental characteristics of Japanese and Caucasian individuals are different (e.g., Japanese have a lower BMI, a higher incidence of stroke, longer longevity, and eat less saturated fat and more soy protein and fish), if these characteristics in Japanese were selected in a sensitivity analysis, a strong inverse association between saturated fat intake and the risk of stroke might be observed. It is known that along with saturated fat, the intake of animal protein, fish oils, sodium, potassium, dairy calcium, and alcohol, and smoking, are also associated with the risk of stroke29), and they become confounding factors when examining the effects of saturated fat on the risk of stroke between Japanese and non-Japanese.
Difference in the Range of Intake of Saturated Fat May be a Cause of Differential Effects of Saturated Fat on the Risk of Stroke between Ethnic Japanese and Non-Japanese
The difference in the risk of stroke in response to dietary saturated fat observed between ethnic Japanese and non-Japanese may be due to a difference in the range of the amount of saturated fat intake, as previously suggested by Iso et al.4) and Yamagishi et al.7). Under this hypothesis, the effect of saturated fat on the risk of stroke was non-linear: in the range of a low intake of saturated fat, the relationship was linear (decrease), but in the range of a high intake of saturated fat, the relationship plateaued. We also plotted the intake of saturated fat and the crude incidence/mortality rates of intracerebral hemorrhage in studies used in our meta-analysis on the basis of saturated fat weight (Fig. 5A) or the percent of total energy intake (en %) (Fig. 5B). The results were similar to those of previous studies4, 7). If we assumed that the dose-dependent effects of saturated fat on the risk of intracerebral hemorrhage applied to both Japanese and non-Japanese, the incidence/mortality rates would appear to reach a plateau around 20 g/day or 8 en %, respectively. However, this is not conclusive because there were no groups with a high intake of saturated fat in the Japanese nor groups with a low intake of saturated fat in the non-Japanese.
Fig. 5.
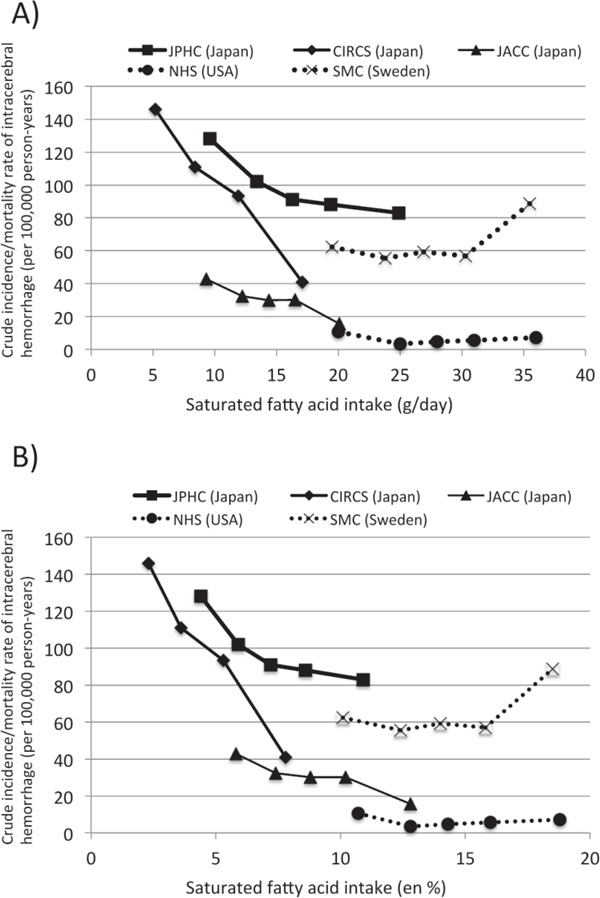
Comparison of the effects of the dose of saturated fat on crude incidence/mortality rates (per 100 000 person-years) of intracerebral hemorrhage in studies used in this metaanalysis, expressed as the weight of saturated fat intake per day (A) and the percent of total energy intake (en %) of saturated fat (B). JPHC: Japan Public Health-based Cohort Study7), CIRCS: Circulatory Risk in Communities Study4), JACC: Japan Collaborative Cohort Study6), NHS: Nurses' Health Study15), SMC: Swedish Mammography Cohort24). The JACC was a mortality study. Note that the crude incidence/mortality rates were not adjusted by age and confounders; therefore, these rates should not be compared between studies.
A similar plot was conducted for ischemic stroke (Fig. 6). In contrast to intracerebral hemorrhage, there was a substantial overlap in the groups with low intake of saturated fat between ethnic Japanese and non-Japanese. On the basis of saturated fat weight (Fig. 6A) or en % (Fig. 6B), an increased incidence/mortality of ischemic stroke in the group with a low intake of saturated fat was observed in the Japanese (JPHC, LSS, and JACC studies) but not in the non-Japanese (MDC-M, MDC-W, and WHI-OS studies). This suggested that the increased incidence/mortality of ischemic stroke in the group with a low intake of saturated fat might be specific to ethnic Japanese, in which lacunar stroke is predominant (see the last section of the Discussion).
Fig. 6.
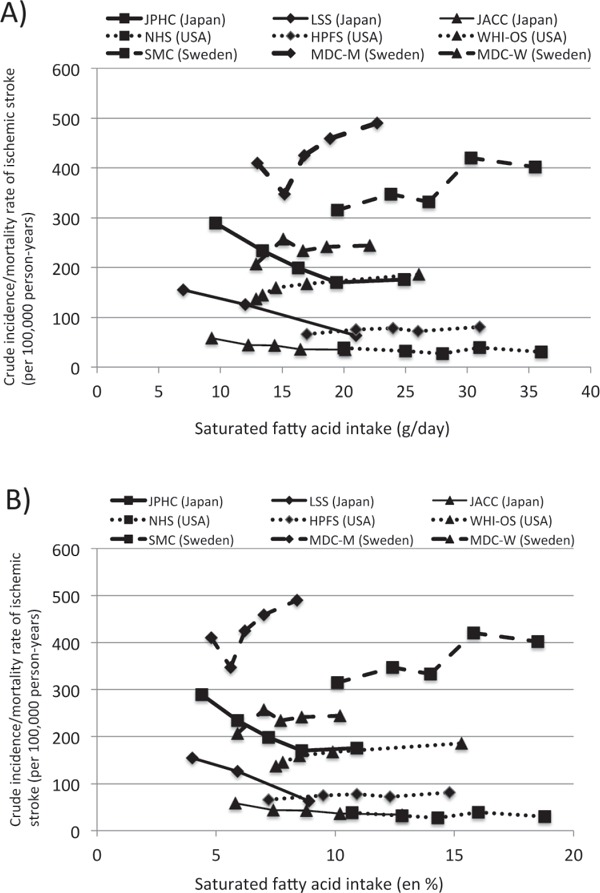
Comparison of the effects of the dose of saturated fat on crude incidence/mortality rates (per 100 000 person-years) of ischemic stroke in the studies used in this meta-analysis, expressed as the weight of saturated fat intake per day (A) and the percent of total energy intake (en %) of saturated fat (B). JPHC: Japan Public Health-based Cohort Study7), LSS: Hiroshima and Nagasaki/Life Span Study5), JACC: Japan Collaborative Cohort Study6), NHS: Nurses' Health Study15), HPFS: Health Professional Follow Up Study21), WHI-OS: Women's Health Initiative Observational Study22), SMC: Swedish Mammography Cohort24), MDC: Malmo Diet and Cancer Cohort23). The JACC was a mortality study. The MDC had two studies; men (MDC-M) and women (MDC-W). The Shibata study was not included because the actual incidence of ischemic stroke was not reported3). The Framingham Heart Study was not included because the absolute amount of saturated fat intake was not reported17). Note that the crude incidence/mortality rates were not adjusted by age and confounders; therefore, these rates should not be compared between studies.
Does a Genetic Difference Affect the Risk of Stroke in Response to Dietary Saturated Fat ?
Studies on second-generation ethnic Japanese living in the US might provide a clue to whether the favorable effects of saturated fats on intracerebral hemorrhage and ischemic stroke in Japanese were due to genetic or environmental factors, because these Japanese individuals tend to consume a large amount of saturated fat. Two studies answered this question, but they were excluded from the meta-analysis because the Honolulu Heart Program investigated total stroke25) and because the diagnoses of intracranial hemorrhage and thromboembolic stroke in the Ni-Hon-San Study were made based on the clinical findings only and not CT or MRI2). In addition, the number of participants in these cohort studies might be too small (7088 men in the Honolulu Heart Program; 7895 men in the Ni-Hon-San Study) to detect the effects of different doses of saturated fat intake on the risk of stroke. Furthermore, both studies used a 24-hour recall nutrition survey rather than a food-frequency questionnaire (FFQ). A 24-hour recall nutrition survey is less suitable than FFQ to estimate habitual intakes in nutrition. However, these 2 studies are worthwhile for examining the genetic differences in the risk of total stroke because both intracerebral hemorrhage and ischemic stroke in ethnic Japanese showed a similar response to saturated fats in our analysis.
The Honolulu Heart Program began in the early 1960s among men of Japanese ancestry who resided on the island of Oahu25, 30). They found intake of saturated fat in these men was related inversely to stroke mortality during 10 years of surveillance25). However, the relationship was not linear: a marked increase in stroke death was observed in the group with the lowest saturated fat intake (< 10 g/day), but no substantial difference in stroke death was observed in the groups with other levels of saturated fat intake (> 10 g/day)25). Although the mean intake of saturated fat in these men was 31.8 ± 15.4 g/day (12.3 ± 4.0% as a percentage of calories)30), which is higher than that of most Japanese living in Japan (median intake of saturated fat, 16.3 g/day)7) and that of male non-Japanese living in the US (24 g/day)21), this data suggested that in Japanese, similar to non-Japanese, intake of over 10 g of saturated fat did not substantially affect the risk of stroke.
The Ni-Hon-San Study was begun in 1965 on men residing in Hiroshima and Nagasaki, Japan and in Japanese-American men residing in Hawaii and San Francisco in whom the difference in genetic factors is minimized but the variation in environmental and lifestyle factors is large2). As part of the Ni-Hon-San Study, the stroke incidence of Japanese was compared in the Japan and Hawaii cohorts2). The age-adjusted incidence of total stroke among the Japanese living in Japan was 2.7 times greater than that of the Japanese living in Hawaii2). The combination of the animal protein and saturated fat intake was significantly inversely associated with the incidence of total stroke among the Japanese living in Hawaii, and this association was also suggested among the Japanese living in Japan2). The total calorie, animal protein, and saturated fat intakes were larger in the Japanese living in Hawaii than in those living in Japan (the age-adjusted mean saturated fat intake of the Japanese living in Hawaii was 55 g/day, whereas that of the Japanese living in Japan was 18 g/day), and the distribution of participants in the group in Hawaii with a low saturated fat intake was very low (1.3% in the group with intake of < 5 g/day vs. 4.1% in the group with intake of 5–14 g/day)2). Among Japanese living in the US, an inverse association was observed between the intake of saturated fat and the risk of stroke. However, non-Japanese living in the US did not show this inverse association (as estimated by other studies), suggesting that a reduction in stroke incidence in response to saturated fat in the range of high saturated fat intake might be observed in ethnic Japanese.
Taken together with these two studies of Japanese living in the US, although there were groups with high saturated fat intake among the Japanese living in Hawaii, it is not clear whether the Japanese showed a plateau or a decrease in the range of high saturated fat intake.
After the submission of this manuscript, the association between the intake of saturated fat and the risk of total stroke was reported in a large prospective study (The Prospective Urban Rural Epidemiology [PURE] study) in 18 countries from 5 continents31). This study included the Asian countries of Bangladesh, China, India, Malaysia, and Pakistan, which are characterized by middle- or low-income subjects and a dietary pattern of a higher carbohydrate/lower fat intake, and thus, groups with a lower intake of saturated fat were included in the analysis. Unexpectedly, the effect of saturated fat intake on the risk of stroke was linear (decreased) in the range of high saturated fat intake. From the lowest to highest quintile, the median intake of saturated fat was 2.8, 4,9, 7.1, 9.5, and 13.2 en%, and the HRs for the incidence of stroke were 1, 1.10, 1.01, 0.93, and 0.79, respectively (trend P = 0.0498). These data suggested that in ethnic Asians, a higher intake of saturated fat might reduce the risk of stroke linearly. A further analysis that separates ethnic and stroke subtypes in this cohort may clarify this finding.
Intervention Study
Cohort studies could not lead to the conclusion that dietary saturated fat exerts an inhibitory effect on the risk of stroke in Japanese. To prove causality, intervention studies are required, but no such studies have examined the effects of increasing or decreasing the saturated fat intake on the risk of stroke in Japanese. Only one intervention study examined the incidence of hemorrhagic and ischemic stroke in non-Japanese. This was a large randomized controlled trial of 48,835 postmenopausal women of 50 to 79 years of age who participated in the Women's Health Initiative Dietary Modification Trial32). Women were randomly assigned to an intervention or comparison group in a free-living setting. The intervention group received intensive behavior modification designed to reduce the total fat intake to 20% of calories and increase the vegetable/fruit intake to 5 servings/day and the grain intake to at least 6 servings/day for 8.1 years. By year 6, the mean saturated fat intake decreased by 2.9% of the energy intake in the intervention group; thus the total saturated fat intake became 9.5% of the energy intake. However, this diet had no significant effect on the incidence of hemorrhagic stroke (HR, 0.90; 95% CI, 0.66–1.22) or ischemic stroke (HR, 1.01; 95% CI, 0.86–1.18). These data support the results of a cohort study of non-Japanese in whom a lower saturated fat intake did not increase the risks of hemorrhagic or ischemic stroke.
Animal Studies
The stroke-prone spontaneously hypertensive rat (SHRSP) is a unique genetic model of stroke, especially of hemorrhage and lacunar infarction, due to its severe hypertension33). Stroke is prevented in SHRSPs by improving their diet through such means as sodium restriction and potassium supplementation and by feeding them diets containing proteins, some amino acids and fatty acids, and dietary fiber34). As for dietary fats, SHRSPs fed a high-fat/cholesterol diet (20% suet, 5% cholesterol, and 2% cholic acid, in wt/wt) did not show an increased lifespan but did show a reduced incidence of cerebral lesions in comparison to SHRSPs fed a normal laboratory stock diet35). SHRSPs fed a milk-fat rich diet (20% milk fat, in wt/wt) did not show an increased lifespan but did show a reduced incidence of cerebrovascular disease in comparison to SHRSPs fed a regular stock diet (Funahashi SP diet including 4.5% crude fat in wt/wt)36). The mean survival time of SHRSPs fed various dietary oils was longest in rats fed a diet high in saturated fatty acids under a 10% (wt/wt) oil-fed condition with 1% NaCl solution37). Under this same condition with a 0.25% NaCl solution, the mean survival duration was longest in SHRSPs that were fed butter in comparison to those fed perilla-lard, lard, margarine, or hydrogenated soy38). Specific fats may also affect the lifespan of SHRSPs. The addition of 1% (wt/wt) palmitoleic acid (C16:1) delayed the development of stroke in salt-loaded SHRSPs relative to the addition of 1% (wt/wt) palmitic, 1% oleic, or 1% linolenic acids, possibly by maintaining the integrity of the vascular smooth muscle cells34, 39). Undecylenic acid (C11:1) also prolonged the lifespan in salt-loaded SHRSPs34).
However, these studies did not examine the effect size of dietary fat to delay the onset of stroke relative to those of protein and carbohydrate. Under ad libitum or iso-energy feeding conditions, when the amount of a specific macronutrient is changed to examine its effects, the amounts of the other macronutrients are obligatorily changed. Thus, it is difficult to examine the specific effects of one macronutrient. To discern the macronutrients responsible for an increased incidence of stroke, the amount of one macronutrient was fixed, and the effect of different ratios of the other two macronutrients on stroke incidence was compared. It was found that the proportion of protein in the diet, but not that of carbohydrate or fat, was a primary determinant of the onset of stroke40). In a further study, peptides in milk protein, but not fat, might be responsible for delaying the onset of stroke41). The amount of protein was a key to the favorable effects of dietary fats. When the protein proportion was low (10% of total calories), increasing dietary fat might delay the onset of stroke40), whereas when the protein proportion was high (20% of total calories), increasing dietary fat did not delay the onset of stroke40, 41).
Animal experiments suggest that the amount of protein may influence the effects of saturated fat on the risk of stroke. Japanese eat less protein than non-Japanese; thus, increasing their saturated fat intake may reduce their risk of stroke.
Possible Reasons for the Reduction in Risk of Ischemic Stroke by Saturated Fatty Acids in Japanese
Cerebral infarction (or ischemic stroke), ICD-10 code I63, includes infarction due to both thrombosis (mostly due to arteriosclerosis) and embolism (mostly due to heart disease). The etiologies of heart diseases (valvular disease, myocardial infarction, bacterial endocarditis, and atrial fibrillation) might be different from those of thrombosis due to atherosclerosis. Thus, the risk factors may differ in cerebral infarction caused by thrombosis and embolism.
Pathologically, cerebral arteries are affected by atherosclerosis and arteriolosclerosis29). Atherosclerosis is observed typically in large arteries such as the carotid arteries and basal cerebral arteries and is characterized by lipid accumulation with proliferative changes leading to plaque formation (a cause of infarction due to thrombosis)29). In contrast, arteriolosclerosis is observed in small penetrating arterioles in basal ganglia of the brain and is characterized by the necrosis or apoptosis of smooth muscle cells within the media, leading to the formation of microaneurysms (a cause of intracerebral hemorrhage) and/or fibrous proliferative changes (a cause of lacunar stroke)42). The major risk factors for atherosclerosis include dyslipidemia, diabetes mellitus, hypertension, and smoking, whereas the main risk factor for arteriolosclerosis is hypertension29). Thus, it is conceivable that the etiology of intracerebral hemorrhage and lacunar stroke might be the same. Cerebral infarction found in Japan is more commonly related to arteriolosclerosis in the small intraparenchymal vessels rather than to atherosclerosis of the circle of Willis and its major branches43, 44). In a recent study in Japan7), among 520 patients with ischemic stroke, 224 experienced lacunar infarction, 155 embolic infarction, and 111 large-artery occlusive infarction, and the intake of saturated fat was inversely associated with the incidence of lacunar infarction (trend P = 0.02) but not that of embolic infarction (trend P = 0.74) and large-artery occlusive infarction (trend P = 0.55). In epidemiological studies, cerebral infarction included lacunar infarction, the etiology of which is similar to that of intracerebral hemorrhage. This may cause a dietary saturated fat-induced reduction in the risk of ischemic stroke and hemorrhage in Japanese.
Conclusion
The results of this review and meta-analysis suggested that in ethnic Japanese, a diet high in saturated fat is associated with a low risk of intracerebral hemorrhage and ischemic stroke. However, this favorable effect of saturated fat was not observed in non-Japanese. This may be due to differences in the range of intake of saturated fat, genetic susceptibility, the incidence of lacunar infarction, and/or confounding factors such as intake of dietary protein, vitamins, and minerals, which may be associated with dietary saturated fat. An intervention study in Japanese may clarify this important issue.
COI
A lecture fee from Ono Pharmaceutical Co., Ltd. to OE.
References
- 1). Yamagishi K, Iso H, Tsugane S. Saturated fat intake and cardiovascular disease in Japanese population. J Atheroscler Thromb. 2015; 22: 435-439 [DOI] [PubMed] [Google Scholar]
- 2). Takeya Y, Popper JS, Shimizu Y, Kato H, Rhoads GG, Kagan A. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: incidence of stroke in Japan and Hawaii. Stroke. 1984; 15: 15-23 [DOI] [PubMed] [Google Scholar]
- 3). Seino F, Date C, Nakayama T, Yoshiike N, Yokoyama T, Yamaguchi M, Tanaka H. Dietary lipids and incidence of cerebral infarction in a Japanese rural community. J Nutr Sci Vitaminol (Tokyo). 1997; 43: 83-99 [DOI] [PubMed] [Google Scholar]
- 4). Iso H, Sato S, Kitamura A, Naito Y, Shimamoto T, Komachi Y. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am J Epidemiol. 2003; 157: 32-39 [DOI] [PubMed] [Google Scholar]
- 5). Sauvaget C, Nagano J, Hayashi M, Yamada M. Animal protein, animal fat, and cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke. 2004; 35: 1531-1537 [DOI] [PubMed] [Google Scholar]
- 6). Yamagishi K, Iso H, Yatsuya H, Tanabe N, Date C, Kikuchi S, Yamamoto A, Inaba Y, Tamakoshi A, JACC Study Group Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am J Clin Nutr. 2010; 92: 759-765 [DOI] [PubMed] [Google Scholar]
- 7). Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue M, Tsugane S, JPHC Study Group Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: the JPHC Study. Eur Heart J. 2013; 34: 1225-1232 [DOI] [PubMed] [Google Scholar]
- 8). Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63: 2960-2984 [DOI] [PubMed] [Google Scholar]
- 9). Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010; 91: 535-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J, Anand SS. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015; 351: h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Cheng P, Wang J, Shao W, Liu M, Zhang H. Can dietary saturated fat be beneficial in prevention of stroke risk? A meta-analysis. Neurol Sci. 2016 [DOI] [PubMed] [Google Scholar]
- 12). Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981; 12: I13-44 [PubMed] [Google Scholar]
- 13). Iso H, Rexrode K, Hennekens CH, Manson JE. Application of computer tomography-oriented criteria for stroke subtype classification in a prospective study. Ann Epidemiol. 2000; 10: 81-87 [DOI] [PubMed] [Google Scholar]
- 14). Iso H, Jacobs DR, Jr., Goldman L. Accuracy of death certificate diagnosis of intracranial hemorrhage and nonhemorrhagic stroke. The Minnesota Heart Survey. Am J Epidemiol. 1990; 132: 993-998 [DOI] [PubMed] [Google Scholar]
- 15). Iso H, Stampfer MJ, Manson JE, Rexrode K, Hu F, Hennekens CH, Colditz GA, Speizer FE, Willett WC. Prospective study of fat and protein intake and risk of intra parenchymal hemorrhage in women. Circulation. 2001; 103: 856-863 [DOI] [PubMed] [Google Scholar]
- 16). Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998; 279: 1477-1482 [DOI] [PubMed] [Google Scholar]
- 17). Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA. 1997; 278: 2145-2150 [PubMed] [Google Scholar]
- 18). Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327: 557-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007; 335: 914-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. United Kingdom: John Wiley & Sons, Ltd; 2009 [Google Scholar]
- 21). He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ. 2003; 327: 777-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Yaemsiri S, Sen S, Tinker L, Rosamond W, Wassertheil-Smoller S, He K. Trans fat, aspirin, and ischemic stroke in postmenopausal women. Ann Neurol. 2012; 72: 704-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Wallstrom P, Sonestedt E, Hlebowicz J, Ericson U, Drake I, Persson M, Gullberg B, Hedblad B, Wirfält E. Dietary fiber and saturated fat intake associations with cardiovascular disease differ by sex in the Malmo Diet and Cancer Cohort: a prospective study. PLoS One. 2012; 7: e31637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Larsson SC, Virtamo J, Wolk A. Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis. 2012; 221: 282-286 [DOI] [PubMed] [Google Scholar]
- 25). McGee D, Reed D, Stemmerman G, Rhoads G, Yano K, Feinleib M. The relationship of dietary fat and cholesterol to mortality in 10 years: the Honolulu Heart Program. Int J Epidemiol. 1985; 14: 97-105 [DOI] [PubMed] [Google Scholar]
- 26). Atkinson C, Whitley E, Ness A, Baker I. Associations between types of dietary fat and fish intake and risk of stroke in the Caerphilly Prospective Study (CaPS). Public Health. 2011; 125: 345-348 [DOI] [PubMed] [Google Scholar]
- 27). Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol. 2012; 176: 1185-1192 [DOI] [PubMed] [Google Scholar]
- 28). Johnsen SP, Overvad K, Sorensen HT, Tjonneland A, Husted SE. Predictive value of stroke and transient ischemic attack discharge diagnoses in The Danish National Registry of Patients. J Clin Epidemiol. 2002; 55: 602-607 [DOI] [PubMed] [Google Scholar]
- 29). Iso H. Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb. 2011; 18: 83-88 [DOI] [PubMed] [Google Scholar]
- 30). McGee DL, Reed DM, Yano K, Kagan A, Tillotson J. Ten-year incidence of coronary heart disease in the Honolulu Heart Program. Relationship to nutrient intake. Am J Epidemiol. 1984; 119: 667-676 [DOI] [PubMed] [Google Scholar]
- 31). Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A, Amma LI, Avezum A, Chifamba J, Diaz R, Khatib R, Lear S, Lopez-Jaramillo P, Liu X, Gupta R, Mohammadifard N, Gao N, Oguz A, Ramli AS, Seron P, Sun Y, Szuba A, Tsolekile L, Wielgosz A, Yusuf R, Hussein Yusufali A, Teo KK, Rangarajan S, Dagenais G, Bangdiwala SI, Islam S, Anand SS, Yusuf S, Prospective Urban Rural Epidemiology (PURE) study investigators Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017 [DOI] [PubMed] [Google Scholar]
- 32). Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006; 295: 655-666 [DOI] [PubMed] [Google Scholar]
- 33). Okamoto K, Yamori Y, Nagaoka A. Establishment of the stroke-prone spontaneously hypertensive rat. Circ Res. 1974; 33/34: I143-153 [Google Scholar]
- 34). Yamori Y. Experimental evidence for dietary prevention of cardiovascular diseases. Clin Exp Pharmacol Physiol. 1989; 16: 303-307 [DOI] [PubMed] [Google Scholar]
- 35). Yamori Y, Horie R, Ohtaka M, Nara Y, Fukase M. Effect of hypercholesterolemic diet on incidence of cerebrovascular and myocardial lesions in spontaneously hypertensive rats (shr). Clinical and experimental pharmacology and physiology. 1976: 205-208 [Google Scholar]
- 36). Ikeda K, Mochizuki S, Nara Y, Horie R, Yamori Y. Effect of milk protein and fat intake on blood pressure and the incidence of cerebrovascular diseases in stroke-prone spontaneously hypertensive rats (SHRSP). J Nutr Sci Vitaminol (Tokyo). 1987; 33: 31-36 [DOI] [PubMed] [Google Scholar]
- 37). Ratnayake WM, Plouffe L, Hollywood R, L'Abbe MR, Hidiroglou N, Sarwar G, Mueller R. Influence of sources of dietary oils on the life span of stroke-prone spontaneously hypertensive rats. Lipids. 2000; 35: 409-420 [DOI] [PubMed] [Google Scholar]
- 38). Tatematsu K, Hirose N, Ichikawa Y, Fujii Y, Takami A, Okuyama H. Nutritional evaluation of an inter-esterified perilla oil and lard in comparison with butter and margarine based on the survival of stroke-prone spontaneously hypertensive (SHRSP) rats. Journal of health science. 2004; 50: 108-111 [Google Scholar]
- 39). Yamori Y, Nara Y, Tsubouchi T, Sogawa Y, Ikeda K, Horie R. Dietary prevention of stroke and its mechanisms in stroke-prone spontaneously hypertensive rats--preventive effect of dietary fibre and palmitoleic acid. J Hypertens Suppl. 1986; 4: S449-452 [PubMed] [Google Scholar]
- 40). Chiba T, Itoh T, Tabuchi M, Satou T, Ezaki O. Dietary protein, but not carbohydrate, is a primary determinant of the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke. 2009; 40: 2828-2835 [DOI] [PubMed] [Google Scholar]
- 41). Chiba T, Itoh T, Tabuchi M, Ooshima K, Satou T, Ezaki O. Delay of stroke onset by milk proteins in stroke-prone spontaneously hypertensive rats. Stroke. 2012; 43: 470-477 [DOI] [PubMed] [Google Scholar]
- 42). Konishi M, Iso H, Komachi Y, Iida M, Shimamoto T, Jacobs DR, Jr., Terao A, Baba S, Sankai T, Ito M. Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries. The Akita Pathology Study. Stroke. 1993; 24: 954-964 [DOI] [PubMed] [Google Scholar]
- 43). Resch JA, Okabe N, Loewenson RB, Kimoto K, Katsuki S, Baker AB. Pattern of vessel involvement in cerebral atherosclerosis. A comparative study between a Japanese and Minnesota population. J Atheroscler Res. 1969; 9: 239-250 [DOI] [PubMed] [Google Scholar]
- 44). Mitsuyama Y, Thompson LR, Hayashi T, Lee KK, Keehn RJ, Resch JA, Steer A. Autopsy study of cerebrovascular disease in Japanese men who lived in Hiroshima, Japan, and Honolulu, Hawaii. Stroke. 1979; 10: 389-395 [DOI] [PubMed] [Google Scholar]


