Abstract
Background
In order to develop new larvicidal agents derived from phytochemicals, the larvicidal activity of fifty molecules that are constituent of essential oils was evaluated against Culex quinquefasciatus Say. Terpenes, terpenoids and phenylpropanoids molecules were included in the in vitro evaluation, and QSAR models using genetic algorithms were built to identify molecular and structural properties of biological interest. Further, to obtain structural details on the possible mechanism of action, selected compounds were submitted to docking studies on sterol carrier protein-2 (SCP-2) as possible target.
Results
Results showed high larvicidal activity of carvacrol and thymol on the third and fourth larval stage with a median lethal concentration (LC50) of 5.5 and 11.1 µg/mL respectively. Myrcene and carvacrol were highly toxic for pupae, with LC50 values of 31.8 and 53.2 µg/mL. Structure–activity models showed that the structural property π-bonds is the largest contributor of larvicidal activity while ketone groups should be avoided. Similarly, property–activity models attributed to the molecular descriptor LogP the most contribution to larvicidal activity, followed by the absolute total charge (Qtot) and molar refractivity (AMR). The models were statistically significant; thus the information contributes to the design of new larvicidal agents. Docking studies show that all molecules tested have the ability to interact with the SCP-2 protein, wherein α-humulene and β-caryophyllene were the compounds with higher binding energy.
Conclusions
The description of the molecular properties and the structural characteristics responsible for larvicidal activity of the tested compounds were used for the development of mathematical models of structure–activity relationship. The identification of molecular and structural descriptors, as well as studies of molecular docking on the SCP-2 protein, provide insight on the mechanism of action of the active molecules, and the information can be used for the design of new structures for synthesis as potential new larvicidal agents.
Electronic supplementary material
The online version of this article (10.1186/s13065-018-0425-2) contains supplementary material, which is available to authorized users.
Keywords: QSAR, Essential oils, Larvicidal activity, Sterol carrier protein-2, Terpenes
Introduction
More than half of the global human population is exposed to the risk of infection spread by mosquitoes; including Culex spp., Anopheles spp. and Aedes spp. that are considered a public health problem, sin are vectors of pathogenic parasites. Lymphatic filariasis uses Culex quinquefasciatus Say (Diptera: Culicidae) as vector; it is one of the leading causes of global morbidity, with close to 150 million infected, especially in tropical climates [1]. Culex quinquefasciatus is present in most tropical regions of the world; it is commonly found in many urban areas and has been reported as resistant to registered insecticides [2].
The control of mosquito larvae and pupae currently relies on the use of synthetic chemical insecticides [3]. However, prolonged use of these synthetic pesticides has caused numerous problems, such as the development of resistance [4], undesirable effects on non-target organisms, effects on wildlife, damage to human health and other negative impacts on the environment [5–7]. Several studies have searched for natural products derived from plants as possible mosquito control environmentally-friendly strategy; reports include the larvicidal action of essential oils (EOs) and their constituents [8, 9]. EOs can be alternative pest control agents, because some of their compounds have proven to be highly selective, easily removable, biodegradable, with low or no toxicity against mammals and are effective against a full spectrum of mosquito pests [10, 11]. Also EOs are characterized by reduced effects on non target organisms and minimal environmental persistence [12]. With few exceptions, some of the purified terpenoid constituents of EOs are moderately toxic to mammals, but the oils themselves or their compounds are mostly non toxic to mammals, birds, and fish [12].
EOs are heterogeneous mixtures of organic chemical compounds [13] mainly terpenoids and phenylpropanes, but low molecular weight aliphatic compounds, acyclic esters or lactones may also be present [14]. The EOs chemical composition is affected by diverse factors, including plant species and subspecies, geographical location, harvested time, the part of the plant used and the extraction methods employed to obtain the EO [15]. In spite of several studies on the larvicidal activity of EOs and their constituents, little is known on the mechanism of action exerted by terpenoids and phenylpropanoids on mosquito larvae. This has motivated the study of the molecular properties, reactivity or structural modulation of essential oil chemical components in order to minimize synthetic and biological evaluation effort for the development of new compounds with potential larvicidal activity.
Computer assisted prediction of the biological activity of specific chemical compounds considering their chemical structure is now a common technique used in drug discovery [16, 17]. Quantitative structure–activity relationship (QSAR) and quantitative property–activity relationship (QPAR) studies can provide information to understand the relationship between molecule’s chemical structure and biological activity [18]. Also, molecular docking is an in silico technique used to estimate the strength of the protein–ligand interaction, to determine biding poses and free energy values [19]. Docking describe ligand binding to a receptor through noncovalent interactions which is commonly used to explore the ligand recognition on targets for new drug development [20].
This article describes the larvicidal activity of fifty compounds against larvae and pupae of Culex quinquefasciatus (Diptera: Culicidae). Terpenes, terpenoids and others related compounds constituents of different EOs were evaluated in this work. Likewise, the present work reports the theoretical characterization of the molecular and electronic properties of experimentally tested molecules. QSAR/QPAR models and docking studies are also included to emphasize the molecular and structural properties that are essential in the larvicidal activity.
Materials and methods
Compounds tested
Fifty compounds were evaluated to determine their larvicidal activity against larvae (stair III and IV) and pupae of Culex quinquefasciatus Say (Diptera: Culicidae). Compounds were purchased from a Sigma-Aldrich (St. Louis, MI, USA) distributor, and its chemical structure is shown in Fig. 1.
Fig. 1.

(1) p-Anisaldehyde, (2) Canphor, (3) (3) Carene, (4) Carvacrol, (5) Carveol, (6) Carvomenthol, (7) Carvone, (8) Carvotanacetol, (9) β-Caryophyllene, (10) Citronellal, (11) β-Citronellol, (12) m-Cresol, (13) o-Cresol, (14) Cuminaldehyde, (15) p-Cimene, (16) t-Dihydrocarvone, (17) 3,4-Dimethylcumene, (18) Eucalyptol, (19) Geranial, (20) Geraniol, (21) Germacrene-D, (22) α-Humulene, (23) Hydrocarvone, (24) Hydrodihydrocarvone, (25) 3-Isopropylphenol, (26) Isoborneol, (27) Isopulegol, (28) t-Isopulegone, (29) Lavandullol, (30) Limonene, (31) Linalool, (32) Menthol, (33) Menthone, (34) Myrcene, (35) Neoisopulegol, (36) Perillaldehyde, (37) β-Phellandrene, (38) α-Pinene, (39) β-Pinene, (40) Pulegone, (41) Rotundifolone, (42) Sabinene (43) α-Terpinene, (44) γ-Terpinene, (45) 4-Terpineol, (46) α-Terpineol, 47) β-Terpineol, 48) γ-Terpineol, (49) Terpinolene, (50) Thymol
Insect cultures and rearing conditions
Larvae of Cx. quinquefasciatus were collected from water tanks in the Sanctorum Cemetery in Mexico City, Mexico (19°27′17″N, 99°12′47″W) and identified using Harwood and James descriptions [21]. Groups of 50 individuals of first and second instar larvae were placed in glass bottles with purified water, maintained at 26 ± 2° C with a natural photoperiod and supplied with 3:1 powdered mixture of dog food and baking powder. The third instar emerging larvae were then separated by groups of 10 individuals in 100 mL tubes with distilled water [22].
Larvicidal activity bioassays and statistical analysis
Bioassays were done according to the World Health Organization (WHO) protocol with few modifications [23]. Third and fourth instar larvae as well as pupae, were used for testing. Five groups of 20 larvae were isolated in beakers of 250 mL, exposed to different concentrations of the tested compounds and maintained in starvation throughout the experimental period; the surviving larvae were counted in order to record larval mortality. The compounds were diluted in dimethyl sulfoxide (DMSO) (Sigma, 472301) before being added to the aqueous medium which contained the larvae. Temephos H at 0.1 ppm (commercial concentration) was used as a standard for comparison. Larvae were considered dead if they were immobile and unable to reach the water surface [24]. Lethal concentrations (LC50) was calculated using Probit analysis. Data were processed using MS Excel 2010 and SAS v. 9 (Proc Probit) computer programs.
DFT study and descriptors calculations
Computational studies were carried out using the Spartan 03 [25] and Gaussian 09 quantum chemistry computer programs [26]. The molecular structures were analyzed by a conformational analysis of each molecule in gas phase using the mechanics force field SYBYL [27]. The minimum energy conformation was selected in order to obtain the geometry optimization using the density functional theory (DFT). The equilibrium geometries of the molecules in the electronic ground state were determined with the Becke three-parameter hybrid functional combined with Lee–Yang–Parr correlation functional (B3LYP) [28, 29]. The basis set 6-311G(d,p) was used for the geometry optimization and vibrational frequency calculations and the 6-311+G(d,p) was applied for vertical excitation energy calculations [30–32]. Analytical frequency calculations were carried out, where the absence of imaginary frequencies confirmed that the stationary points correspond to the global minima of the potential energy hypersurfaces.
The Koopmans theorem [33] was applied for calculations of the chemical reactivity descriptors such as: the ionization potential (I), electron affinity (A), electronegativity (χ), chemical potential (μ), hardness (ɳ), softness (σ), global electrophilicity (ω), as well as the electronic parameters of, EHOMO (energy of highest occupied molecular orbital), ELUMO (energy of the lowest unoccupied molecular orbital) and band gap (GAPE) were calculated. All molecules were analyzed in the gas and aqueous phase. The polarizable continuum model (PCM) was used to model the solvent effects [34].
Structure, constitutional, physicochemical and topological descriptors were generated using Dragon 5.0 software [35] using the optimized structure in the aqueous phase.
Structure–property–larvicidal activity models
QSAR/QPAR studies was carried out using all biological activities obtained in vitro and the calculated theoretical descriptors; the analysis was carried out using genetic algorithms with the Mobydigs Software [36]. The quality of the model was considered statistically satisfactory based on the determination coefficient (R2), leave-one-out cross-validated explained variance (Q2), standard deviation (s) and the ANOVA (F) of the model.
Molecular docking studies on protein SCP-2
The sequence of sterol carrier protein (SCP-2) of Cx. quinquefasciatus (GenBank: AAO43438.1) was obtained from the database of the National Center for Biotechnology Information (NCBI). The protein was modeled through Swiss-Model server [37, 38], using as template the sterol carrier protein of Aedes aegypti (PDB: 1PZ4) [39] reported in the RCSB Protein Data Bank. The final model was subjected to Ramachandran analysis using the Rampage server [40]. Docking analysis was done using the AutoDock4 software [41]. For the docking the active site was defined considering the residues within a grid of 60 A° × 60 A° × 60 A° centered in the active site, with an initial population of 100 randomly placed individuals and a maximum number of 1.0 × 107 energy evaluations. Active site was determined under the description made by Dyer et al. [39]. Compounds for docking were drawn in Gauss view before docking, the compounds were subjected to energy minimization using the hybrid functional B3LYP with a 6, 311G(d,p) basis set. The Kd and ΔG (Kcal/mol) values were obtained from the conformation with the lowest minimum free energy of the ligand coupled on the protein targets. The figures were prepared with ChemBioOffice [42] for the structures and Chimera [43] for the proteins and ligands.
Results and discusion
Larvicidal activity and quantitative structure–larvicidal activity relationship
Chemical compounds known to be constituents of EOs demonstrated larvicidal activity against III and IV stairs of Cx. quinquefasciatus; activity against pupae was moderate, with higher concentrations of the compounds required to reach LC50; LC50 values as shown in Table 1. In all experiments, 100% of the larvae remained active in the negative control; DMSO larvicidal activity was also determined, and concentration of 1000 µg/mL had no larvicidal effect; therefore, larvicidal activity can be attributed entirely to the compounds, and not the solvent used.
Table 1.
Larvicidal activity of the terpenes, terpenoids and related compounds against Cx. quinquefasciatus
| Assays | Larvicidal activity (µg/mL) | ||||
|---|---|---|---|---|---|
| III | IV | Pupaes | |||
| Molecules | Classification | LC50 | LC50 | LC50 | |
| 1 | p-Anisaldehyde | Benzaldehyde | 18.0 (15.5–20.4) | 18.8 (16.9–20.6) | 96.4 (92.5–100.2) |
| 2 | Canphor | Bicyclic monoterpenoid | 22.3 (21.6–23.9) | 25.8 (23.6–27.9) | 245.1 (234.6–255.5) |
| 3 | 3-Carene | Bicyclic monoterpene | 24.7 (23.7–25.7) | 25.5 (24.3–26.7) | 105.5 (101.8–109.1) |
| 4 | Carvacrol | Cyclic monoterpenoid | 5.5 (5.28–5.72) | 7.7 (7.3–8.1) | 53.2 (51.8–54.5) |
| 5 | Carveol | Cyclic monoterpenoid | 103.0 (99.4–109.9) | 104.6 (102.0–107.2) | 249.0 (241.8–256.1) |
| 6 | Carvomenthol | Cyclic monoterpenoid | 198.2 (183.69–212.71) | 219.8 (206.6–232.9) | 452.2 (435.2–469.1) |
| 7 | (+)-Carvone | Cyclic monoterpenoid | 150.2 (149.0–151.4) | 150.2 (145.5–154.8) | 500.6 (495.0–506.1) |
| 8 | Carvotanacetol | Cyclic monoterpenoid | 152.3 (148.2–156.8) | 198.3 (192.1–204.44) | 245.1 (238.1–252.0) |
| 9 | β-Caryophyllene | Bicyclic sesquiterpene | 45.6 (43.8–47.2) | 47.7 (42.2–52.9) | 222.3 (216.8–27.7) |
| 10 | Citronellal | Acyclic monoterpenoid | 105.3 (98.3–102.3) | 124.9 (123.2–125.6) | 549.2 (557.35–565.5) |
| 11 | β-Citronellol | Acyclic monoterpenoid | 90.4 (88.9–91.9) | 94.8 (93.4–95.2) | 203.1 (198.44–207.76) |
| 12 | m-Cresol | Phenolic derivative | 60.0 (58.8–61.2) | 60.6 (59.3–61.9) | 107.7 (104.94–110.4) |
| 13 | o-Cresol | Phenolic derivative | 54.8 (53.6–56.0) | 54.4 (53.8–54.0) | 105.6 (103.4–107.7) |
| 14 | Cuminaldehyde | Benzaldehyde | 23.0 (22.0–24.0) | 23.9 (22.0–25.8) | 95.4 (91.1–99.6) |
| 15 | p-Cimene | Cyclic monoterpene | 23.1 (22.3–24.9) | 24.0 (23.8–26.2) | 306.3 (298.4–314.1) |
| 16 | trans-Dihydrocarvone | Cyclic monoterpene | 345.0 (340.8–350.1) | 361.3 (346.2–366.4) | 708.6 (698.1–719.1) |
| 17 | 3,4-Dimethylcumene | Phenolic derivative | 35.6 (33.5–37.7) | 47.7 (46.2–49.2) | 105.5 (101.9–109.1) |
| 18 | Eucalyptol | Bicyclic monoterpenoid | 48.0 (47.9–49.1) | 44.4 (43.3–45.5) | 92.9 (86.2–99.6) |
| 19 | Geranial | Acyclic monoterpenoid | 52.2 (51.1–53.3) | 53.4 (49.9–56.8) | 193.9 (186.8–200.9) |
| 20 | Geraniol | Acyclic monoterpenoid | 20.4 (19.78–21.02) | 20.4 (19.4–21.3) | 104.6 (101.9–107.2) |
| 21 | Germacrene-D | Sesquiterpene | 45.4 (44.3–46.6) | 45.6 (46.71–47.49) | 229.0 (222.7–235.2) |
| 22 | α-Humulene | Bicyclic sesquiterpene | 100.5 (98.2–102.7) | 101.8 (100.0–103.5) | 508.3 (497.17–519.43) |
| 23 | Hydrocarvone | Cyclic monoterpene | 1351.6 (1228.68–1474.5) | 1470.9 (1347.9–1592.9) | > 2000 |
| 24 | Hydrodihydrocarvone | Cyclic monoterpenemonoterpene | 1416.5 (1152.4–1680.1) | 1628.2 (1364.6–1889.3) | > 2000 |
| 25 | 3-Isopropylphenol | Cyclic monoterpene | 21.3 (20.9–21.6) | 23.1 (21.2–24.9) | 100.2 (96.4–104.4) |
| 26 | Isoborneol | Bicyclic monoterpenoid | 91.9 (89.7–94.0) | 97.1 (94.1–100.1) | 206.1 (199.7–213.5) |
| 27 | Isopulegol | Cyclic monoterpene | 247.4 (234.4–250.9) | 297.3 (290.2–304.3) | 610.8 (604.6–616.9) |
| 28 | trans-Isopulegone | Cyclic monoterpene | 529.1 (510.1–537.1) | 538.8 (530.7–546.8) | 908.6 (896.2–920.9) |
| 29 | Lavandullol | Acyclic monoterpenoid | 52.2 (51.0–53.3) | 56.5 (53.3–59.9) | 238.7 (224.6–252.7) |
| 30 | Limonene | Cyclic monoterpene | 24.2 (23.4–24.9) | 27.3 (23.3–28.2) | 98.4 (95.4–101.4) |
| 31 | Linalool | Acyclic monoterpenoid | 26.8 (26.0–27.5) | 30.7 (29.7–31.6) | 249.0 (241.8–256.1) |
| 32 | Menthol | Cyclic monoterpenoid | 443.6 (432.3–443.2) | 404.1 (381.1–427.0) | 529.1 (521.0–537.1) |
| 33 | Menthone | Cyclic monoterpenoid | 500.6 (495.0–506.1) | 508.9 (500.8–516.9) | 878.5 (867.4–889.5) |
| 34 | Myrcene | Acyclic monoterpene | 19.5 (18.5–20.4) | 19.1 (18.0–20.2) | 31.8 (30.2–33.2) |
| 35 | Neoisopulegol | Cyclic monoterpenoid | 458.4 (450.2–466.6) | 554.2 (545.6–562.7) | 908.6 (896.2–920.9) |
| 36 | (−)-Perillaldehyde | Cyclic monoterpenoid | 95.9 (94.8–97.0) | 115.8 (113.0–118.6) | 429.1 (422.9–435.22) |
| 37 | Phellandrene | Cyclic monoterpene | 490.7 (483.1–498.2) | 554.3 (545.8–563.0) | 908.6 (896.3–920.9) |
| 38 | α-Pinene | Bicyclic monoterpene | 24.4 (23.2–25.5) | 25.5 (22.0–28.97) | 98.4 (95.4–101.4) |
| 39 | β-Pinene | Bicyclic monoterpene | 19.6 (18.82–20.38) | 24.3 (22.8–25.7) | 96.9 (89.9–103.9) |
| 40 | (+)–Pulegone | Cyclic monoterpenoid | 168.7 (665.8–171.59) | 188.1 (185.29–190.91) | 496.2 (490.4–501.9) |
| 41 | Rotundifolone | Cyclic monoterpenoid | 58.9 (57.8–59.9) | 62.5 (61.5–63.5) | 287.4 (279.4–295.3) |
| 42 | Sabinene | Bicyclic monoterpene | 53.7 (51.9–55.4) | 59.0 (58.3–60.7) | 268.0 (262.5–273.0) |
| 43 | α-Terpinene | Cyclic monoterpene | 13.8 (12.9–14.7) | 13.6 (12.8–14.3) | 209.5 (204.0–214.9) |
| 44 | γ-Terpinene | Cyclic monoterpenemonoterpene | 45.4 (44.3–46.5) | 56.8 (55.7–57.9) | 287.4 (280.2–294.6) |
| 45 | 4-Terpineol | Cyclic monoterpenoid | 94.2 (91.1–97.3) | 97.7(90.6–104.8) | 201.8 (195.6–208.0) |
| 46 | α-Terpineol | Cyclic monoterpenoid | 95.9 (93.8–98.0) | 98.4 (95.3–101.4) | 206.1 (198.4–213.7) |
| 47 | β-Terpineol | Cyclic monoterpenoid | 101.3 (99.5–103.0) | 107.4 (103.9–110.8) | 508.3 (497.1–519.43) |
| 48 | γ-Terpineol | Cyclic monoterpenoid | 100.5 (98.3–102.7) | 103.6 (100.0–109.9) | 4965.5 (4949.1–4981.9) |
| 49 | Terpinolene | Cyclic monoterpene | 20.4 (19.6–21.2) | 18.6 (16.9–20.2) | 107.4 (103.9–110.8) |
| 50 | Thymol | Cyclic monoterpenoid | 11.1 (10.28–11.9) | 12.2 (11.7–12.7) | 111.4 (108.5–114.2) |
| Tx | Temephos H | Organophosphorus | 2.1 (1.8–2.5) | 5.6 (4.1–6.7) | 34.0 (29.1–39.0) |
In parenthesis, 95% confidence intervals, compounds activity is considered significantly different when the 95% CI fail to overlap
EOs are aromatic extracts obtained from plant material that are complex mixtures of volatile secondary metabolites [44]. Some of the compounds present in EOs are terpenes (molecules formed of isoprene units) [45], terpenoids (terpenes with oxygen on its structure) [45] and phenylpropanoids [47]. In the present report, carvacrol and thymol (terpenoids found mainly in the EO of oregano) were the most active molecules with a LC50 of 7.7 and 8.4 μg/mL respectively, against larvae at fourth stage. Myrcene presented a relevant activity against pupae with a LC50 of 31.8 μg/mL. Cheng et al. reported the results of screening EOs and suggested that oils with LC50 values > 100 ppm should not be considered active, whereas those with LC50 values < 50 ppm could be regarded as highly active [48]. Our results agree with reports of the larvicidal activity of constituents of oregano EO; the reports demonstrate that these compounds have fumigant and repellent activity [49–53].
In relation to chemical structure and larvicidal activity, results have been grouped considering the main chemical moiety of the tested compounds in monocyclic-terpenes, monocyclic-terpenoids, bicyclic-terpenes and bicyclic-terpenoids, and phenylpropanes. β-Caryophyllene, a bicyclic sesquiterpene, showed the lower larvicidal activity with a LC50 of 57.7 μg/mL against fourth instar and 222.3 μg/mL against pupae, Doria et al. also report low larvicidal activity of β-caryophyllene against Aedes aegypti [54]. Sabineno, a bicyclic monoterpene, also had a low activity, with LC50 values of 59.0 μg/mL for fourth instar and 258 μg/mL against pupae. β-Pinene and 3-carene presented a LC50 of 19.6 and 24.7 μg/mL respectively against the fourth stair being the most active of the bicyclic terpenes. Eucalyptol was the bicyclic terpenoid most active against pupae, the only activity lower than 100 μg/mL of all bicyclic compounds evaluated.
Table 2 include the QSAR models of larvicidal activity against the fourth instar with greater statistical significance. The models were built based on structural descriptors; models 1 and 2 describe the biological activity of the fifty molecules evaluated, and includes the number of total tertiary carbons (sp3) (nCt) and the number of non-aromatic conjugated carbons (sp2) (nCconj) as the structural descriptors that contribute the most to the biological activity, whereas the number of ketones (nRCO) and number of ethers (nROR) showed an inverse relationship with larvicidal activity. The structural descriptors that were less significant, including molecules without benzene ring (models 1 and 2, Table 2) were present in the tested molecules with the lowest biological activity. Sabinene and β-caryophyllene are examples of molecules with no benzene rings and presence of ketone groups. In fact the keto group reduces the activity of carvone more than a half as compared to limonene, which does not have keto groups in its structure.
Table 2.
Summary of the statistics quantitative structure–larvicidal activity relationship models for activity against fourth instar of Cx. quinquefasciatus
| Statistical parameter | IV instar | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| n | 50 | 50 | 47 | 47 | 39 | 39 |
| Q2 | 0.793 | 0.75.34 | 0.781 | 0.759 | 0.851 | 0.832 |
| R2 | 0.828 | 0.78.73 | 0.881 | 0.858 | 0.965 | 0.957 |
| F | 14.5 | 11.1 | 21.8 | 21.3 | 49.2 | 39.4 |
| s | 0.291 | 0.301 | 0.231 | 0.234 | 0.137 | 0.152 |
| Descriptors | Contributions | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| nCt | 0.0679 | WC | WC | WC | WC | WC |
| nCconj | 0.0631 | 0.04241 | 0.052 | 0.0444 | 0.3304 | 0.3606 |
| nR = Cp | WC | 0.0803 | WC | WC | WC | WC |
| nRCO | − 0.5006 | − 0.5641 | − 0.491 | − 0.48 | − 0.7285 | − 0.6265 |
| nROR | − 0.34331 | WC | − 0.2618 | − 0.2579 | − 0.503 | − 0.5205 |
| nArOH | WC | 0.1229 | 0.1518 | WC | 0.6552 | 0.6582 |
| nOH | WC | WC | WC | 0.0187 | WC | WC |
| Intercept | − 1.5373 | − 1.644 | − 1.6531 | − 1.6723 | − 2.80322 | − 2.8386 |
n, number of systems evaluated; Q2, the square of the coefficient of cross-validation; R2, the square of the correlation coefficient; s, standard deviation; F, Fisher statistic; WC, without contribution; nCt, number of total tertiary C (sp3); nCconj, number of non-aromatic conjugated C (sp2); nR = Cp, number of terminal primary C (sp2); nRCO, number of ketones (aliphatic); nROR, number of ethers; nArOH, number of phenolic groups; nOH, number of a hydroxyls
Models 3 and 4 (Table 2) were constructed based on the larvicidal activity of 47 evaluated molecules, excluding the sesquiterpenes β-caryophyllene (9), germacrene (21) and α-humulene (22) from the analysis. The models showed the same relationship with the nCconj, nRCO, nROR descriptors and the number of phenolic groups (nArOH) and the number of hydroxyl groups (nOH) as descriptors directly related to the biological activity. This is consistent with the most biologically-active molecules: carvacrol and thymol. In monocyclic terpenoids and monocyclic terpenes, increasing the number of double bonds also increased the larvicidal activity. Menthol has a LC50 of 38.1 μg/mL against fourth instar larvae, while thymol had an activity of 12.2 μg/mL. The structural difference between these two compounds is the phenolic group in thymol as compared to menthol that only has the hydroxyl group; p-Cymene has the benzene group without hydroxyl group with an activity of 24.0 μg/mL; this demonstrate the importance of the phenolic group in the larvicidal activity. Carvacrol, an isomer of thymol, has a LC50 of 7.7 μg/mL; therefore, the position of the hydroxyl group plays an important role in the larvicidal activity.
For acyclic terpenes and terpenoids, higher larvicidal activity was observed in compounds with a higher number of double bonds and increased lipophilicity. Ketone acyclic terpenes were the compounds with lowest larvicidal activity; substitution of the ketone group by the hydroxyl group increased the biological activity considerably. Citronellol was the alcohol terpene with lower activity against the fourth instar and pupae. Geraniol has one double bond more than citronellol, and this structural difference increase the larvicidal activity. Linalool also presents one double bond more than citronellol, and this differential structure is reflected in an increase in larvicidal activity, however the position of the hydroxyl group changes from a primary to a secondary alcohol; this difference could be responsible for the lower biological activity shown. On the other hand, myrcene, an acyclic molecule with no oxygen in its structure, has the highest larvicidal activity and is the only compund with significant activity against pupae, with a LD50 of 19.1 μg/mL against the fourth instar larvae and 31.8 μg/mL against pupae. Myrcene has three double bonds in its structure, and since the lipophilicity is increased in the absence of oxygen, these is an important trait for their potential activity. Accordingly, Lucia et al. consider that the octanol water partition coefficient (LogP) is an important molecular property in the larvicidal activity of monoterpenes [55].
In models 5 and 6, the sesquiterpenes and all acyclic monotepenes were excluded. The relations of the descriptors are maintained although their values increase considerably, demonstrating that nRCO and nROR obstruct the activity of monoterpenes, so that in order to potentiate the activity of the compounds as larvicides agents, these functional groups must be avoided. On the other hand, the nArOH excels on the nCconj as the descriptor of greatest contribution in larvicidal activity, an issue discussed previously. The values of structural descriptors for each target system are confined in Table 3. A plot of the predicted activity versus experimental activity for molecules using a training set for structure–activity relationship models is shown in Fig. 2. Experimental and predicted LogLC50 values are shown in Additional file 1: Table S1, while the constitutional descriptors can be observed in Additional file 1: Table S2.
Table 3.
Structural descriptors calculated
| Mol. | nCs | nCt | nCconj | nR = Cp | nR = Cs | nR = Ct | nRCO | nArOH | nOH | nHDon | nHAcc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 2 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 3 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 5 | 3 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 |
| 6 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 7 | 2 | 1 | 3 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 |
| 8 | 3 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 9 | 4 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| 10 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| 11 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 14 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 15 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 3 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| 17 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | 2 | 0 | 3 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 |
| 20 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 1 |
| 21 | 4 | 2 | 4 | 1 | 3 | 2 | 0 | 0 | 0 | 0 | 0 |
| 22 | 4 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 0 | 0 |
| 23 | 2 | 2 | 3 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 |
| 24 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 |
| 25 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 26 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 27 | 4 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 28 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 29 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 |
| 30 | 3 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| 31 | 2 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 |
| 32 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 33 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 34 | 2 | 0 | 4 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 35 | 4 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 36 | 3 | 1 | 3 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 |
| 37 | 2 | 1 | 4 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 38 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 39 | 3 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 40 | 3 | 1 | 3 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 |
| 41 | 3 | 1 | 3 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 2 |
| 42 | 3 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 43 | 2 | 1 | 4 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 44 | 2 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 45 | 3 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 46 | 3 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 47 | 4 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 48 | 4 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 |
| 49 | 3 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| 50 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
nCs, Number of total secondary C (sp3); nCt, number of total tertiary C (sp3); nCconj, Number of non-aromatic conjugated C (sp2); nR = Cp, number of terminal primary C (sp2); nR = Cs, number of aliphatic secondary C(sp2); nR = Ct, number of aliphatic tertiary C(sp2); nRCO, number of ketones (aliphatic); nArOH, number of aromatic hydroxyls; nOH, number of a hydroxyls; nHDon, number of donor atoms for H-bonds; nHAcc, number of acceptor atoms for H-bonds
Fig. 2.
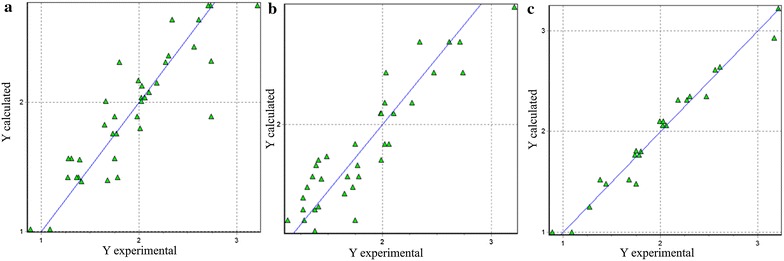
Predicted versus experimental larvicidal activity from structural–activity relationship models. a Model 1, b model 3, c model 5
Quantitative property–larvicidal activity relationship and DFT study
The models that describe the relationship between the molecular properties and biological activity demonstrate that the octanol–water partition coefficient (MlogP) descriptor is the largest contributor to the larvicidal activity. Lucia et al. developed a QSAR model based on six monoterpenes and they found that when vapor pressure and lipophilicity values decreased, the larvicidal activity against A. aegypti also diminished. The strong effect of the octanol–water partition coefficient can be explained considering that the main conduit for component entrance to the organism is tactile (external cuticle) [55]. Therefore, the partition occurs between the hydrophilic environment (water) and the lipophilic environment (larval epicuticle); therefore, molecule hydrophobicity plays an important role in the intoxication of the larva [56].
Table 4 includes the QPAR models of larvicidal activity against the fourth instar with greater statistical significance. Like QSAR models, QPAR models 1 and 2 were constricted based on all the evaluated compounds, in the models 3 and 4 the sesquiterpenos were excluded and the models 5 and 6 were constructed excluding sesquiterpenes and acyclic monoterpenes. The predicted activity versus experimental activity for molecules using a training set for structure–activity relationship models is shown in Fig. 3. Experimental and predicted LogLC50 values of QPAR models are shown in Additional file 1: Table S3.
Table 4.
Summary of the statistics quantitative property–larvicidal activity relationship models for activity against fourth instar of Cx. quinquefasciatus
| Statistical parameter | IV instar | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| n | 50 | 50 | 47 | 47 | 39 | 39 |
| Q2 | 0.759 | 0.630 | 0.761 | 0.751 | 0.840 | 0.818 |
| R2 | 0.829 | 0.812 | 0.880 | 0.880 | 0.929 | 0.917 |
| F | 20.9 | 20.2 | 24.1 | 23.8 | 34.3 | 29.6 |
| s | 0.293 | 0.297 | 0.022 | 0.021 | 0.151 | 0.162 |
| Descriptors | Contributions | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| J | − 2.3271 | − 1.6812 | WC | WC | − 0.0638 | WC |
| MlogP | 0.3632 | 0.3222 | WC | WC | 1.5415 | 1.1347 |
| TIE | 0.0843 | 0.0929 | 0.16824 | 0.1684 | 0.2377 | 0.0467 |
| AMR | 0.0441 | WC | WC | WC | WC | WC |
| Qtot | WC | WC | 0.4735 | 0.5324 | WC | WC |
| BAC | WC | − 0.0535 | WC | WC | WC | WC |
| Hy | WC | WC | − 0.7359 | − 0.4735 | WC | WC |
| ƞ | WC | WC | 0.3068 | WC | WC | WC |
| m | WC | WC | WC | 0.0927 | 0.5698 | 0.6654 |
| E HOMO | WC | WC | WC | WC | 0.2377 | 0.2486 |
| Intercept | − 0.3266 | − 0.3421 | − 2.3992 | − 2.3891 | 7.8613 | 4.817 |
n, number of systems evaluated; Q2, the square of the coefficient of cross-validation; R2, the square of the correlation coefficient; s, standard deviation; F, Fisher statistic; WC, without contribution; J, Balaban-like index; MlogP, Moriguchi octanol–water partition coeff. (logP); TIE, E-state topological parameter; AMR, Ghose–Crippen molar refractivity; Qtot, total absolute charge; BAC, Balaban centric index; Hy, hydrophilic factor; η, chemical hardness; EHOMO, energy of the HOMO orbital; m, dipole moment
Fig. 3.

Predicted versus experimental larvicidal activity from property–activity relationship models. a Model 1, b model 3, c model 5
The lipophilic character of terpenes and their derivatives have been widely discussed as a key factor in the antimicrobial and larvicidal properties of these compounds [14–16, 44, 45]; however, it does not finish describing their larvicidal behavior. Sesquiterpenes, for example, have high MlogP values and are not the most active compounds.
Some QPAR models consider molar refractivity (AMR) and absolute total charge (Qtot) as descriptors that contribute to larvicidal activity. Qtot is a measure of the weak intermolecular interactions which provides information on the electrical charges of the molecules and is considered as the driving force of electrostatic interactions, important for the interaction of the component with its biological target [57]. Myrcene, the most active acyclic terpene, is the terpene with largest number of double bonds, more MlogP and lowest Qtot, also it had the lowest AMR. Molar refractivity (AMR) descriptor is related to specific interactions with a target molecule and the electronic effects in the biological–chemical interaction, mainly for allosteric effects of interactions between the ligand-receptor [58]: therefore, it demonstrates the importance of interaction with a specific enzyme, pools of metabolites, or signaling pathways [59]. Hanch and Verma proposed a QSAR model for complex triorganotin with larvicidal activity reported by Eng et al., its models included hydrophobicity (Hy) and molar refractivity (AMR) as the most important parameters for the description of larvicidal activity [60, 61]. In these results, when MlogP was not included in the models the Hy presented in inverse relation to the larvicidal activity. The values of molecular and physicochemical descriptors for each compound are included in Table 5.
Table 5.
Molecular and physicochemical descriptors calculated
| Mol. | Qpos | Qneg | Qtot | Ui | Hy | AMR | TPSA (tot) | MlogP | AlogP |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.28 | − 1.28 | 2.56 | 3 | − 0.768 | 39.112 | 26.3 | 1.49 | 1.573 |
| 2 | 1.659 | − 1.659 | 3.318 | 1 | − 0.877 | 44.492 | 17.07 | 2.357 | 1.936 |
| 3 | 1.413 | − 1.413 | 2.825 | 1 | − 0.96 | 44.722 | 0 | 3.374 | 2.873 |
| 4 | 1.5 | − 1.5 | 2.999 | 2.807 | − 0.294 | 46.984 | 20.23 | 2.813 | 3.243 |
| 5 | 1.234 | − 1.234 | 2.468 | 1.585 | − 0.294 | 47.995 | 20.23 | 2.25 | 2.401 |
| 6 | 1.249 | − 1.249 | 2.497 | 0 | − 0.294 | 47.445 | 20.23 | 2.502 | 2.779 |
| 7 | 1.665 | − 1.665 | 3.331 | 2 | − 0.877 | 47.174 | 17.07 | 2.153 | 2.361 |
| 8 | 1.704 | − 1.704 | 3.408 | 1 | − 0.294 | 48.218 | 20.23 | 2.357 | 2.597 |
| 9 | 1.926 | − 1.926 | 3.853 | 1.585 | − 0.975 | 62.851 | 0 | 4.375 | 4.297 |
| 10 | 1.288 | − 1.288 | 2.576 | 1.585 | − 0.877 | 49.297 | 17.07 | 2.642 | 3.019 |
| 11 | 1.791 | − 1.791 | 3.582 | 1 | − 0.294 | 50.486 | 20.23 | 2.749 | 3.049 |
| 12 | 0.884 | − 0.884 | 1.768 | 2.807 | − 0.158 | 32.793 | 20.23 | 1.859 | 2.049 |
| 13 | 0.867 | − 0.867 | 1.734 | 2.807 | − 0.158 | 32.793 | 20.23 | 1.859 | 2.049 |
| 14 | 1.444 | − 1.444 | 2.887 | 3 | − 0.877 | 46.84 | 17.07 | 2.723 | 2.784 |
| 15 | 1.315 | − 1.315 | 2.631 | 2.807 | − 0.96 | 45.29 | 0 | 3.562 | 3.51 |
| 16 | 1.283 | − 1.283 | 2.566 | 1.585 | − 0.877 | 46.298 | 17.07 | 2.25 | 2.401 |
| 17 | 0.905 | − 0.905 | 1.81 | 2.807 | − 0.965 | 50.331 | 0 | 3.854 | 3.997 |
| 18 | 1.422 | − 1.422 | 2.844 | 0 | − 0.96 | 43.799 | 0 | 4.431 | 3.077 |
| 19 | 1.283 | − 1.283 | 2.566 | 2 | − 0.877 | 50.199 | 17.07 | 2.545 | 3.19 |
| 20 | 1.684 | − 1.684 | 3.367 | 1.585 | − 0.294 | 51.182 | 20.23 | 2.642 | 2.934 |
| 21 | 1.478 | − 1.478 | 2.956 | 2 | − 0.977 | 70.55 | 0 | 4.534 | 5.135 |
| 22 | 1.446 | − 1.446 | 2.892 | 2 | − 0.977 | 71.549 | 0 | 4.534 | 5.035 |
| 23 | 1.596 | − 1.596 | 3.191 | 1.585 | − 0.244 | 49.154 | 37.3 | 1.369 | 1.274 |
| 24 | 1.612 | − 1.612 | 3.223 | 1 | − 0.244 | 48.278 | 37.3 | 1.477 | 1.313 |
| 25 | 1.06 | − 1.06 | 2.12 | 2.807 | − 0.257 | 41.943 | 20.23 | 2.51 | 2.757 |
| 26 | 1.157 | − 1.157 | 2.313 | 0 | − 0.294 | 45.314 | 20.23 | 2.502 | 1.975 |
| 27 | 1.232 | − 1.232 | 2.463 | 1 | − 0.294 | 47.222 | 20.23 | 2.357 | 2.583 |
| 28 | 1.286 | − 1.286 | 2.571 | 1 | − 0.877 | 46.52 | 17.07 | 2.357 | 2.597 |
| 29 | 1.344 | − 1.344 | 2.688 | 1.585 | − 0.325 | 54.812 | 20.23 | 2.933 | 3.105 |
| 30 | 1.438 | − 1.438 | 2.877 | 1.585 | − 0.96 | 46.48 | 0 | 3.267 | 3.503 |
| 31 | 1.882 | − 1.882 | 3.763 | 1.585 | − 0.294 | 50.206 | 20.23 | 2.642 | 2.735 |
| 32 | 1.772 | − 1.772 | 3.545 | 0 | − 0.294 | 47.445 | 20.23 | 2.502 | 2.779 |
| 33 | 1.288 | − 1.288 | 2.575 | 1 | − 0.877 | 46.52 | 17.07 | 2.357 | 2.597 |
| 34 | 1.526 | − 1.526 | 3.053 | 2 | − 0.96 | 48.379 | 0 | 3.562 | 3.688 |
| 35 | 1.27 | − 1.27 | 2.54 | 1 | − 0.294 | 47.222 | 20.23 | 2.357 | 2.583 |
| 36 | 1.231 | − 1.231 | 2.461 | 2 | − 0.877 | 47.272 | 17.07 | 2.153 | 2.668 |
| 37 | 0.89 | − 0.89 | 1.78 | 1.585 | − 0.96 | 47.553 | 0 | 3.267 | 3.449 |
| 38 | 1.398 | − 1.398 | 2.796 | 1 | − 0.96 | 44.722 | 0 | 3.374 | 2.873 |
| 39 | 0.933 | − 0.933 | 1.866 | 1 | − 0.96 | 43.65 | 0 | 3.374 | 2.927 |
| 40 | 1.224 | − 1.224 | 2.448 | 1.585 | − 0.877 | 47.129 | 17.07 | 2.25 | 2.752 |
| 41 | 1.236 | − 1.236 | 2.472 | 1.585 | − 0.807 | 46.637 | 29.6 | 1.369 | 1.824 |
| 42 | 1.47 | − 1.47 | 2.941 | 1 | − 0.96 | 43.65 | 0 | 3.374 | 2.927 |
| 43 | 1.386 | − 1.386 | 2.772 | 1.585 | − 0.96 | 47.553 | 0 | 3.267 | 3.449 |
| 44 | 0.892 | − 0.892 | 1.784 | 1.585 | − 0.96 | 47.553 | 0 | 3.267 | 3.449 |
| 45 | 1.257 | − 1.257 | 2.515 | 1 | − 0.294 | 48.307 | 20.23 | 2.357 | 2.55 |
| 46 | 1.247 | − 1.247 | 2.494 | 1 | − 0.294 | 48.461 | 20.23 | 2.357 | 2.415 |
| 47 | 1.288 | − 1.288 | 2.577 | 1 | − 0.294 | 47.388 | 20.23 | 2.357 | 2.469 |
| 48 | 1.195 | − 1.195 | 2.39 | 1 | − 0.294 | 48.194 | 20.23 | 2.357 | 2.61 |
| 49 | 1.369 | − 1.369 | 2.738 | 1.585 | − 0.96 | 47.286 | 0 | 3.267 | 3.643 |
| 50 | 1.523 | − 1.523 | 3.045 | 2.807 | − 0.294 | 46.984 | 20.23 | 2.813 | 3.243 |
Qpos, total positive charge; Qneg, total negative charge; Qtot, total absolute charge (electronic charge index-ECI); Ui, unsaturation index; Hy, hydrophilic factor; AMR, Ghose–Crippen molar refractivity; TPSA, topological polar surface area; MlogP, Moriguchi octanol–water partition coeff.; AlogP, Ghose–Crippen octanol–water partition coeff
The quantum-chemical parameters, such as: chemical hardness (η), dipole moment (m) and energy of the HOMO orbital (EHOMO), were considered as descriptors directly related to biological activity by the models. These descriptors, related to chemical reactivity, are derived from the information provided by molecular orbitals. Some authors have suggested that the presence of a free hydroxyl group and a delocalized electron system in terpenes are critical for their antibacterial activity [62]. This proposal is important when the chemical reactivity of carvacrol and thymol with respect to carvomenthol and menthol is compared. Phenolic group reduces the energy values of the frontier orbitals, whereas the hydroxyl groups by itself increase the η, making carvomenthol and menthol less reactive and also less active. However, η or chemical softness (S) cannot be determinants of biological activity, since p-cymene presents these values closer to thymol and carvacrol and yet has less activity than menthol and carvomenthol. Thus, the hydroxyl group alone is also important in the larvicidal activity, a factor considered in the QSAR models. The values of the chemical reactivity descriptors are shown in Table 6.
Table 6.
Chemical reactivity descriptors calculated
| Mol. | E HOMO | E LUMO | GAP E | I | A | χ | µ | n | σ | m |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − 8.954 | 2.096 | 11.050 | 8.954 | − 2.096 | − 3.429 | 3.429 | 5.525 | 0.181 | 5.429 |
| 2 | − 10.343 | 3.969 | 14.312 | 10.343 | − 3.969 | − 3.187 | 3.187 | 7.156 | 0.140 | 3.927 |
| 3 | − 8.971 | 4.13 | 13.102 | 8.973 | − 4.13 | − 2.421 | 2.421 | 6.551 | 0.153 | 0.178 |
| 4 | − 8.351 | 4.112 | 12.463 | 8.351 | − 4.112 | − 2.119 | 2.119 | 6.232 | 0.16 | 1.672 |
| 5 | − 9.356 | 4.94 | 14.296 | 9.356 | − 4.94 | − 2.207 | 2.207 | 7.148 | 0.139 | 1.96 |
| 6 | − 10.751 | 6.545 | 17.296 | 10.751 | − 6.545 | − 2.102 | 2.102 | 8.648 | 0.115 | 1.751 |
| 7 | − 9.308 | 2.797 | 12.106 | 9.308 | − 2.797 | − 3.255 | 3.255 | 6.053 | 0.165 | 3.989 |
| 8 | − 8.945 | 5.270 | 14.215 | 8.945 | − 5.270 | − 1.837 | 1.837 | 7.108 | 0.140 | 1.864 |
| 9 | − 8.646 | 4.517 | 13.162 | 8.646 | − 4.517 | − 2.064 | 2.064 | 6.581 | 0.152 | 0.711 |
| 10 | − 9.080 | 4.409 | 13.490 | 9.080 | − 4.409 | − 2.335 | 2.335 | 6.745 | 0.148 | 2.873 |
| 11 | − 8.808 | 4.832 | 13.641 | 8.808 | − 4.832 | − 1.988 | 1.988 | 6.820 | 0.147 | 2.137 |
| 12 | − 8.545 | 3.951 | 12.496 | 8.545 | − 3.951 | − 2.297 | 2.297 | 6.248 | 0.160 | 1.368 |
| 13 | − 8.481 | 4.075 | 12.555 | 8.481 | − 4.075 | − 2.202 | 2.202 | 6.277 | 0.159 | 1.921 |
| 14 | − 9.180 | 1.997 | 11.177 | 9.180 | − 1.997 | − 3.591 | 3.591 | 5.588 | 0.179 | 4.237 |
| 15 | − 8.542 | 4.17 | 12.712 | 8.542 | − 4.17 | − 2.186 | 2.186 | 6.356 | 0.157 | 0.054 |
| 16 | − 9.491 | 3.82 | 13.311 | 9.491 | − 3.82 | − 2.835 | 2.835 | 6.656 | 0.15 | 3.599 |
| 17 | − 8.432 | 4.303 | 12.735 | 8.432 | − 4.303 | − 2.064 | 2.064 | 6.367 | 0.157 | 0.328 |
| 18 | − 10.288 | 5.493 | 15.782 | 10.288 | − 5.493 | − 2.397 | 2.397 | 7.891 | 0.127 | 1.727 |
| 19 | − 9.05 | 2.340 | 11.390 | 9.050 | − 2.346 | − 3.360 | 3.360 | 5.701 | 0.18 | 4.732 |
| 20 | − 8.859 | 4.724 | 13.583 | 8.859 | − 4.724 | − 2.068 | 2.068 | 6.792 | 0.147 | 2.409 |
| 21 | − 8.268 | 4.476 | 12.744 | 8.268 | − 4.476 | − 1.896 | 1.896 | 6.372 | 0.157 | 0.39 |
| 22 | − 8.704 | 4.909 | 13.613 | 8.704 | − 4.909 | − 1.897 | 1.897 | 6.807 | 0.147 | 0.206 |
| 23 | − 7.752 | 6.518 | 14.271 | 7.753 | − 6.518 | − 0.617 | 0.617 | 7.136 | 0.14 | 3.777 |
| 24 | − 10.387 | 4.417 | 14.804 | 10.387 | − 4.417 | − 2.985 | 2.985 | 7.402 | 0.135 | 2.525 |
| 25 | − 8.523 | 3.985 | 12.508 | 8.523 | − 3.985 | − 2.268 | 2.268 | 6.254 | 0.159 | 1.397 |
| 26 | − 10.727 | 5.844 | 16.571 | 10.727 | − 5.844 | − 2.441 | 2.441 | 8.286 | 0.121 | 1.855 |
| 27 | − 9.46 | 5.094 | 14.554 | 9.461 | − 5.094 | − 2.183 | 2.183 | 7.278 | 0.137 | 3.754 |
| 28 | − 10.473 | 3.961 | 14.434 | 10.474 | − 3.961 | − 3.256 | 3.256 | 7.217 | 0.138 | 3.506 |
| 29 | − 8.98 | 3.88 | 12.86 | 8.98 | − 3.88 | − 2.55 | 2.55 | 6.43 | 0.16 | 2.32 |
| 30 | − 8.745 | 4.901 | 13.646 | 8.746 | − 4.901 | − 1.922 | 1.922 | 6.824 | 0.146 | 0.586 |
| 31 | − 9.082 | 4.442 | 13.524 | 9.082 | − 4.442 | − 2.320 | 2.320 | 6.762 | 0.148 | 1.361 |
| 32 | − 10.918 | 5.517 | 16.435 | 10.918 | − 5.517 | 2.7 | − 2.7 | 8.218 | 0.121 | 2.047 |
| 33 | − 10.711 | 3.419 | 14.130 | 10.712 | − 3.419 | − 3.645 | 3.645 | 7.066 | 0.141 | 3.632 |
| 34 | − 8.519 | 5.024 | 13.542 | 8.519 | − 5.024 | − 1.747 | 1.747 | 6.771 | 0.148 | 0.751 |
| 35 | − 9.605 | 4.832 | 14.437 | 9.605 | − 4.832 | − 2.386 | 2.386 | 7.219 | 0.138 | 2.324 |
| 36 | − 9.442 | 2.596 | 12.038 | 9.442 | − 2.596 | − 3.422 | 3.422 | 6.019 | 0.166 | 3.631 |
| 37 | − 7.764 | 3.901 | 11.674 | 7.764 | − 3.901 | − 1.926 | 1.926 | 5.837 | 0.171 | 0.516 |
| 38 | − 8.695 | 5.170 | 13.865 | 8.695 | − 5.170 | − 1.763 | 1.763 | 6.933 | 0.144 | 0.176 |
| 39 | − 8.695 | 5.170 | 13.865 | 8.695 | − 5.170 | − 1.763 | 1.763 | 6.933 | 0.144 | 0.164 |
| 40 | − 9.146 | 3.51 | 12.656 | 9.146 | − 3.51 | − 2.818 | 2.818 | 6.328 | 0.158 | 3.559 |
| 41 | − 9.391 | 2.798 | 12.189 | 9.392 | − 2.798 | − 3.296 | 3.296 | 6.095 | 0.164 | 3.748 |
| 42 | − 8.885 | 4.062 | 12.947 | 8.885 | − 4.062 | − 2.411 | 2.411 | 6.474 | 0.154 | 0.841 |
| 43 | − 9.001 | 3.052 | 12.053 | 9.001 | − 3.052 | − 2.975 | 2.975 | 6.027 | 0.165 | 0.802 |
| 44 | − 7.64 | 3.378 | 11.018 | 7.641 | − 3.378 | − 2.131 | 2.131 | 5.509 | 0.181 | 0.648 |
| 45 | − 9.355 | 5.102 | 14.457 | 9.355 | − 5.102 | − 2.126 | 2.126 | 7.229 | 0.138 | 1.841 |
| 46 | − 9.016 | 3.878 | 12.895 | 9.016 | − 3.878 | − 2.569 | 2.569 | 6.447 | 0.155 | 1.901 |
| 47 | − 9.892 | 3.097 | 12.989 | 9.892 | − 3.097 | − 3.397 | 3.397 | 6.495 | 0.153 | 1.691 |
| 48 | − 9.371 | 3.852 | 13.222 | 9.371 | − 3.852 | − 2.759 | 2.759 | 6.611 | 0.151 | 1.772 |
| 49 | − 8.475 | 4.996 | 13.471 | 8.475 | − 4.996 | − 1.739 | 1.739 | 6.735 | 0.148 | 0.198 |
| 50 | − 8.325 | 4.145 | 12.470 | 8.325 | − 4.145 | − 2.09 | 2.09 | 6.235 | 0.161 | 1.765 |
EHOMO, energy of the HOMO orbital; ELUMO, energy of the LUMO orbital; GapE, ELUMO–EHOMO; I, ionization potential; A, electron affinity; μ, chemical potential; χ, electronegativity; ƞ, Chemical hardness σ, chemical softness; m, dipole moment
A study conducted with sesquiterpenes found that the repellent activity of these compounds was related primarily to the vapor pressure (VP) and electronic properties as LUMO energies [63], so that in their models, repellent activity increased as polarizability decreased, while high LUMO energies maintained a relationship with activity. This relationship is consistent with results applied to monoterpenes and their derivatives. The HOMO orbital is used as an indicator of the highest electron density area, so that these zones exhibit a favorable region to be attacked by electrophiles [64]. Figures 4 and 5 shows the mapping of the HOMO orbitals on the most active molecules, while Additional file 1: Figure S1 shows the mapping of LUMO orbitals.
Fig. 4.

The contour plots of LUMO orbitals of the most active molecules. (1) p-Anisaldehyde, (2) Canphor, (3) 3-Carene, (4) Carvacrol, (9) β-Caryophyllene, (10) Citronellal, (11) β-Citronellol, (12) m-Cresol, (13) o-Cresol, (14) Cuminaldehyde, (15) p-Cimene, (17) 3,4-Dimethylcumene, (18) Eucalyptol, (19) Geranial, (20) Geraniol
Fig. 5.

The contour plots of LUMO orbitals of the most active molecules (conti…). (21) Germacrene-D, (22) α-Humulene, (25) 3-Isopropylphenol, 26) Isoborneol, (29) Lavandullol, (30) Limonene, (31) Linalool, (34) Myrcene, (38) α-Pinene, (41) Rotundifolone, (42) Sabinene, (43) α-Terpinene, (46) α-Terpineol, (48) γ-Terpineol, (49) Terpinolene, (50) Thymol
The models presented demonstrated that the lipophilic character as well as the electronic properties conferred by phenolic groups are important for the larvicidal activity. The models also propose topological descriptors as factors driving the activity, especially when comparing among isomers. The position of the hydroxyl in the thymol molecule favors higher values of the Balaban index (J), E-state topological parameter (TIE), centralization (CENT), variation (VAR) and radial centric information index (ICR), with respect to carvacrol, as observed in models that incorporate this descriptors. Raising J and TIE increases the biological activity and explains the difference in activities between carvacrol and thymol. Distance-based index, J [65], strongly reflects the molecular branch, based on the sum of the distances from one atom to another in the conformation of the molecule and its value depends on three-dimensional conformation [66], while TIE [67, 68] use electronic and topological organization to define the intrinsic atom state and the perturbations of this state induced by other atoms. The values calculated of topological descriptors are listed in Table 7.
Table 7.
Topological descriptors calculated
| Mol. | J | TIE | UNIP | CENT | VAR | BAC | Lop | ICR | CSI | ECC | PHI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.174 | 10.042 | 21 | 40 | 14 | 9 | 1.261 | 1.922 | 100 | 52 | 2.185 |
| 2 | 2.396 | 18.594 | 16 | 70 | 13 | 17 | 0.845 | 1.322 | 73 | 36 | 1.135 |
| 3 | 2.037 | 10.135 | 17 | 46 | 12 | 10 | 0.853 | 1.571 | 84 | 40 | 1.073 |
| 4 | 2.396 | 14.877 | 21 | 73 | 14 | 18 | 1.16 | 1.868 | 99 | 52 | 2.3 |
| 5 | 2.396 | 18.074 | 21 | 73 | 14 | 18 | 1.16 | 1.868 | 99 | 52 | 2.504 |
| 6 | 2.396 | 19.928 | 21 | 73 | 14 | 18 | 1.16 | 1.868 | 99 | 52 | 2.941 |
| 7 | 2.396 | 18.471 | 21 | 73 | 14 | 18 | 1.16 | 1.868 | 99 | 52 | 2.281 |
| 8 | 2.396 | 18.314 | 21 | 73 | 14 | 18 | 1.16 | 1.868 | 99 | 52 | 2.717 |
| 9 | 2.059 | 19.097 | 29 | 144 | 20 | 17 | 0.788 | 1.531 | 148 | 72 | 2.328 |
| 10 | 3.1 | 25.28 | 26 | 102 | 22 | 29 | 2.187 | 2.231 | 122 | 70 | 5.82 |
| 11 | 3.1 | 24.613 | 26 | 102 | 22 | 29 | 2.187 | 2.231 | 122 | 70 | 6.24 |
| 12 | 2.231 | 6.729 | 13 | 18 | 6 | 5 | 0.875 | 1 | 54 | 28 | 1.31 |
| 13 | 2.279 | 7.081 | 12 | 24 | 6 | 5 | 0.875 | 1 | 54 | 28 | 1.31 |
| 14 | 2.243 | 13.72 | 23 | 71 | 19 | 14 | 1.273 | 1.936 | 113 | 59 | 2.425 |
| 15 | 2.26 | 9.324 | 18 | 60 | 14 | 11 | 1.185 | 1.971 | 88 | 46 | 2.103 |
| 16 | 2.396 | 20.345 | 21 | 73 | 14 | 18 | 1.16 | 1.868 | 99 | 52 | 2.483 |
| 17 | 2.396 | 11.627 | 21 | 73 | 14 | 18 | 1.16 | 1.868 | 99 | 52 | 2.33 |
| 18 | 2.369 | 11.204 | 14 | 48 | 8 | 10 | 0.853 | 0.722 | 58 | 28 | 1.068 |
| 19 | 3.1 | 21.175 | 26 | 102 | 22 | 29 | 2.187 | 2.231 | 122 | 70 | 5.452 |
| 20 | 3.1 | 20.675 | 26 | 102 | 22 | 29 | 2.187 | 2.231 | 122 | 70 | 5.858 |
| 21 | 2.45 | 24.92 | 38 | 158 | 22 | 18 | 1.029 | 1.506 | 171 | 88 | 4.873 |
| 22 | 2.453 | 23.917 | 41 | 103 | 18 | 17 | 0.769 | 0.918 | 166 | 85 | 4.379 |
| 23 | 2.512 | 28.931 | 23 | 102 | 18 | 27 | 1.124 | 1.855 | 110 | 58 | 2.383 |
| 24 | 2.512 | 31.085 | 23 | 102 | 18 | 27 | 1.124 | 1.855 | 110 | 58 | 2.573 |
| 25 | 2.32 | 11.61 | 17 | 64 | 12 | 11 | 1.185 | 1.522 | 80 | 42 | 2.073 |
| 26 | 2.396 | 17.859 | 16 | 70 | 13 | 17 | 0.845 | 1.322 | 73 | 36 | 1.258 |
| 27 | 2.437 | 20.001 | 20 | 80 | 16 | 18 | 1.16 | 1.936 | 95 | 50 | 2.717 |
| 28 | 2.437 | 20.893 | 20 | 80 | 16 | 18 | 1.16 | 1.936 | 95 | 50 | 2.695 |
| 29 | 3.631 | 30.981 | 25 | 146 | 24 | 42 | 1.888 | 1.959 | 118 | 68 | 5.733 |
| 30 | 2.26 | 11.025 | 18 | 60 | 14 | 11 | 1.185 | 1.971 | 88 | 46 | 2.311 |
| 31 | 3.376 | 29.883 | 24 | 96 | 18 | 37 | 1.859 | 1.936 | 109 | 63 | 4.126 |
| 32 | 2.437 | 20.222 | 20 | 80 | 16 | 18 | 1.16 | 1.936 | 95 | 50 | 2.941 |
| 33 | 2.437 | 20.893 | 20 | 80 | 16 | 18 | 1.16 | 1.936 | 95 | 50 | 2.695 |
| 34 | 3.033 | 15.472 | 22 | 72 | 16 | 28 | 1.922 | 1.971 | 98 | 57 | 4.649 |
| 35 | 2.437 | 20.001 | 20 | 80 | 16 | 18 | 1.16 | 1.936 | 95 | 50 | 2.717 |
| 36 | 2.243 | 16.093 | 23 | 71 | 19 | 14 | 1.273 | 1.936 | 113 | 59 | 2.641 |
| 37 | 2.26 | 10.108 | 18 | 60 | 14 | 11 | 1.185 | 1.971 | 88 | 46 | 2.311 |
| 38 | 2.156 | 10.238 | 16 | 44 | 10 | 10 | 0.853 | 1 | 74 | 35 | 1.073 |
| 39 | 2.156 | 10.914 | 16 | 44 | 10 | 10 | 0.853 | 1 | 74 | 35 | 1.073 |
| 40 | 2.437 | 18.381 | 20 | 80 | 16 | 18 | 1.16 | 1.936 | 95 | 50 | 2.483 |
| 41 | 2.044 | 19.068 | 23 | 86 | 16 | 18 | 1.126 | 1.959 | 114 | 55 | 1.464 |
| 42 | 2.106 | 10.81 | 15 | 64 | 13 | 11 | 1.185 | 1.571 | 80 | 39 | 1.073 |
| 43 | 2.26 | 10.108 | 18 | 60 | 14 | 11 | 1.185 | 1.971 | 88 | 46 | 2.311 |
| 44 | 2.26 | 9.965 | 18 | 60 | 14 | 11 | 1.185 | 1.971 | 88 | 46 | 2.311 |
| 45 | 2.481 | 20.27 | 19 | 87 | 18 | 18 | 1.16 | 1.981 | 97 | 51 | 2.382 |
| 46 | 2.394 | 19.231 | 20 | 84 | 18 | 18 | 1.16 | 1.936 | 99 | 52 | 2.382 |
| 47 | 2.362 | 20.311 | 22 | 66 | 14 | 18 | 1.16 | 1.936 | 99 | 52 | 2.382 |
| 48 | 2.362 | 18.58 | 22 | 66 | 14 | 18 | 1.16 | 1.936 | 99 | 52 | 2.382 |
| 49 | 2.26 | 9.616 | 18 | 60 | 14 | 11 | 1.185 | 1.971 | 88 | 46 | 2.311 |
| 50 | 2.437 | 15.124 | 20 | 80 | 16 | 18 | 1.16 | 1.936 | 95 | 50 | 2.3 |
J, Balaban-like index; TIE, E-state topological parameter; UNIP, unipolarity; CENT, centralization; VAR, variation; BAC, Balaban centric index; LOP, lopping centric index; ICR, radial centric; CSI, eccentric connectivity index; ECC, eccentricity; PHI, Kier flexibility index
Docking studies on sterol carrier protein-2 (SCP-2)
The mechanism of action of the larvicidal and repellent activity exerted by EOs and their constituents is not fully described. Inhibition of the acetylcholinesterase (AChE) enzyme has been frequently proposed, a similar neurotoxic effect produced by organophosphorus and carbamate incesticides [69, 70]. Similar results have been reported when flies and cockroaches are exposed to eugenol and α-terpineol [71]. However, some authors agree that in most cases there is no relationship between inhibition of AChE and larvicidal effects of terpenes and derivatives [72, 73].
Priestley et al. proposes that EOs and their constituents act on GABA receptors, as indicated by their results when exposing Drosophila melanogaster to thymol [74]. In addition, Kumar et al. have reported that terpenes present in Calotropis gigantea have larvicidal activity due to the ability to block the sterol carrier protein (AeSCP-2) [75], which is partially responsible for intracellular cholesterol transport in insects [76]. The larvaes during the feeding step contain high concentrations of SCP-2 because they depend on exogenous sources of cholesterol for biosynthesis of steroid derivatives [77]. Therefore, compounds that can inhibit this protein have a high potential as vector control agents.
With the purpose of estimating the interactions (theoretical affinity) of the evaluated compounds on sterol carrier protein (SCP-2) a docking study was carried out. The crystal structure of AeSCP-2 (Aedes agypti Sterol Carrier Protein ID-PBD: 1PZ4) was used for docking studies and to build its homologous enzyme from Culex quinquefasciatus. The SCP-2 sequence of Culex quinquefasciatus reported in the NCBI (GenBank: AA043438.1) presented a percentage of identity of 99.09% with AeSCP-2. Figure 6 shows the tridimensional (3D) model of SCP-2 and the corresponding Ramachandran plot used for evaluation. The analysis of the free energy values of the molecular interaction between the terpenes on SCP-2 enzyme showed that all the compounds bind strongly inside the active site with a similar binding mode; binding energies (ΔG) for each molecule are shown in Table 8.
Fig. 6.

Results of the construction homology of the sterol carrier protein (SCP-2). a Model of sterol carrier protein (SCP-2). b Ramachandran plot corresponding for the model of SCP-2
Table 8.
Docking results by SCP-2 from Culex quinquefasciatus
| Molecules | ΔG (kcal) | Interaction with amino acids | |
|---|---|---|---|
| 1 | p-Anisaldehyde | − 5.72 | N23, R24, Q25, V26, L102, F105 |
| 2 | Canphor | − 5.86 | L16, Q25,V26 |
| 3 | 3-Carene | − 6.17 | I19, N23, R24, Q25, V26 |
| 4 | Carvacrol | − 6.88 | I19, R24, Q25, V26, L48, L102, F105 |
| 5 | Carveol | − 5.22 | R24, Q25, V26, L102, F105 |
| 6 | Carvomenthol | − 5.69 | I19, R24, Q25, V26, Q25 |
| 7 | (+)-Carvone | − 6.62 | R15, I19, D20, R24, N23, Q25, V26 |
| 8 | Carvotanacetol | − 5.32 | I19, R24, Q25, V26, Q25 |
| 9 | β-Caryophyllene | − 7.87 | R15, L16, I19, V26, L48, L102, F105 |
| 10 | Citronellal | − 4.16 | I19, D20, N23, R24, Q25, V26 |
| 11 | β-Citronellol | − 5.29 | I19, D20, N23, R24, Q25, V26, L48 |
| 12 | m-Cresol | − 6.26 | I19, R24*, Q25*, F105 |
| 13 | o-Cresol | − 6.11 | I19, R24, Q25, F105 |
| 14 | Cuminaldehyde | − 5.72 | N23, R24, Q25, V26, L48 |
| 15 | p-Cymene | − 5.28 | D20, N23, R24, Q25, V26 |
| 16 | t-Dihydrocarvone | − 5.97 | R15*, I19, D20, R24, N23, Q25, V26, F105 |
| 17 | 3,4-Dimethylcumene | − 5.22 | D20, N23, R24, Q25, V26 |
| 18 | Eucalyptol | − 5.03 | R15, L16, L102 |
| 19 | Geranial | − 5.96 | I19, D20, N23, R24, Q25, V26 |
| 20 | Geraniol | − 5.96 | I19, D20, N23, R24, Q25, V26, L48, L102, F105 |
| 21 | Germacrene-D | − 7.65 | R15, L16, I19, V26, L48, L102, F105 |
| 22 | α-Humulene | − 7.87 | R15, L16, I19, V26, L48, L102 |
| 23 | Hydrocarvone | − 5.72 | I19, D20, R24, N23, Q25, V26 |
| 24 | Hydrodihydrocarvone | − 5.81 | R15, I19, D20, R24, N23, Q25, V26 |
| 25 | 3-Isopropylphenol | − 5.22 | D20, N23, R24, Q25, V26 |
| 26 | Isoborneol | − 5.21 | R15, L16, L102 |
| 27 | Isopulegol | − 6.26 | I19, D20, R24, N23, Q25, V26, L48, L102 |
| 28 | t-Isopulegone | − 6.44 | R15*, I19, D20, R24, N23, Q25, V26, L48 |
| 29 | Lavandullol | − 4.72 | D20, N23, R24, Q25 |
| 30 | Limonene | − 5.81 | I19, N23, R24, Q25, V26, L48, L102 |
| 31 | Linalool | − 5.76 | I19, D20, N23, R24, Q25, V26 |
| 32 | Menthol | − 5.69 | I19, R24, Q25, V26, Q25 |
| 33 | Menthone | − 5.51 | R15, I19, R24, N23, Q25, V26 |
| 34 | Myrcene | − 6.05 | I19, N23, R24, Q25, L102, F105 |
| 35 | Neoisopulegol | − 6.34 | I19, N23, R24, Q25, V26 |
| 36 | (− )-Perillaldehyde | − 5.95 | R15, L16, I19, N23, R24, Q25, V26 |
| 37 | Phellandrene | − 5.1 | D20, N23, R24, Q25, V26 |
| 38 | α-Pinene | − 5.85 | R15, I19, N23, R24, Q25, V26, L48 |
| 39 | β-Pinene | − 5.96 | R15, I19, N23, R24, Q25, V26, L48 |
| 40 | (+)-Pulegone | − 6.51 | R15, I19, N23, R24, Q25, V26 |
| 41 | Rotundifolone | − 6.33 | I19, R24, Q25, V26, L48 |
| 42 | Sabinene | − 5.5 | I19, N23, R24,Q25, V26 |
| 43 | α-Terpinene | − 6.76 | I19, N23, R24, V26, L48 |
| 44 | γ-Terpinene | − 6.85 | I19, N23, R24, Q25, V26, L48 |
| 45 | 4-Terpineol | − 5.77 | R15, I19, R24, V26, L102 |
| 46 | α-Terpineol | − 5.46 | I19, R24, V26, L102 |
| 47 | β-Terpineol | − 5.13 | I19, D20, R24, N23, Q25, V26 |
| 48 | γ-Terpineol | − 5.14 | I19, D20, R24, N23, Q25, V26 |
| 49 | Terpinolene | − 6.01 | I19, R24, V26, L48, L102, F105 |
| 50 | Thymol | − 6.66 | I19, D20, N23, R24, Q25, V26, L48 |
* Hydrogen bonds interaction
Results showed that monoterpenes and monoterpenoids with the highest larvicidal activity were also the compounds with better binding energy values, being carvacrol the most active followed by α-terpinene and terpinolene. Another important observation is that monoterpenes and monoterpenoides with the highest larvicidal activity are capable of interact with the Phe105 residue.
All cyclic terpenes and cyclic terpenoids interact with Arg24 and Val26 by hydrophobic interactions; only terpinene, terpinolene and carvacrol have interaction with the Phe105 residue. In these compounds, the greater number of π conjugated bonds, provides better interaction with SCP-2 (Fig. 7a). Carvone interacts to a lesser extent than limonene with the SCP-2 protein, since the keto group present in carvone makes the molecule more hydrophilic and therefore does not interact with and Leu48 residues Leu102, as does limonene (Fig. 7b). Results agree with QSAR descriptors related to their poor biological activity.
Fig. 7.

Interaction of cyclic terpenes and terpenoids with SCP-2. a Interaction of carvacrol (green), α-Terpinene (orange) and terpinolene (yellow). b Interaction of limonene (brown) and carvona (green). c Interaction of p-cimene (yellow), menthol (pink) and thymol (purple)
The relevance of the phenol group is observed when the binding energies of cymene, menthol, thymol and carvacrol are compared. Cymene binding energy is − 5.28 kcal, while menthol is − 5.69 kcal, this energy difference can be attributed to the hydroxyl group; on the other hand, thymol has a binding energy of − 6.66 kcal, which shows that the phenolic group is also important. This characteristics are also observed when comparing the bonding energy of carvomenthol and carvacrol. These results are consistent with the QSAR models also included in this work. The structural difference between the aromatic ring present in thymol and menthol without π bonds, generates a change in the arrangement of the later in the SCP-2 protein active site. It can be observed that the larger aliphatic chain in para position of cymene and thymol is in the direction of Phe105 residue, but does not interact with it, while the menthol is in the opposite position; however the hydroxyl group is kept in the same coordinates as for thymol (Fig. 7c). This is because the hydroxyl group of thymol and menthol are capable of forming hydrogen bonds with the amino group of Arg24 residue.
The position of the hydroxyl group in the phenolic group is also relevant. The hydroxyl group in the meta position of carvacrol leaves more exposed to larger aliphatic chain, which interacts with the Phe105 residue; the results is an increased biological activity as well as a more favorable binding energy as compared to thymol. The hydroxyl group of carvacrol can form hydrogen bonds with the amino group in Arg24 and with the amino group of the peptide bond between Gln25 and Val26 residues. The isopropyl group, on the other hand, also plays a fundamental role in the recognition of monoterpenes; for example, m-cresol and o-cresol, does not have the isopropyl residue and have no affinity on the SCP-2. This observation also agrees with the QSAR models, which propose that nCt are important in biological activity.
The results on acyclic terpenes denote the importance of π bonds despite not being aromatic moieties. Citronellol, the molecule with lower number of π bonds, is an acyclic terpene less able to interact with SCP-2 and is also the molecule with lower larvicidal activity. On the other hand, myrcene has the highest number of π bonds, presented the highest larvicidal activity and is also the best to interact with SCP-2. Geraniol and myrcene are the acyclic terpenes with the higher larvicidal activity and both interact with the Phe105 residue (Additional file 1: Figure S2). All acyclic terpenes, except those with ketone groups, are capable of interact with residues Ile19, Asn23, Arg24 and Gln25. Geraniol has the ability to form a hydrogen bond with the amino group of the backbone between the Ile19 and Asn20.
Anisaldehyde presented a binding energy of − 5.72 kcal/mol and was able to interact with the Phe105 residue and form a hydrogen bond with the amino group of Arg24 (Additional file 1: Figure S3a). The cuminaldehyde does not interact with the Phe105 residue and was not able to form hydrogen bonds. Sesquiterpenes presented the highest affinity on the SCP-2 active site, presented interactions with the Phe105 residue and with the hydrophobic pocket (Additional file 1: Figure S3b).
Conclusions
The larvicidal activity of terpenes and terpenoids was analyzed by LC50 determination for different stairs of Culex quinquefasciatus Say. The description of the molecular properties and the structural characteristics responsible for larvicidal activity of the tested compounds, were used for the development of mathematical models of structure–property–activity relationship. The docking studies were able to show that molecular and structural descriptors provide evidence of SCP-2 as a possible biological target, an important protein in cholesterol and fatty acid catabolism, which cleaves the 3-oxoacyl-CoAs of methyl-branched fatty acid and bile acid intermediates. However experimental studies should be conducted to elucidate this effect.
Additional file
Additional file 1. Additional tables and figures.
Authors’ contributions
BNT and LEST coordinated the larvicidal bioassay. SAO developed the larvicidal bioassays. LMRV coordinated the first electronic structure calculations and advised SAO on the analysis of the results and development of the QSAR model. SAO and JCB developed the docking studies. JCB and LEST revised the first draft. GVNM and SAO wrote the manuscript. GVNM conceived the study and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgements
SAO wishes to acknowledge the support of Eric Contreras-Suarez, Manuel Villanueva-García and Alejandro D. Camacho during the experimental development of this work.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data is available from the authors by request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
SAO which to thank the Consejo Nacional de Ciencia y Tecnología for his graduate studies scholarship (Fellowship No. 278488).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13065-018-0425-2) contains supplementary material, which is available to authorized users.
Contributor Information
S. Andrade-Ochoa, Email: s_andrade_rat@hotmail.com
J. Correa-Basurto, Email: corrjose@gmail.com
L. M. Rodríguez-Valdez, Email: lmrodrig@uach.mx
L. E. Sánchez-Torres, Email: luviasanchez@hotmail.com
B. Nogueda-Torres, Email: bnogueda@yahoo.com
G. V. Nevárez-Moorillón, Phone: +526142366000, Email: vnevare@uach.mx
References
- 1.Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 2.Raghavendra K, Barik TK, Bhatt RM, Srivastava HC, Sreehari U, Dash AP. Evaluation of the pyrrole insecticide chlorfenapyr for the control of Culex quinquefasciatus Say. Acta Trop. 2011;118:50–55. doi: 10.1016/j.actatropica.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu ZL, Liu QZ, Du SS, Deng ZW. Mosquito larvicidal activity of alkaloids and limonoids derived from Evodia rutaecarpa unripe fruits against Aedes albopictus (Diptera: Culicidae) Parasitol Res. 2012;111:991–996. doi: 10.1007/s00436-012-2923-9. [DOI] [PubMed] [Google Scholar]
- 4.Ocampo CB, Salazar-Terreros MJ, Mina NJ, McAllister J, Brogdon W. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Trop. 2011;118:37–44. doi: 10.1016/j.actatropica.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Regnault-Roger C, Vincent C, Arnason JT. Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol. 2012;57:405–424. doi: 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- 6.Miranda JEM, Navickiene HMD, Nogueira-Couto RH, De Bartolo S, Kato MJ, Bolzani VS, Furlan M. Susceptibility of Apis mellifera (Hymenoptera: Apidae) to pellitorine, an amide isolated from Piper tuberculatum (Piperaceae) Apidologie. 2003;34:409–415. doi: 10.1051/apido:2003036. [DOI] [Google Scholar]
- 7.Lin CY, Wu DC, Yu JZ, Chen BH, Wang CL, Ko WH. Control of silverleaf whitefly, cotton aphid and kanzawa spider mite with oil and extracts from seeds of sugar apple. Neotrop Entomol. 2009;38:531–536. doi: 10.1590/S1519-566X2009000400016. [DOI] [PubMed] [Google Scholar]
- 8.Pushpanathan T, Jebanesan A, Govindarajan M. Larvicidal, ovicidal and repellent activities of Cymbopogan citratus Stapf (Graminae) essential oil against the filarial mosquito Culex quinquefasciatus (Say) (Diptera:Culicidae) Trop Biomed. 2006;23:208–212. [PubMed] [Google Scholar]
- 9.Pitasawat B, Champakaew D, Choochote W, Jitpakdi A, Chaithong U, Kanjanapothi D, Chaiyasit D. Aromatic plant-derived essential oil: an alternative larvicide for mosquito control. Fitoterapia. 2007;78(3):205–210. doi: 10.1016/j.fitote.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Michaelakis A, Papachristos D, Kimbaris A, Koliopoulos G, Giatropoulos A, Polissiou MG. Citrus essential oils and four enantiomeric pinenes against Cx. pipiens (Diptera: Culicidae) Parasitol Res. 2009;105(3):769–773. doi: 10.1007/s00436-009-1452-7. [DOI] [PubMed] [Google Scholar]
- 11.Rahuman AA, Bagavan A, Kamaraj C, Saravanan E, Zahir AA, Elango G. Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol Res. 2009;104:1365–1413. doi: 10.1007/s00436-009-1337-9. [DOI] [PubMed] [Google Scholar]
- 12.Isman MB. Botanical insecticides, deterrents, and repellents inmodern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 13.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Celikel N, Kavas G. Antimicrobial properties of some essential oils against some pathogenic microorganisms. Czech J Food Sci. 2008;26:174–181. doi: 10.17221/1603-CJFS. [DOI] [Google Scholar]
- 15.Rasooli I, Gachkar L, Yadegari D, Bagher-Rezaei M, Taghizadeh M, Alipoor-Astaneh S. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007;102:898–904. doi: 10.1016/j.foodchem.2006.06.035. [DOI] [Google Scholar]
- 16.Poroikov VV, Filimonov DA, Ihlenfeldt WD, Gloriozova TA, Lagunin AA, Borodina YV, Nicklaus MC. PASS biological activity spectrum predictions in the enhanced open NCI database browser. J Chem Inform Comput Sci. 2003;43(1):228–236. doi: 10.1021/ci020048r. [DOI] [PubMed] [Google Scholar]
- 17.Geronikaki AA, Dearden JC, Filimonov D, Galaeva I, Garibova TL, Gloriozova T, Vlad L. Design of new cognition enhancers: from computer prediction to synthesis and biological evaluation. J Med Chem. 2004;47(11):2870–2876. doi: 10.1021/jm031086k. [DOI] [PubMed] [Google Scholar]
- 18.Subramaniam R, Rao G. 2D QSAR studies of some novel quinazolinone derivatives as antitubercular agents. J Comput Method Mol Design. 2011;3:69–82. [Google Scholar]
- 19.Yuriev E, Agostino M, Ramsland PA. Challenges and advances in computational docking: 2009 in review. J Mol Recognit. 2011;24(2):149–164. doi: 10.1002/jmr.1077. [DOI] [PubMed] [Google Scholar]
- 20.Kumalo HM, Bhakat S, Soliman ME. Theory and applications of covalent docking in drug discovery: merits and pitfalls. Molecules. 2015;20(2):1984–2000. doi: 10.3390/molecules20021984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harwood RF, James MT. Entomología médica y veterinaria. México: LIMUSA; 1987. pp. p201–p203. [Google Scholar]
- 22.Andrade-Ochoa S, Sánchez-Aldana D, Chacón-Vargas KF, Rivera-Chavira BE, Sánchez-Torres LE, Camacho AD, Nogueda-Torres B, Nevárez-Moorillón GV. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): synergism–antagonism effects. Insects. 2018;9(1):25. doi: 10.3390/insects9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO (2007) WHO/CDS/WHOPES/GCDPP/2005.13. Guidelines for laboratory and field testing of mosquito larvicides. Accessed 7 Feb 2018
- 24.Ajaiyeoba EO, Sama W, Essien EE, Olayemi JO, Ekundayo O, Walker TM, Setzer WN. Larvicidal activity of turmerone-rich essential oils of Curcuma longa. Leaf and rhizome from Nigeria on Anopheles gambiae. Pharm Biol. 2008;46(4):279–282. doi: 10.1080/13880200701741138. [DOI] [Google Scholar]
- 25.Deppmeier BJ, Driessen AJ, Hehre TS, Hehre WJ, Johnson JA, Klunzinger PE, Jianguo Y. Spartan ‘08, build 132. Irvine: Wavefunction Inc.; 2009. [Google Scholar]
- 26.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Ukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Anayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 09, revision A02. Wallingford CT: Gaussian Inc.; 2009. [Google Scholar]
- 27.Mayo SL, Olafson BD, Goddard WA. DREIDING: a generic force field for molecular simulations. J Phys Chem. 1990;94(26):8897–8909. doi: 10.1021/j100389a010. [DOI] [Google Scholar]
- 28.Sobolewski AL, Domcke W. Ab initio study of the energetic of photoinduced electron and proton transfer processes in a bioinspired model of photochemical water splitting. Chem Phys Lett. 2009;479:144–148. doi: 10.1016/j.cplett.2009.07.098. [DOI] [Google Scholar]
- 29.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98(7):5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- 30.Young DC. Computational chemistry: a practical guide for applying techniques to real-world problems. New York: Wiley; 2001. [Google Scholar]
- 31.Cramer CJ. Essentials of computational chemistry: theories and models. Chichester: Wiley; 2004. [Google Scholar]
- 32.Schafer A, Huber C, Ahlrichs R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys. 1994;100(8):5829–5835. doi: 10.1063/1.467146. [DOI] [Google Scholar]
- 33.Koopmans T. Über die zuordnungwellenfunktionen von und zu den eigenwerteneinzelnen elektronenátomoseines. Physica. 1934;1(1):104–113. doi: 10.1016/S0031-8914(34)90011-2. [DOI] [Google Scholar]
- 34.Miertus S, Scrocco E, Tomasi J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem Phys. 1981;55:117–129. doi: 10.1016/0301-0104(81)85090-2. [DOI] [Google Scholar]
- 35.Talete srl (2006) Dragon for Windows (software for Molecular Descriptor Calculations) Version 5.4. http://www.talete.mi.it/
- 36.Todeschini R, Consonni V, Mauri A, Pavan M. MobyDigs software for regression and classification models by genetic algorithms. Data Handling Sci Technol. 2003;23:141–167. doi: 10.1016/S0922-3487(03)23005-7. [DOI] [Google Scholar]
- 37.Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30(S1):S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 38.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyer DH, Lovell S, Thoden JB, Holden HM, Rayment I, Lan Q. The structural determination of an insect sterol carrier protein-2 with a ligand-bound C16 fatty acid at 1.35-Å resolution. J Biol Chem. 2003;278(40):39085–39091. doi: 10.1074/jbc.M306214200. [DOI] [PubMed] [Google Scholar]
- 40.Lovell C, Davis IW, Arendall WB, de Bakker PIW, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Protein Struct Funct Genet. 2002;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 41.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;16:2785e2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerwin SM. ChemBioOffice ultra 2010 suite. J Am Chem Soc. 2010;132(7):2466–2467. doi: 10.1021/ja1005306. [DOI] [PubMed] [Google Scholar]
- 43.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 44.Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 45.Ávila L, Baquero E, Viña A, Murrillo E. Actividad antibacteriana de Diplostephium tolimense cuatrec (Asteraceae) frente a Staphylococcus aureus. Vitae. 2006;3(1):55–60. [Google Scholar]
- 46.Calsamiglia S, Busquet M, Cardozo PW, Castillejos L, Ferret A, Fandino I. The use of essential oils in ruminants as modifiers of rumen microbial fermentation. Structure. 2007;100:5. doi: 10.3168/jds.2006-644. [DOI] [PubMed] [Google Scholar]
- 47.Koroch AR, Juliani HR, Zygadlo JA. Bioactivity of essential oils and their components. In: Flavours and fragrances. Berlin: Springer; 2007. pp. p87–p115. [Google Scholar]
- 48.Cheng SS, Chang HT, Chang ST, Tsai KH, Chen WJ. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour Technol. 2003;89:99–102. doi: 10.1016/S0960-8524(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 49.Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour Technol. 2005;96(16):1749–1757. doi: 10.1016/j.biortech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Tiwary M, Naik SN, Tewary DK, Mittal PK, Yadav S. Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. J Vector Dis. 2007;44(3):198. [PubMed] [Google Scholar]
- 51.Tunc I, Berger BM, Erler F, Dagli F. Ovicidal activity of essential oils from five plants against two stored-products insects. J Stored Prod Res. 2000;36:161–168. doi: 10.1016/S0022-474X(99)00036-3. [DOI] [Google Scholar]
- 52.Erler F, Tunc I. Monoterpenoids as fumigants against greenhouse pests: toxic, development and reproduction inhibiting effects. Pflanzenkr Pflanzenschutz. 2005;112:181–192. [Google Scholar]
- 53.Erler F. Fumigant activity of six monoterpenoids from aromatic plants in Turkey against the two stored-product insects confused flour beetle, Tribolium confusum, and Mediterranean flour moth, Ephestia kuehniella. Pflanzenkr Pflanzenschutz. 2005;112:602–611. [Google Scholar]
- 54.Dória GA, Silva WJ, Carvalho GA, Alves PB, Cavalcanti SC. A study of the larvicidal activity of two Croton species from northeastern Brazil against Aedes aegypti. Pharm Biol. 2010;48(6):615–620. doi: 10.3109/13880200903222952. [DOI] [PubMed] [Google Scholar]
- 55.Lucia A, Zerba E, Masuh H. Knockdown and larvicidal activity of six monoterpenes against Aedes aegypti (Diptera: Culicidae) and their structure–activity relationships. Parasitol Res. 2013;112(12):4267–4272. doi: 10.1007/s00436-013-3618-6. [DOI] [PubMed] [Google Scholar]
- 56.Fahmy MAH, Mllipudi NM, Roy Fukuto R. Selective toxicity of N. N’-Thiodicarbamates. J Agric Food Chem. 1978;26(3):550–557. doi: 10.1021/jf60217a021. [DOI] [PubMed] [Google Scholar]
- 57.Karelson M, Lobanov VS, Katritzky AR. Quantum-chemical descriptors in QSAR/QSPR studies. Chem Rev. 1996;96(3):1027–1044. doi: 10.1021/cr950202r. [DOI] [PubMed] [Google Scholar]
- 58.Hansch C, Steinmetz WE, Leo AJ, Mekapati SB, Kurup A, Hoekman D. On the role of polarizability in chemical–biological interactions. J Chem Inform Comput Sci. 2003;43(1):120–125. doi: 10.1021/ci020378b. [DOI] [PubMed] [Google Scholar]
- 59.Dambolena JS, Zygadlo JA, Rubinstein HR. Antifumonisin activity of natural phenolic compounds. A structure–property–activity relationship study. Int J Food Microbiol. 2011;145(1):140–146. doi: 10.1016/j.ijfoodmicro.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Hansch C, Verma RP. Larvicidal activities of some organotin compounds on mosquito larvae: a QSAR study. Eur J Med Chem. 2009;44:260–273. doi: 10.1016/j.ejmech.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 61.Eng G, Song X, Duong Q, Strickman D, Glass J, May L. Synthesis, structure characterization and insecticidal activity of some triorganotin dithiocarbamates. Appl Organomet Chem. 2003;17:218–225. doi: 10.1002/aoc.423. [DOI] [Google Scholar]
- 62.Ultee A, Bennik MHJ, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68(4):1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paluch G, Grodnitzky J, Bartholomay L, Coats J. Quantitative structure−activity relationship of botanical sesquiterpenes: spatial and contact repellency to the yellow fever mosquito, Aedes aegypti. J Agric Food Chem. 2009;57(16):7618–7625. doi: 10.1021/jf900964e. [DOI] [PubMed] [Google Scholar]
- 64.Andrade-Ochoa S, Nevárez-Moorillón GV, Sánchez-Torres LE, Villanueva-García M, Sánchez-Ramírez BE, Rodríguez-Valdez LM, Rivera-Chavira BE. Quantitative structure–activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complem Altern Med. 2015;15(1):332. doi: 10.1186/s12906-015-0858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu K, Liu M, Das KC, Gutman I, Furtula B. A survey on graphs extremal with respect to distance-based topological indices. MATCH Commun Math Comput Chem. 2014;71:461–508. [Google Scholar]
- 66.Balaban AT. Highly discriminating distance-based topological index. Chem Phys Let. 1982;89(5):399–404. doi: 10.1016/0009-2614(82)80009-2. [DOI] [Google Scholar]
- 67.Platt JR. Influence of neighbor bonds on additive bond properties in paraffins. J Chem Phys. 1947;15(6):419–420. doi: 10.1063/1.1746554. [DOI] [Google Scholar]
- 68.Schultz HP, Schultz EB, Schultz TP. Topological organic chemistry. 4. Graph theory, matrix permanents, and topological indices of alkanes. J Chem Inf Comput Sci. 1992;32(1):69–72. doi: 10.1021/ci00005a011. [DOI] [Google Scholar]
- 69.Isman M. Plant essential oil for pest and disease management. Crop Prot. 2000;19:603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- 70.Houghton PJ, Ren Y, Howes MJ. Acetylcholinesterase inhibitors from plants and fungi. Nat Prod Rep. 2006;23(2):181–199. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]
- 71.Enan E. Insecticidal activity of essential oils: octopaminergic sites of action. Comp Biochem Physiol C. 2001;130(3):325–337. doi: 10.1016/s1532-0456(01)00255-1. [DOI] [PubMed] [Google Scholar]
- 72.Seo SM, Jung CS, Kang J, Lee HR, Kim SW, Hyun J, Park IK. Larvicidal and acetylcholine esterase inhibitory activity of apiaceae plant essential oils and their constituents against Aedes albopictus, and formulation development. J Agric Food Chem. 2015;63(45):9977–9986. doi: 10.1021/acs.jafc.5b03586. [DOI] [PubMed] [Google Scholar]
- 73.Yeom HJ, Jung CS, Kang J, Kim J, Lee JH, Kim DS, Park IK. Insecticidal and acetylcholine esterase inhibition activity of Asteraceae plant essential oils and their constituents against adults of the German cockroach (Blattella germanica) J Agric Food Chem. 2015;63(8):2241–2248. doi: 10.1021/jf505927n. [DOI] [PubMed] [Google Scholar]
- 74.Priestley CM, Williamson EM, Wafford KA, Satelle DB. Thymol, a constituent of thyme essential oils, is a positive modulator of human GABA and a homo-oligosteric GABA receptor from Drosophila melanogaster. Br J Clin Pharmacol. 2003;140:1363–1372. doi: 10.1038/sj.bjp.0705542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar PS, Chezhian A, Raja PS, Sathiyapriya J. Computational selections of terpenes present in the plant Calotropis gigantea as mosquito larvicide’s by blocking the sterol carrying protein, AeSCP-2. Bangladesh J Pharmacol. 2012;7(1):1–5. doi: 10.3329/bjp.v7i1.10156. [DOI] [Google Scholar]
- 76.Larson R, Wessely V, Jiang Z, Lan Q. Larvicidal activity of sterol carrier protein-2 inhibitor in four species of mosquitoes. J Med Entomol. 2008;45:439–444. doi: 10.1093/jmedent/45.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kitamura T, Kobayashi S, Okada M. Regional expression of the transcript encoding sterol carrier protein x related thiolaseand its regulation by homeotic genes in the midgut of Drosophila embryos. Dev Growth Differ. 1996;38:373–381. doi: 10.1046/j.1440-169X.1996.t01-3-00005.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional tables and figures.
Data Availability Statement
Data is available from the authors by request.


