Abstract
The impact of tumor infiltrating lymphocytes (TILs) on survival was confirmed in various cancer types. Our study aims to investigate the prognostic role of TILs on survival in patients with metastatic urothelial carcinoma (mUC) receiving platinum based chemotherapy. Patients who were diagnosed to have pathologically proved mUC between 1997 and 2016 and received palliative chemotherapy with platinum based regimen were recruited into our study. Kaplan-Meier curves and Cox regression analysis were constructed for overall survival (OS). A total of 259 mUC patients were enrolled into our study with median age 63 years and median follow-up visit 13.5 months. Of these patients, 179 (69%) had intense TILs and 80 (31%) had non-intense TILs. The median OS were 15.7 vs. 6.7 months (P = < 0.001) for patients with intense TILs and non-intense TILs, respectively. Subgroup analysis showed that TILs was both prognostically significant no matter for urothelial carcinoma of bladder and upper tract urothelial carcinoma. Multivariate analysis showed that TILs were strongly prognostic factors related to OS. Our study suggested mUC patients with intense TILs were independently associated with survival. Based on our study, TILs is clinically useful for outcomes anticipation and risk stratification, as well as patients counseling.
Introduction
Urothelial carcinoma is the leading malignancy of genitourinary tract1. The only curative treatment is radical surgery. However, disease recurrence is usually developed in the future, resulting in poor prognosis. Palliative chemotherapy with platinum based regimen remains the golden standard for patients with advanced, recurrent metastatic urothelial carcinoma (mUC)2. For fit patients with mUC, Methotrexate/vinblastine/doxorubicin/cisplatin(MVAC) and gemcitabine/cisplatin are two commonly used regimens3. The outstanding phase III study conducted by von der Maase et al. demonstrated that the median survival was 14.0 months for GC and 15.2 months for MVAC (hazard ratio [HR]: 1.09; 95% CI: 0.88 to 1.34; P = 0.66), respectively and concludes that GC provided a similar survival advantage as that of MVAC with a better safety profile as well as a improved tolerability4. For unfit patients, carboplatin was used instead of cisplatin in case of renal toxicity. EORTC study 30986 exhibited the efficacy of carboplatin based regimen with a median survival ranging from 8.1 to 9.3 months5. Although recent advances of anti-cancer modality, the mortality rate has not changed over the past two decades substantially with the median overall survival of no more than 15 months6. Several publications made great efforts to explore the reliable prognostic factors for outcomes prediction. It has been well understanding that histological variants7, lymphovascular invasion8, positive surgical margin9, hepatic metastases10–12 and more than one metastatic site12 were all significant to OS. However, these prognosticators merely based on tumor behaviors, excluding the immunologic reaction between host and tumor.
Undoubtedly, tumor microenvironment is closely related to treatment outcomes13. There is increasing evidence that the interaction between immune cells and tumor cells is critical for the development and progression of cancer14. It is worth noting that tumor-infiltrating lymphocytes (TILs) play an important role in that situation. TILs are frequently found in tumors, suggesting that tumors trigger an immune response in the host15. The impact of TILs on survival was confirmed in various cancer types16–21. Furthermore, the association between TILs and treatment response was also emphasized in several publications22,23. Given that the understandings of the relationship between TILs and prognosis in patients with mUC were limited, there is an urgent need to determine the clinical relevance of TILs in patients with mUC. Therefore, our study aims to identify the prognostic impact of TILs on survival in patients with mUC receiving platinum based chemotherapy.
Result
Baseline Characteristics
The median age was 63 years and median follow-up visit was 13.5 months. As for location of primary tumor, 166 patients had upper tract urothelial carcinoma (UTUC) and 93 patients have urothelial carcinoma of bladder (UCB). Table 1 summarized the clinical characteristics of our patients. The median age at diagnosis was 62 years. The majority of our patients were male in gender (65%), with a good ECOG performance status (0–1) (81%) and fit renal function (68%). Nearly two third of our patients had primary tumor in their upper urinary tract. Most patients (91%) underwent nephroureterectomy or cystectomy for tissue proof while the remains (9%) received transurethral resection of bladder tumor. After metastasis, half of our patients had single metastatic site and 81% received cisplatin based chemotherapy as first line treatment. All these specimens were sent for TILs assessment. After stratified by TILs, 179 patients (69%) had intense TILs and 80 (31%) had non-intense TILs. As compared to those with non-intense TILs, patients with intense TILs tended to have more female in gender (42% vs. 20%, P = 0.052), older ages (65% vs. 45%, P = 0.073) and single metastatic site (56% vs. 35%, P = 0.068). Apart from these, there were no significant differences in performance status, renal function, location of primary tumor, first-line chemotherapy regimen for metastatic disease and cycles of first-line chemotherapy.
Table 1.
Clinical characteristics of 259 patients with metastatic urothelial carcinoma.
| Intense TIL | Non-intense TIL | p value | |||
|---|---|---|---|---|---|
| N = 179 (%) | N = 80 (%) | ||||
| Gender | 0.052 | ||||
| Male | 103 | 58% | 64 | 80% | |
| Female | 79 | 42% | 16 | 20% | |
| Age | 0.073 | ||||
| ≤60 | 62 | 35% | 44 | 55% | |
| >60 | 117 | 65% | 36 | 45% | |
| ECOG Performance status | 0.548 | ||||
| 0–1 | 142 | 79% | 68 | 85% | |
| ≥2 | 37 | 21% | 12 | 15% | |
| Clearance of creatinine (mL/min) | 0.378 | ||||
| ≥60 | 116 | 65% | 60 | 75% | |
| <60 | 63 | 35% | 20 | 25% | |
| Location of primary tumors | 0.238 | ||||
| Upper urinary tract | 122 | 68% | 44 | 55% | |
| Bladder | 57 | 32% | 36 | 45% | |
| Previous radical surgery | 0.657 | ||||
| Yes | 167 | 93% | 69 | 86% | |
| No | 12 | 7% | 11 | 14% | |
| Number of metastatic sites | 0.068 | ||||
| 1 | 101 | 56% | 28 | 35% | |
| >1 | 78 | 44% | 52 | 65% | |
| First-line chemotherapy regimen | 0.865 | ||||
| Cisplatin based | 146 | 82% | 65 | 81% | |
| Carboplatin based | 33 | 18% | 15 | 19% | |
| Cycles of first-line chemotherapy | 0.178 | ||||
| <3 | 37 | 21% | 28 | 35% | |
| ≧3 | 142 | 79% | 52 | 65% | |
TIL, tumor infiltrating lymphocyte; UC, urothelial carcinoma; NLR, neutrophil to lymphocyte ratio.
Survival outcomes
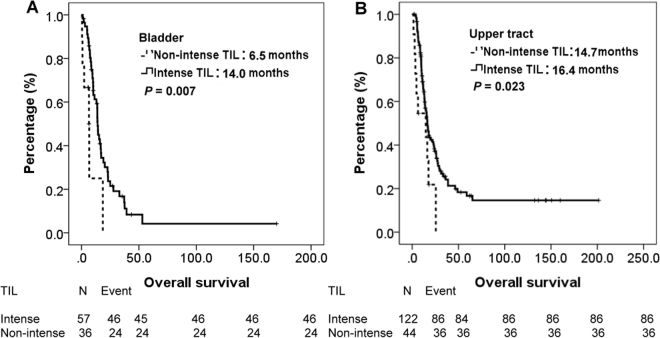
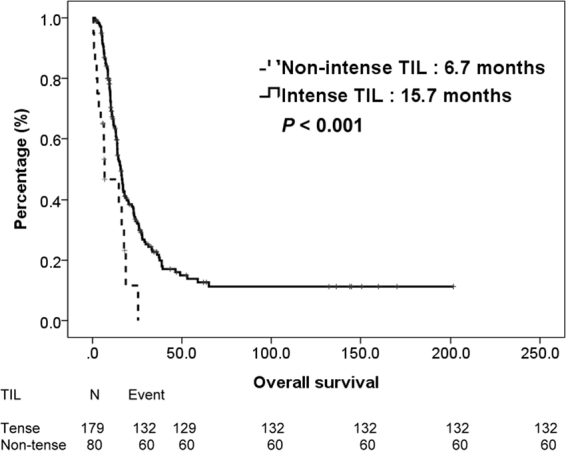
During this period, 192 (74%) patients died and tumor was the major reason of their death. All patients were initially divided into two groups, stratified by TILs. The median OS were 15.7 vs. 6.7 months (P = < 0.001) for patients with intense TILs and non-intense TILs, respectively. The survival curves of intense TILs and non-intense TILs were depicted in Fig. 1. Then, we categorized all patients into UTUC group and UCB group according to their primary tumor location. For patients with UCB, the median OS were 14.0 vs. 6.5 months (P = 0.007) for patients with intense TILs and non-intense TILs, respectively. For patients with UTUC, the median OS were 16.4 vs. 14.7 months (P = 0.023) for patients with intense TILs and non-intense TILs, respectively. Figure 2 plotted the survival curves of UCB and UTUC, stratified by TILs.
Figure 1.
Kaplan-Meier overall survival curve of 259 patients with mUC, stratified by TILs.
Figure 2.
Kaplan-Meier overall survival curve of patients with UCB and UTUC, stratified by TILs.
Univariate and multivariate Cox regression analyses
The univariate and multivariate analyses of OS in all patients are shown in Tables 2 and 3. In univariate analysis, age (≦60 vs. >60), gender (female vs. male), performance status (≦1 vs. >1), location of primary tumor (UTUC vs. UCB), number of metastatic sites (1 vs. ≧2) and TILs (intense vs. non-intense) had statistically impact. Moreover, TILs was classified into sTILs and itTILs according to the predominant area of lymphocyte infiltration. Subgroup analysis showed sTILs has a strong significance for survival (P = < 0.001), rather than itTILs (P = 0.148). After adjustment of confounding factors, multivariate analysis showed that female (HR: 0.65, 95% CI: 0.45–0.93, P = 0.020), performance status ≦1 (HR: 0.52, 95% CI: 0.34–0.79, P = 0.003), single metastatic sites (HR: 0.64, 95% CI: 0.46–0.89, P = 0.009) and TILs (HR: 0.41, 95% CI: 0.23–0.74, P = 0.003) were strongly prognostic factors related to OS. Age and location of primary tumor failed to be significant with OS in the final fitted Cox model.
Table 2.
Univariate Cox regression analysis of parameters associated with overall survival.
| Variables | Univariate | |
|---|---|---|
| HR (95% CI) | P value | |
| Age, ≦60 vs. >60 | 0.71 (0.51–1.00) | 0.050 |
| Gender, Female vs. Male | 0.64 (0.45–0.89) | 0.009 |
| ECOG PS, ≦1 vs. >1 | 0.61 (0.42–0.89) | 0.010 |
| Renal function, ≧60 vs. <60 | 0.92 (0.65–1.30) | 0.626 |
| UTUC vs. UCB | 0.70 (0.50–0.98) | 0.036 |
| Number of metastatic site, 1 vs. >1 | 0.65 (0.47–0.90) | 0.009 |
| Chemotherapy, cisplatin vs. carboplatin | 0.61 (0.27–1.39) | 0.237 |
| TIL, intense vs non-intense | 0.41 (0.24–0.71) | <0.001 |
| Stroma TIL, intense vs non-intense | 0.30 (0.19–0.48) | <0.001 |
| Intratumor TIL, intense vs non-intense | 0.79 (0.57–1.09) | 0.148 |
ECOG PS = Eastern Cooperative Oncology Group performance status, UTUC = upper tract urothelial carcinoma, UCB = urothelial carcinoma of bladder, PUC = pure urothelial carcinoma, VUC = variant urothelial carcinoma, TIL = tumor-infiltrating lymphocyte, HR = hazzard ratio, CI = confidence interval.
Table 3.
Multivariate Cox regression analysis of parameters associated with overall survival.
| Variables | Univariate | |
|---|---|---|
| HR (95% CI) | P value | |
| Age, ≦ 60 vs. >60 | 0.72 (0.49–1.06) | 0.094 |
| Gender, Female vs. Male | 0.65 (0.45–0.93) | 0.020 |
| ECOG PS, ≦ 1 vs. >1 | 0.52 (0.34–0.79) | 0.003 |
| UTUC vs. UCB | 0.71 (0.50–1.00) | 0.050 |
| Number of metastatic site, 1 vs. >1 | 0.64 (0.46–0.89) | 0.009 |
| TIL, intense vs non-intense | 0.41 (0.23–0.74) | 0.003 |
ECOG PS = Eastern Cooperative Oncology Group performance status, UTUC = upper tract urothelial carcinoma, UCB = urothelial carcinoma of bladder, PUC = pure urothelial carcinoma, VUC = variant urothelial carcinoma, TIL = tumor-infiltrating lymphocyte, HR = hazzard ratio, CI = confidence interval.
Discussion
To the best of our knowledge, this is the first study focusing on the prognostic impact of TILs on survival in patients with mUC receiving platinum based chemotherapy. Most recent publications demonstrated that TILs predicts a favorable prognosis in patients with organ confined urothelial carcinoma24,25. Our study suggested mUC patients with intense TILs were independently associated with better outcomes. Subgroup analysis showed that TILs was both significant for mUC patients no matter for primary tumor origin from UTUC or UCB. Furthermore, after adjustment of confounding factors, TILs remained a significant prognostic factor for OS in mUC patients. When categorizing TILs according to the area of lymphocyte infiltration, we disclosed that sTILs is more crucial than itTILs for survival of patients with mUC. This conclusion is consistent with previous literatures with regard to other types of cancers26,27. Thus, based on our conclusion, TILs might be clinically useful for outcomes anticipation and risk stratification, as well as patients counseling.
Several studies had demonstrated that TILs has prognostic significance in patients with various types of cancers, including breast, melanoma, lung, ovarian and anal caner et al.16–21, as well as mUC in our study. Furthermore, TILs has also shown to be associated with response to chemotherapy and prognosis28. The explanation of this observation remains undetermined. Current evidences showed that TILs represents an interaction between our immune system and tumor microenvironment. All immune cells may be present in the tumor microenvironment, including macrophages, neutrophil granulocytes, dendritic cells, mast cells, natural killer cells, naive and memory B lymphocytes and effector T cells (T helper cells; regulatory T cells; and cytotoxic T cells)29. The area infiltrated is quite widespread and can be located in the central zone of tumor, invasive margin, or peripheral tumor stroma. The immune contexture is composed of the immune cell type, density, and location, as well as the function and shaped with chemokines and cytokines30. As the advances of immuno-oncolgy, it is necessary for better understanding of the immunologic characteristics of the microenvironment and development of therapeutic strategies that might be beneficial to these patients31. Our study provided a rational toward the investigation of relationship between tumor microenvironment and prognosis in patients with mUC.
Several molecular on the surface of TILs have also been investigated. Liu et al. assessed FOXP3+ TILs by immunohistochemistry on tissue microarrays constructed from a well-defined cohort of 3992 breast cancer patients and found that the prognostic value of FOXP3+ TILs in breast cancer differs depending on their ER and HER2 expression status and CD8+ T-cell infiltration32. Webb et al. investigated TILs in a large collection of primary ovarian tumors and confirmed that CD103+ TILs is strongly associated with patient survival in ovary cancer, which may serve as a useful marker for enriching the most beneficial subsets for immunotherapy19. Djenidi et al. analyzed TILs on 126 lung cancer patients by multicolor flow cytometry and demonstrated that CD8+CD103+ TILs is a prognostic factor for survival33. Zhang et al. evaluated 162 specimens of upper tract urothelial carcinoma and reported PD-L1+ TILs independently predicted longer survival34. Wang et al. also estimated CD8+CD103+ TILs in patients with bladder cancer and suggested that CD8+CD103+ TILs might have a significant role in tumor immunity25. Patschan et al. reviewed 296 tissue microarray of muscle invasive bladder cancer and reported that a higher CD68/CD3 ratio of TILs was correlated with a bad prognosis35. Furthermore, Bellmunt et al. reviewed 160 specimens of mUC and concluded that PD-L1+TILs was significantly associated with longer survival36. In this study, we presented the prognostic role of TILs on patient of mUC. Further evaluation of molecular on TILs is ongoing.
With recent approvals for immune checkpoint inhibitors (ICI) such as cytotoxic T lymphocyte associated antigen 4 inhibitors and programmed cell death protein 1 inhibitors in caners harboring programmed death ligand 1 (PD-L1) overexpression, microsatellite instability and high mutation load, many questions regarding the biomarker for the optimal use of ICI that block these pathways are raising23. At the beginning, the expression of PD-L1 on immune cells or tumor cells was thought to be most reliable. However, evidences were not solid yet. TILs were an emerging prognosticator with encouraging results. Tomioka et al. investigated the status of TILs and PD-L1 in patients with triple negative breast cancer and found that patients with low TILs had higher probability of PD-L1 expression which could be the therapeutic target for ICI22. Loi et al. also focused on TILs in the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy and demonstrated that MEK inhibition upregulated PD-L1 expression in TNBC cells with lower TILs, suggesting the possible combination use of MEK inhibitor and ICI37. Moreover, new or increased TILs following anti-PD1 therapy in melanoma with initial low TILs have also been observed which resulted in tumor regression38,39. Base on abovementioned evidences, the importance of TILs should be emphasized and hopefully can be a strong predictor of ICI.
There are several potential limitations in our work, which are inherent to any retrospective studies. In this present study, we only recruited mUC patients after prior radical surgery. Therefore, our result can not be applied to patients with de novo mUC. Furthermore, a single institutional experience, divergent treatments for metastatic disease and irregular follow-up interval also limit our study. In spite of a retrospective study with inevitable selection bias, we emphasize the significant impact of TILs in patient with mUC after prior radical surgery. Further prospective, multi-institutional studies are warranted to confirm our observations.
Conclusions
In conclusion, TILs serves as a significant prognostic factor in patients with recurrent mUC after prior radical surgery. Our study suggested mUC patients with intense TILs were independently associated with lower NLR and better OS. Subgroup analysis showed that TILs was both significant for mUC patients with primary tumor origin from UTUC and UCB. Our findings highlight the clinical value of TILs which may useful for outcomes anticipation and risk stratification, as well as patients counseling.
Materials and Methods
Patients
This retrospective study was approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital and informed consent was exempt from requiring. All methods were performed in accordance with the relevant guidelines and regulations. No datasets were generated or analyzed during the current study. This study was performed by histopathological analyses of tissue samples obtained from patients at our institution. Patients who were diagnosed to have pathologically proved mUC between 1997 and 2016 were recruited into our study. Clinical characteristic of our patients were collected by chart review. All patients have pathologically confirmed urothelial carcinoma. Perioperative chemotherapy and radiotherapy were permitted in our study. Exclusion criteria were not pathologically confirmed, insufficient specimens for TILs assessment and non-platinum based chemotherapy for metastatic disease. The consort flow diagram of our study was presented as Supplementary Figure 1. Initially, 308 mUC patients were identified. Of them, 22 specimens generating from biopsy were too small to perform TILs assessment and 11 surgical specimens can not be obtained. Meanwhile, 16 patients were treated with non-platinum based chemotherapy. Finally, a total of 259 mUC patients were enrolled into our study.
Assessment of TILs
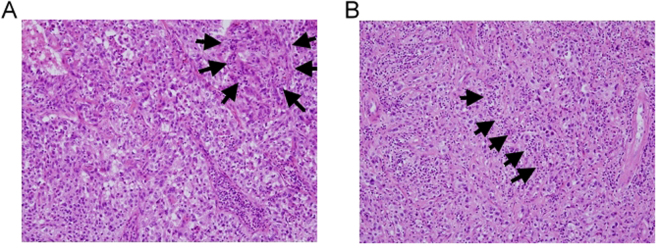
Full-face hematoxilin and eosin stained (HES) slides of primary tumors were retrieved for the evaluation of percentage of TILs. The analysis was based on the recommendations by an International TILs Working Group 201440. All pathologic slides were evaluated by two dedicated urologic pathologists (H.S. Huang and P.H. Lee) independently, who were blinded to clinical information, including treatment allocation and outcomes. Our patients were stratified according to the percentage of TILs labeled as intense TILs and non-intense TILs, respectively. Intense TILs was defined as more than 10% of lymphocyte infiltration, while non-intense TILs was defined as less than 10% infiltration. According to the recommendations by the international TILs Working Group 2014, no formal recommendation for a clinically relevant TILs threshold can be given at this stage. Therefore, we set the cut-off value at 10% according to previous literatures41. TILs was also classified into intratumoral (itTILs) and stromal (sTILs) according to their predominant area of infiltration. itTILs were defined as lymphocytes infiltrating within tumor nests and in direct contact with the tumor cells, whereas sTILs were defined as lymphocytes infiltration surrounding tumor stroma. Figure 3 exhibited the picture of intense itTILs and intense sTILs, respectively.
Figure 3.
Representative urothelial carcinoma specimens stained with haematoxylin and eosin (H&E 100X) demonstrating examples with (A) intense intratumoral tumor infiltrating lymphocytes (itTILs) (black arrows), (B) intense stromal tumor infiltrating lymphocytes (sTILs) (black arrows).
Statistical analysis
Patients were divided according to the percentage of TILs. All the clinical characteristics were analyzed with Pearson χ2 test between both groups. Survival outcomes were presented with Kaplan-Meier curves to estimate overall survival (OS) of all patients. Subgroup analysis was also performed according to location of primary tumor, labeling as upper tract urothelial carcinoma (UTUC) and urothelial carcinoma of bladder (UCB). OS was calculated from the diagnosis of metastatic disease until the date of death or the last contact when the patients were still alive at the time of the follow-up visit. Univariable and multivariable Cox regression analysis were constructed for survival and presented with hazard ratio (HR) and 95% confidence intervals (CIs). All statistical tests were two-sided. P-values < 0.05 were considered to be statistically significant.
Electronic supplementary material
Acknowledgements
The authors would like to thank the genitor-urologic cancer-working group at our cancer center for their gratuitous assistance of patient record examining and formatting. The study was approved by the Institutional Review Board (No. 102–1425B).
Author Contributions
Conceptualization: M.C.H. Methodology: H.S.H., and P.H.L. Formal Analysis: M.C.H. Investigation: H.Y.L.S., P.H.C., C.H.H. and C.H.C. Writing – Original Draft: H.S.H. and P.H.L. Writing – Review and Editing: H.Y.L.S., P.H.C., C.H.H. and C.H.C. Supervision: M.C.H.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25944-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Witjes JA, et al. Updated 2016 EAU guidelines on muscle-invasive and Metastatic Bladder Cancer. Eur Urol. 2017;71:462–75. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J Clin Oncol. 2000;18:3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 4.von der Maase H, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 5.De Santis M, et al. Randomized Phase II/III Trial Assessing Gemcitabine/Carboplatin and Methotrexate/Carboplatin/Vinblastine in Patients With Advanced Urothelial Cancer Who Are Unfit for Cisplatin-Based Chemotherapy: EORTC Study 30986. Journal of Clinical Oncology. 2012;30:191–9. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galsky M, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2012;23:406–10. doi: 10.1093/annonc/mdr156. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh M-C, et al. The Prognostic Impact of Histopathological Variants in Patients with Advanced Urothelial Carcinoma. Plos One. 2015;10:e0129268. doi: 10.1371/journal.pone.0129268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku JH, et al. Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Eur. J. Cancer. 2013;49:2665–80. doi: 10.1016/j.ejca.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Colin P, et al. Influence of positive surgical margin status after radical nephroureterectomy on upper urinary tract urothelial carcinoma survival. Ann. Surg. Oncol. 2012;19:3613–20. doi: 10.1245/s10434-012-2453-9. [DOI] [PubMed] [Google Scholar]
- 10.Bajorin DF, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–81. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi S, et al. Prognostic factors for metastatic urothelial carcinoma undergoing cisplatin-based salvage chemotherapy. Jpn J Clin Oncol. 2013;43:923–8. doi: 10.1093/jjco/hyt096. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka N, et al. Patient characteristics and outcomes in metastatic upper tract urothelial carcinoma after radical nephroureterectomy: the experience of Japanese multi-institutions. BJU Int. 2013;112:E28–34. doi: 10.1111/bju.12133. [DOI] [PubMed] [Google Scholar]
- 13.Solinas C, et al. The immune infiltrate in prostate, bladder and testicular tumors: An old friend for new challenges. Cancer Treatment Reviews. 2017;53:138–45. doi: 10.1016/j.ctrv.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 14.de Visser KE, Eichten A, LM C. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 15.Gooden MJM, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams S, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancers From Two Phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. Journal of Clinical Oncology. 2014;32:2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff SL, et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. Journal of Clinical Oncology. 2016;34:2389–97. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bremnes RM, et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non–Small Cell Lung Cancer. Journal of Thoracic Oncology. 2016;11:789–800. doi: 10.1016/j.jtho.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Webb JR, et al. Tumor-Infiltrating Lymphocytes Expressing the Tissue Resident Memory Marker CD103 Are Associated with Increased Survival in High-Grade Serous Ovarian Cancer. Clinical Cancer Research. 2013;20:434–44. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert DC, et al. Tumour-infiltrating lymphocyte scores effectively stratify outcomes over and above p16 post chemo-radiotherapy in anal cancer. British Journal of Cancer. 2016;114:134–7. doi: 10.1038/bjc.2015.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward MJ, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. British Journal of Cancer. 2013;110:489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomioka N, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC) Breast Cancer. 2017;25:34–42. doi: 10.1007/s12282-017-0781-0. [DOI] [PubMed] [Google Scholar]
- 23.Topalian SL, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature Reviews Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baras AlexanderS, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. OncoImmunology. 2016;5:e1134412. doi: 10.1080/2162402X.2015.1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, et al. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. The Journal of Urology. 2015;194:556–62. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 26.Perez EdithA, et al. Association of Stromal Tumor-Infiltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA Oncol. 2016;2:56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loi S, et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin With Doxorubicin-Based Chemotherapy: BIG 02-98. Journal of Clinical Oncology. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 28.Loi SSN, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 29.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 30.Galon J, Fridman WH, F P. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–6. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 31.Badoual C, et al. Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head Neck. 2010;32:946–58. doi: 10.1002/hed.21346. [DOI] [PubMed] [Google Scholar]
- 32.Shuzhen Liu WilliamD, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Research. 2014;16:432–43. doi: 10.1186/s13058-014-0432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djenidi F, et al. CD8+CD103+ Tumor–Infiltrating Lymphocytes Are Tumor-Specific Tissue-Resident Memory T Cells and a Prognostic Factor for Survival in Lung Cancer Patients. The Journal of Immunology. 2015;194:3475–86. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, et al. Prognostic significance of PD-L1 expression on tumor cells and tumor-infiltrating mononuclear cells in upper tract urothelial carcinoma. Medical Oncology. 2017;34:94–9. doi: 10.1007/s12032-017-0941-2. [DOI] [PubMed] [Google Scholar]
- 35.Sjödahl G, et al. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors11Grant support: The Swedish Cancer Society, the Swedish research council, the Nilsson Cancer foundation, the BioCARE Strategic Cancer Research program, the Lund Medical Faculty, and FoU Landstinget Kronoberg and Södra Regionvårdnämnden. Urologic Oncology: Seminars and Original Investigations. 2014;32:791–7. doi: 10.1016/j.urolonc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Bellmunt J, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Annals of Oncology. 2015;26:812–7. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 37.Loi S, et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clinical Cancer Research. 2015;22:1499–509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salgado R, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Annals of Oncology. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rouanne Mathieu, et al. Association of stromal lymphocyte infiltration with tumor invasion depth and high-grade T1 bladder cancer. J Clin Oncol. 2018;36:488–488. doi: 10.1200/JCO.2018.36.6_suppl.488. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.