Abstract Abstract
The rice crop and associated ecosystems constitute a rich mosaic of habitats that preserve a rich biological diversity. Spiders are an abundant and successful group of natural predators that are considered efficient in the biocontrol of the major insect pests in agroecosystems. Spider diversity in different stages of the rice crop growth from eastern Uruguay was analysed. Field study was developed on six rice farms with rotation system with pasture, installed during intercropping stage as cover crop. Six rice crops distributed in three locations were sampled with pitfall and entomological vaccum suction machine. Sixteen families, representing six guilds, were collected. Lycosidae, Linyphiidae, Anyphaenidae and Tetragnathidae were the most abundant families (26%, 25%, 20% and 12%, respectively) and comprised more than 80% of total abundance. Other hunters (29%), sheet web weavers (25%) and ground hunters (24%) were the most abundant guilds. Species composition along different crop stages was significantly different according to the ANOSIM test. The results showed higher spider abundance and diversity along the crop and intercrop stages. This study represents the first contribution to the knowledge of spider diversity associated with rice agroecosystem in the country.
Keywords: Agroecology, Araneae , diversity, guilds composition, rice crop
Introduction
The study of biological diversity associated with agroecosystems has focused the attention of biologists in the last decades to produce sustainable crops. One of them has been the cultivation of rice, which is the most ancient form of intensive agriculture in the world (Fernando 1977). During 2016, more than 159 million hectares were cultivated in more than 100 countries (FAOSTAT 2016). Irrigated rice crops are seasonal temporary wetland ecosystems with a variable degree of agronomical management (Bambaradeniya et al. 2004). Throughout a single cultivation period, rice agroecosystem presents three major temporary ecological phases: aquatic, semiaquatic and dry (Fernando 1995).
Uruguay produced 1,348,301 tonnes of rice, becoming the seventh exporter country inthe world during the 2013/2014 season (ACA 2015). According to the Khush classification (Khush 1984), Uruguay cultivates rice in irrigated environments with a standing layer of water of 5 to 10 cm of depth. Rice cultivation in the country is mostly based on a rotational production system with perennial pastures, consisting in two years of rice crop, followed by four years of pastures, integrated with livestock production (ACA 2013). One of the more important cultivated areas is the eastern region of the country, located within the Bañados del Este, a wetland region which belongs to an internationally protected area by the RAMSAR convention. In this region of the country, rice cultivation area is commonly surrounded by others crops or pasture areas or remnants of native ecosystems like patches of riparian forests and wetlands (PROBIDES 1999).
The rice crop and associated ecosystems (natural environments or other crops) constitute a rich mosaic of habitats that preserve a high biological diversity (Roger 1996). This disturbance promotes intensive changes in the ecotones like processes of colonisation, migration, reproduction and higher rates of growth of organisms (Bambaradeniya 2000). Furthermore, the flood production system provides a temporal environment that is beneficial for the conservation of invertebrates and vertebrate species (Heong et al. 1991, Schoenly et al. 1996). Currently, the International Rice Research Institute (IRRI) aims to develop ecological engineering methods to strengthen the diversity of natural enemies and to increase the ecosystem services they provide (Norton et al. 2010). Some important components of this diversity are certain arthropod groups which participate as regulators of insect pest populations (Roger 1996), giving an ecological service provided by biodiversity of the rice ecosystem itself (Heinrichs and Barrion 2004, Naeem et al. 1999). Spiders constitute a megadiverse order with high value as biological control agents against the major insect pests in agroecosystems (Riechert and Lockley 1984, Norma-Rashid et al. 2014, Pompozzi et al. 2014). The ecosystem service provided by spiders as generalist predators in agroecosystems is supported in part by the availability of alternative habitats from which they take refuge and recolonise the crop after cultivation and the following growth stages (Hibbert and Buddle 2008).
A few studies related to spider diversity in agroecosystems and adjacent environments have been carried out in Uruguay (Simó et al. 2011, Jorge et al. 2013). Despite the fact that rice has been grown in Uruguay for so many years, there are no data about the spider fauna associated with this crop in the country and the impact on insect pest communities. Considering the role of this group, the knowledge of the assemblages of spider species present in the different rice phenological stages is crucial for the ecological management of the crop.
In the present paper, we study the spider diversity in different stages of the rice crop from eastern Uruguay, with the aim of identifying the changes along the crop cycle and to provide baseline information as a tool for evaluation of the impact of management practices on crop sustainability.
Materials and methods
Study area and crop management
The main area for rice production in Uruguay is located in the eastern region of the country (ACA 2015). It belongs to Laguna Merín basin, with rice and livestock farming as main production activities. The field study was conducted in rice farms of first and second year crop in a system rotation with pasture, installed during intercropping stage as cover crop. Sampling periods were performed considering intercropping and the phenological stages of the crop where the main rice pests are detected: post-seeding, tillering and grain filling. Post-seeding is an early stage during the first 20 to 30 days of the crop where the early seedling growth occurs. Tillering is a flooded stage that comprises the vegetative growth of the plant. Grain filling is also a flooded stage where immature grains of rice arise. Pastures were composed of a mix of Lotus corniculatus L. (Fabaceae), Lolium multiflorum Lam. (Poaceae) and Trifolium repens L. (Fabaceae). Native vegetation patches present in the study area correspond to the type riparian forest habitat with an average vegetation height of 4 metres. The most common floristic composition of this area is the hydrophitic species as Phyllantus sellowianus Müll. Arg. (Phyllanthaceae) immediate to the water line and an intermediate edge of transition species to pasture represented by Eryththrina cristagalli L. (Fabaceae), Scutia buxifolia Reiss. (Rhamnaceae), Celtis tala Gillies ex Planch. (Cannabaceae), Schinus longifolius (Lindl.) Speg. (Anacardiaceae), Myrcianthes cisplatensis (Cambess.) O. Berg (Myrtaceae), Tillandsia sp. (Bromeliaceae) (Muñoz et al. 2011). Three collecting sites were selected: Julio María Sanz (33°11'54.99"S, 54°5'12.30"W), El Tigre (33°13'27.80"S, 53°59'38.84"W) and General Enrique Martínez (33°12'8.15"S, 53°50'47.98"W) located in Treinta y Tres Department, eastern Uruguay (Fig. 1). According to the Köppen-Geiger classification, Uruguay belongs to the Cfa climate type, which corresponds to temperate climate without a dry season and the hottest month (January) with the temperature above 22°C (Peel et al. 2007). The soil type is a melanised solod of the “La Charqueada” Soil Unit. The crop was treated only with herbicide previous to sowing; no insecticide was applied on rice and pasture (Altamirano et al. 1976).
Figure 1.
A) Sampling locations Julio María Sanz (1), El Tigre (2) and General Enrique Martínez (3). B) View of the rice crop (RC) usually surrounded by native vegetation patches (NV)
Spider collection and data analysis
From November 2013 to November 2015, two rice paddies on each of the three collecting sites were sampled seasonally (one rice paddy of first and other of second year), resulting in six rice paddies in the whole work. Spiders were sampled with pitfall traps and an entomological vacuum suction machine. Fifteen pitfall traps were installed for each crop and set for a week. Each trap consisted of a 400 ml cup containing 100 ml conservative mix (8.5 volumes of distilled water, 1.5 volumes of acetic acid 4%, 1 volume NaCl). Ground and vegetation from the surrounded area of each pitfall trap (3 to 4 metres away) was sampled during one minute with the vacuum suction machine (fifteen samples per paddy). The collected material was kept in 70% ethanol. Specimens were identified at family and species/morphospecies level using keys and taxonomic revisions from literature (Grismado et al. 2014). Vouchers were deposited in the arachnological collection of Facultad de Ciencias, Universidad de la República, Uruguay. Only adults were considered for species/morphospecies identification. Guilds were assigned following Cardoso et al. (2011). EstimateS 9.1.0 (Collwell 2013) was used to calculate species accumulation curves for each collecting method and analytical richness estimators (Chao 1, Jacknife 1 and Bootstrap) in order to evaluate the sampling effort. Total number of spiders per sampling moment were compared using generalised linear-mixed model with Poisson distribution (PROC GLIMMIX, S.A.S. Institute 2009). Means were separated using Tukey-Kramer (p<0.05). To test statistic differences intaxonomic composition between the sampling moments, we used ANOSIM and SIMPER analysis performed with PAST 3.14 software (Hammer et al. 2001).
Results
A total of 16 families, 61 species/morphospecies and six guilds of spiders were registered (Table 1). From the 2088 spiders collected, 945 were adults (45%) and 1143 juveniles (55%). The most abundant spider families were Lycosidae, Linyphiidae, Anyphaenidae and Tetragnathidae (26%, 25%, 20% and 12%, respectively) that represented more than 80% of total relative abundance (Table 2).
Table 1.
Families and species collected in rice fields.
| Family | Species/morphospecies |
| Anyphaenidae | Acanthoceto acupicta |
| Arachosia magna | |
| Sanogasta maculatipes | |
| Araneidae | Alpaida veniliae |
| Alpaida versicolor | |
| Argiope argentata | |
| Araneidae sp1 | |
| Larinia bivittata | |
| Corinnidae | Mazax cf ramirezi |
| Ctenidae | Asthenoctenus borellii |
| Gnaphosidae | Camilina chilensis |
| Apopyllus silvestri | |
| Gnaphosidae sp1 | |
| Linyphiidae | Linyphiidae sp1 to sp9 |
| Scolecura propinqua | |
| Sphecozone ignigena | |
| Sphecozone sp1 | |
| Sphecozone sp2 | |
| Tutaibo sp1 | |
| Tutaibo sp2 | |
| Lycosidae | Agalenocosa velox |
| Allocosa sp1 | |
| Allocosa sp2 | |
| Allocosa sp3 | |
| Diapontia uruguayensis | |
| Lobizon corondaensis | |
| Lobizon humilis | |
| Lycosa cf thorelli | |
| Lycosa u-album | |
| Lycosinae sp1 | |
| Lycosinae sp2 | |
| Lycosa auroguttata | |
| Schizocosa malitiosa | |
| Miturgidae | Miturgidae sp1 |
| Oxyopidae | Oxyopes salticus |
| Pholcidae | Pholcidae sp1 |
| Salticidae | Hisukattus transversalis |
| Dendryphantes mordax | |
| Salticidae sp1 | |
| Salticidae sp2 | |
| Salticidae sp3 | |
| Tetragnathidae | Glenognatha lacteovitatta |
| Leucage volupis | |
| Tetragnatha sp1 | |
| Tetragnatha sp2 | |
| Theridiidae | Steatoda ancorata |
| Theridiidae sp1 | |
| Theridiidae sp2 | |
| Thymoites sp1 | |
| Thomisidae | Thomisidae sp1 |
| Thomisidae sp2 | |
| Titanoecidae | Goeldia luteipes |
| Trachelidae | Meriola cetiformis |
Table 2.
Family relative abundances during different crop stages.
| Family | Sampling moment | ||||||||
| post-seeding | tillering 1 | grain filling 1 | intercrop 1 | tillering 2 | grain filling 2 | intercrop 2 | Subtotal | % | |
| Anyphaenidae | 25 | 116 | 144 | 6 | 24 | 67 | 33 | 415 | 19.88 |
| Araneidae | 0 | 6 | 12 | 30 | 7 | 12 | 9 | 76 | 3.64 |
| Corinnidae | 2 | 0 | 0 | 0 | 1 | 0 | 2 | 5 | 0.24 |
| Ctenidae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.05 |
| Gnaphosidae | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 5 | 0.24 |
| Linyphiidae | 100 | 129 | 26 | 91 | 37 | 11 | 122 | 516 | 24.71 |
| Lycosidae | 30 | 197 | 53 | 32 | 65 | 59 | 114 | 550 | 26.34 |
| Miturgidae | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 3 | 0.14 |
| Oxyopidae | 0 | 4 | 1 | 1 | 1 | 1 | 24 | 32 | 1.53 |
| Pholcidae | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.05 |
| Salticidae | 9 | 35 | 49 | 2 | 4 | 11 | 25 | 135 | 6.47 |
| Tetragnathidae | 23 | 106 | 24 | 18 | 25 | 39 | 10 | 245 | 11.73 |
| Theridiidae | 2 | 8 | 16 | 9 | 4 | 1 | 11 | 51 | 2.44 |
| Thomisidae | 2 | 3 | 1 | 4 | 0 | 26 | 7 | 43 | 2.06 |
| Titanoecidae | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 4 | 0.19 |
| Trachelidae | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 6 | 0.29 |
| 198 | 606 | 330 | 193 | 173 | 229 | 359 | 2088 | 100 | |
Hisukattus transversalis Galiano, 1987, Lycosa auroguttata (Keyserling, 1891), Lobizon corondaensis (Mello-Leitão, 1941), Agalenocosa velox (Keyserling, 1891), Sphecozone ignigena (Keyserling, 1886), Camillina chilensis (Simon, 1902), Mazax ramirezi (Rubio & Danişman, 2014) and Arachosia magna are registered for the first time for Uruguay (Table 1).
Spider abundance adjusted to Poisson distribution and presented statistical differences between sampling periods, showing higher values in the intercrop stages and the lower values immediately after seeding (F= 24.22, df=562 p>0.0001, Fig. 2A). The discrete variable (number of spiders per sample) was adjusted to Binomial, Negative Binomial and Poisson distribution. The indicators used to compare the adjustments were the Akaike (AIC), Bayesian (BIC) criteria and the logarithm of the Maximum Likelihood (-2LMV). Poisson distribution had the best values for all the indicatiors mentioned above in all cases. Species richness per sampling period was higher at tillering of the first year and intercrop of the second year sampling (F=7.16, df=6, p<0.0036; Fig. 2B). Considering sampling done during crop presence, tillering stage richness values were higher than the grain filling stage.
Figure 2.

A) Mean number of spiders per sample. Different letters indicate significant differences compared by Tukey-Kramer test (F=24.22, df=562, p<0.0001). B) Mean spider species richness. Different letters indicate significant differences compared by Tukey_Kramer test (F=7.16, df=6, p<0.0036). Sampling periods: 1:post-seeding,; 2 and 5: tillering, 3 and 6: grain filling; 4 and 7: intercrop.
Other hunters, sheet web weavers and ground hunters were the more abundant guilds with 29%, 25% and 24% of relative abundance respectively (Fig. 3).
Figure 3.

Spider guilds relative abundances for the whole collecting period. OH: other hunters, AH: ambush hunters, SWW: sheet web weavers, SP: specialists, SW: space weavers, GH: ground hunters, OW: orb web weavers.
Species accumulation curve was non-asymptotic, indicating that there could be additional species to be sampled (Fig. 4). Richness estimators showed that at least 67% of the total expected species were sampled (Incidence based estimators: Jack 2: 91.17, 67%; Chao 2: 82.67, 74%; Bootstrap 69.85, 87%; Jack 1: 80.5, 76%; Abundance based estimator Chao 1: 79.04; 77%). Singletons represented 27.8% of the species collected, doubletons 13.1%, uniques 32.8% and duplicates 14.7%.
Figure 4.

Species accumulation curves of observed (S) and corrected richness (S est: 500 randomisations), singletons, doubletons, uniques and duplicates from the forty samples from different sampling moments: 1-6: post-seeding, 7-12, 23-28: tillering, 13-18, 29-34: grain filling, 19-22, 35-40: intercrop.
According to the SIMPER test comparing between collecting moments, Lycosa thorelli (Keyserling, 1877), Glenognatha lacteovitatta (Mello-Leitao, 1944), Scolecura propinqua (Millidge, 1991), Tetragnatha sp. 1, Diapontia uruguayensis (Keyserling, 1877), Sphecozone ignigena (Keyserling, 1886) and Sanogasta maculatipes (Keyserling, 1891) contributed to 57% of the observed dissimilarity (Suppl. material 1). Relative abundances of species by crop stage showed different patterns according to the species considered (Fig. 5). Arachosia magna (Rubio and Ramírez, 2015), Acanthoceto acupicta (Nicolet 1849) and Apopyllus silvestri (Simon 1905) were collected only in the rice crop. Meanwhile Oxyopes salticus (Hentz 1845), Alpaida versicolor (Keyserling 1877), Salticidae sp. 1 and Salticidae sp. 2, were collected only during the intercrop stage. Species composition for collecting moments were significantly different according to the ANOSIM test (R=0.544, p=0.0001 Jaccard index, R=0.433, p=0.0001 Morisita index).
Figure 5.
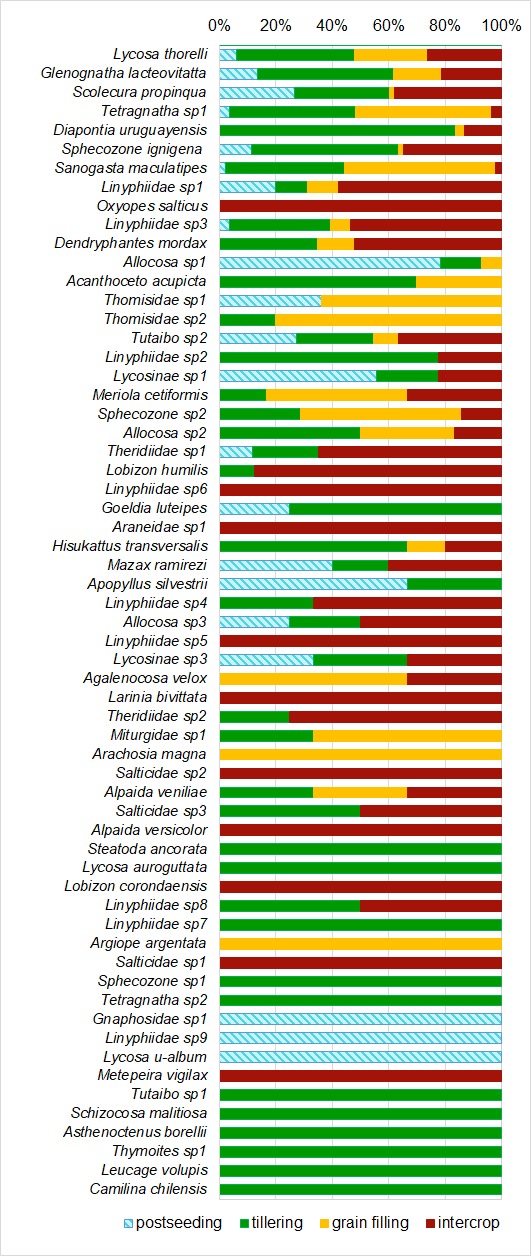
Relative abundances of the more abundant species according to crop stage.
Discussion
Total number of spiders per sample was lower in the early crop stages and increasing to the end of the crop cycle. Recently tilled fields had low vegetation complexity and represent a critical period for predator’s establishment (Ryptstra et al. 1999). The recovery of spider populations after disturbances in the field is achieved by reproduction, but immigration of surrounding habitats is also very important (Thorbek and Topping 2005). Therefore, surrounding habitats like pastures, other crops and riparian vegetation patches could serve as a reservoir of species that can recolonise the rice crop after the tillage or other management disturbances (Thorbek and Bilde 2004). Considering species richness during the crop cycle, the higher values observed at the tillering stages could be explained by the high intensity of spider colonisation from neighbouring environments. Beltramo et al. 2006 observed in sosybean crops from Argentina, that after soil disturbance, spiders with aerial dispersion promote recolonisation from surrounding habitats.
In this study, we confirm that rice crops serve as reservoirs for spider species that where recorded at different regional environments.
A. magna was reported in grasses and near streams from Argentina (Rubio and Ramírez 2015). According to this, the species was registered only for the grain filling stage, when rice plants are in flooded ground.
A. velox was registered in flooded grasslands from Argentina (Piacentini 2014). Similarly, in the rice crop, the species was collected in grain filling (flooded area) and intercrop stages (pastures).
L. corondaensis was reported for woodlands neighbouring grasslands from Argentina (Piacentini and Grismado 2009). Althougth a few specimens were collected in this study, they were registered in pasture and flooded stages of the rice crop.
Mazax ramirezi was collected in pitfall traps for grasslands from Buenos Aires, Argentina. In this study, all the exemplars were also obtained with this type of traps and the species was found throughout the rice cycle.
Lobizon humilis (Mello-Leitao, 1944) (Lycosidae) was registered mainly during the intercrop stage. The species was reported from Argentina (Piacentini and Grismado 2009) and prefers open grasslands (Rubio et al. 2008). Other records from data collection in Uruguay are associated with rocky hills and wetlands (Simó et al. 2015). Sphecozone ignigena (Linyphiidae) has been collected in rice crops in southern Brazil (Rodrigues et al. 2008). In this study, it was registered in all crop and intercrop stages, being more abundant during rice presence.
Sanogasta maculatipes (Anyphaenidae) was more abundant at tillering and grain filling stages in the rice cultivation, as it was also reported from Brazilian crops (Rodrigues et al. 2008). The species constructs refuges on foliage and grasses (Ramírez 2003) which explains its scarcity in the post-seeding stage of the crop, where vegetation complexity is scarce. Diapontia uruguayensis (Lycosidae) was registered throughout the whole cycle, but it was more abundant during the tillering stage when water has just arrived to the crop. This agrees with the fact that the species usually lives in association with water streams or flooded soils (Piacentini et al. 2017). The presence of Asthenoctenus borellii Simon, 1897 (Ctenidae) and Apopyllus silvestri (Gnaphosidae) during postseed and tillering stages was expected, considering these species have been reported in Uruguay from native but also from disturbed environments (Simó et al. 2000, Costa et al. 2006)
Glenognatha lacteovitatta (Tetragnathidae) was reported for alfalfa and wheat crops from Argentina (Armendano and González 2010, Armendano and González 2011). The species was found in all the stages surveyed in this study which suggests it colonises the initial stages of the crop from the surrounding habitats. Goeldia luteipes (Titanoecidae) was similar, being recorded in post-seeding and tillering stages.
The family Linyphiidae presented high species diversity and it was the second more abundant in this study. This result agrees with the results of Rodrigues et al. (2013) in rice crops from southern Brazil. Sphecozone ignigena was reported from rice crops in southern Brazil (Rodrigues et al. 2013) and, in the present study, it was recorded along the crop and the intercrop stages. This suggests that this species and other linyphiids represent an important part of the spiders colonising rice seedlings after crop installation.
The range percentage obtained for the richness estimators was nearly 70% to 87%, indicating that a comprehensive inventory would be reached (Cardoso 2009) and additional species are pending sampling.
Except for sensing web spiders, all the guilds proposed by Cardoso et al. (2011) were registered in this study. Other hunters (29%), sheet web weavers (25%) and ground hunters (25%) were the most abundant guilds in the crop. Other hunters were mostly represented by Anyphaenidae and Salticidae. Previous studies related to spider guilds structure in rice crop (Uetz et al. 1999, Rodrigues et al. 2008) registered ground hunters and sheet web weavers as the more abundant guilds. In the present study, they ranked as second and third most abundant guilds, mostly composed of Lycosidae and Linyphiidae, respectively. These families are commonly abundant in agroecosystems in many parts of the world and are mentioned as potential pest control agents (Nyffeler and Sunderland 2003). According to Jocqué and Dippenaar-Schoeman (2007), lycosids are supposed to have co-evolved with grasslands, which could explain the abundance of this group in the rotation system with pastures used in Uruguay, representing a fourth part of the total abundance at family level (Table 2). Additionally, the use of pitfall traps in this study can also explain the greater abundance of this family. These traps are considered a good method for collecting dwelling spiders (Green 1999). Linyphiids also represented a fourth part of the total abundance at family level in this study (Table 2). They are abundant in moderate temperatures and high humidity regions, where they spin sheet webs in tall herbs or close to the ground (Nyffeler and Sunderland 2003). The fourth mostabundant guild was the group of orb web weavers mostly represented by Glenognatha lacteovitatta (Tetragnathidae). This species was reported as a common species in alfalfa and wheat in Argentina (Armendano and González 2010, Armendano and González 2011). Some species of Glenognatha construct webs close to the soil surface (Hormiga and Döbel 1990) and specimens of this genus were reported in rice crops in Arkansas (Heiss and Meisch 1985). Ballooning present in linyphiid and tetragnathid species represent a good ability for dispersion, colonisation and survival to water contact (Hayashi et al. 2015), which is an advantageous characteristic for living in rice paddy fields.
This study represents the first contribution to the knowledge of spider diversity associated with rice crop agroecosystems in Uruguay. The results showed a high spider abundance and diversity throughout the crop and intercrop stages. Future research should focus on successional changes in the mosaic of landscapes of the region and evaluate the effects of management strategies on biodiversity, in order to promote its conservation and assure a sustainable rice crop production through natural biological control.
Supplementary Material
Dissimilarity Matrix SIMPER Test
L.Bao, M.Simó
Data type: Excel file
Brief description: Dissimilarity Matrix of SIMPER Test obtained with PAST 3.14 software (Hammer et al. 2001).
File: oo_198147.xlsx
Acknowledgements
To Héctor Da Fonseca, Jorge and Raúl Servetto, who kindly allowed us to conduct the research on their farms; and to Ing. Agr. Marcelo Segovia for his help on Charqueada farm selection and providing farm management information.
Funding program
Fellowship ANII POSNAC_2012_4459 L. Bao.
Hosting institution
Unidad de Entomología. Facultad de Agronomía. Universidad de la República. Garzón 780. CP 12900. Montevideo. Uruguay.
Funding Statement
Fellowship ANII POSNAC_2012_4459 L. Bao.
Funding program
Fellowship ANII POSNAC_2012_4459 L. Bao.
Hosting institution
Unidad de Entomología. Facultad de Agronomía. Universidad de la República. Garzón 780. CP 12900. Montevideo. Uruguay.
Author contributions
The paper was originally conceived by LB and MS. The study was designed by LB, MC and EC, LB, LC, SM and MPC carried out the field work. MS, LB, JG and AL identified the exemplars and performed the curatorial work. LB, MS and MC analyzed the data. LB and MS wrote the final version of the manuscript. All authors read and approved the final manuscript
References
- ACA Guía de buenas prácticas en el cultivo de arroz en Uruguay. http://www.aca.com.uy/manual-de-buenas-practicas-agricolas. [2018-02-26T00:00:00+02:00];
- ACA Informe arrocero – febrero 2015. http://www.uruguayxxi.gub.uy/informacion/wp-content/uploads/sites/9/2015/06/Informe-arrocero-Febrero-2015-Uruguay-XXI.pdf. [2018-02-01T00:00:00+02:00];
- Altamirano A., Da Silva H., Durán A., Echeverría A., Panario D., Puentes R. Clasificación de Suelos. Vol. 1. Ministerio de Agricultura y Pesca; Montevideo: 1976. 96 [Google Scholar]
- Armendano A., González A. Comunidad de arañas (Arachnida, Araneae) del cultivo de alfalfa (Medicago sativa) en Buenos Aires, Argentina. Revista de Biología Tropical. 2010;58(2):757–767. [PubMed] [Google Scholar]
- Armendano A., González A. Spider fauna associated with wheat crops and adjacent habitats in Buenos Aires, Argentina. Revista Mexicana de Biodiversidad. 2011;82:1176–1182. [Google Scholar]
- Bambaradeniya C. N.B. Ecology and biodiversity in an irrigated rice field ecosystem in Sri Lanka. PhD Thesis. University of Peradeniya; Sri Lanka: 2000. 525 [Google Scholar]
- Bambaradeniya C. N. B., Edirisinghe J. P., De Silva D. N., Gunatilleke C. V. S, Ranawana K. B., Wiekoon S. Biodiversity associated with an irrigated rice agro-ecosystem in Sri Lanka. Biodiversity and Conservation. 2004;13:1715–1753. doi: 10.1023/B:BIOC.0000029331.92656.de. [DOI] [Google Scholar]
- Beltramo J., Bertolaccini I., González A. Spiders of soybean crops in Santa Fe Province, Argentina: Influence of surrounding spontaneous vegetation on lot colonization. Brazilian Journal of Biology. 2006;66(3):891–898. doi: 10.1590/s1519-69842006000500015. [DOI] [PubMed] [Google Scholar]
- Cardoso P. Standardization and optimization of arthropod inventories—the case of Iberian spiders. Biodiversity Conservation. 2009;18:3949–3962. doi: 10.1007/s10531-009-9690-7. [DOI] [Google Scholar]
- Cardoso P., Pekár S., Jocqué R., Coddington J. A. Global patterns of guild composition and functional diversity of spiders. PLoS ONE. 2011;6(6):e21710. doi: 10.1371/journal.pone.0021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collwell R. K. EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. Persistent URL 2013
- Costa F. G., Simó M., Aisenberg A. Composición y ecología de la fauna epígea de Marindia (Canelones, Uruguay) con especial énfasis en las arañas: un estudio de dos años con trampas de intercepción. In: Menafra R., Rodríguez-Gallego L., Scarabino F., Conde D., editors. Bases para la conservación y el manejo de la costa uruguaya. Vida Silvestre Uruguay; Montevideo: 2006. [Google Scholar]
- FAOSTAT FAOSTAT Database. http://www.fao.org/faostat/ [2017-12-28T00:00:00+02:00];
- Fernando C. H. Investigation on the aquatic fauna of tropical rice fields with special reference to South East Asia. Geo-Eco-Trop. 1977;3:169–188. [Google Scholar]
- Fernando C. H. Rice fields are aquatic, semi-aquatic, terrestrial and agricultural: a complex and questionable limnology. In: Timotius K. H., Goltenboth F., editors. Tropical Limnology. Vol. 1. Satya Wacana University Press; Salatiga: 1995. 121-148 [Google Scholar]
- Green J. Sampling method and time determines composition of spider collections. Journal of Arachnology. 1999;27:176–182. [Google Scholar]
- Grismado C. J., Ramírez M. J., Izquierdo M. A. Araneae: Diversidad y clave de identificación de familias de la Argentina. In: Claps L. E., Roig-Juñent S. A., editors. Biodiversidad de artrópodos argentinos. Vol. 3. Universidad Nacional de Tucumán. Facultad de Ciencias Naturales; San Miguel de Tucumán: 2014. 55-93 [Google Scholar]
- Hammer Ř., Harper D. A.T., Ryan P. D. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4(1):9. [Google Scholar]
- Hayashi M., Bakkali M., Hyde A., Goodacre S. Sail or sink: novel behavioural adaptations on water in aerially dispersing species. BMC Evolutionary Biology. 2015;15:118. doi: 10.1186/s12862-015-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs E. A., Barrion A. T. Rice-feeding Insects and Selected Natural Enemies in West Africa: Biology, Ecology, Identification. International Rice Research Institute (IRRI); Manila: 2004. [Google Scholar]
- Heiss J. S., Meisch M. V. Spiders (Araneae) associated with rice in Arkansas with notes on species compositions of populations. The Southwestern Naturalist. 1985;30(1):119–127. doi: 10.2307/3670665. [DOI] [Google Scholar]
- Heong K. L., Aquino G. B., Barrion A. T. Arthropod community structures of rice ecosystems in the Philippines. Bulletin of Entomological Research. 1991;81:407–416. doi: 10.1017/S0007485300031977. [DOI] [Google Scholar]
- Hibbert A. C., Buddle C. M. Assessing the dispersal of spiders within agricultural fields and an adjacent mature forest. The Journal of Arachnology. 2008;36(1):195–198. doi: 10.1636/T07-14SC.1. [DOI] [Google Scholar]
- Hormiga G., Döbel H. A new Glenognatha (Araneae, Tetragnathidae) from New Jersey, with redescriptions of G. centralis and G. minuta. Journal Arachnology. 1990;18:195–204. [Google Scholar]
- Jocqué R,, Dippenaar-Schoeman AS. Spider Families of the World. Musée Royal de l’Afrique Central; Tervuren: 2007. 336 [Google Scholar]
- Jorge C., Laborda A., Simó M. Las arañas en plantaciones de Pinus taeda: su potencial uso como controladores biológicos. INIA, Serie Técnica. 2013;209:15–22. [Google Scholar]
- Khush G. S. In: Terminology for rice growing environments. International Research Institute; Manila: 1984. Terminology for rice growing environments. [Google Scholar]
- Muñoz J., Ross P., P. Cracco. Flora indígena del Uruguay: árboles y arbustos ornamentales. Editorial Hemisferio Sur. Hemisferio Sur; Montevideo: 2011. 320. Spanish. [Google Scholar]
- Naeem S., Chair F. S., Chapin III., Costanza R., Ehrlich P. R., Golley F. B., Hooper D. U., Lawton J. H., O´Neil R. V., Mooney H. A., Sala O. E., Symstad A. J., Tilman D. Biodiversity Ecosystem Functioning: Maintaining Natural Life Support Processes. Issues in Ecology. 1999;4:Ecological Society of America, Washington DC. [Google Scholar]
- Norma-Rashid Y., Azizi W. M., Zainudin W., Dzulhelmi M. N., Masduki N. Spiders as Potential Ecofriendly Predators Against Pests. In: Sahayaraj K., editor. Basic and Applied Aspects of Biopesticides. Springer; India: 2014. 245-254 [Google Scholar]
- Norton G. W., Heong K. L., Johnson D., Savary S. Rice pest management: issues and opportunities. In: Pandey S., Byerlee D., Dawe D., Dobermann A., Mohanty S., Rozelle S., Hardy B., editors. Rice in the global economy: strategic research and policy issues for food security. IRRI; Los Banos: 2010. 477 [Google Scholar]
- Nyffeler M., Sunderland K. D. Composition, abundance and pest control potential of spider communities in agroecosystems: A comparison of European and US studies. Agriculture Ecosystems and Environment. 2003;95:579–612. doi: 10.1016/S0167-8809(02)00181-0. [DOI] [Google Scholar]
- Peel M. C., Finlayson B. L., McMahon T. A. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences. 2007;11:1633–1644. doi: 10.5194/hess-11-1633-2007. [DOI] [Google Scholar]
- Piacentini L. N., Grismado C. J. Lobizon and Navira, two new genera of wolf spiders from Argentina (Aranea: Lycosidae) Zootaxa. 2009;2195:1–33. [Google Scholar]
- Piacentini L. N. A taxonomic review of the wolf spider genus Agalenocosa Mello-Leitão (Araneae, Lycosidae) Zootaxa. 2014;3790(1):1–35. doi: 10.11646/zootaxa.3790.1.1. [DOI] [PubMed] [Google Scholar]
- Piacentini L. N., Scioscia C. L., Carbajal M. N., Ott R., Brescovit A. D., Ramírez M. J. A revision of the wolf spider genus Diapontia Keyserling, and the relationships of the subfamily Sosippinae (Araneae: Lycosidae) Arthropod Systematics and Phylogeny. 2017;75(3):387–415. [Google Scholar]
- Pompozzi G. A., Ferretti N. E., Copperi M. S., Simó M., Ferrero A. A. Arthropod fauna of winter wheat of southwest Buenos Aires Province, Argentina. Munis Entomology and Zoology. 2014;9(1):182–190. [Google Scholar]
- PROBIDES . Plan Director. Reserva de Biosfera Bañados del. PROBIDES; Este, Uruguay. Rocha, UY: 1999. [Google Scholar]
- Ramírez M. The spider subfamily Amaurobioidinae (Araneae, Anyphaenidae): a phylogenetic revision at the generic level. Bulletin of the American Museum of Natural History. 2003;277:262. [Google Scholar]
- Riechert S. E., Lockley T. Spiders as biological control agents. Annual Review of Entomology. 1984;29:299–320. doi: 10.1146/annurev.en.29.010184.001503. [DOI] [Google Scholar]
- Rodrigues E. N., Mendoça M., Ott R. Fauna de aranhas (Arachnida, Araneae) em diferentes estágios do cultivo de arroz irrigado em Cachoeirinha, RS, Brasil. Iheringia, Série Zoologia. 2008;98(3):362–371. doi: 10.1590/S0073-47212008000300011. [DOI] [Google Scholar]
- Rodrigues E. N., Mendonça Jr M. S., Fritz L. L., Heinrichs E. A., Fiuza L. Effect of the insecticide Lambda-cyhalothrin on rice spider populations in southern Brazil. Zoologia. 2013;30(6):615–622. doi: 10.1590/S1984-46702013005000010. [DOI] [Google Scholar]
- Roger P. A. Biology and management of the floodwater ecosystem in rice fields. IRRI; P. O. Box 933, Manila 1099: 1996. 250 [Google Scholar]
- Rubio G. D., Minoli I., Piacentini L. N. Patrones de abundancia de cinco especies de arañas lobo (Araneae: Lycosidae) en dos ambientes del Parque Nacional Mburucuyá, Corrientes, Argentina. Brenesia. 2008;67:59–67. [Google Scholar]
- Rubio G. D., Ramírez M. J. Taxonomic revision of the American spider genus Arachosia (Araneae: Anyphaenidae) Zootaxa. 2015;3932:1–105. doi: 10.11646/zootaxa.3932.1.1. [DOI] [PubMed] [Google Scholar]
- Ryptstra A. L., Carter P. E., Balfour R. A., Marshall S. D. Architectural features of agricultural habitats and their impact on the spider inhabitants. Journal of Arachnology. 1999;27(1):371–377. [Google Scholar]
- S.A.S. Institute . SAS/STAT 9.2 User’s Guide. SAS Publishing, Cary, North Carolina; 2009. 524 [Google Scholar]
- Schoenly K., Cohen J. E., Heong K. L., Aquino G. B., Barrion A. T., Arida G. Food web dynamics of irrigated rice fields at five elevations in Luzon, Philippines. Bulletin of Entomological Research. 1996;86:451–466. doi: 10.1017/S0007485300035033. [DOI] [Google Scholar]
- Simó M., Vázquez V., Useta G. Estudio comparativo de la fenología y el hábitat de Ctenus taeniatus Keyserling 1891 y Asthenoctenus borellii Simon 1897 en el Uruguay (Araneae, Ctenidae) Boletín de la Sociedad Zoológica del Uruguay. 2000;2:12–32. [Google Scholar]
- Simó M., Laborda A., Jorge C., Castro M. Las arañas en agroecosistemas: bioindicadores terrestres de calidad ambiental. Revista del Laboratorio tecnológico del Uruguay. INNOTEC. 2011;6:51–55. [Google Scholar]
- Simó M., Nuñez M., Ojeda L., Laborda A., Queirolo D. Knowing the biological linkage: spider composition and guilds in a hill range of northern Uruguay. Boletín de la Sociedad Zoológica del Uruguay. 2015;24(2):117–129. [Google Scholar]
- Thorbek P, Bilde T. Declines of generalist arthropod predators due to mortality, emigration, or habitat disruption after tillage and grass cutting. Journal of Applied Ecology. 2004;41:526–538. [Google Scholar]
- Thorbek P., Topping C. J. The influence of landscape diversity and heterogeneity on spatial dynamics of agrobiont linyphiid spiders: an individual-based model. BioControl. 2005;50:1–33. doi: 10.1007/s10526-004-1114-8. [DOI] [Google Scholar]
- Uetz G. W., Halaj J., Cady A. B. Guild structure of spiders in major crops. Journal of Arachnology. 1999;27:270–280. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dissimilarity Matrix SIMPER Test
L.Bao, M.Simó
Data type: Excel file
Brief description: Dissimilarity Matrix of SIMPER Test obtained with PAST 3.14 software (Hammer et al. 2001).
File: oo_198147.xlsx



