Abstract
NVP-BEZ235 is a dual phosphoinositide 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) inhibitor. A dual approach targeting more than one downstream effector is a promising strategy for treating cancers. The aim of this study was to evaluate the effect of NVP-BEZ235 in treating FaDu hypopharyngeal squamous cell carcinoma (HSCC), either alone or in combination with cisplatin. We found mTOR expression was higher in patients with HSCC. In the in vitro study, treatment with NVP-BEZ235 alone attenuated cell proliferation and suppressed p-p70S6K and p-4E-BP1 expression in FaDu cells. When NVP-BEZ235 was combined with Cisplatin, apoptosis was induced more effectively than with either drug alone. In mice with a FaDu xenograft, cotreatment with NVP-BEZ235 and Cisplatin engendered synergistic effects and produced a greater antitumor response than did treatment with either drug alone. Resected tumor samples also showed decreased p-p70S6K expression. Collectively, these data demonstrate that NVP-BEZ235 inhibits HSCC growth through phospho-p70S6K suppression and has a synergistic effect with Cisplatin in treating HSCC. The data also provide a strategy for more effective HSCC treatment.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer around the world. HNSCC affects nearly 600,000 new patients every year and its mortality rate is approximately 50%. Hypopharyngeal squamous cell carcinoma (HSCC), a less prevalent cancer arising from the mucosa of the upper aerodigestive tract, accounts for 3–5% of all HNSCC cases1. Most patients with HSCC are diagnosed only in the advanced stage. In a large series study2, approximately 70–85% of the patients were at Stage III or IV at presentation, with 5-year overall survival rates of approximately 15–45%.
Platinum combination regimens are considered the standard first-line treatment for patients with inoperable, recurrent, or metastatic HNSCC3,4. However, if cis-diamminedichloridoplatinum (CDDP)-based chemotherapy fails, these patients must receive salvage total laryngectomy. If they were still inoperable, only limited therapeutic options are available and most could receive only palliative radiation or supportive care5–7. Treatment failure is mostly due to the development of CDDP resistance, which compromises the treatment outcome and patient survival. Therefore, novel agents that can significantly enhance the effects of existing chemotherapeutic drugs with less toxicity are needed for possible organ preservation.
With progress in cancer research, targeted therapy is becoming a first- or second-line treatment option for various malignant diseases including HSCC8. Many preclinical studies have demonstrated synergistic antitumor effects when combining targeted therapy with CDDP9,10. The phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt)-mammalian target of rapamycin (mTOR) intracellular signaling pathway is influential in various physiological activities, including cellular survival, proliferation, migration and differentiation, as well as in protein synthesis, angiogenesis, and glucose metabolism. In addition, the PI3K–Akt–mTOR pathway is associated with many oncogenic processes and is one of the most frequently dysregulated signaling pathways in cancer, including hypopharyngeal cancer11. The PI3K-Akt-mTOR pathway provides unique opportunities for anticancer therapy, because it is often constitutively activated in human cancer cells. Therefore, targeting of PI3K-Akt-mTOR signaling could be a reasonable strategy in the treatment of hypopharyngeal cancer where systemic therapy is effective, especially in advanced disease. NVP-BEZ235 is an imidazo[4,5-c]quinoline derivative that inhibits PI3K and mTOR kinase activity by binding to the adenosine triphosphate-binding cleft of these enzymes5. NVP-BEZ235 is a dual PI3K-mTOR inhibitor. Because targeting more than one downstream effectors may delay or even prevent therapy resistance, the dual approach is promising12. NVP-BEZ235 exhibited antitumor activity in lung cancer13,14, cells of human glioma15,16, breast cancer17,18, melanoma19, pancreatic cancer20,21, sarcoma12,22, nasopharyngeal cancer23,24, and hepatoma25–27. NVP-BEZ235 as a PI3K-mTOR inhibitor is currently in Phase I/II clinical trials and has shown great promise in treating solid tumors in preclinical mouse models15.
In this study, we demonstrated the effect of NVP-BEZ235 on PI3K-Akt-mTOR signaling in FaDu cells both in vitro and in vivo. Furthermore, we demonstrated that Cisplatin and NVP-BEZ235 synergistically inhibited the proliferation of FaDu cell lines with an enhanced reduction of mTOR signaling and p70S6K levels. Cisplatin and NVP-BEZ235 treatment also synergistically inhibited FaDu cells in vivo by exhibiting a significant reduction in tumor burden and p-p70S6K expression after treatment.
Results
Analysis of mTOR expression in patients with HSCC by using real-time quantitative reverse transcriptase-polymerase chain reaction
Cancerous and noncancerous tissues from 12 patients with HSCC were examined for the expression of mTOR by using real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) in order to elucidate whether the expression levels of mTOR were altered in cancerous tissues. Our data revealed that the mTOR expression levels were significantly upregulated in hypopharyngeal cancer (p = 0.030). Specifically, mTOR levels in advanced hypopharyngeal cancerous tissues showed a 5.15-fold increase compared with those in early hypopharyngeal cancerous tissues (p = 0.020) (Fig. 1).
Fig. 1. mTOR expression in HSCC.
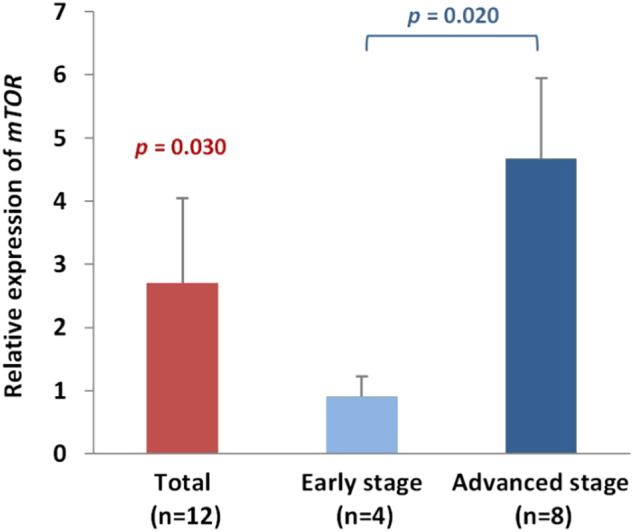
mTOR expression was upregulated in cancerous tissues of HSCC (p = 0.030) and was higher in advanced hypopharyngeal cancerous tissues compared with early hypopharyngeal cancerous tissues (p = 0.020). The y-axis represents the fold change in the mTOR expression level of cancerous tissues relative to noncancerous parts. The relative expression level in cancerous tissues was calculated by the comparative Ct (ΔΔCt method). The mean mTOR expression level in noncancerous parts was assigned a value of 1, whereas the mTOR expression level in cancerous tissues was calibrated to obtain the fold change in cancerous tissues
NVP-BEZ235 downregulated PI3K-Akt-mTOR signaling pathway in FaDu HSCC cells: suppression of p-mTOR and p-p70S6K
We investigated whether NVP-BEZ235 could inhibit the phosphorylation of downstream targets of the mTOR pathway. In NVP-BEZ235-treated FaDu cells, mTOR, p70S6K, and 4E-BP1 phosphorylation was effectively reduced within 30 min, but the corresponding total proteins were not affected (Fig. 2). The phospho-p70S6K level was completely suppressed for 3 days; the phospho-mTOR (p-mTOR) and phospho-4E-BP1 levels were suppressed for 1 day.
Fig. 2. NVP-BEZ235 inhibited downstream targets of mTOR pathway.
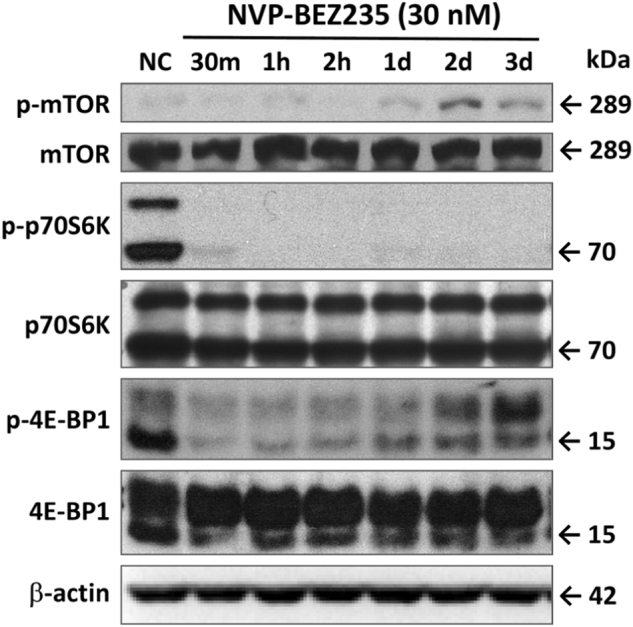
FaDu HSCC cells were cultured in six-well plates and treated with 30 nM NVP-BEZ235 for 30 min (30 m), 1 h (1h), 2 h (2h), 1 day (1d), 2 days (2d), and 3 days (3d). Protein extracts of cells were analyzed by Western blotting with antibodies against p-mTOR, mTOR, phospho-p70S6K (p-p70S6K), p70S6K, phospho-4E-BP1 (p-4E-BP1), 4E-PB1, and β-actin
NVP-BEZ235 and Cisplatin combination synergistically inhibited proliferation of FaDu HSCC cells and induced cell apoptosis
The median-effect principle is often used to analyze concentration–response data for a single drug or a combination of drugs. In this principle, the potency (Dm or half maximal inhibitory concentration [IC50]) and the shape of the concentration–effect curve (m value) are considered. However, to analyze whether NVP-BEZ235 has a synergistic effect, IC20 (20% inhibitory concentration) values were determined to choose a constant Cisplatin concentration ratio.
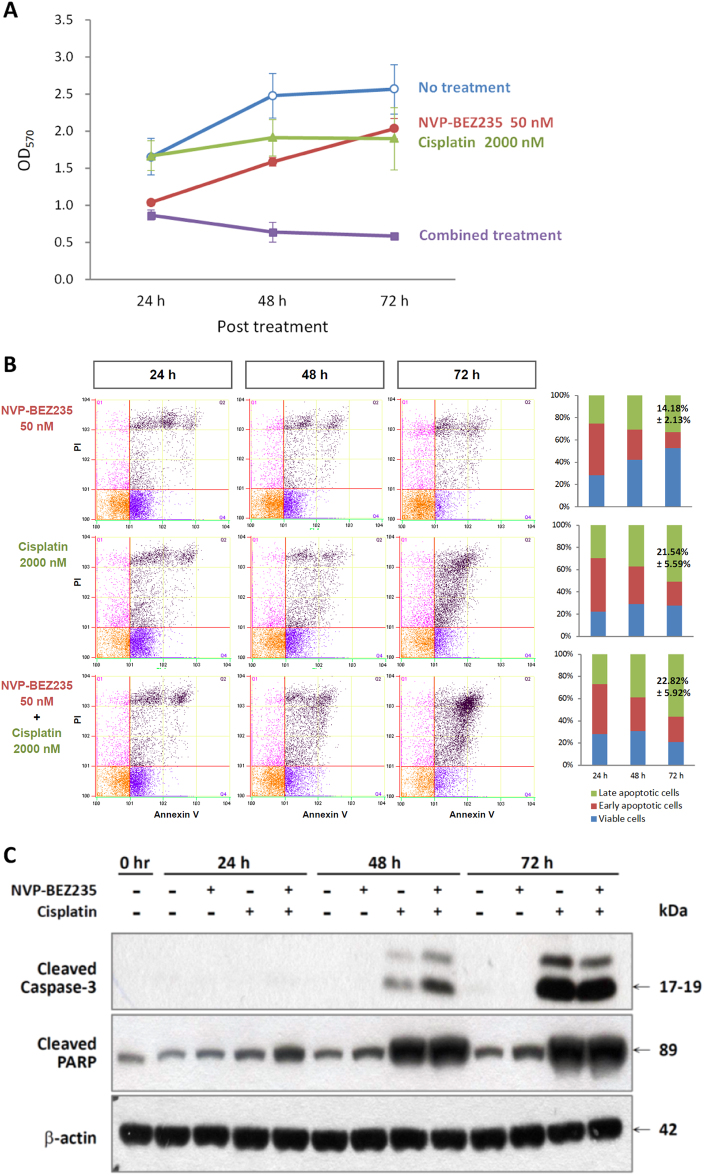
We assessed the antiproliferative potential of Cisplatin and NVP-BEZ235 on FaDu cells. We performed a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and manually counted viable cells to determine the effects of NVP-BEZ235 on cell growth. After 72 h of treatment, the combined treatment with NVP-BEZ235 and Cisplatin had significantly inhibited the growth of FaDu cell lines compared with the treatment with NVP-BEZ235 or Cisplatin alone (Fig. 3a).
Fig. 3. NVP-BEZ235 and Cisplatin synergistically inhibited cell proliferation and induced apoptosis of FaDu HSCC cells.
a Inhibitory effects of NVP-BEZ235, Cisplatin, or a combination of NVP-BEZ235 and Cisplatin on FaDu HSCC cells were assessed by MTT methods after 24, 48, and 72 h of treatment. Data presented are the mean and SE of three independent experiments. b Combination of NVP-BEZ235 and Cisplatin synergistically induced apoptosis. FaDu HSCC cells were treated with NVP-BEZ235, Cisplatin, or a combination of NVP-BEZ235 and Cisplatin for 72 h; the percentages of apoptotic cells were determined by Annexin V/PI staining followed by flow cytometric analysis. c Combination of NVP-BEZ235 and Cisplatin synergistically induced cleavages of Caspase 3 and PARP, as determined by Western blotting
The combined treatment of FaDu cells with NVP-BEZ235 and Cisplatin markedly increased the numbers of late apoptotic cells (22.82% ± 5.92%) compared with the untreated control (8.22% ± 1.26%) and treatment with Cisplatin (21.54% ± 5.59%) or NVP-BEZ235 (14.18% ± 2.43%) alone (p < 0.05; Fig. 3b), as demonstrated by flow cytometric analysis after Annexin V–Fluorescein isothiocyanate (FITC)/propidium iodide (PI) double staining. The combined treatment of FaDu cells with NVP-BEZ235 and Cisplatin also enhanced the cleavage of the apoptosis-related proteins caspase-3 and PARP. The cleavage of caspase-3 and PARP increased significantly at 48 and 24 h, respectively, compared with NVP-BEZ235 alone and Cisplatin alone (Fig. 3c). These data suggest that NVP-BEZ235 could sensitize Cisplatin-induced proliferation inhibition and apoptosis.
Combination of NVP-BEZ235 and Cisplatin-induced cell cycle arrest in G2/M phase
To study the antiproliferative mechanism of NVP-BEZ235 in HSCC cells, we tested whether NVP-BEZ235 treatment affects the cell cycle. FaDu cells were cultured with 50 nM NVP-BEZ235, 2000 nM Cisplatin, or a combination of the two for 72 h, and cell cycle arrest was analyzed using flow cytometry. As shown in Fig. 4, the combined treatment resulted in a marked increase in cells in the G2/M phase (61.01% ± 11.02%) compared with NVP-BEZ235 (19.50% ± 1.17%) or Cisplatin (49.50% ± 1.20%) alone at 72 h. These results suggest that NVP-BEZ235 may enhance the cytostatic effect of Cisplatin by promoting G2/M phase accumulation and inhibiting cell cycle progression.
Fig. 4. Effect of NVP-BEZ235 and Cisplatin on cell cycle progression.
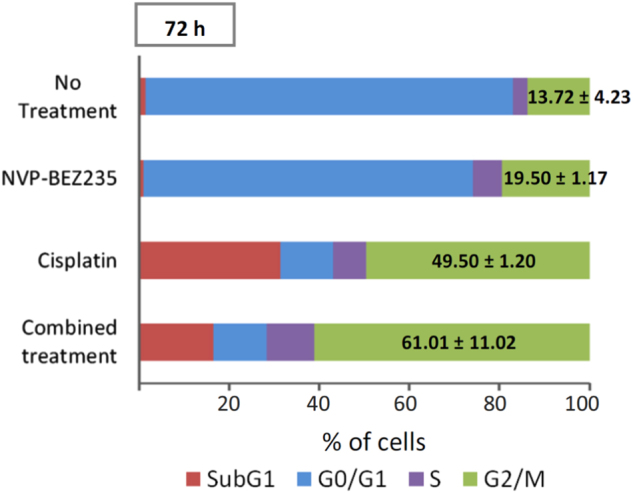
FaDu HSCC cells were treated with NVP-BEZ235, Cisplatin, or a combination of NVP-BEZ235 and Cisplatin for 72 h; the proportion of cells in each phase of the cell cycle was calculated as the percentage of the total cell population. Data presented are the means and SE of three independent experiments
NVP-BEZ235 showed synergistic antitumor activity with Cisplatin and suppressed phosphor-p70S6K in in vivo xenograft models
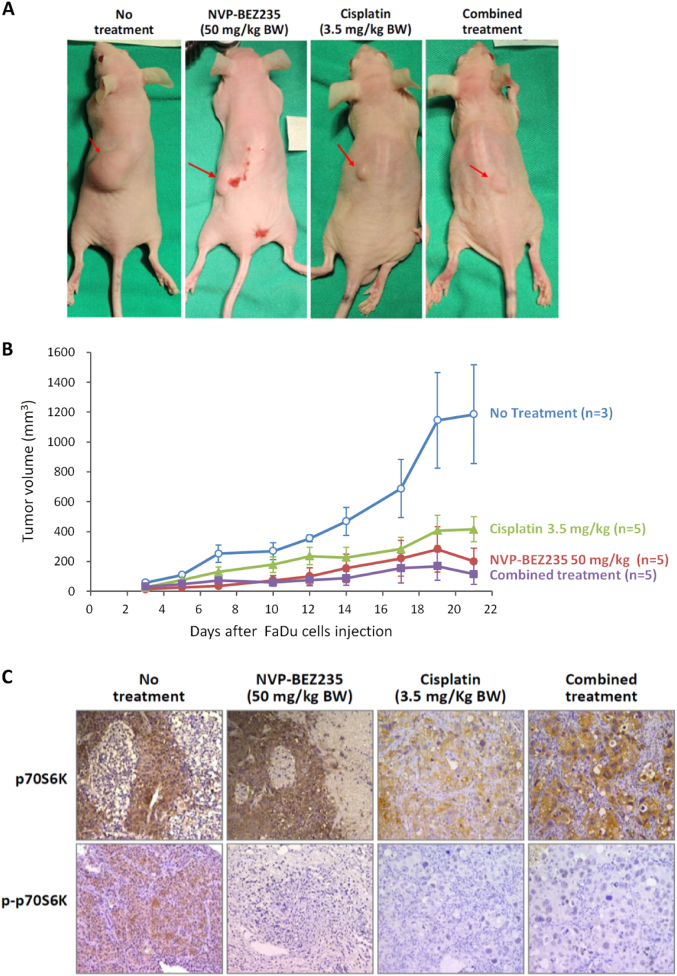
To examine the antitumor effects of NVP-BEZ235 in vivo, we established a nude mouse (BALB/cAnN.Cg) model by using the FaDu cell xenograft. Because the in vitro study showed that the effect of the combined treatment on cell apoptosis was superior to that of single treatment involving either Cisplatin or NVP-BEZ235 alone, we anticipated that the combined treatment would further suppress tumor growth in vivo. To confirm our assumptions, FaDu cells were injected subcutaneously into nude mice to establish xenograft tumors. The mice were then treated with NVP-BEZ235 (50 mg/kg) by oral gavage, Cisplatin (3.5 mg/kg) by intraperitoneal injection, or a combination of the two for 21 consecutive days. As shown in Fig. 5a, the combined treatment resulted in more significant tumor suppression compared with monotherapy. Quantitative analysis indicated that the tumor volume in the group subjected to the combined treatment was less than 50% of the volumes in the groups subjected to treatment with NVP-BEZ235 or Cisplatin alone. The results of the Western blot analysis from the xenograft tumor showed that the drug combination led to an enhanced reduction in phosphor-p70S6K (Fig. 5c).
Fig. 5. Antitumor effect of NVP-BEZ235 and its synergy with Cisplatin in FaDu HSCC xenograft.
a, b Antitumor effects of NVP-BEZ235, Cisplatin, and a combination of NVP-BEZ235 and Cisplatin in FaDu tumor xenografts determined by calculating tumor volumes. c Immunohistochemical staining of p70S6K and p-p70S6K. Combination of NVP-BEZ235 and Cisplatin inhibited p-p70S6K expression in FaDu tumor xenografts
Discussion
This is the first study to investigate the effect of NVP-BEZ235 therapy on FaDu (HSCC) cells. NVP-BEZ235 is a novel orally available dual PI3K-mTOR inhibitor, which is currently used in Phase I/II clinical trials and was revealed to control solid tumors in preclinical mouse models16. The PI3K-Akt-mTOR signaling pathway and its abnormal activation were reported to be influential in the progression, metastasis, and chemoresistance of a variety of tumors28.
Our in vitro results demonstrated that NVP-BEZ235 could significantly suppress mTOR, 4EBP1, and p70S6K activities. Moreover, p-mTOR and phospho-4EBP1 were each suppressed in the initial 24 h, but phospho-p70S6K did not return to the normal level for 72 h. A previous study reported that phospho-p70S6K downregulated by targeted therapy may benefit patients through the inhibition of tumor growth as well as metastasis29. Our results also show that either NVP-BEZ235 alone or Cisplatin alone could inhibit cell proliferation and induce apoptosis, but the combination of NVP-BEZ235 and Cisplatin could synergistically induce FaDu cell cycle arrest in the G2/M phase and cell apoptosis more significantly, thereby reducing proliferation and survival more effectively. Cancer drugs exert antitumor effects through cell cycle arrest which is one of the important antitumor mechanisms. The mechanism underlying NVP-BEZ235-induced apoptosis of HSCC cells is not entirely clear; thus, additional in-depth studies should be conducted in this regard. Previous studies have reported that the S6 kinase (S6K) plays various roles in different mechanisms of apoptosis30,31. In the PI3K-Akt-mTOR pathway, mTOR activation results in the phosphorylation of numerous substrates, including the phosphorylation of S6K by the mammalian target of rapamycin complex 1. The effect of NVP-BEZ235 on apoptosis of FaDu cells is associated with the phosphorylation of S6K, which may play important roles in this effect. NVP-BEZ235 exhibits the antitumor effect not only by inhibiting Akt survival pathway but also by promoting cell apoptosis. These raise the possibility of using the combination treatment to develop a promising therapeutic strategy to enhance the effects of chemotherapy and improve clinical outcomes for patients with head and neck cancer.
We observed that mice fed oral NVP-BEZ235 may exhibit a reduction in body weight. The PI3K-Akt pathway promotes cell survival and inhibits apoptosis; moreover, the alteration of intracellular signaling through the PI3K-Akt pathway promotes proliferation and sustains a higher demand of transformed cells for metabolic input by upregulating glycolysis through the Warburg effect32. NVP-BEZ235 will thus have a potential to affect glucolysis of tumor and normal cells. The dose of NVP-BEZ235, however, requires further investigation to ensure the basic glycolytic pathway is unaffected.
Grade 3–4 adverse effects associated with NVP-BEZ235 were reported by Carlo and colleagues33 in 50% of patients (5/10), without observing objective responses in the study group. A Phase Ib study of NVP-BEZ235, a dual inhibitor of PI3K and mTOR, was conducted in patients with advanced renal cell carcinoma. Most of the toxicity-related incidences, including fatigue, diarrhea, nausea, and mucositis, have been described as dose limiting. Thus, the finding that a combination of PI3K and mTOR blockade resulted in a high frequency of adverse events is not unexpected34. In in vivo studies, mice could survive well after adjusting to the NVP-BEZ235 dose. The tumor volume was also reduced successfully in these animals; however, their body weight should be carefully monitored.
Hypopharyngeal cancer often involves the larynx and requires oncologic management; however, most cases are diagnosed at more advanced tumor stages and have a higher incidence of neck nodal metastasis. According to the treatment guideline, hypopharyngeal cancers are suggested to be treated with total laryngectomy followed by postoperative radiotherapy (RT)35. To preserve the larynx among patients with advanced hypopharyngeal cancer, the combination of targeted therapy and RT compared with the use of RT alone was reported to be encouraging36. Molecular targeting agents have the potential to increase laryngeal preservation. Compared with other HNSCCs, HSCC needs more powerful targeting agents for laryngeal preservation. Therefore, NVP-BEZ235, cetuximab, and other targeting agents warrant further investigation.
This study has a limitation. Because injecting tumor cells into the hypopharynx is difficult, we injected the xenograft tumor into the flanks of mice in the in vivo study, which is not an appropriate site for FaDu cell growth.
In conclusion, NVP-BEZ235 alone could inhibit the proliferation and induce the apoptosis of FaDu cells. When NVP-BEZ235 was combined with Cisplatin, the two could synergistically inhibit the proliferation of FaDu cells by inducing cell cycle arrest in the G2/M phase through deregulating the PI3K-Akt-mTOR pathway; therefore, they could inhibit proliferation and induce apoptosis more effectively. Our study results suggest that NVP-BEZ235 could effectively sensitize Cisplatin-induced proliferation inhibition and apoptosis. The study results further confirm the effect of NVP-BEZ235 on FaDu cells and provide evidence for the clinical application of a combination of NVP-BEZ235 and Cisplatin in the treatment of hypopharyngeal cancer.
Materials and methods
Patients and samples
This study enrolled 12 male patients, aged 41–68 years (mean ± standard deviation, 52.3 ± 9.5), diagnosed with HSCC undergoing surgery at the Department of Otolaryngology, Kaohsiung Chang Gung Memorial Hospital, from 2009 to 2012. Clinical pathologic characteristics, including patients’ age, sex, TNM (Tumor, lymph node, metastasis) staging, and survival are presented in Table 1. Tumor samples and the adjacent noncancerous tissues were obtained immediately after resection and snap frozen in liquid nitrogen and stored until RNA extraction. Prior to tissue acquisition, informed consent was obtained from all patients. This study was approved by the Institutional Review Board of the Kaohsiung Chang Gung Memorial Hospital (IRB No. 100-4455A3).
Table 1.
Characteristics of patients with HSCC
| Characteristic | No. of patients |
|---|---|
| Sex | |
| Male | 12 |
| Female | 0 |
| Median age, y (range) | 50.0 (41–68) |
| Staging | |
| I | 0 |
| II | 4 |
| III | 0 |
| IV | 8 |
| T stage | |
| T0 | 1 |
| T1 | 0 |
| T2 | 5 |
| T3 | 2 |
| T4a | 4 |
| T4b | 0 |
| N stage | |
| N0 | 5 |
| N1 | 2 |
| N2a | 0 |
| N2b | 4 |
| N2c | 1 |
| Two-year survival | |
| Expired | 6 |
| Survived | 6 |
qRT-PCR analysis of mTOR expression
The total RNA of cancerous and noncancerous tissues was obtained from patients with HSCC and from FaDu cells by using TRIzol reagent (Invitrogen; Life Technologies, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. The 10-μl reaction volume contained 25 ng cDNA, 0.5 μl mTOR gene expression assay (Hs00234508_ml, Applied Biosystems) and 5 μl 2 × TaqMan Universal PCR Master Mix (Applied Biosystems) and was run in an ABI 7500 Fast Real-Time System (Applied Biosystems). The thermal cycling parameters of PCR were 95 °C for 10 min followed by 40 cycles of 95 °C for 20 s and 60 °C for 1 min. The expression level of the mTOR gene was normalized to the internal control ACTB to obtain the relative threshold cycle (ΔCt). The relative expression in cancerous tissue compared with noncancerous tissue (ΔΔCt) is calculated as follows: ΔΔCt = [ΔCt (cancerous tissue – ACTB) – ΔCt (noncancerous tissue – ACTB)]. The fold change in cancerous tissue compared with untreated noncancerous tissue was then calculated using the 2−ΔΔCt method.
Cell culture
Human FaDu HSCC cells used in this study were purchased from the Food Industry Research and Development Institute, Hsinchu, Taiwan. The cells were maintained in minimum essential medium (MEM; Invitrogen) containing 10% fetal bovine serum and grown at 37 °C with 5% CO2.
MTT assay
FaDu cells were treated with various concentrations of NVP-BEZ235 or Cisplatin or phosphate-buffered saline (PBS; as control) and the percentages of metabolically active cells were determined based on mitochondrial conversion of MTT to formazan. The assay included the following steps: 5000 cells in 100 μl subjected to different treatments were plated in triplicates in a 96-well plate and culture media were replaced with MEM (without phenol) containing 0.02% MTT (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 4 h. The medium was then replaced with 200 μl dimethyl sulfoxide per well. The absorbance was read at 570 nm using a 96-well format DTX880 Multimode Detector (Beckman Coulter, Brea, CA, USA). The background absorbance was measured in wells containing only the dye solution and culture medium. Data presented were the absorbance values subtracted by the background absorbance values and the mean of the triplicates.
Flow cytometric analysis
Flow cytometric analysis of stained cells was performed on a Beckman Coulter flow cytometer (Beckman Coulter, Fullerton, CA, USA). Dual staining of cells with Annexin V and PI was used to assess the percentages of apoptotic cells. Cells (1 × 106) were washed in cold PBS and resuspended in 200 μl staining solution [10 mM 4-(2-hydroxyethyl)- 1-piperazineethane- sulfonic acid (HEPES) pH 7.4, 140 mM NaOH, and 2.5 mM CaCl2] containing 5 μl of Annexin V-FITC and 10 μ of 20 mg/ml PI (BD Pharmingen, Franklin Lakes, NJ, USA) for 15 min at room temperature in the dark. Percentages of viable cells (double negative for Annexin V and PI), early apoptotic cells (Annexin V-positive and PI-negative cells), and late apoptotic cells (double positive for Annexin V and PI) were calculated. For cell cycle analysis, cells (5 × 105) were washed in PBS, fixed with cold 70% ethanol, and incubated with 25 μg RNAse A (Sigma-Aldrich) for 1 h at 37 °C. Prior to analysis, 25 μl (5 mg/ml) of PI (Sigma-Aldrich) was added and the percentages of the cell population in sub-G1, G1, S, or G2/M phases were calculated from histograms.
Western blotting
Samples were extracted in radioimmunoprecipitation assay (RIPA) buffer [20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM disodium ethylenediaminetetraacetate dihydrate (Na2EDTA), 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin]. For Western blotting, 30 μg of total lysates were separated by 8–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Millipore, Darmstadt, Germany). After blocking with nonfat dry milk for 1 h, the membranes were incubated overnight with primary antibodies at 1:1000 dilutions. Primary antibodies and antibodies against phosphorylated epitopes used in this study were mTOR (Cell Signaling Technologies, Danvers, MA, USA), phospho-mTOR (p-mTOR; Ser2448) (Cell Signaling), p70S6K (Sigma-Aldrich), phospho-p70S6K (p-p70S6K; Thr389) (Cell Signaling), caspase 3 (Cell Signaling), cleaved caspase 3 (Cell Signaling), poly (ADP-ribose) polymerase (PARP), (Cell Signaling) and cleaved PARP (Cell Signaling). β-actin (1:5000 dilution; Sigma-Aldrich) was used as internal control. Secondary antibodies were horseradish peroxidase (HRP)-conjugated goat antimouse immunoglobulin G (IgG; Sigma-Aldrich) and goat anti-rabbit IgG (Sigma-Aldrich). The membranes were briefly incubated with Western Lightning Plus-ECL enhanced chemiluminescence substrate (PerkinElmer, Waltham, MA) to visualize the proteins.
Xenograft studies
Male BALB/cAnN.Cg nude mice (age: 4–6 weeks; wt: 18–20 g) were purchased from the National Laboratory Animal Breeding and Research Center, Taipei, Taiwan. The mouse xenograft experimental procedures conducted in this study were approved by The Institutional Animal Care and Use Committee of Kaohsiung Chang Gung Memorial Hospital (IACUC No. 2014121610). BALB/cAnN.Cg nude mice were housed in pathogen-free conditions and experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals under the supervision of authorized investigators. FaDu cells (5 × 106) were inoculated subcutaneously into the back of nude mice under 2–4% isoflurane inhalational anesthesia. Tumors were measured in length and width using calipers every 2–3 days for 3 weeks. Mice were assigned randomly to receive NVP-BEZ235 (oral administration: 50 mg/kg/day for 3 weeks), Cisplatin (intraperitoneal injections: 3.5 mg/kg/day twice a week for 3 weeks), a combination of the two at the same dose and schedule, or placebo for negative control. Tumor material was harvested for immunohistological studies by formalin fixation.
Immunohistochemical staining
Polyclonal antibodies against p70S6K (Sigma-Aldrich) and phospho-p70S6K (Cell Signaling) were used as the primary antibodies. The tumor sections were incubated with primary antibodies (1:100 dilutions) for 1 h and then incubated with biotinylated goat anti-rabbit antibodies for 30 min. An HRP-diaminobenzidine staining kit (Sigma-Aldrich) was used to visualize the specific binding of the secondary antibody to the primary antibody and the stained sections were examined using a Zeiss microscope (Zeiss, Gottingen, Germany).
Statistical analysis
The data sets of MTT assay, apoptotic cell detection, cell cycle analysis, and tumor volumes of mice included at least three biological replicates, and the data were expressed as the mean ± standard deviation. For qRT-PCR, the values of ΔCt were used for the statistical analysis of gene expression. A two-sample t test was used to determine statistical significance and null hypotheses of no difference were rejected if p values were <.05. SPSS (version 15.0; SPSS, Chicago, IL, USA) was used for all statistical analyses.
Acknowledgements
This work was supported in part by grants from Chang Gung Memorial Hospital [grant numbers CMRPG6F0511, CMRPG6G0341, CMRPG6G0521, CMRPD8E0171, CMRPD8D0292, CMRPD8F0761]; and the Ministry of Science and Technology of Taiwan [grant numbers MOST 100-2314-B-182A-023, MOST 101-2314-B-182A-051, MOST 103-2314-B-182A-063, MOST 103-2320-B-182-023, MOST 104-2320-B-182-018].
Authors contributions
C-M. H., S-F. L., and M-Y. Y. conceived the experiments and wrote the manuscript. C-M. H., P-M. L., M-S. T. performed the experiments and statistical analysis. C-M. H., Y-T. T., and C-H. T. provided the clinical samples and data. All authors reviewed and approved the final manuscript. This manuscript was edited by Wallace Academic Editing.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by I. Lavrik
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sheng-Fung Lin, Email: shlintw@yahoo.com.tw.
Ming-Yu Yang, Email: yangmy@mail.cgu.edu.tw.
References
- 1.Hall SF, Groome PA, Irish J, O’Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. 2008;118:1362–1371. doi: 10.1097/MLG.0b013e318173dc4a. [DOI] [PubMed] [Google Scholar]
- 2.Takes RP, et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck. 2012;34:270–281. doi: 10.1002/hed.21613. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, et al. Cisplatin, 5-fluorouracil, and cetuximab (PFE) with or without cilengitide in recurrent/metastatic squamous cell carcinoma of the head and neck: results of the randomized phase I/II ADVANTAGE trial (phase II part) Ann. Oncol. 2014;25:682–688. doi: 10.1093/annonc/mdu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, et al. Platinum-based chemotherapy plus cetuximab first-line for Asian patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: results of an open-label, single-arm, multicenter trial. Head Neck. 2015;37:1081–1087. doi: 10.1002/hed.23707. [DOI] [PubMed] [Google Scholar]
- 5.Sosa AE, et al. Outcome of patients treated with palliative weekly paclitaxel plus cetuximab in recurrent head and neck cancer after failure of platinum-based therapy. Eur. Arch. Otorhinolaryngol. 2014;271:373–378. doi: 10.1007/s00405-013-2537-6. [DOI] [PubMed] [Google Scholar]
- 6.Péron J, et al. Paclitaxel and cetuximab combination efficiency after the failure of a platinum-based chemotherapy in recurrent/metastatic head and neck squamous cell carcinoma. Anticancer Drugs. 2012;23:996–1001. doi: 10.1097/CAD.0b013e328358d226. [DOI] [PubMed] [Google Scholar]
- 7.Chinn SB, Darr OA, Peters RD, Prince ME. The role of head and neck squamous cell carcinoma cancer stem cells in tumorigenesis, metastasis, and treatment failure. Front. Endocrinol. 2012;3:90. doi: 10.3389/fendo.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon duG, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of Cisplatin in bladder cancer cells. Int. J. Oncol. 2014;45:1027–1035. doi: 10.3892/ijo.2014.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacchi A, et al. Synergistic antitumor activity of Cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin. Cancer Res. 2006;12:175–182. doi: 10.1158/1078-0432.CCR-05-1147. [DOI] [PubMed] [Google Scholar]
- 10.Vassilopoulos A, et al. Synergistic therapeutic effect of Cisplatin and phosphatidylinositol 3-kinase (PI3K) inhibitors in cancer growth and metastasis of Brca1 mutant tumors. J. Biol. Chem. 2014;289:24202–24214. doi: 10.1074/jbc.M114.567552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson DR, Mell LK, Cohen EE. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015;51:291–298. doi: 10.1016/j.oraloncology.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Gobin B, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer Lett. 2014;344:291–298. doi: 10.1016/j.canlet.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Herrera VA, Zeindl-Eberhart E, Jung A, Huber RM, Bergner A. The dual PI3K/mTOR inhibitor BEZ235 is effective in lung cancer cell lines. Anticancer Res. 2011;31:849–854. [PubMed] [Google Scholar]
- 14.Xu CX, et al. The combination of RAD001 and NVP-BEZ235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS ONE. 2011;6:e20899. doi: 10.1371/journal.pone.0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang WJ, et al. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol. Sin. 2013;34:681–690. doi: 10.1038/aps.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gil del Alcazar CR, et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014;20:1235–1248. doi: 10.1158/1078-0432.CCR-13-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuger S, et al. Novel PI3K and mTOR inhibitor NVP-BEZ235 radiosensitizes breast cancer cell lines under normoxic and hypoxic conditions. Breast Cancer. 2014;8:39–49. doi: 10.4137/BCBCR.S13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung E, Kim JE, Rewcastle GW, Finlay GJ, Baguley BC. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol. Ther. 2011;11:938–946. doi: 10.4161/cbt.11.11.15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sznol JA, Jilaveanu LB, Kluger HM. Studies of NVP-BEZ235 in melanoma. Curr. Cancer Drug. Targets. 2013;13:165–174. doi: 10.2174/1568009611313020006. [DOI] [PubMed] [Google Scholar]
- 20.Venkannagari S, et al. Superior efficacy of co-treatment with dual PI3K/mTOR inhibitor NVP-BEZ235 and pan-histone deacetylase inhibitor against human pancreatic cancer. Oncotarget. 2012;3:1416–1427. doi: 10.18632/oncotarget.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awasthi N, Yen PL, Schwarz MA, Schwarz RE. The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J. Cell. Biochem. 2012;113:784–791. doi: 10.1002/jcb.23405. [DOI] [PubMed] [Google Scholar]
- 22.Manara MC, et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin. Cancer Res. 2010;16:530–540. doi: 10.1158/1078-0432.CCR-09-0816. [DOI] [PubMed] [Google Scholar]
- 23.Ma BB, et al. Preclinical evaluation of the mTOR-PI3K inhibitor BEZ235 in nasopharyngeal cancer models. Cancer Lett. 2014;343:24–32. doi: 10.1016/j.canlet.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Yang F, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 has a therapeutic potential and sensitizes Cisplatin in nasopharyngeal carcinoma. PLoS ONE. 2013;8:e59879. doi: 10.1371/journal.pone.0059879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda M, Shimomura M, Kobayashi K, Kojima S, Nakatsura T. Growth inhibition by NVP-BEZ235, a dual PI3K/mTOR inhibitor, in hepatocellular carcinoma cell lines. Oncol. Rep. 2011;26:1273–1279. doi: 10.3892/or.2011.1370. [DOI] [PubMed] [Google Scholar]
- 26.Chang Z, et al. Dual PI3K/mTOR inhibitor NVP-BEZ235-induced apoptosis of hepatocellular carcinoma cell lines is enhanced by inhibitors of autophagy. Int. J. Mol. Med. 2013;31:1449–1456. doi: 10.3892/ijmm.2013.1351. [DOI] [PubMed] [Google Scholar]
- 27.Kirstein MM, et al. Activity of the mTOR inhibitor RAD001, the dual mTOR and PI3-kinase inhibitor BEZ235 and the PI3-kinase inhibitor BKM120 in hepatocellular carcinoma. Liver Int. 2013;33:780–793. doi: 10.1111/liv.12126. [DOI] [PubMed] [Google Scholar]
- 28.Broek RV, Mohan S, Eytan DF, Chen Z, Van Waes C. The PI3K/Akt/mTOR axis in head and neck cancer: functions, aberrations, crosstalk, and therapies. Oral. Dis. 2015;21:815–825. doi: 10.1111/odi.12206. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, et al. p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma. Oncotarget. 2016;7:36539–36550. doi: 10.18632/oncotarget.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miwa S, et al. Caffeine induces apoptosis of osteosarcoma cells by inhibiting AKT/mTOR/S6K, NF-kappaB and MAPK pathways. Anticancer Res. 2012;32:3643–3649. [PubMed] [Google Scholar]
- 31.Tomioka H, et al. Inhibition of the mTOR/S6K signal is necessary to enhance fluorouracil-induced apoptosis in gastric cancer cells with HER2 amplification. Int. J. Oncol. 2012;41:551–558. doi: 10.3892/ijo.2012.1485. [DOI] [PubMed] [Google Scholar]
- 32.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 33.Carlo MI, et al. A Phase Ib Study of BEZ235, a dual inhibitor of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncologist. 2016;21:787–788. doi: 10.1634/theoncologist.2016-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pongas G, Fojo T. BEZ235: when promising science meets clinical reality. Oncologist. 2016;21:1033–1034. doi: 10.1634/theoncologist.2016-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edson MA, et al. Outcomes for hypopharyngeal carcinoma treated with organ-preservation therapy. Head Neck. 2016;38(Suppl 1):E2091–E2099. doi: 10.1002/hed.24387. [DOI] [PubMed] [Google Scholar]
- 36.Bonner J, et al. Cetuximab and radiotherapy in laryngeal preservation for cancers of the larynx and hypopharynx: a secondary analysis of a randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 2016;142:842–829. doi: 10.1001/jamaoto.2016.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]