Abstract
Background
Psidium guineense, known as Araçá, is a Brazilian botanical resource with commercial application perspectives, based on the functional elements of its fruits and due to the use of its leaves as an anti-inflammatory and antibacterial agent. The essential oils of leaves of twelve specimens of Araçá were analyzed by GC and GC-MS to identify their volatile constituents and associate them with the biological activities reputed to the plant.
Results
In a total of 157 identified compounds, limonene, α-pinene, β-caryophyllene, epi-β-bisabolol, caryophyllene oxide, β-bisabolene, α-copaene, myrcene, muurola-4,10(14)-dien-1-β-ol, β-bisabolol, and ar-curcumene were the primary components in descending order up to 5%. Hierarchical Cluster Analysis (HCA) and Principal Component Analysis (PCA) displayed three different groups with the following chemical types: limonene/α-pinene, β-bisabolene/epi-β-bisabolol, and β-caryophyllene/caryophyllene oxide. With the previous description of another chemical type rich in spathulenol, it is now understood that at least four different chemotypes for P. guineense should occur.
Conclusions
In addition to the use of the Araçá fruits, which are rich in minerals and functional elements, it should be borne in mind that the knowledge of the chemical composition of the essential oils of leaves of their different chemical types may contribute to the selection of varieties with more significant biological activity.
Keywords: Psidium guineense, Myrtaceae, essential oil composition, chemical variability
Background
Myrtaceae comprises 132 genera and 5671 species of trees and shrubs, which are distributed mainly in tropical and subtropical regions of the world, particularly South America, Australia and Tropical Asia [1]. It is one of the most prominent families in Brazil, represented by 23 genera and 1034 species, with occurrence in all regions of the country [2, 3]. Psidium is a genus with at least 60 to 100 species, occurring from Mexico and Caribbean to Argentina and Uruguay. Therefore, it is naturally an American genus, although P. guajava, P. guineense and P. cattleyanum are subtropical and tropical species in many other parts of the world [4].
Psidium guineense Swartz [syn. Guajava guineensis (Sw.) Kuntze, Myrtus guineensis (Sw.) Kuntze, Psidium araca Raddi, P. guyanense Pers., P. laurifolium O. Berg, P. rotundifolium Standl., P. sprucei O. Berg, among others [5] (www.tropicos.org/Name/22102032) is a native shrub or small tree up to about 6 m high occurring in all Brazilian biomes, commonly known as Araçá. It has a berry-type fruit with yellow, red or purple peel and whitish pulp, rich in minerals and functional elements, such as vitamin C and phenolic compounds [6–9]. The leaves and pulp of Araçá have been used as an anti-inflammatory remedy for wound healing and oral antibacterial agent [10, 11], as well as it presented antibacterial activity against pathogenic microorganisms [11–13]. Some essential oils of Araçá were previously described: Foliar oil from a specimen growing in Arizona, USA, with predominance of β-bisabolene, α-pinene and limonene [14]; foliar oil from a specimen collected in Roraima, Brazil, with β-bisabolol, epi-α-bisabolol and limonene as the main constituents [15]; and another foliar oil from a specimen sampled in Mato Grosso do Sul Brazil, where spathulenol was the primary volatile compound [16].
The present work aimed at investigating the variability of the chemical composition of the essential oils of different specimens of Psidium guineense, occurring in the Amazon region, to contribute to the knowledge of its chemical types.
Experimental
Plant material
The leaf samples of twelve Psidium guineense specimens were collected in Pará state, Brazil. Collection site and voucher number of each specimen are listed in Table 1. The plant vouchers after the identification were deposited in the Herbaria of Embrapa Amazônia Oriental, in Belém (IAN) and Santarém (HSTM), Pará state, Brazil. The leaves were dried for two days in the natural environment and, then, subjected to essential oil distillation.
Table 1.
Identification data and collection site of the specimens of Psidium guineense
| Samples | Collection site | Herbarium Nº | Local coordinates |
|---|---|---|---|
| PG-01 | Curuçá, PA, Brazil | IAN-195396 | 0°72’65” S/47°84’07” W |
| PG-02 | Curuçá, PA, Brazil | IAN-195397 | 0°43’40” S/47°50’58” W |
| PG-03 | Curuçá, PA, Brazil | IAN-195398 | 0°72’67” S/47°85’13” W |
| PG-04 | Curuçá, PA, Brazil | IAN-195399 | 0°72’57” S/47°84’84” W |
| PG-05 | Curuçá, PA, Brazil | IAN-195400 | 0°72’57” S/47°84’07” W |
| PG-06 | Santarém, PA, Brazil | HSTM-3611 | 2°27’48.7” S/54°44’04” W |
| PG-07 | Monte Alegre, PA, Brazil | HSTM-6763 | 1°57’24.9” S/54°07’07.8” W |
| PG-08 | Monte Alegre, PA, Brazil | HSTM-6763 | 1°57’24.9” S/54°07’07.8” W |
| PG-09 | Santarém, PA, Brazil | HSTM-6775 | 2°25’14.6” S/54°44’25.8” W |
| PG-10 | Santarém, PA, Brazil | HSTM-3603 | 2°25’08.4” S/54°44’28.3” W |
| PG-11 | Santarém, PA, Brazil | HSTM-6769 | 2°29’16.8” S/54°42’07.9” W |
| PG-12 | Ponta de Pedras, PA, Brazil | HSTM-6759 | 2°31’08.3” S/54°52’25.8” W |
Isolation and analysis of the composition of oils
The leaves were ground and submitted to hydrodistillation using a Clevenger-type apparatus (3 h). The oils were dried over anhydrous sodium sulfate, and their yields were calculated by the plant dry weight. The moisture content of the samples was calculated using an Infrared Moisture Balance for water loss measurement. The procedure was performed in duplicate.
The oils were analyzed on a GCMS-QP2010 Ultra system (Shimadzu Corporation, Tokyo, Japan), equipped with an AOC-20i auto-injector and the GCMS-Solution software containing the NIST (Nist, 2011) and FFNSC 2 (Mondello, 2011) libraries [17, 18]. A Rxi-5ms (30 m x 0.25 mm; 0.25 μm film thickness) silica capillary column (Restek Corporation, Bellefonte, PA, USA) was used. The conditions of analysis were: injector temperature of 250 °C; Oven temperature programming of 60-240 °C (3 °C/min); Helium as carrier gas, adjusted to a linear velocity of 36.5 cm/s (1.0 mL/min); split mode injection for 1 μL of sample (oil 5 μL : hexane 500 μL); split ratio 1:20; ionization by electronic impact at 70 eV; ionization source and transfer line temperatures of 200 and 250 °C, respectively. The mass spectra were obtained by automatic scanning every 0.3 s, with mass fragments in the range of 35-400 m/z. The retention index was calculated for all volatile components using a homologous series of C8-C20 n-alkanes (Sigma-Aldrich, USA), according to the linear equation of Van den Dool and Kratz (1963) [19]. The quantitative data regarding the volatile constituents were obtained by peak-area normalization using a GC 6890 Plus Series, coupled to FID Detector, operated under similar conditions of the GC-MS system. The components of oils were identified by comparing their retention indices and mass spectra (molecular mass and fragmentation pattern) with data stored in the GCMS-Solution system libraries, including the Adams library (2007) [20].
Statistical analysis
The multivariate analysis was performed using as variables the constituents with content above than 5%. For the multivariate analysis, the data matrix was standardized by subtracting the mean and then dividing it by the standard deviation. For hierarchical cluster analysis, the complete linkage method and the Euclidean distance were used. Minitab software (free 390 version, Minitab Inc., State College, PA, USA), was used for these analyzes.
Results and discussion
Yield and composition of the oils
Psidium guineense is a botanical resource that presents commercial application perspectives, based on its fruits and functional elements, as well as due to the use of its leaves as anti-inflammatory and antibacterial agent [6–14]. For this study were selected twelve Araçá specimens, with occurrence in various localities of Pará state (PA), Brazil (see Table 1), and which showed different composition for the leaf oils. The yields of the oils from these twelve Araçá samples ranged from 0.1 to 0.9%, where the higher yields were from specimens sampled in the Northeast of Pará, Brazil (0.4-0.9%), and the lower yields were from plants collected in the West of Pará, Brazil (0.1-0.3%). The identification of the constituents of the oils by GC and GC-MS was 92.5% on average, with a total of 157 compounds, where limonene (0.3-47.4%), α-pinene (0.1-35.6%), β-caryophyllene (0.1-24.0%), epi-β-bisabolol (6.5-18.1%), caryophyllene oxide (0.3-14.1%), β-bisabolene (0.1-8.9%), α-copaene (0.3-8.1%), myrcene (0.1-7.3%), muurola-4,10(14)-dien-1-β-ol (1.6-5.8%), β-bisabolol (2.9-5.6%), and ar-curcumene (0.1-5.0%) were the primary components, in descending order up to 5% (see Figure 1 and Table 2). In general, the constituents identified in oils belong to the terpenoids class, with the following predominance: monoterpene hydrocarbons (0.9-76.9%), oxygenated sesquiterpenes (5.2-63.5%), sesquiterpene hydrocarbons (5.6-46.7%), and oxygenated monoterpenes (1.9-8.8%).
Fig. 1.
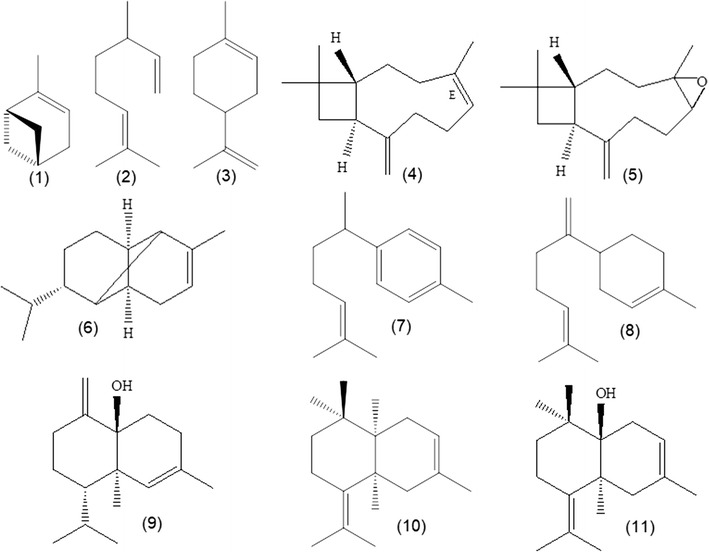
Main constituents identified in the oils of P. guineense: (1) α-pinene, (2) myrcene, (3) limonene, (4) β-caryophyllene, (5) caryophyllene oxide, (6) α-copaene, (7) ar-curcumene, (8) β-bisabolene, (9) muurola-4,10(14)-dien-1-β-ol, (10) epi-β-bisabolol, (11) β-bisabolol
Table 2.
Yield and volatile composition of twelve essential oil samples of P. guineense
| RI(C) | RI(L) | Constituents (%) | PG-01 | PG-02 | PG-03 | PG-04 | PG-05 | PG-06 | PG-07 | PG-08 | PG-09 | PG-10 | PG-11 | PG-12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 848 | 846a | (2E)-Hexenal | 0.3 | 0.1 | ||||||||||
| 850 | 850a | (3Z)-Hexenol | 0.2 | 0.1 | 0.1 | 0.1 | ||||||||
| 933 | 932a | α-Pinene | 35.6 | 26.1 | 17.7 | 13.4 | 34.0 | 26.4 | 2.0 | 0.8 | 1.0 | 1.3 | 0.1 | 0.6 |
| 946 | 948a | α-Fenchene | 0.1 | 0.1 | 0.1 | |||||||||
| 957 | 952a | Benzaldehyde | 0.3 | 0.5 | 1.1 | 0.8 | 0.9 | 0.6 | 0.1 | 0.4 | 0.3 | 0.3 | 0.5 | 0.1 |
| 977 | 974a | β-Pinene | 2.1 | 1.8 | 1.4 | 1.3 | 1.7 | 3.9 | 0.1 | 0.3 | ||||
| 985 | 981a | 6-methyl-5-Hepten-2-one | 0.2 | 0.1 | 0.1 | 0.4 | 0.1 | 0.1 | 0.1 | |||||
| 990 | 988a | Myrcene | 0.2 | 1.4 | 1.2 | 1.4 | 1.3 | 1.6 | 0.1 | 0.1 | 0.6 | 0.7 | 0.1 | 7.3 |
| 1005 | 1003a | p-Mentha-1(7),8-diene | 0.5 | 0.9 | 1.0 | 0.7 | 0.3 | 0.1 | 0.2 | 0.7 | 1.2 | 0.1 | ||
| 1016 | 1014a | α-Terpinene | 0.1 | 0.1 | 0.1 | |||||||||
| 1023 | 1020a | p-Cymene | 0.3 | 0.5 | 1.0 | 0.7 | 1.4 | 0.5 | 0.2 | 0.3 | 0.4 | 0.3 | 0.1 | 0.6 |
| 1028 | 1024a | Limonene | 3.7 | 30.7 | 30.4 | 26.5 | 37.2 | 14.0 | 4.3 | 9.6 | 23.4 | 47.4 | 0.3 | 5.4 |
| 1031 | 1032b | 1,8-Cineole | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 1.7 | 0.8 | |
| 1035 | 1032a | (Z)-β-Ocimene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||
| 1046 | 1044a | (E)-β-Ocimene | 0.1 | 0.2 | 0.1 | 0.1 | 0.8 | 0.1 | 0.1 | |||||
| 1057 | 1054a | γ-Terpinene | 0.6 | 0.4 | 0.7 | 0.6 | 0.3 | 0.9 | 0.2 | 0.2 | 0.1 | 0.1 | ||
| 1088 | 1086a | Terpinolene | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | ||||
| 1100 | 1095a | Linalool | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | ||||
| 1114 | 1114a | endo-Fenchol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| 1116 | 1113b | 4,8-dimethyl-(E)-Nona-1,3,7-triene | 0.1 | 0.1 | ||||||||||
| 1120 | 1122b | trans-p-Mentha-2,8-dien-1-ol | 0.1 | 0.1 | 0.1 | |||||||||
| 1125 | 1122a | α-Campholenal | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||
| 1130 | 1131b | Limona ketone | 1.6 | |||||||||||
| 1134 | 1133a | cis-p-Mentha-2,8-dien-1-ol | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||
| 1138 | 1136a | trans-p-Menth-2-en-1ol | 0.1 | |||||||||||
| 1139 | 1135a | trans-Pinocarveol | 0.4 | 0.1 | 0.4 | 0.1 | 0.4 | 0.4 | 0.2 | |||||
| 1148 | 1145a | Camphene hydrate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | ||||||
| 1161 | 1165b | Hydrocinnamaldehyde | 0.9 | 1.5 | 0.5 | |||||||||
| 1166 | 1165a | Borneol | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | ||||||
| 1177 | 1174a | Terpinen-4-ol | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | 0.1 | |||||
| 1186 | 1187a | trans-p-Mentha-1(7),8-dien-2-ol | 0.1 | 0.1 | 0.4 | 0.2 | ||||||||
| 1187 | 1189a | trans-Isocarveol | 0.4 | 0.2 | ||||||||||
| 1191 | 1186a | α-Terpineol | 1.0 | 0.6 | 1.3 | 0.4 | 1.0 | 1.7 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | |
| 1218 | 1215a | trans-Carveol | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| 1221 | 1218a | endo-Fenchyl acetate | 0.7 | 0.2 | 0.4 | 0.3 | 0.4 | 0.7 | ||||||
| 1226 | 1227a | cis-p-Mentha-1(7),8-dien-2-ol | 0.4 | 0.2 | 0.1 | 0.3 | 0.1 | |||||||
| 1243 | 1239a | Carvone | 0.1 | 0.1 | 0.1 | |||||||||
| 1267 | 1261a | cis-Chrysanthenyl acetate | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.1 | ||||||
| 1286 | 1287a | Bornyl acetate | 1.5 | 0.6 | 0.7 | 0.5 | 0.9 | 1.5 | 0.1 | 0.1 | ||||
| 1300 | 1298a | trans-Pinocarvyl acetate | 1.5 | 0.3 | 0.3 | 0.2 | 0.8 | 1.6 | ||||||
| 1324 | 1322a | Methyl geranate | 0.2 | 0.6 | 0.6 | 0.4 | 0.3 | 0.3 | 0.9 | 2.0 | 0.3 | |||
| 1326 | 1324a | Myrtenyl acetate | 0.1 | 0.2 | ||||||||||
| 1336 | 1335a | δ-Elemene | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 2.3 | ||||||
| 1338 | 1339a | trans-Carvyl acetate | 0.1 | 0.1 | 0.2 | 0.1 | ||||||||
| 1364 | 1359a | Neryl acetate | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||
| 1367 | 1369a | Cyclosativene | 0.1 | 0.1 | 0.1 | |||||||||
| 1378 | 1374a | α-Copaene | 8.1 | 6.2 | 8.1 | 7.2 | 3.0 | 3.7 | 4.2 | 4.7 | 2.5 | 1.1 | 0.3 | |
| 1383 | 1379a | Geranyl acetate | 0.1 | 1.1 | 1.0 | 1.7 | 0.6 | 0.8 | 0.2 | 0.2 | 1.9 | 0.5 | 0.8 | |
| 1401 | 1401a | iso-Italicene | 0.5 | 0.6 | 0.6 | 0.2 | 0.1 | |||||||
| 1406 | 1405a | Sesquithujene | 0.1 | 0.1 | 0.1 | |||||||||
| 1412 | 1410a | α-Cedrene | 0.8 | 0.8 | 1.0 | 0.4 | 0.5 | |||||||
| 1416 | 1407a | Acora-3,7(14)-diene | 0.9 | 0.6 | 1.0 | 0.5 | ||||||||
| 1423 | 1417a | β-Caryophyllene | 6.1 | 2.8 | 0.1 | 0.1 | 0.8 | 5.2 | 1.4 | 1.0 | 0.9 | 1.1 | 24.0 | |
| 1426 | 1419a | β-Cedrene | 0.1 | 0.3 | 0.1 | 0.1 | ||||||||
| 1431 | 1430a | β-Copaene | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | |||||||
| 1435 | 1434a | γ-Elemene | 0.2 | |||||||||||
| 1436 | 1432a | trans-α-Bergamotene | 0.3 | 0.3 | 0.3 | 0.2 | ||||||||
| 1436 | 1435b | Perillyl acetate | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.4 | ||||
| 1440 | 1439a | Aromadendrene | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | ||||||
| 1441 | 1439a | Phenyl ethyl but-2-anoate | 0.4 | |||||||||||
| 1444 | 1440a | (Z)-β-Farnesene | 0.2 | |||||||||||
| 1444 | 1442a | Guaia-6,9-diene | 0.3 | |||||||||||
| 1447 | 1445a | epi-β-Santalene | 0.1 | 0.1 | 0.1 | |||||||||
| 1452 | 1449a | Amorpha-4,11-diene | 0.3 | 0.3 | ||||||||||
| 1452 | 1453a | Geranyl acetone | 0.1 | 0.2 | ||||||||||
| 1455 | 1452a | α-Humulene | 0.9 | 0.7 | 0.3 | 0.5 | 0.1 | 0.9 | 0.4 | 0.1 | 0.2 | 2.8 | ||
| 1458 | 1454a | (E)-β-Farnesene | 1.0 | 0.1 | 0.5 | 0.2 | 0.3 | 0.1 | ||||||
| 1460 | 1457a | β-Santalene | 1.2 | 1.1 | 0.5 | 0.5 | ||||||||
| 1461 | 1460a | allo-Aromadendrene | 0.2 | 0.2 | 0.3 | 0.3 | 0.1 | 0.1 | ||||||
| 1464 | 1464a | α-Acoradiene | 1.3 | 1.1 | 1.3 | 0.6 | 0.7 | |||||||
| 1467 | 1469a | β-Acoradiene | 0.4 | 0.3 | 0.4 | 0.2 | 0.2 | |||||||
| 1471 | 1471a | 4,5-di-epi-Aristolochene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| 1474 | 1474a | 10-epi-β-Acoradiene | 0.4 | 0.3 | 0.4 | 0.2 | ||||||||
| 1477 | 1475a | γ-Gurjunene | 0.3 | 0.3 | ||||||||||
| 1477 | 1476a | β-Chamigrene | 1.0 | |||||||||||
| 1479 | 1478a | γ-Muurolene | 0.4 | 0.8 | 0.1 | 0.3 | 0.5 | 0.2 | 0.1 | |||||
| 1479 | 1481a | γ-Curcumene | 0.4 | 1.1 | 0.8 | 0.7 | ||||||||
| 1482 | 1479a | ar -Curcumene | 5.0 | 4.6 | 2.5 | 0.6 | 1.6 | 0.1 | ||||||
| 1486 | 1481a | γ-Himachalene | 1.0 | 0.9 | 0.4 | |||||||||
| 1488 | 1488a | β-Selinene | 0.7 | 0.8 | 1.0 | 3.8 | 0.5 | 3.2 | 3.0 | 3.7 | 0.1 | 3.2 | ||
| 1495 | 1493a | α-Zingiberene | 0.4 | 0.3 | 0.7 | |||||||||
| 1497 | 1498a | α-Selinene | 0.9 | 3.7 | 0.3 | 2.7 | 4.3 | 2.4 | 3.2 | |||||
| 1502 | 1500a | α-Muurolene | 0.4 | 0.3 | 0.5 | 0.5 | 0.1 | 0.2 | 0.3 | 0.4 | 0.2 | 0.1 | 0.2 | |
| 1502 | 1506a | (Z)-α-Bisabolene | 0.1 | 0.8 | 0.3 | 1.0 | 0.7 | 0.6 | 0.1 | |||||
| 1509 | 1505a | (E,E)-α-Farnesene | 2.6 | |||||||||||
| 1509 | 1511a | δ-Amorphene | 0.4 | |||||||||||
| 1510 | 1508b | β-Bisabolene | 0.1 | 8.9 | 4.0 | 6.4 | 5.2 | 4.0 | ||||||
| 1512 | 1514a | β-Curcumene | 2.0 | 0.1 | 3.6 | 2.9 | 2.5 | |||||||
| 1516 | 1513a | γ-Cadinene | 0.3 | 0.3 | 0.3 | 0.4 | 0.1 | 0.2 | 2.9 | 0.5 | 0.2 | |||
| 1516 | 1514a | (Z)-γ-Bisabolene | 0.9 | 1.1 | 1.0 | 1.0 | ||||||||
| 1519 | 1520a | 7-epi-α-Selinene | 0.1 | 0.1 | 0.1 | |||||||||
| 1522 | 1524a | δ-Cadinene | 1.0 | 1.9 | 1.7 | 2.6 | 0.3 | 0.7 | 0.8 | 2.7 | 1.9 | 0.7 | ||
| 1525 | 1521a | β-Sesquiphellandrene | 1.8 | |||||||||||
| 1532 | 1529a | (E)-γ-Bisabolene | 2.7 | 2.3 | 2.0 | 1.4 | 0.1 | |||||||
| 1534 | 1533a | trans-Cadina-1,4-diene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| 1534 | 1536a | Italicene ether | 0.2 | 0.5 | 0.2 | 0.5 | ||||||||
| 1539 | 1540a | 10-epi-cis-Dracunculifoliol | 0.1 | 0.4 | 0.1 | |||||||||
| 1543 | 1540b | (E)-α-Bisabolene | 0.8 | 0.6 | 0.4 | 0.4 | ||||||||
| 1543 | 1545a | Selina-3,7(11)-diene | 0.8 | |||||||||||
| 1544 | 1544a | α-Calacorene | 0.2 | 0.3 | 0.1 | 0.3 | 0.7 | |||||||
| 1559 | 1559a | Germacrene B | 0.1 | 1.1 | 0.4 | |||||||||
| 1565 | 1561a | E-Nerolidol | 0.3 | 0.1 | 0.4 | 0.2 | 0.1 | 1.0 | 1.3 | 0.9 | 2.2 | 0.2 | ||
| 1570 | 1571a | Caryolan-8-ol | 0.4 | |||||||||||
| 1572 | 1570a | Caryophyllenyl alcohol | 0.3 | 0.2 | ||||||||||
| 1579 | 1578b | ar-Tumerol | 0.3 | 0.6 | 0.1 | |||||||||
| 1580 | 1577a | Spathulenol | 0.7 | 0.4 | 0.6 | |||||||||
| 1584 | 1590a | Globulol | 0.1 | 0.4 | ||||||||||
| 1585 | 1586a | Gleenol | 0.3 | |||||||||||
| 1586 | 1582a | Caryophyllene oxide | 2.5 | 0.7 | 0.6 | 2.7 | 1.0 | 0.3 | 1.2 | 14.1 | ||||
| 1589 | 1590a | β-Copaen-4-α-ol | 0.5 | 0.1 | 0.2 | 0.3 | 0.2 | 0.8 | ||||||
| 1594 | 1592a | Viridiflorol | 0.2 | 0.9 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | ||
| 1596 | 1595a | Cubeban-11-ol | 0.1 | 0.2 | ||||||||||
| 1599 | 1600a | Guaiol | 0.5 | |||||||||||
| 1601 | 1600a | Cedrol | 0.4 | 0.4 | 0.5 | 0.8 | ||||||||
| 1609 | 1619a | (Z)-8-hydroxy-Linalool | 0.9 | 0.7 | 0.1 | |||||||||
| 1611 | 1613b | Humulene Epoxide | 0.4 | 0.1 | 0.1 | 0.1 | 1.0 | |||||||
| 1615 | 1613b | Copaborneol | 0.4 | |||||||||||
| 1617 | 1618a | 1,10-di-epi-Cubenol | 0.2 | 1.7 | ||||||||||
| 1625 | 1622a | 10-epi-γ-Eudesmol | 1.3 | 1.0 | 1.7 | 0.7 | 2.1 | |||||||
| 1630 | 1627a | epi-Cubenol | 1.5 | 3.4 | 0.7 | 0.5 | ||||||||
| 1631 | 1632a | α-Acorenol | 1.5 | 1.1 | 1.8 | 1.2 | 4.3 | |||||||
| 1632 | 1630a | Muurola-4,10(14)-dien-1-β-ol | 5.8 | 2.4 | 3.6 | 2.3 | 1.6 | 2.6 | ||||||
| 1635 | 1636a | β-Acorenol | 0.4 | 0.5 | 0.3 | 0.8 | ||||||||
| 1637 | 1636a | Gossonorol | 1.0 | 1.6 | 0.5 | 0.3 | 1.1 | |||||||
| 1639 | 1638a | Caryophylla-4(12),8(13)-dien-5β-ol | 1.3 | 0.3 | 0.3 | 2.1 | 1.5 | |||||||
| 1639 | 1642b | Caryophylla -4(12),8(13)-dien-5α-ol | 3.1 | |||||||||||
| 1641 | 1638a | epi-α-Cadinol | 1.9 | 1.8 | 1.7 | 1.7 | 0.6 | 1.3 | 1.1 | 1.6 | 0.4 | 0.8 | 1.4 | |
| 1645 | 1640a | epi-α-Murrolol | 1.1 | 0.9 | 1.2 | 0.3 | 2.6 | |||||||
| 1646 | 1640a | Hinesol | 0.6 | 1.8 | 0.7 | 0.4 | 1.1 | |||||||
| 1649 | 1644a | α-Muurolol | 1.2 | 1.1 | 0.4 | 0.8 | 1.1 | 1.0 | 1.6 | 3.1 | ||||
| 1653 | 1649a | β-Eudesmol | 0.1 | 0.1 | 0.2 | 0.1 | 0.7 | |||||||
| 1654 | 1652a | α-Cadinol | 1.8 | 2.0 | 1.8 | 0.5 | 0.4 | 2.4 | ||||||
| 1655 | 1651a | Pogostol | 3.8 | 4.8 | 0.1 | |||||||||
| 1659 | 1658a | Selin-11-en-4α-ol | 4.2 | 3.7 | 4.4 | |||||||||
| 1659 | 1668b | Intermedeol | 0.2 | 0.5 | ||||||||||
| 1660 | 1656a | α-Bisabolol Oxide B | 2.3 | |||||||||||
| 1671 | 1670a | epi -β-Bisabolol | 8.1 | 6.5 | 9.5 | 8.2 | 18.1 | |||||||
| 1674 | 1674a | β-Bisabolol | 2.9 | 1.9 | 3.6 | 3.9 | 5.6 | |||||||
| 1675 | 1671a | 14-hydroxy-9-epi-β-Caryophyllene | 1.4 | 0.7 | 1.3 | |||||||||
| 1677 | 1675a | Cadalene | 0.1 | 0.6 | ||||||||||
| 1678 | 1674a | Helifolenol A | 0.6 | 0.2 | ||||||||||
| 1680 | 1679a | Khusinol | 0.3 | 0.2 | ||||||||||
| 1685 | 1683a | epi-α-Bisabolol | 1.0 | 0.8 | 1.3 | 1.2 | 2.5 | |||||||
| 1687 | 1685a | α-Bisabolol | 2.8 | 4.0 | 2.6 | 2.2 | 3.4 | |||||||
| 1692 | 1692a | Acorenone | 0.2 | |||||||||||
| 1696 | 1696b | Juniper camphor | 0.8 | |||||||||||
| 1698 | 1700a | Eudesm-7(11)-en-4-ol | 0.1 | 0.1 | ||||||||||
| 1714 | 1713a | (2E,6Z)-Farnesal | 0.2 | 1.3 | 1.5 | 2.7 | 0.2 | 1.0 | 0.4 | 2.8 | ||||
| 1721 | 1722a | (2Z,6E)-Farnesol | 3.7 | 4.6 | 0.2 | 0.1 | ||||||||
| 1722 | 1724a | (2E,6E)-Farnesol | 0.4 | 2.2 | 1.1 | 0.2 | 0.9 | 0.3 | 4.9 | |||||
| 1741 | 1740a | (2E,6E)-Farnesal | 0.3 | 1.9 | 2.1 | 3.6 | 0.4 | 0.7 | 1.4 | 0.6 | 3.8 | |||
| 1751 | 1751a | Xanthorrhizol | 0.1 | 0.1 | ||||||||||
| 1757 | 1753a | Isobaeckeol | 0.2 | |||||||||||
| 1767 | 1768a | β-Bisabolenal | 0.1 | 0.2 | 0.2 | 0.1 | ||||||||
| 1841 | 1832b | Farnesyl acetate | 0.1 | 0.3 | 0.2 | 0.1 | ||||||||
| 1843 | 1845a | (2E,6E)-Farnesyl acetate | 0.7 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| 1962 | 1958a | Geranyl benzoate | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | |||||||
| Monoterpenes hydrocarbons | 42.9 | 61.6 | 54.0 | 45.5 | 76.9 | 48.1 | 7.8 | 11.0 | 26.4 | 51.1 | 0.9 | 14.6 | ||
| Oxygenated monoterpenes | 6.6 | 3.9 | 7.5 | 4.7 | 6.5 | 8.8 | 1.9 | 4.5 | 3.9 | 2.8 | 3.2 | 1.4 | ||
| Sesquiterpene hydrocarbons | 19.5 | 14.6 | 14.0 | 21.1 | 5.6 | 18.6 | 46.7 | 28.0 | 34.3 | 21.3 | 20.7 | 40.1 | ||
| Oxygenated sesquiterpenes | 21.8 | 15.1 | 17.8 | 22.5 | 5.2 | 15.9 | 31.2 | 36.5 | 30.2 | 23.0 | 63.5 | 33.6 | ||
| Others | 0.3 | 1.8 | 2.1 | 2.4 | 1.9 | 0.8 | 0.4 | 0.9 | 0.5 | 0.8 | 0.6 | 0.8 | ||
| Total (%) | 91.1 | 97.0 | 95.4 | 96.2 | 96.1 | 92.2 | 88.0 | 80.9 | 95.3 | 99.0 | 88.9 | 90.5 | ||
| Yield of oil (%) | 0.6 | 0.6 | 0.6 | 0.9 | 0.4 | 0.3 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | ||
Comparing these results with the composition of other essential oils described for the same plant, a specimen of P. guineense sampled in Arizona, USA, has also been found to contain β-bisabolene, α-pinene, and limonene as its primary constituents [14]. In addition, the oil from another specimen collected in Roraima, Brazil, presented β-bisabolol as the main component, followed by limonene and epi-α-bisabolol [15]. On the other hand, a specimen sampled in Mato Grosso do Sul, Brazil, presented an essential oil with a very high value of spathulenol [16]. Therefore, it is possible that there is a significant variation in the essential oils of different types of Araçá.
Variability in oils composition
The multivariate analysis of PCA (Principal Component Analysis) (Fig. 2) and HCA (Hierarchical Cluster Analysis) (Fig. 3) were applied to the primary constituents present in oils (content ≥ 5.0%), for the evaluation of chemical variability among the P. guineense specimens.
Fig. 2.
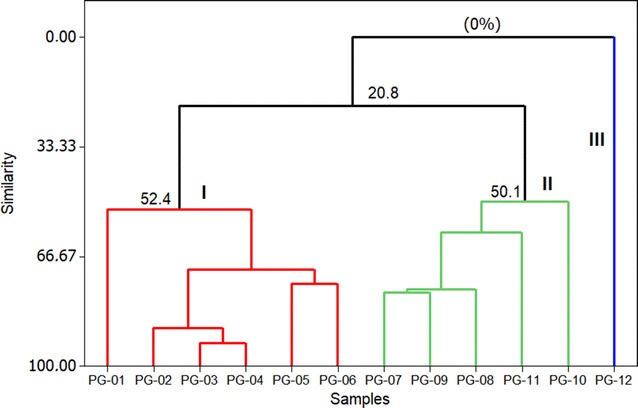
Dendrogram representing the similarity relation in the oils composition of P. guineense
Fig. 3.
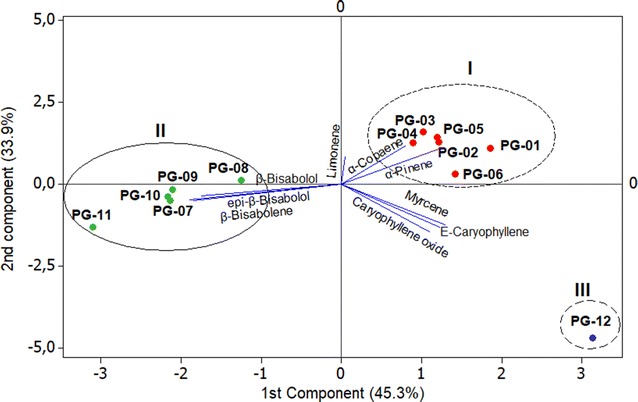
Biplot (PCA) resulting from the analysis of the oils of P. guineense
The HCA analysis performed with complete binding and Euclidean distance showed the formation of three different groups. These were confirmed by the PCA analysis, which accounted for 79.5% of the data variance. The three groups were classified as:
Group I Characterized by the presence of the monoterpenes α-pinene (13.4-35.6%) and limonene (3,7-37,2%), composed by the specimens PG-01 to PG-06, collected in Curuçá (PG -01 to PG-05) and Santarém (PG-06), Pará state, Brazil, with 49.2% similarity between the samples.
Group II Characterized by the presence of the sesquiterpenes β-bisabolene (4.0-8.9%) and epi-β-bisabolol (6.5-18.1%), consisting by PG-07 to PG-10 specimens collected in Monte Alegre (PG-07 and PG-08) and Santarém (PG-09 and PG-10), Pará State, Brazil, with 50.3% similarity between samples.
Group III Characterized by the presence of a significant content of β-caryophyllene (24.0%) and caryophyllene oxide (14.1%), constituted by the PG-12 specimen, collected in the city of Ponta de Pedras, Pará state, Brazil, which presented zero% similarity with the other groups.
Thus, based on the study of these essential oils, the multivariate analysis (PCA and HCA) has suggested the existence of three chemical types among the twelve specimens of P. guineense collected in different locations of the Brazilian Amazon. It would then be the chemical types α-pinene/limonene (Group I), β-bisabolene/epi-β-bisabolol (Group II) and β-caryophyllene/caryophyllene oxide (Group III). Taking into account that two essential oils with a predominance of α-pinene/limonene and β-bisabolene/epi-β-bisabolol, respectively, were previously described [14, 15], it is understood that adding these two chemical types to that one rich in β-caryophyllene + caryophyllene oxide, which was a product of this study, besides the other chemical type with a high value of spathulenol, before reported by Nascimento and colleagues (2018) [16], will be now, at least, four chemical types known for the P. guineense essential oils.
Several studies have demonstrated the anti-inflammatory activities of limonene, α-pinene and β-caryophyllene, the primary constituents found in the oils of P. guineense presented in this paper. Limonene showed significant anti-inflammatory effects both in vivo and in vitro, suggesting a beneficial role as a diet supplement in reducing inflammation [21]; limonene decreased the infiltration of peritoneal exudate leukocytes and reduced the number of polymorphonuclear leukocytes, in the induced peritonitis [22]. α-Pinene presented anti-inflammatory effects in human chondrocytes, exhibiting potential anti-osteoarthritic activity [23], and in mouse peritoneal macrophages induced by lipopolysaccharides [24], being, therefore, a potential source for the pharmaceutical industry. The anti-arthritic and the in vivo anti-inflammatory activities of β-caryophyllene was evaluated by molecular imaging [25].
Conclusion
In addition to the great use of the fruits of P. guineense, which are rich in minerals and functional elements, it is understood that the knowledge of the chemical composition of the essential oils of leaves of their different chemical types may contribute to the selection of varieties with more significant biological activity. The study intended to address this gap.
Authors’ contributions
PLBF participated in the collection and preparation the plants to the herbaria, run the laboratory work, analyzed the data and contributed to the drafted paper. RCS helped with lab work. JKRS guided the lab work and data analysis. CS identified the plants and managed their introduction in herbaria. RHVM helped with lab work and data analysis. JGSM proposed the work plan, guided the laboratory work and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank CAPES, a Brazilian Government’s research funding agency, for its financial support.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- HCA
Hierarchical Cluster Analysis
- PCA
Principal Component Analysis
- GC
Gas chromatography
- GC-MS
Gas chromatography-Mass spectrometry
- IAN
Herbarium of Embrapa Amazônia Oriental
- HSTM
Herbarium of Santarém
References
- 1.Govaerts R, Sobral M, Ashton P, Barrie F, Holst B, Landrum L, Lucas E, Matsumoto K, Mazine F, Proença C, Soares-Silva L, Wilson P, Niclughdha E: World checklist of selected plant families – Myrtaceae. Kew: The Board of Trustees of the Royal Botanic Gardens; 2013. http://wcsp.science.kew.org.
- 2.Landrum LR, Kawasaki ML. The genera of Myrtaceae in Brazil: an illustrated synoptic treatment and identification keys. Brittonia. 1997;49:508–536. doi: 10.2307/2807742. [DOI] [Google Scholar]
- 3.Sobral M, Proença C, Souza M, Mazine F, Lucas E: Myrtaceae. Lista de espécies da flora do Brasil. Rio de Janeiro: Jardim Botânico do Rio de Janeiro; 2015. http://floradobrasil.jbrj.gov.br.
- 4.Landrum LR. The genus Psidium (Myrtaceae) in the state of Bahia, Brazil. Canotia. 2017;13:1–101. [Google Scholar]
- 5.Missouri Botanical Garden. www.tropicos.org/Name/22102032.
- 6.Caldeira SD, Hiane PA, Ramos MIL, Ramos Filho MM. Caracterização físico-química do Araçá (Psidium guineense Sw.) e do Tucumá (Vitex cymosa Bert.) do Estado de Mato Grosso do Sul. B CEPPA. 2004;22:145–154. [Google Scholar]
- 7.Genovese MI, Pinto MS, Gonçalves AESS, Lajolo FM. Bioactive compounds and antioxidant capacity of exotic fruits and commercial frozen pulps from Brazil. Food Sci Technol Int. 2008;14:207–214. doi: 10.1177/1082013208092151. [DOI] [Google Scholar]
- 8.Gordon A, Jungfer E, da Silva BA, Maia JGS, Marx F. Phenolic constituents and antioxidant capacity of four underutilized fruits from the Amazon region. J Agric Food Chem. 2011;59:7688–7699. doi: 10.1021/jf201039r. [DOI] [PubMed] [Google Scholar]
- 9.Rivero-Maldonado G, Pacheco D, Martín LM, Sánchez-Urdaneta A, Quirós M, Ortega J, Colmenares C. Bracho B (2013) Flavonoides presentes en especies de Psidium (Myrtaceae) de Venezuela. Rev Fac Agron. 2013;30:217–230. [Google Scholar]
- 10.Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz-Junior M, Tien OS, Kakimari SH, Reis MS. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia. 2002;73:69–91. doi: 10.1016/S0367-326X(01)00362-8. [DOI] [PubMed] [Google Scholar]
- 11.Vieira TI, Gondim BLC, Santiago BM, Valença AMG. In vitro antibacterial and non-stick activity of extracts from leaves of Psidium guineense Sw. and Syzygium cumini (L.) Skeels on oral microorganisms. Rev Gaúcha Odontol. 2012;60:359–365. [Google Scholar]
- 12.Anesini C, Perez C. Screening of plants used in Argentine folk medicine for antimicrobial activity. J Ethnopharmacol. 1993;39:119–128. doi: 10.1016/0378-8741(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes TG, Mesquita ARC, Randau KP, Franchitti AA, Ximenes EA. In vitro synergistic effect of Psidium guineense (Swartz) in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus strains. Sci World J. 2012;158237:7p. doi: 10.1100/2012/158237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker O, Maciarelloa MJ, Landrumb LR. Volatile leaf oils of American Myrtaceae. III. Psidium cattleianum Sabine, P. friedrichsthalianum (Berg) Niedenzu, P. guajava L., P. guineense Sw., and P. sartorianum (Berg) Niedenzu. J Essent Oil Res. 1995;7:187–190. doi: 10.1080/10412905.1995.9698497. [DOI] [Google Scholar]
- 15.da Silva JD, Luz AIR, da Silva MHL, Andrade EHA, Zoghbi MGB, Maia JGS. Essential oils of leaves and stems of four Psidium spp. Flav Fragr J. 2003;18:240–243. doi: 10.1002/ffj.1219. [DOI] [Google Scholar]
- 16.do Nascimento KF, Moreira FMF, Santos JA, Kassuia CAL, Croda JHR, Cardoso CAL, Vieira MC, Ruiz ALTG, Foglio MA, de Carvalho JE, Formagio ASN. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw and spathulenol. J Ethnopharmacol. 2017;210:351–358. doi: 10.1016/j.jep.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 17.NIST - National Institute of Standards and Technology (2011) Mass Spectral Library (NIST/EPA/NIH, v.2.0d). The NIST Mass Spectrometry Data Center, Gaithersburg.
- 18.Mondello L. Flavors and fragrances of natural and synthetic compounds, Mass Spectral Database (FFNSC 2) New York: John Wiley & Sons Inc; 2011. [Google Scholar]
- 19.Van Den Dool H, Kratz PDJA. Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 20.Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- 21.d’Alessio PA, Ostan R, Bisson J-F, Schulzke JD, Ursini MV, Béné MC. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013;92:1151–1156. doi: 10.1016/j.lfs.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Kummer R, Estevão-Silva CF, Bastos RL, Rocha BA, Spironello RA, Yamada AN, Bersani-Amado CA, Cuman RKN. Alpha-pinene reduces in vitro and in vitro leukocyte migration during acute inflammation. Int Journal applied Res Nat Prod. 2015;8:12–17. [Google Scholar]
- 23.Rufino AT, Ribeiro M, Judas F, Salgueiro L, Lopes MC, Cavaleiro C, Mendes AF. Anti-inflammatory and chondroprotective activity of (+)-pinene structural and enantiomeric selectivity. J Nat Prod. 2014;77:264–269. doi: 10.1021/np400828x. [DOI] [PubMed] [Google Scholar]
- 24.Kim DS, Lee HJ, Han YH, Kee JY, Kim HJ, Shin HJ, Lee BS, Kim SH, Kim SJ, Park SH, Choi BM, Park SJ, Um JY, Hong SH. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-kB pathway in mouse peritoneal macrophages. Am J Chin Med. 2015;43:731–742. doi: 10.1142/S0192415X15500457. [DOI] [PubMed] [Google Scholar]
- 25.Dahham SS, Tabana YM, Khadeer Ahamed MB, Abdul Majid AMS. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Molecules & Medicinal Chemistry. 2015;1(e1001):6p. [Google Scholar]


