Abstract
Background
To evaluate the diagnostic performances of detecting circulating tumor cells (CTCs) and tumor cells in bronchoalveolar lavage fluid (BALF) for peripheral lung cancer.
Methods
A total of 247 patients with lung cancer and 70 cases with benign lung disease were recruited in this study. Peripheral blood and BALF samples were collected, in which the tumor cells were enriched by negative immunomagnetic selection and detected by fluorescence in situ hybridization (FISH) of chromosome enumeration probe 8 (CEP8). The levels of tumor-associated markers (e.g., CEA, CA125, and NSE) in peripheral blood plasma were measured by using electrochemiluminescence.
Results
The numbers of CTCs detected in peripheral blood were significantly higher in patients with lung cancer than those with benign lung disease (5.78±0.57 vs. 1.13±0.39, Z=−8.64, P<0.01). Similarly, tumor cells count in BALF of malignancy were higher than that of benign lesions (6.76±0.89 vs. 0.89±0.23, Z=−6.254, P<0.01). However, as for patients with lung cancer and benign lung disease, the numbers of tumor cells in peripheral blood were comparable with those in BALF (both P>0.05). Detecting CTCs and tumor cells in BALF had similar areas under curves (AUC =0.871 and 0.963, respectively; P>0.05) in discriminating benign lesions from lung cancer (sensitivity 83.8% and 92.6%, specificity 86.5% and 99.9%, respectively), both of which were larger than those of NSE, CEA, and CA125 (AUC =0.564, 0.512 and 0.554, respectively; all P<0.05). The diagnostic performances of discriminating benign lesions and lung cancer in BALF and peripheral blood were both in concordance with that of histopathology (kappa values 0.662 and 0.569, respectively; both P<0.001).
Conclusions
Detecting tumor cells in peripheral blood and BALF may sensitive to identify benign and malignant peripheral lung lesions and be of value for early diagnosis of lung cancer.
Keywords: Lung cancer, circulating tumor cell (CTC), bronchoalveolar lavage, diagnosis
Introduction
Lung cancer is the malignancy with the highest morbidity and mortality in the world. Approximately 18% of cancer deaths are associated with lung cancer (1,2). The 5-year survival rate of lung cancer patients is low which is frequently associated with late diagnosis (3). Therefore, early diagnosis of lung cancer is of clinical importance for improving long-term prognosis. High resolution computed tomography (HRCT), which can display the morphology and location of small peripheral nodes, is the most common technique for early diagnosis of lung cancer. However, a CT examination confers limited value for distinguishing benign lung lesion from malignancy. Although a high magnitude of lung cancer-related tumor marker (e.g., CEA, CA125, and NSE) usually suggest a lung cancer diagnosis, the sensitivity and specificity are not yet high enough for early screening and diagnosis of lung cancer in clinical practice.
The clinical significance of circulating tumor cells (CTCs) has been widely studied (4-7). CTC refers to a group of tumor cell that detach from the primary or metastatic foci of solid tumors, and spontaneously enter the peripheral blood circulation, whereas CTC is commonly absent in normal individuals. CTCs detection, a novel non-invasive method for identifying of malignant lesion, is reportedly to be applicable for the diagnosis of solid malignant tumors (8). The chromosome enumeration probe 8 (CEP8) were amplificated frequently in lung cancer specimens with 81% amplification percentage (9). In addition, CTCs detection can specifically identify chromosome amplification in tumor cells detach from lung cancer lesions. Hence, this detection is also called a “liquid biopsy”.
Bronchoalveolar lavage, rather than ultra-fine bronchoscope or conventional biopsy forceps, is available to reach lung periphery by directly washing out and obtaining detached tumor cells from pulmonary lesions. Therefore, we hypothesize that bronchoalveolar lavage fluid (BALF) might have better local detection for lung cancer with equal or higher diagnostic sensitivity when compared with blood circulation. In this study, we detected CTCs in peripheral blood and tumor cells in BALF by negative enrichment and immune fluorescence in situ hybridization (imFISH). Furthermore, we sought to compare the diagnostic performances of detecting tumor cells in peripheral blood and BALF, as well as serum tumor markers, for diagnosis of lung cancer, and explore whether determination of tumor cells in BALF be of superior to that in peripheral blood.
Methods
Subjects
Patients who received bronchoscopy in the First Affiliated Hospital of Guangzhou Medical University between August 2015 and January 2016 were enrolled in this study. All the patients received chest CT examination to confirm the lung lesions adjacent to bronchi. We excluded patients who recently received radiotherapy, chemotherapy or immunotherapy, and had non-pulmonary primary tumor.
All patients got confirmed diagnosis by transbronchial lung biopsy, transthoracic needle lung biopsy and surgical lung biopsy. Lung cancer patients with distant metastasis were diagnosed by chest CT, magnetic resonance imaging (MRI), single photon emission CT (SPECT)/CT, and/or positron emission tomography-CT (PET-CT).
Collection and processing of peripheral blood and BALF
Five mL of peripheral blood was collected in a vacuum blood collection tube with acid citrate dextrose (ACD) anticoagulant (containing 0.8 mL of anticoagulant). BALF collection was performed before transbronchoscopic lung biopsy or brushing. Radial probe endobronchial ultrasound (ME-1, OLYMPUS, Japan) was performed to target the lung lesions, followed by a suction catheter was inserted into the target subsegmental bronchus to collect approximately 20 mL of BALF specimens (recovery rate >40%). In addition, the tumor related markers (CEA, CA125 and NSE) in peripheral blood were measured using electrochemiluminescence with an electrochemistry luminescence immunity analyzer (Cabas6000-e601, Roche, Swiss). Tumor cells in peripheral blood and BALF were enriched and identified by using a CEP8 amplificated CTC detection kit (Cyttel®, Cyttel Bio, China) as described in previous report (9,10).
Identification of tumor cells
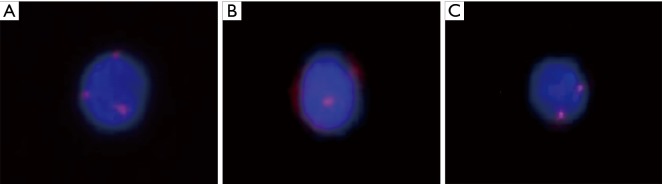
The identification criteria were described in the previous study (9). Positive cells: number of FISH signal dots ≥3 (i.e., hyperdiploid), CD45 negative, and DAPI positive. Negative cells: (I) number of FISH signal dots <3, CD45 positive, and DAPI positive; (II) number of FISH signal dots <3, CD45 negative, and DAPI positive (Figure 1).
Figure 1.
Examples of positive and negative cells. CEP8: orange; CD45: red; DAPI: blue; positive cells: number of FISH signal dots ≥3 (i.e., hyperdiploid), CD45 negative, and DAPI positive. (A) Positive cell (CTC); (B) negative cell (leukocyte); (C) negative cell (leukocyte). FISH, fluorescence in situ hybridization; CEP8, chromosome enumeration probe 8; CTC, circulating tumor cell.
Statistical methods
Statistical analyses were performed using the SPSS 16.0 statistical software (SPSS Inc., Chicago, USA). Quantitative data were expressed as the mean ± standard deviation (SD). For the comparison of quantitative data, when two groups of independent samples both followed a normal distribution, the t-test was performed; otherwise, the rank sum test was performed. If the difference values did not follow a normal distribution, the sign rank sum test of paired data was performed. The sensitivity and specificity were analyzed using the receiver operating characteristic (ROC) curve. The differences of the areas under curves (AUCROC) were calculated by using the MedCalc15.2 software. P<0.05 indicated statistical significance.
Results
Baseline characteristics
A total of 324 patients were recruited in this study, 247 patients were pathologically diagnosed with lung cancer (126 cases had tumor metastasis), 70 patients with benign lung diseases, whereas 7 patients were undiagnosed and excluded. A total of 224 males and 94 females were enrolled with the mean age 56.6±12.0 years. Ultimately, 302 cases received CTCs detection, and 75 cases received a detection of tumor cells in BALF for some patients refused to receive bronchoscopy or take a blood sample (Table 1, Figure 2).
Table 1. Baseline characteristics.
| Characteristics | Value (%) |
|---|---|
| Number of cases | 317 |
| Sex (M/F) | 1.8 |
| Age (years) | 56.55±12.04 |
| Lung cancer | 248 |
| Adeno carcinoma | 151 |
| Squamous carcinoma | 69 |
| Adenosquamous carcinoma | 2 |
| Small cell lung cancer | 13 |
| Others | 13 |
| Staging | |
| I | 24 |
| II | 16 |
| III | 71 |
| IV | 124 |
M, male; F, female.
Figure 2.
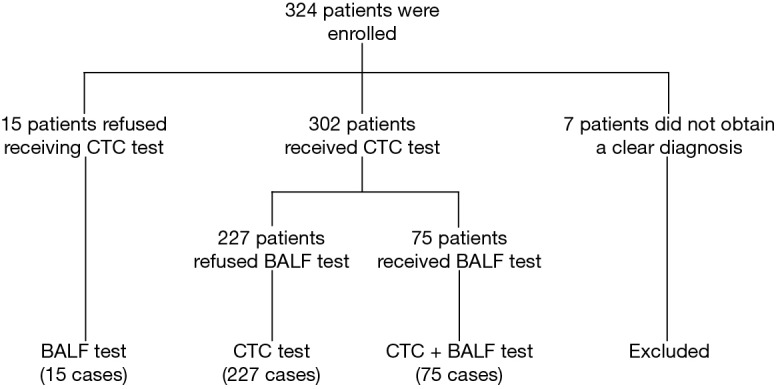
Flow chart of the study. CTC, circulating tumor cell; BALF, bronchoalveolar lavage fluid.
Comparison of the numbers of CTCs and tumor cells in the BALF in benign and malignant lung lesions
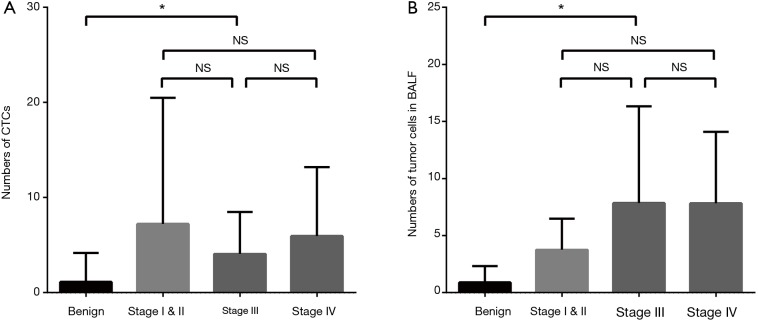
The detection rate of CTCs in lung cancer patients was 90.5% (218/241), which was comparable with that of tumor cells in BALF (46/51, 90.2%). Numbers of tumor cells detected in peripheral blood and BALF were higher in lung cancer compared with benign lung lesions (Z=−8.64, P<0.01 and Z=−6.254, P<0.01, respectively) (Figure 3). In term of patients with lung cancer, tumor cells detected in BALF was markedly higher than that of CTCs in peripheral blood (6.76±0.89 vs. 5.78±0.57, Z=−2.39, P=0.016). However, counts of tumor cells in BALF and peripheral blood did not differ significantly in patients with benign lung disease (1.13±0.39 vs. 0.89±0.23, P>0.05) (Figure 3). Tumor cells detected in peripheral blood and BALF of benign lesions and different stages of lung cancer are shown in Figure 4.
Figure 3.
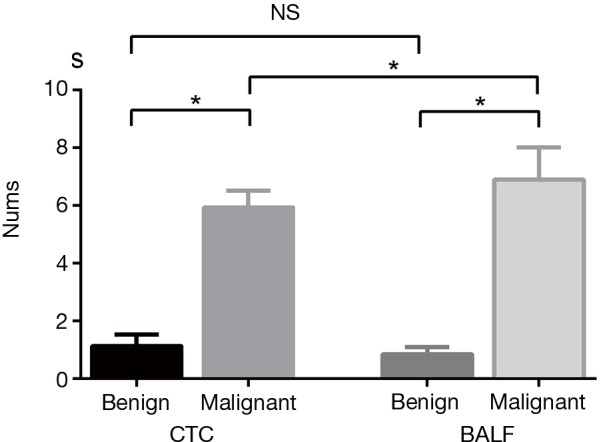
Comparison between numbers of tumor cells in lung cancer and benign lesions in patients underwent CTC and BALF detection. *, P<0.05; NS, non-significance. CTC, circulating tumor cell; BALF, bronchoalveolar lavage fluid.
Figure 4.
Numbers of tumor cells in benign lung lesions, stage I, II, III and IV lung cancers. (A) Detected in peripheral blood; (B) detected in BALF. *, P<0.05; NS, non-significance. BALF, bronchoalveolar lavage fluid; CTC, circulating tumor cell.
Comparison of the diagnostic performance for lung cancer between tumor cells and tumor markers detection
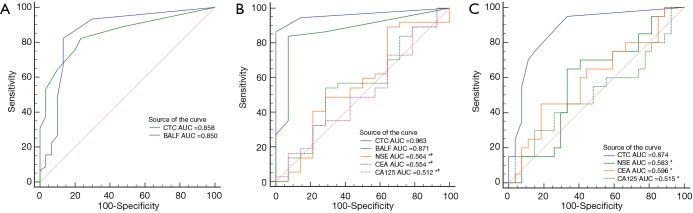
Based upon the pathological diagnosis for benign and malignant lesions, the diagnostic performance for lung cancer between tumor cells detection and tumor markers detection are shown in Table 2. The diagnostic performances of tumor cells detection in peripheral blood and BALF (AUCROC =0.871 and 0.963) were significant higher than that of NSE, CEA and CA125 (all AUCROC <0.6, all P<0.05), whereas no significant difference existed between CTCs and tumor cells in BALF detection (AUCROC =0.858 and 0.850, respectively; P=0.894 among) (Figure 5).
Table 2. Comparison of the diagnostic performance for lung cancer between the tumor cells and tumor markers detection.
| Methods | Cases | Cut-off value | AUCROC | 95% CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| BALF | 90 | 1.5 | 0.880 | 0.808–0.952 | 84.3 | 82.1 | 86.4 | 83.0 |
| CTC | 302 | 1.5 | 0.856 | 0.794–0.917 | 82.2 | 86.9 | 85.2 | 58.6 |
| NSE | 254 | 19.07 | 0.658 | 0.554–0.761 | 51.6 | 75.8 | 66.5 | 22.9 |
| CEA | 249 | 4.85 | 0.700 | 0.604–0.796 | 52.2 | 80.6 | 67.5 | 22.3 |
| CA125 | 251 | 20.32 | 0.688 | 0.603–0.774 | 62.5 | 72.7 | 72.7 | 28.2 |
CTC, circulating tumor cell; ROC, receiver operating characteristic; AUC, areas under curves; PPV, positive predictive value; NPV, negative predictive value; BALF, bronchoalveolar lavage fluid.
Figure 5.
Comparison between ROC curves in different diagnostic methods. (A) Comparison of CTC and BALF detection in 75 patients; (B) comparison among CTC, BALF, NSE, CEA and CA125 detection in 51 patients; (C) comparison among CTC, NSE, CEA and CA125 detection in 20 stage I lung cancer patients and 27 lung benign disease patients. Compared with CTC, *P<0.05; compared with BALF, #P<0.05. CTC, circulating tumor cell; ROC, receiver operating characteristic; AUC, areas under curves; NPV, negative predictive value; BALF, bronchoalveolar lavage fluid.
CTCs detection had a great diagnostic performance in discriminating stage I lung cancer from benign lesions (AUCROC =0.887, 95% CI: 0.802–0.952, sensitivity 0.829 and specificity 0.869), and was significantly higher than those of NSE, CEA and CA125 (AUCROC =0.874 vs. 0.583, 0.596 and 0.515 respectively, all P<0.05) (Figure 5). However, both CTCs and tumor cells in BALF conferred limited values in identifying lung cancer with (stage III and IV) and without tumor metastasis (stage I and II) (AUCROC =0.506 and 0.688, both P>0.05).
Multivariate logistic analysis showed that numbers of tumor cell in peripheral blood and BALF, as dependent variables, were included in the equation (both P<0.05), which predicted the diagnosis of lung cancer: Logit (P) = 0.443 × BALF + 0.213 × CTCs − 1.749 (AUCROC =0.886, 95% CI: 0.810–0.962, sensitivity 0.933 and specificity 0.767).
Concordance of tumor cells detection and pathology to diagnosis of lung cancer
According to the cut-off value (tumor cells count ≥2) as a diagnosis criterion of lung cancer, detection of tumor cells in both peripheral blood and BALF were in concordance with pathological diagnosis (kappa =0.569, P<0.001 and kappa =0.662, P<0.001, respectively).
Discussion
In this study tumor cells were obtained by negative enrichment and identified by imFISH in peripheral blood and BALF, and their diagnosis performances for peripheral lung cancer were compared with those of lung cancer-related tumor markers’ levels (CEA, CA125, and NSE) in plasma. The results demonstrated that tumor cells count ≥2 in peripheral blood and BALF both had better diagnostic performances in discriminating malignant from benign lung lesions compared with peripheral blood tumor markers. Moreover, CTCs detection performed a well diagnostic significance in identification of stage I lung cancer from benign lung lesions.
Conventional CTC detection studies were mainly based upon technologies such as CellSearchTM Assay and CTC-Chip (9,10). The mechanisms involve targeting epithelial cell adhesion molecules (EpCAM) on the surface of tumor cells as antigen to design antibodies for capturing CTCs. However, EpCAM would get loss since tumor cells undergo the epithelial mesenchymal transition (EMT) process, thus resulting in possible CTCs lost (9). In this study, we specifically obtained CTCs with amplification of chromosome 8 (CEP8) by negative enrichment and identifying them by imFISH combined of CEP8/CD45/DAPI markers. As the blood-based marker CD45 was targeted, the influences of blood-based markers were excluded during the process of tumor cells detection. Therefore, theoretically, the sensitivity and specificity of FISH could be increased (11). A previous small sample study (9) indicated that this method had high sensitivity and specificity in the diagnosis of lung cancer and cervical cancer.
In this study, CTCs could be detected in peripheral blood with positive rate of 90.5% (218/241) in lung cancer patients. The higher number of CTCs in lung cancer than that in benign lung lesions suggested that CTCs in peripheral blood could help to identify benign and malignant lung lesions. It was consistent with the previous study of detecting CTCs in lung cancer (9). In term of biopsy-proven early stage lung cancer, CTCs detection remained high diagnostic performance (AUC =0.874) in discriminating stage I lung cancer from benign lesions, which superior to CEA, NSE and CA125 in peripheral blood. The explanation might be that tumor cells detaching from lesions and entering the blood circulation probably occur in early stage lung cancer, even though the absent of notable abnormities in HRCT. Under the condition of escaping from immune clearance, tumor cells usually aggregate and colonize in other organ through peripheral blood to form metastatic foci (12-15). Studies showed that even small amount of CTCs that detached from primary lesions survived without being recognized and cleared by the immune system, distant metastasis might occur at some time or other (16).
This study was the first to report the detection tumor cells with amplification of CEP8 by negative enrichment and identifying them by imFISH combined of CEP8/CD45/DAPI markers in BALF. Compared to the conventional smear or immunofluorescence method for determination of detached cells in BALF, this method could eliminate a large amount of inflammatory cells in BALF specimens before hybridization staining and identifying detached tumor cells. This lead to higher sensitivity and specificity for diagnosis of lung cancer. The capture rates of conventional CTCs detection methods were approximately 50–60% in late-stage breast cancer, colorectal cancer and prostate cancers (17), whereas only 20–30% in non-small cell lung cancer (17,18). In this study, the capture rate of tumor cells in BALF of patients with lung cancer patients reached 90.2%, which was similar to that in peripheral blood CTCs detection (90.5%). Diagnostic performance of tumor cells detection in BALF was similar with that of CTC detection. Intriguingly, the number of tumor cells detected in BALF were greater than that of CTCs. It might be explained that lavage fluid directly reached the tumor lesions, which resulted in easier detection of detached tumor cells in BALF rather than in peripheral blood. Thus, theoretically, diagnostic performance of tumor cell detection in BALF would be better than CTCs in early stage lung cancer. However, compared with CTC detection, the diagnostic performances were not improved by detecting tumor cells in BALF in discriminating stage I and II lung cancer from benign lung lesions in this study. Briefly, diagnostic performances were similar between two methods in this study. Whether the diagnostic performance of tumor cells detection in BALF is superior to that of CTCs detection for early stage lung cancer still awaits further prospective and large sample studies.
Our results showed that the diagnostic performances of CTCs and BALF in discriminating stage I lung cancer from benign lesions were significantly higher than those of serum tumor markers (CEA, NSE, CA125). The reason might be that serum tumor markers are metabolic products, commonly produced and released by tumor cells, and could be detected in some benign lesions, thus leading a high false negative/positive rate (18,19). In contrast, tumor cells detection in peripheral blood and BALF have the advantage of specifically targeting and detecting tumor cells with higher sensitivity and specificity over serum tumor markers.
Some limitations should be considered in this study. First, this was a single-center study with small samples size, particularly in early stage cancer, leading to limited generalizability of the study results. Second, the diagnostic reference values of tumor cells in peripheral blood and BALF are still absent. Third, whether tumor cell count is variable in different pathological types or genotypes of lung cancer remains unclear. This study did not perform correlation analysis to provide explanations.
Conclusions
In summary, tumor cells detection in peripheral blood and BALF had similar diagnostic performances for lung cancer, and may be sensitive to discriminate malignancy from benign lung lesions and make early diagnosis of lung cancer.
Acknowledgements
We thank Prof. Zhou Cheng-Zhi (State Key Laboratory of Respiratory Disease, National Clinical Research center for Respiratory Disease, Guangzhou Institute of Respiratory Disease, First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China) for his recommendation and assistance for patients’ recruitment.
Funding: This study was supported by Special Funds for Research Public Welfare Research and Capacity Building in Guangdong Province (No. 2014A020215036).
Ethical Statement: Permission was obtained from ethics committee of First Affiliated Hospital of Guangzhou Medical University (2015-31) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153-6. 10.1002/ijc.1440 [DOI] [PubMed] [Google Scholar]
- 2.Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. 10.1097/JTO.0b013e318252cfef [DOI] [PubMed] [Google Scholar]
- 3.Gasparri R, Romano R, Sedda G, et al. Diagnostic biomarkers for lung cancer prevention. J Breath Res 2018;12:027111. 10.1088/1752-7163/aa9386 [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- 5.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. 10.1200/JCO.2007.15.8923 [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. 10.1158/1078-0432.CCR-08-0872 [DOI] [PubMed] [Google Scholar]
- 7.Uenosono Y, Arigami T, Kozono T, et al. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer 2013;119:3984-91. 10.1002/cncr.28309 [DOI] [PubMed] [Google Scholar]
- 8.Nurwidya F, Zaini J, Putra AC, et al. Circulating Tumor Cell and Cell-free Circulating Tumor DNA in Lung Cancer. Chonnam Med J 2016;52:151-8. 10.4068/cmj.2016.52.3.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning N, Zhan T, Zhang Y, et al. Improvement of specific detection of circulating tumor cells using combined CD45 staining and fluorescence in situ hybridization. Clin Chim Acta 2014;433:69-75. 10.1016/j.cca.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Ge F, Cui W, et al. Lung cancer circulating tumor cells isolated by the EpCAM-independent enrichment strategy correlate with Cytokeratin 19-derived CYFRA21-1 and pathological staging. Clin Chim Acta 2013;419:57-61. 10.1016/j.cca.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 11.Lu G, Muddasani R, Orlowski RZ, et al. Plasma cell enrichment enhances detection of high-risk cytogenomic abnormalities by fluorescence in situ hybridization and improves risk stratification of patients with plasma cell neoplasms. Arch Pathol Lab Med 2013;137:625-31. 10.5858/arpa.2012-0209-OA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross K, Pailler E, Faugeroux V, et al. The potential diagnostic power of circulating tumor cell analysis for non-small-cell lung cancer. Expert Rev Mol Diagn 2015;15:1605-29. 10.1586/14737159.2015.1111139 [DOI] [PubMed] [Google Scholar]
- 13.Peck K, Sher YP, Shih JY, et al. Detection and quantitation of circulating cancer cells in the peripheral blood of lung cancer patients. Cancer Res 1998;58:2761-5. [PubMed] [Google Scholar]
- 14.Hosseini H, Obradovic MM, Hoffmann M, et al. Early dissemination seeds metastasis in breast cancer. Nature 2016. [Epub ahead of print]. 10.1038/nature20785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper KL, Sosa MS, Entenberg D, et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 2016. [Epub ahead of print]. 10.1038/nature20609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Zhou F, Li X, et al. Folate Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1163-71. 10.1097/JTO.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 17.Bach PB, Kelley MJ, Tate RC, et al. Screening for lung cancer: a review of the current literature. Chest 2003;123:72s-82s. 10.1378/chest.123.1_suppl.72S [DOI] [PubMed] [Google Scholar]
- 18.Milman N, Pedersen LM. The serum ferritin concentration is a significant prognostic indicator of survival in primary lung cancer. Oncol Rep 2002;9:193-8. [PubMed] [Google Scholar]
- 19.Lee JH, Chang JH. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, neuron-specific enolase, and cytokeratin 19 fragments in patients with effusions from primary lung cancer. Chest 2005;128:2298-303. 10.1378/chest.128.4.2298 [DOI] [PubMed] [Google Scholar]