Abstract
Background
Radical resection is the cornerstone for patients with early stage of non-small cell lung cancer (NSCLC). However, fatal disease recurs in about 30–70% of resected cases. The circulating tumor cells (CTCs) is one of the main causes of recurrence of cancer. Circulating tumor DNA (ctDNA) is also a potential predictive biomarker of recurrence in patients with early stage NSCLC. A meta-analysis was conducted to identify the prognostic value of the CTCs and ctDNA in predicting the disease recurrence after surgery of NSCLC patients.
Methods
Electronic databases were comprehensively searched for eligible studies. A random effects model was used. The primary endpoint was the hazards ratio (HR) for the disease-free survival (DFS) between CTCs/ctDNA positive and negative groups. The relative risks (RR) of one and two-year recurrence rate between CTCs/ctDNA positive and negative groups were also calculated.
Results
A total of 5 studies involving 351 patients were included, in which 3 were studies on CTCs and 2 were ctDNA. Our result revealed that positive peripheral blood CTCs (HR, 3.37; 95% CI: 2.28–4.96; P<0.001) and ctDNA (HR, 8.15; 95% CI: 2.11–31.50; P=0.002) indicated poor prognosis for DFS. One (68% vs. 18.2%; RR 3.28; P<0.001) and two (76% vs. 44%; RR 1.80; P=0.06) years recurrence rate were higher in CTCs positive group compared with the negative group, respectively. The same result was also observed in ctDNA positive versus negative groups of 1 (77.9% vs. 8.3%; RR 9.05; P=0.001) and 2 (85.6% vs. 8.3%; RR 9.63; P<0.001) years recurrence rate.
Conclusions
Both postoperative CTCs and ctDNA are promising predictive biomarkers of early tumor recurrence in NSCLC patients. In addition, detection based on ctDNA seems to be more sensitive than CTCs.
Keywords: Non-small cell lung cancer (NSCLC), circulating tumor cell (CTC), circulating tumor DNA (ctDNA), recurrence
Introduction
Radical resection is the cornerstone for patients with early stage of non-small cell lung cancer (NSCLC). NCCN guidelines have recommended that patients require follow-up with CT ± contrast scans every 6 months for the first 2 to 3 years after radical surgery of NSCLC (1). However, tumor relapse occurs in about 30–70% of resected cases (2). Early detection of recurrence of primary lung cancer after surgery is associated with improved outcomes and survival in patients at early stage. With the rapid development of precision medicine and individual therapy in lung cancer, liquid biopsy, a very promising detection method of oncological research, could predict recurrence 1 to 2 years earlier than radiographic progression (3).
Circulating tumor cells (CTCs), originating from the tumor site and migrating to peripheral blood, is one of the “liquid biopsy” approach and a source of valuable tumor markers (4). Tumor cells can often be found in the circulation of patients with advanced cancer. The presence of CTCs is consistently associated with poor outcome with shorter disease free survival (DFS) (5), lower progression-free survival (PFS) and overall survival (OS) (6,7).
Circulating tumor DNA (ctDNA) is also a potential predictive biomarker of recurrence in patients with early stage NSCLC (8). ctDNA can reflect the tumor burden, and its levels decreased after radical surgery and generally increased as new lesions became apparent upon radiological examination, which may help to provide the genetic follow-up data (9). Postoperative ctDNA detection of breast and colorectal tumors has been shown to be used as an indicator to identify patients at high risk of recurrence and the presence of ctDNA after completion of resection was associated with an inferior recurrence-free survival (10,11).
Recently, an increasing interest has been focused on the predictive value of CTCs and ctDNA, which may serve as new indicators for discovering recurrence and metastasis of tumors. With the aim of evaluating the predictive value of the CTCs and ctDNA on the recurrence of tumor, we conducted a meta-analysis comparing the DFS for post-operative NSCLC patients.
Methods
Search strategy
The following electronic databases were searched up to 13 January 2018: The Cochrane Library, PubMed, Science Direct, Google Scholar, Embase. In order to search the eligible studies, we set no restrictions for language or publications status. The following words were used as algorithms: non-small cell lung cancer, CTCs, ctDNA, DFS, recurrence. To locate trials that were unpublished or failed to be identified by keyword searching, the body and reference sections of the studies were checked to find potentially eligible articles. We also scanned the poster and presentation of international conference to capture the target.
Study selection
All the studies were selected on the following inclusion criteria: (I) studies that reported the recurrence of the early stage NSCLC; (II) CTCs/ctDNA were used to diagnose disease and evaluate the tumor relapse; (III) studies in which the sufficient information are provided to estimate hazard ratio (HR) of DFS and 1 or 2 years recurrence rate. The exclusion criteria was as following: (I) reviews, letters, case reports, expert opinion, editorials; (II) all publications regarding in vitro/ex vivo studies, cell lines, and human xenografts; (III) studies in which the same population or overlapping data were used.
Although the language restrictions were not set initially, we only conducted the review of the studies published in English language. After the exclusion of non-relevant and redundant publications from different databases, we collected studies and evaluated in full-text by taking the inclusion and exclusion criteria mentioned above.
Data extraction
The eligible data was independently reviewed and extracted by two authors (Bo Wang, Zhichao Liu). Once there were disagreements the third author (Hengrui Liang) was consulted. Information was recorded into a table format from the all studies: the first author name, year of publication, sample source, number of CTC/ctDNA positive and negative cases, follow up (month), gender, age, smoker, adenocarcinoma, tumor size, cancer tumor node metastasis stage, PET-CT (SUV). We also conducted investigation of heterogeneity which was evaluated to determine whether the data from studies could be used in meta-analysis.
Quality assessment of the included studies
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in demonstrating the standard methodology and results (12). Quality assessment of included studies was assessed using the Joanna Briggs Institute Prevalence Critical Appraisal Tool to assess the quality of the studies. Any disagreement was resolved via discussion among the authors (13).
Statistical analysis
We used STATA12.0, Review Manager5.3 to analyze the data. A random effects model was used as the statistical heterogeneity assessment by using the Cochrane’s Q statistic and I2 test (14). The primary endpoint was the hazards ratio (HR) for the DFS between CTCs/ctDNA positive and negative groups. HR was calculated based on the method of Parmar et al.’s study if it was unavailable from original paper (15,16). The relative risks (RR) of one and two year recurrence rate between CTC/ctDNA positive and negative groups were also calculated.
Results
Identification of relevant studies
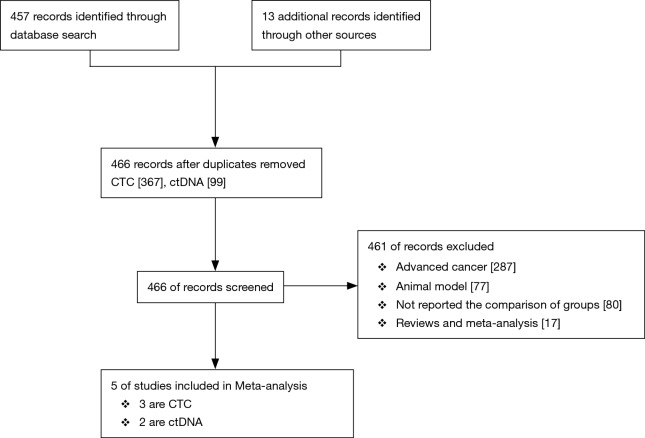
Initially there were 457 records identified through database searching together with 13 additional records from other sources. After duplicating there were 466 records (including 367 records related to CTCs and 99 records related to ctDNA) remained and screened by two authors independently. Ultimately, 5 eligible articles (3 were studies on CTC and 2 were on ctDNA) included in and 351 patients were involved totally (17-20) (Figure 1).
Figure 1.
Flow diagram detailing the search strategy and identification of studies.
Study characteristics
Table 1 noted the detailed information of included studies. The studies were conducted in two different countries with the periods ranged from 2001 to 2017. One study related to ctDNA was a conference abstract. Two articles were about CTCs and the others focused on ctDNA. The detection rate of CTCs and ctDNA in all studies are 57.3% and 56.9%, respectively. All studies gained 7 to 10 stars in study quality assessment on a scale of 0 to 10 with Joanna Briggs Institute Prevalence Critical Appraisal Tool.
Table 1. Characteristics and demographics of the included studies.
| Author | Year | (+) | (−) | Follow up (month) | Female (%) | Age (years) | Smoker (%) | Adenocarcinoma (%) | Tumor size (cm) | PET (SUV) | I stage (%) | II stage (%) | III stage (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ctDNA | |||||||||||||
| Abbosh et al. | 2017 | 14 | 10 | 25.2 [22.9–31.5] | 33.33 | NG | 95.80 | 66.67 | NG | NG | 41.67 | 41.67 | 16.67 |
| Chaudhuri et al. | 2017 | 19 | 15 | 35.1 [6.9–56] | 32.00 | 66.8 | 88.00 | 49.00 | NG | 17.2 | 19.00 | 17.00 | 64.00 |
| CTCs | |||||||||||||
| Lara et al. | 2016 | 18 | 38 | 16 [3–23] | 10.70 | 67.4 | 94.60 | 44.60 | 3.60 | 13.17 | 46.40 | 39.30 | 14.30 |
| Crosbie et al. | 2016 | 6 | 21 | 22 [1–52] | 46.70 | 67.5 | 100 | 26.70 | 3.97 | 12 | 40.00 | 36.70 | 23.30 |
| Hofman et al. | 2011 | 144 | 66 | 15 [1–28] | 23.00 | 63 | 88.00 | 73.00 | 3.8 | NG | 43.00 | 19.00 | 29.00 |
+, ctDNA or CTCs positive; −, ctDNA or CTCs negative. ctDNA, circulating tumor DNA; CTCs, circulating tumor cells; PET, positron emission tomography; SUV, standard uptake value; NG, not given.
The correlation of CTCs/ctDNA with tumor recurrence in post-operative NSCLC patients
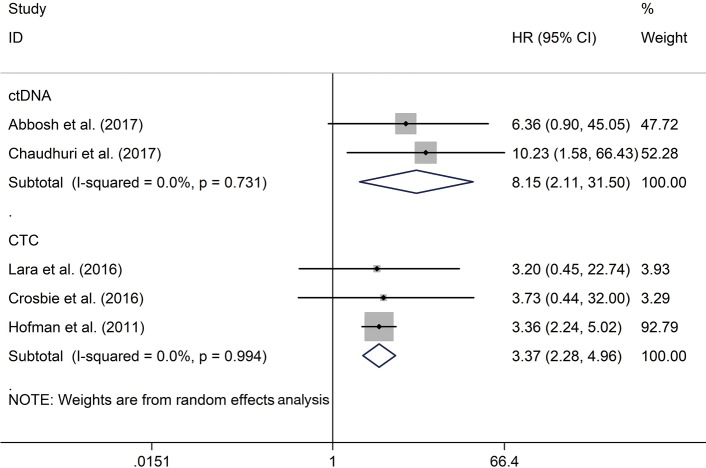
Both positive peripheral blood CTCs (HR, 3.37; 95% CI: 2.28–4.96; P<0.001) and ctDNA (HR, 8.15; 95% CI: 2.11–31.50; P=0.002) indicated poor prognosis for tumor relapse, with the use of random effect model (Figure 2).
Figure 2.
The correlation of CTCs/ctDNA with tumor recurrence in post-operative NSCLC patients. CTCs, circulating tumor cells; ctDNA, circulating tumor DNA; NSCLC, non-small cell lung cancer.
The correlation of CTCs/ctDNA with 1- and 2-year recurrence rate
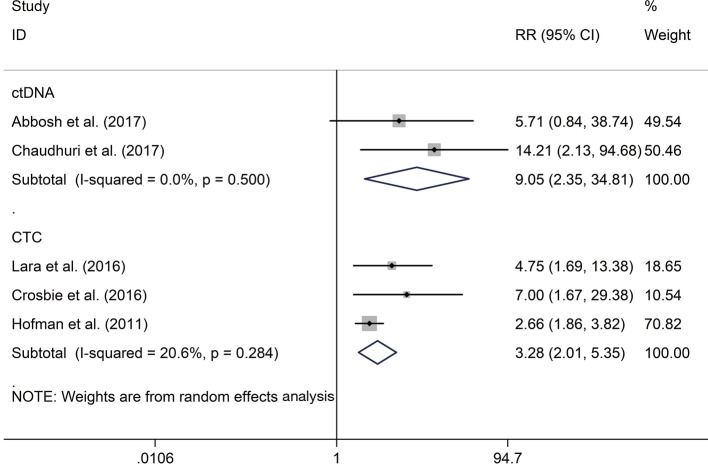
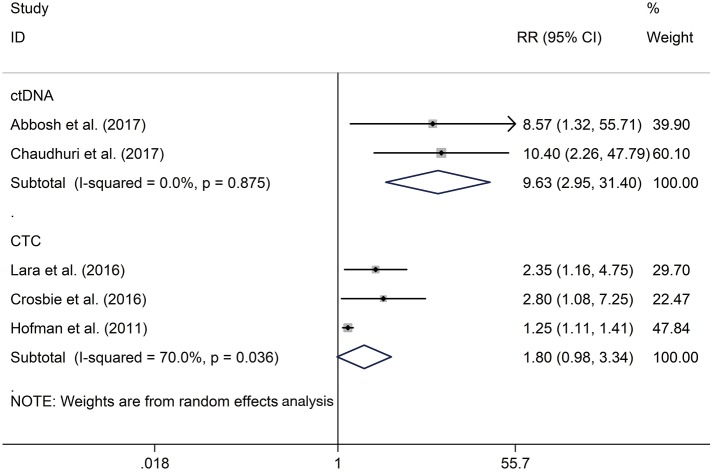
The RR of 1 (68% vs. 18.2%; RR 3.28; P<0.001) and 2 (76% vs. 44%; RR 1.80; P=0.06) years recurrence rate were higher in CTC positive group compared with the negative group, respectively. The same result were also observed in ctDNA positive versus negative groups of 1 (77.9% vs. 8.3%; RR 9.05; P=0.001) (Figure 3) and 2 (85.6% vs. 8.3%; RR 9.63; P<0.001) (Figure 4) years recurrence rate.
Figure 3.
The correlation of CTCs/ctDNA with one-year recurrence rate. CTCs, circulating tumor cells; ctDNA, circulating tumor DNA.
Figure 4.
The correlation of CTCs/ctDNA with two-year recurrence rate. CTCs, circulating tumor cells; ctDNA, circulating tumor DNA.
Discussion
Tumor recurrence after radical surgery of NSCLC patients is common. Regular low-dose computed tomography (LDCT) examination is recommended to monitor the tumor relapse, but there is no clinical biomarker so far that could better stratify high-risk individuals. Our pooled data indicated that positive CTCs (HR, 3.37; P<0.001) and ctDNA (HR, 8.15; P=0.002) indicated worse DFS of NSCLC patients. Higher recurrence rates were observed in CTCs/ctDNA positive group compared with the negative group both at 1 and 2 years after surgery. Thus, the dynamic monitoring of CTCs/ctDNA might be suggested as an evidence for predicting the neoplasm relapse. In addition, the higher HR value in ctDNA studies than in CTC studies, although not a direct comparison, suggested higher sensitivity of ctDNA in detecting micro-residuals.
With the development of molecular diagnosis and target therapy of NSCLC in the past few years, tumor-specific genomic and epigenetic alterations are usually recognized based on tissue either from surgery or biopsy. However, because of temporal and spatial heterogeneity, molecular information based on tissue is inaccuracy and inconvenience (21). Liquid biopsy therefore has been widely concerned by scientists owing to its obvious clinical advantages. CTCs are cells that from a primary tumor and are carried around the body in the circulation, which thus are recognized as seeds for the subsequent metastases in vital distant organs, leading to cancer-related deaths. ctDNA is tumor-derived small fragments of nucleic acid in the bloodstream, which can reflect the entire genetic profile of a tumor. Both CTCs and ctDNA have shown the potential to be a promising clinical biomarker for patient stratification, early diagnosis, disease monitoring and guide for therapy (22).
Nearly 30–70% NSCLC patients had surgery would eventually experience tumor recurrence or metastasis. Adjuvant chemotherapy is commonly recommended to high-risk stage IB and II–IIIA NSCLC patients after surgery owing to the significantly increase of OS rate, but only 4% to 5% of targeted patients would get profit (23). For patients those who harbor EGFR mutation, EGFR-TKIs could also be another candidate (24). However, it is also known that systemic adjuvant therapies own toxicities such as infection, neuropathy and secondary malignant tumor which can even lead to premature deaths. Unfortunately, neither guideline nor in the clinical trials, patients were not selected by clinical biomarkers in predicting their potential risk of recurrence, which probably since there are no enough tissue sample after radical surgery for monitoring the relapse, and liquid biopsy might be helpful.
Increasing evidence suggests that early recurrence of tumor in resected NSCLC patients may arise from CTCs that shed from the primary tumor into the vasculature or lymphatics since the beginning of the malignant process and are carried around the body in the circulation (17-19). Detection of peripheral CTCs may identify recurrence high-risk patients and could be a more specific indication for adjuvant therapy. There are several studies that evaluate the prognostic significance of CTCs detection in resected NSCLC cases after surgery. Hofman and colleagues (5) found the detection rate of CTCs in NSCLC patients after surgery was 49%, which is similar to ours. Furthermore, using ISET technique, they indicated that CTCs count of 50 or more is the independent negative factor for the disease relapse and OS. Another study by Rolle et al. (25) reported 30 patients with post-surgery NSCLC were examined using the MAINTRAC technique. They observed CTCs in 2 weeks and 5 months after surgery. The CTCs count was compared to prognosis and patients with continuously increasing in median CTC-count post-operatively were shown to be at a higher risk of tumor relapse. Sawabata et al. (4) observed that CTCs disappeared about 10 days after surgery was performed. In fact, it is well known that a majority of CTCs can be shed into the vascular or lymph system during operation, but it is still uncertain whether this part of CTCs are involved in the subsequent recurrence or metastasis. Thus Bayarri-Lara et al. (17) thought that in order to get a real assessment of CTCs presence, detection must be performed at least 3 weeks after the operation.
ctDNA, which carried whole genomic information, can be detected in the peripheral blood of patients with advanced cancer, acting as a potential noninvasive source to characterize the somatic mutation features of NSCLC (8). However limited data are available on whether ctDNA analyses would be applicable to early cancer, especially in monitoring tumor relapse, in part because the low tumor burden of micro-metastatic disease after radical surgery, which makes detection of ctDNA challenging. With the fast development of sequencing technique, several scientists tempted to characterize the feature the dynamic change of ctDNA in early stage of malignant tumor. In a prospective cohort of early breast cancer patients, detection of ctDNA in plasma after curative treatment, either at a single postsurgical time point or with serial follow-up plasma samples, predicted metastatic or relapse with high accuracy (HR =25.1; P<0.0001) (10). They show that tracking of ctDNA mutation, especially driver or clone mutation, can detect minimal residual disease noninvasively and identify earlier high risk patients of tumor relapse than the radiological or clinical recurrence. Tie et al. (11) used massively parallel sequencing–based assays to evaluate the ability of ctDNA to detect minimal residual disease in a prospective cohort of 230 stage II colon cancer patients. They concluded the same with previous study that ctDNA detection after surgery performed provided the direct evidence of residual disease and identifies patients at very high risk of recurrence. Compared with CTC, detection of which was restricted by the uneven distribution and rare existence as a complete form in the blood, ctDNA should theoretically have higher sensitivity. This was supported by our current results in terms of HR value comparison. However, limited in the sequencing technique, the false negative rate of ctDNA detection is still high, which need to improve in the future.
Besides CTCs/ctDNA, some epigenetic markers like DNA methylation might also be a candidate to predict the recurrence of disease. With the help of genome-wide array assessment, Kitchen and colleagues (26) performed a study in exploring the novel DNA methylation markers of clinical outcomes for bladder cancer. They found hyper-methylation of CpG cg11850659 and hypo-methylation of CpG cg01149192 in combination predicted HR-NMIBC recurrence and progression within 1 year of diagnosis with 83% sensitivity, 79% specificity, and 83% positive and 79% negative predictive values. Another study also revealed a methylation-independent loss of MYO5B expression in colorectal cancer that matched disease progression (27). Their data identify MYO5B as a powerful prognostic biomarker in colorectal cancer, especially in early stages (stages I and II), which might help stratifying patients with stage II for adjuvant chemotherapy. Hyper-methylated oncologic region in pancreatic cancer also could distinguish early stage I disease from normal tissue and was associated with worse prognosis (28). With the further promotion of technology and practice, tumor relapse could be better predicted in combination of radiological examination, genetic and epigenetic molecular biomarker, and thus the corresponding therapeutic plan could be given timely.
We acknowledge several limitations to our study. First, the small number of studies and samples is the primary limitation of the present article. Second, follow-up time is not enough in all included studies another shortcoming. Third, there is no data available of CTCs/ctDNA both before and after surgery. Last, we were unable to calculate the dose-response curve of the prognostic effect of CTCs/ctDNA. Further studies should focus more on the dynamic change of liquid biopsy result and provide a precise treatment recommendation.
Conclusions
Our study suggested both CTCs and ctDNA might be predictive biomarkers of early tumor recurrence in patients with operable NSCLC. Detection based on ctDNA seems to be more sensitive than CTCs. More prospective clinical study should be done to validate the practicability and economic efficacy of liquid biopsy in predicting tumor relapse.
Acknowledgements
Funding: Chinese National Natural Science Foundation (Grant No. 81501996); Key Project of Guangzhou Scientific Research Project (Grant No. 201804020030); Guangdong Doctoral Launching Program (Grant No. 2014A030310460); Doctoral Launching Program of Guangzhou Medical University (Grant No. 2014C27); and Key Project of Livelihood Technology of Guangzhou (2011Y2-00024).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. 10.6004/jnccn.2017.0050 [DOI] [PubMed] [Google Scholar]
- 2.Früh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol 2008;26:3573-81. 10.1200/JCO.2008.16.2727 [DOI] [PubMed] [Google Scholar]
- 3.Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017;545:446-51. 10.1038/nature22364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawabata N, Okumura M, Utsumi T, et al. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg 2007;55:189-92. 10.1007/s11748-007-0101-2 [DOI] [PubMed] [Google Scholar]
- 5.Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011;17:827-35. 10.1158/1078-0432.CCR-10-0445 [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Liao GQ, He P, Zhu H, et al. Detection of circulating cancer cells in lung cancer patients with a panel of marker genes. Biochem Biophys Res Commun 2008;372:756-60. 10.1016/j.bbrc.2008.05.101 [DOI] [PubMed] [Google Scholar]
- 7.Muinelo-Romay L, Vieito M, Abalo A, et al. Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment. Cancers (Basel) 2014;6:153-65. 10.3390/cancers6010153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu W, Yang Y, Zhang L, et al. Post surgery circulating free tumor DNA is a predictive biomarker for relapse of lung cancer. Cancer Med 2017;6:962-74. 10.1002/cam4.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42. 10.1093/annonc/mds138 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015;7:302ra133. 10.1126/scitranslmed.aab0021 [DOI] [PubMed] [Google Scholar]
- 11.Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 2016;8:346ra92. 10.1126/scitranslmed.aaf6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91-2. 10.1016/j.jcms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014;3:123-8. 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
- 16.Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337-51. 10.1002/sim.1303 [DOI] [PubMed] [Google Scholar]
- 17.Bayarri-Lara C, Ortega FG, Cueto Ladron de Guevara A, et al. Circulating Tumor Cells Identify Early Recurrence in Patients with Non-Small Cell Lung Cancer Undergoing Radical Resection. PloS One 2016;11:e0148659. 10.1371/journal.pone.0148659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crosbie PA, Shah R, Krysiak P, et al. Circulating Tumor Cells Detected in the Tumor-Draining Pulmonary Vein Are Associated with Disease Recurrence after Surgical Resection of NSCLC. J Thorac Oncol 2016;11:1793-7. 10.1016/j.jtho.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. 10.1002/ijc.25819 [DOI] [PubMed] [Google Scholar]
- 20.Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. 10.1056/NEJMoa1616288 [DOI] [PubMed] [Google Scholar]
- 21.Zhang YC, Zhou Q, Wu YL. The emerging roles of NGS-based liquid biopsy in non-small cell lung cancer. J Hematol Oncol 2017;10:167. 10.1186/s13045-017-0536-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y, Qiao G, Xu E, et al. Biomarkers for early diagnosis, prognosis, prediction, and recurrence monitoring of non-small cell lung cancer. Onco Targets Ther 2017;10:4527-34. 10.2147/OTT.S142149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther 2018;18:63-70. 10.1080/14737140.2018.1409624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. 10.1016/S1470-2045(17)30729-5 [DOI] [PubMed] [Google Scholar]
- 25.Rolle A, Gunzel R, Pachmann U, et al. Increase in number of circulating disseminated epithelial cells after surgery for non-small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: A preliminary report. World J Surg Oncol 2005;3:18. 10.1186/1477-7819-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitchen MO, Bryan RT, Emes RD, et al. HumanMethylation450K Array-Identified Biomarkers Predict Tumour Recurrence/Progression at Initial Diagnosis of High-risk Non-muscle Invasive Bladder Cancer. Biomark Cancer 2018;10:1179299X17751920. [DOI] [PMC free article] [PubMed]
- 27.Letellier E, Schmitz M, Ginolhac A, et al. Loss of Myosin Vb in colorectal cancer is a strong prognostic factor for disease recurrence. Br J Cancer 2017;117:1689-701. 10.1038/bjc.2017.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faleiro I, Apolonio JD, Price AJ, et al. The TERT hypermethylated oncologic region predicts recurrence and survival in pancreatic cancer. Future Oncol 2017;13:2045-51. 10.2217/fon-2017-0167 [DOI] [PubMed] [Google Scholar]