Abstract
To make an informed choice to participate in a genome sequencing study that may yield primary and secondary findings, one understands relevant information in the context of personal values. Consent forms to enroll in a sequencing study can be long and complex. The efficacy of the professional encounter to consider the information contained in the consent form and make an informed choice is unknown. Women diagnosed with primary ovarian insufficiency and eligible for a sequencing study were randomized to participate in one of two encounters with a genetic counselor: a consent intervention using a lower literacy, less dense form or a standard consent encounter. Data were complete for 188 of 225 participants. The average time was 32 min for the intervention and 34 min for the standard, with the intervention encounter generating more questions from participants. At six weeks following consent, no differences were found between the two groups in primary outcomes: ‘sequencing benefits’ knowledge (d = 0.12, 95%CI: −0.03,0.27), ‘sequencing limitations’ knowledge (d = 0.04, 95%CI: −0.13,0.21), expected personal benefits (d = −0.01, 95%CI: −0.26,0.23), and decisional conflict (d = 0.04, 95%CI: −0.14,0.21). Although intentions to learn secondary variants were high, only 60% (113) of participants made an informed choice as defined by the multi-dimensional model of informed choice. We found that a modified consent intervention was as effective as a standard encounter and led to more interaction. Our data suggest that making decisions to receive secondary findings may be particularly challenging and in need of further investigation to achieve informed choice.
Introduction
Genome sequencing studies to pursue the cause of rare disorders and increasingly, common conditions, are prevalent [1]. Yet little is known about the most effective ways to consent eligible individuals to participate in this research. Several characteristics of genome sequencing distinguish it from consent to genetic testing; the potential for return of secondary findings and the scope of uncertainties that may be returned. The Presidential Commission for the Study of Bioethical Issues generated a report, “Informed Consent: Privacy and Progress in Whole Genome Sequencing” identifying desirable consent elements [2]. For NIH studies governed by the Common Rule and involving genome sequencing, the Commission recommended that consent forms include descriptions of the following:
Whole genome sequencing and analysis;
How the data will be used in the study and in the future;
The extent to which the participant will have control over future data use;
The benefits, potential risks, and unknown future risks;
What data and information might be returned to the individual.
Investigators have found some consistency between consent forms for genome sequencing used in a clinical setting [3]. They identify a minimum set of elements included in consent forms, which largely match those listed above. However, a review of the content of consent documents for genome sequencing in a research setting revealed significant variability, demonstrating the lack of consensus on essential elements when inviting participants to enter a research study [4]. In their review, Henderson and colleagues note a wide variation in the length (range: 2917–5757 words) and reading level (range: 9.4–11.7 Flesch-Kincaid Grade Level) across nine consent documents. This study found further heterogeneity in the categories of results described in these consent documents, and the handling of these results. For example, some consent forms stated that the result will be returned to participants without attaining participant preferences, whereas other consent forms stated that participant preferences would determine return of results.
While efforts to generate consent documents for genome sequencing address many, if not all, elements recommended by the Presidential Commission, evidence on the effectiveness of such documents in achieving informed consent to participate in genome sequencing for research is wanting. Even less evidence may be found on consenting participants to receipt of secondary findings and whether they made informed choices. Marteau and colleagues define informed choice as having relevant sufficient knowledge about the consequences of a decision and attitudes consistent with one’s decision [5]. Yet it is unclear what constitutes sufficient knowledge to consent to enroll in a genome sequencing study, making it difficult to assess whether informed choice has been achieved.
More broadly beyond genome sequencing studies, efforts have commenced to reduce the complexity of consent information provided to prospective participants in a variety of research contexts [6, 7]. Prior research has found that many individuals fail to comprehend consent information [8], and that consent documents have increased in length over time, with concerns raised that long consent forms are less likely to be read [9]. Further, the consent process can be burdensome for researchers or clinicians who are responsible for enrolling patients.
A valuable contribution to the evidence on the effectiveness of consent encounters can be made by studying the process in real time, within studies recruiting participants for genome sequencing. The National Institute of Child Health and Human Development’s (NICHD) Intramural Research Program has investigated primary ovarian insufficiency (POI) for 26 years (https://clinicaltrials.gov/ct2/show/NCT00001275). POI is a condition of women who develop oligo-amenorrhea for at least 4 months before the age of 40, with elevated serum gonadotropin levels in the menopausal range [10, 11]. The majority of POI cases are sporadic with unknown etiology. A genome sequencing study was approved by the NICHD IRB to identify novel gene variants that may play a role in causing or modifying onset of POI.
To contribute critical evidence to efforts currently underway to reduce the complexity of consent forms, we developed a lower literacy, less dense consent form which was informed by evidence from a variety of sources. We implemented a parallel randomized controlled study of two consent encounters in conjunction with the POI sequencing study. We aimed to determine whether an encounter using a lower literacy, less dense consent form could be used in the POI sequencing study, without compromising effectiveness as compared to an encounter using the standard consent form.
Methods
This study used a randomized two-factor design with randomization to compare a consent intervention to standard practice (protocol # NCT01927770). Participants affected with POI and enrolled in an ongoing POI study (protocol # NCT00001275) were contacted by an NIH investigator (LMN) and informed about their eligibility to independently participate in the parallel consent and genome sequencing studies. Participants were eligible for the consent study if they were adults (≥18 years of age) considering enrollment in the POI genome sequencing study. Participants had to be cognitively competent to consent and fluent in written and spoken English.
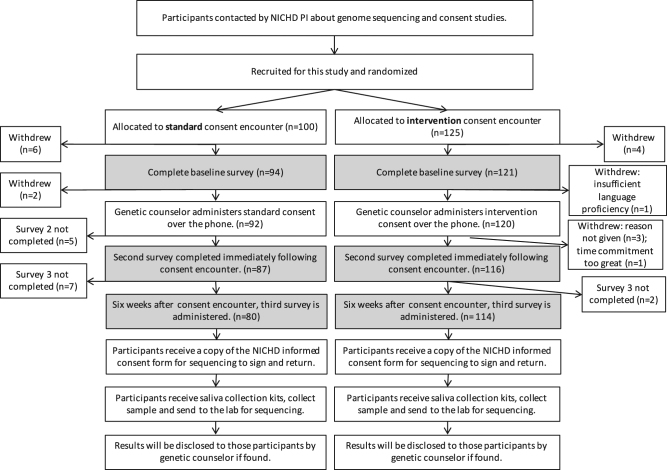
Participants were assigned to an allocation sequence which was randomly generated using an on-line computerized program (GraphPad) to one of two encounters (see Fig. 1). If they chose to participate in the consent study, they were contacted by a research fellow (PC) not involved in the consent process to be scheduled for the consent encounter. Participants were blinded to which encounter they received throughout the study and the randomized assignment was not known to the genetic counselor conducting the consent encounter prior to the day of execution.
Fig. 1.
Participant recruitment flow. Shading indicates where surveys were administered to collect data for this report
On the scheduled day, participants were sent a link to a web-based baseline survey that was completed prior to engaging with a genetic counselor in the consent process. The consent encounters were conducted over the phone by one of two genetic counselors and audio-recorded. In the time 24 h after the encounter, participants completed the second survey, and six weeks later the third survey. The surveys each took 10–15 min to complete.
Consent encounters
Both consent encounters were carefully scripted to follow the less dense or standard form. Both encounters could be implemented by any healthcare provider with appropriate training and familiarity with sequencing, for example, genetic counselors, research nurses, and research coordinators.
Intervention
Development of the novel, evidence-based consent intervention began with eliciting expert opinion from twelve NIH genetic counselors who regularly consent eligible participants to genome sequencing. Counselors reported following the standard NIH consent form closely and addressing any questions. Counselors were interested in a shorter form but felt the majority of information was central for participants to understand to make an informed decision.
To develop the novel consent form, we extracted essential content from the standard NIH consent form using the President’s Commission report for guidance. The consent form text was edited into short sentences with lower literacy language. To pilot test with the target population, we conducted qualitative content and process interviews with the first three participants immediately following the consent intervention. The feedback led to several modifications of the consent form by further simplifying word literacy. Overall the pilot participants had clear and accurate understanding of the essential content and few residual uncertainties. The final consent form is four pages of 1432 words. The Flesch-Kincaid grade level is 7.9 and it has a Flesch reading ease score of 63.6. (See supplementary materials).
Standard
The standard consent form and encounter mimicked usual care by NIH genetic counselors to consent individuals to genome sequencing studies. A group of twelve NIH genetic counselors were interviewed about their process and the forms used for their studies. Most counselors described systematically progressing through the consent form with eligible participants and answering questions. The content of the consent form had been endorsed by the NHGRI IRB for use in sequencing studies (https://www.genome.gov/27565449/the-informed-consent-resource/). As such, this consent encounter represents a usual care arm of the study. The standard consent form is six pages long and contains 2422 words. The Flesch-Kincaid grade level is 9.6 and has a Flesch reading ease score of 55.
Measures: decision to participate in the genome sequencing study
Genome sequencing knowledge scale
We assessed knowledge of genome sequencing using a scale developed by Kaphingst and colleagues [12]. The scale ranks understanding of benefits and limitations of genome sequencing. We used nine items of the Kaphingst et al. sequencing knowledge scale designed to assess knowledge and confidence in one’s understanding [12]. The 9-item knowledge scale was scored by assigning responses of ‘definitely yes’ a value of 2, ‘probably yes’ a value of 1, and ‘uncertain’, ‘probably no’ and ‘definitely no’ a value of 0. Four items were reverse scored so that ‘definitely no’ reflected a correct answer for a value of 2 and ‘probably no’ a value of 1. A mean score was calculated for the two subscales, ‘sequencing limitations’ (5 items) and ‘sequencing benefits’, (4 items). The Cronbach’s alpha for the limitations and benefits subscales was 0.81 and 0.74, respectively.
Perceived benefits
Perceived benefits were assessed at all three time points using a nine-item scale. The scale is scored on a Likert scale ranging from ‘strongly disagree’ (1) to ‘strongly agree’ (7). Higher scores indicate a higher perception of benefits from participating in the study. The Cronbach’s alpha was 0.82.
Intentions to participate
Participants were asked at the six-week follow-up survey “what decision have you made about participating in the POI sequencing study?” with response options ‘I plan to participate’, ‘I do not plan to participate at this time’, and ‘undecided’.
Decisional conflict scale
Decisional conflict was assessed in survey two and three regarding the choice to participate in the POI genome sequencing study. The decisional conflict scale developed by O’Connor examines several elements including lack of information about alternatives and their consequences, unclear values, and lack of support or advice [13]. The 16-item scale is scored on a Likert scale ranging from ‘strongly disagree’ (6) to ‘strongly agree’ (0). A low score on the decisional conflict scale indicates less decisional conflict about choosing whether to participate in genome sequencing. The Cronbach’s alpha was 0.95.
Measures: intentions to learn secondary findings
Attitudes scale
Participant’s attitudes toward learning secondary variant results were measured using a previously published 6-item scale [14]. Participants were asked: ‘for me, learning such a result would be;’ and responded based on a 1–7 rating for ‘a bad thing—not a bad thing,’ ‘not beneficial–beneficial’, ‘harmful–not harmful’, ‘not a good thing–a good thing’, ‘not worthwhile–worthwhile’, ‘unimportant–important.’ Higher scores were indicative of a positive attitude. The Cronbach’s alpha was 0.90.
Intentions to receive secondary variants
Intentions to learn secondary variants were measured by the sum of two questions as previously described in Facio et al. [14]. The participants were asked to think about the results they might receive from genome sequencing and consider if they would want to learn about a gene variant that predisposes them to a disease other than POI. Intentions were measured on a 5-point scale asking whether they intended to learn such a result, from definitely no, scored as ‘1’ to definitely yes, scored as ‘5’ and a 7-point scale asking how likely it is that they would choose to learn about such a result, from extremely unlikely, scored as ‘1’ to extremely likely, scored as ‘7’. The two items were summed to possible total score of 12. The Cronbach’s alpha was 0.69.
Informed choice
We used Marteau et al.’s multi-dimensional model of informed choice (MMIC) to assess participants’ intentions to learn secondary variants [15]. According to the MMIC, sufficient knowledge and behavior consistent with one’s attitude constitutes an informed choice. A lack of relevant knowledge or behavior incongruent with attitudes suggests a less informed choice. At the time of consent, we assessed intentions to learn secondary findings as a predictor of subsequent behavior (learning secondary findings). We defined sufficient knowledge as 75% (a score of 0.75 or greater) on both the benefits and limitations subscales, positive attitude as a score of 6 or greater, and positive intentions as a score of 9 or greater. As there are no published data to guide what constitutes sufficient relevant information for consent, we determined our cut-off through consultation with the same twelve NIH genetic counselors who provided input to the intervention development. Those who made an informed choice had sufficient knowledge with positive attitudes and positive intentions, or sufficient knowledge with negative attitudes and negative intentions to learn secondary findings [15].
Open-ended questions
All three surveys included questions that invited open-ended responses. These questions explored participants’ expectations, concerns, hesitations and further questions regarding participation in a genome sequencing study.
Data analysis
Descriptive statistics were initially calculated for the quantitative measures. Subsequently, t-tests were used to test for differences between the means of the variables. We assessed differences between randomization arms and at the three time points. Chi-squared tests (or Fisher’s exact) were used to compare participant characteristics and informed choice to receive secondary variants of the randomized groups. Statistical analyses were done using SPSS 21.0 (Released 2011. IBM SPSS Statistics for Macintosh, Version 20.0. Armonk, NY: IBM Corp).
Qualitative data were analyzed using content analysis. Two researchers (ET and ARH) developed a codebook for each question. Inter-rater agreement scores were calculated where consistencies were counted only if responses were coded using identical sets of codes [16]. Inter-rater agreement scores were high (89% or above) across all questionnaires and questions. Response proportions (percentages) were calculated using the number who responded to each question as the denominator (rather than the total number of participants) as assumptions cannot be made about the opinions of those who choose not to answer.
Results
Sample and randomization
Two hundred and twenty-five women affected with POI and enrolled in an NICHD natural history study were recruited for this study between December 2013 and January 2016. We used data from 188 of the 225 participants based on scale completion. The participants were on average 40 years old (39.71 ± 6.7), predominantly non-Hispanic white (81.9%), with a college (35.1%) or post graduate (53.2%) degree. Thirty participants (16%) reported having a family history of POI (Supplementary Table 1). Participant characteristics did not vary by randomization arm (all p > 0.05). Slightly fewer participants who did not complete all measures had a college (31.8%) or postgraduate (36.4%) degree compared to those included in final analyses (p = 0.03).
One hundred and ninety-four women completed all phases of the study up to the final survey; however, some returned surveys did not have sufficient data to be included in analysis. Of the 188 participants whose data are reported here, 109 underwent the consent intervention and 79 underwent the standard consent. The difference in number is due to: allocation to group by random number generation prior to participation, with a higher number of participants randomized to the intervention group participating; and a larger number of those randomized to the standard consent insufficiently completing surveys (Fig. 1). We hypothesize that this may be due to participants in the standard arm being less engaged in the consent process and thus, the study of the process than those randomized to the more interactive intervention arm.
Execution of the consent encounters
The average time for the intervention encounter was 32 and 34 min for the standard consent encounter. The time was similar because although the evidence-based written consent form was significantly shorter, it generated more questions from participants resulting in an interactive dialog for a similar length encounter. Although more questions were asked in the intervention encounters, the questions most often asked in both arms pertained to secondary variants.
Outcomes related to the decision to participate in genome sequencing
Sequencing knowledge
Overall, participants had high knowledge of both the limitations (mean, 1.32; SD, 0.58) and benefits (mean, 1.17; SD, 0.51) of sequencing at six weeks (see Table 1 for descriptive statistics by randomized arm). An increase in knowledge of sequencing limitations was observed over time. Among the total 188 participants, knowledge of sequencing limitations increased from a mean of 1.19 at baseline to 1.32 at 6-week follow-up p < 0.001.
Table 1.
Genome sequencing knowledge, perceived benefits, and decisional conflict scores by randomization arm. Total N = 188
| Measure | Intervention | Standard | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||||
| Range | Baseline | Immediate following | 6 weeks | Baseline | Immediate following | 6 weeks | |
| Knowledge subscale 1: sequencing limitations | 0–2 | 1.21 (0.54) | 1.28 (0.55) | 1.33 (0.58) | 1.17 (0.54) | 1.30 (0.59) | 1.29 (0.59) |
| Knowledge subscale 2: sequencing benefits | 0–2 | 1.22 (0.48) | 1.22 (0.48) | 1.22 (0.51) | 1.10 (0.47) | 1.05 (0.45) | 1.10 (0.49) |
| Perceived benefits | 1–7 | 4.74 (0.89) | 4.75 (0.86) | 4.70 (0.87) | 4.81 (0.76) | 4.62 (0.85) | 4.71 (0.84) |
| Decisional conflict | 0–6 | – | 0.80 (0.87) | 0.57 (0.66) | – | 0.67 (0.57) | 0.53 (0.51) |
There were no significant differences in knowledge of the limitations of sequencing between the two types of encounters at any time point. (Table 2) There was a small difference in knowledge of the benefits of sequencing between the two arms immediately after the consent encounter (d = 0.17, 95%CI: 0.03,0.31), though this difference was not observed after six weeks.
Table 2.
Differences on study outcomes between intervention arm and standard arm
| Measure | Baseline | Immediate following | 6 weeks | |||
|---|---|---|---|---|---|---|
| ∆ (95% CI) | p | ∆ (95% CI) | p | ∆ (95% CI) | p | |
| Knowledge subscale 1: sequencing limitations | 0.04 (−0.12 to 0.20) | 0.6 | −0.02 (−0.19 to 1.47) | 0.8 | 0.04 (−0.13 to 0.21) | 0.7 |
| Knowledge subscale 2: sequencing benefits | 0.12 (−0.02 to 0.26) | 0.09 | 0.17 (0.03 to 0.31) | 0.02 | 0.12 (−0.03 to 0.27) | 0.1 |
| Perceived benefits | −0.06 (−0.31 to 0.18) | 0.6 | 0.14 (−0.11 to 0.38) | 0.3 | −0.01 (−0.26 to 0.23) | 0.9 |
| Decisional conflict | − | 0.13 (−0.09–0.36) | 0.2 | 0.04 (−0.14 to 0.21) | 0.7 | |
Perceived benefits and intentions to participate
Overall, participants had relatively high expectations for perceived personal benefit from their genome sequencing results (mean, 4.69; SD, 0.85). No statistically significant differences in perceived personal benefits were observed over time. (Table 1) There were no significant differences in perceived personal benefit between the two arms at any time point. (Table 2)
Most participants (97%, n = 183) planned to enroll in the POI genome sequencing study with 1.6% (n = 3) not planning to enroll and 1.0% (n = 2) undecided.
Decisional conflict
Overall, decisional conflict scores were low (mean, 0.57; SD, 0.61), indicating low conflict about choosing to participate in a genome sequencing study. There were no significant differences in decisional conflict between the two types of encounter. (Table 2). Overall, there was an observed difference in decisional conflict over time. Decisional conflict was slightly higher overall at immediate follow-up (mean, 0.76; SD, 0.76) compared to 6-week follow-up (mean, 0.57; SD, 0.61; p < 0.001) suggesting a decrease in decisional conflict over time.
Outcomes related to the decision to receive secondary variants
Attitudes and intentions to receive secondary variants
Overall, participants had positive attitudes at six weeks toward learning secondary variants (mean, 6.48; SD, 0.93). There were no differences in attitudes between the two consent encounters, nor were there significant differences over time.
Overall, participants’ intentions at six weeks to receive secondary variants were high (mean, 10.94; SD, 1.60). A slight increase in intentions was observed from baseline (mean, 10.66; SD,1.60) to 6 weeks (p = 0.05). There were no differences in intentions to receive secondary variants between the two arms at 6 weeks (d = –0.01, 95%CI: –0.48,0.45).
Informed choice to receive secondary variants
Based on the data from the knowledge, attitudes and intentions scores, 113 (60%) participants made an informed choice to receive secondary variants, three of whom made an informed choice not to learn results (see Table 3). There was no statistically significant difference in informed choice between the two consent arms (p = 0.65).
Table 3.
Informed choice to receive results for secondary variants based on the multi-dimensional measure of informed choice model
| Intervention (N = 109) | Standard (N = 79) | Overall (N = 188) | ||||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||
| Sufficient knowledge (75% and over) | 83 (76) | 55 (70) | 138 (73) | |||
| Positive attitudes | Negative attitudes | Positive attitudes | Negative attitudes | Positive attitudes | Negative attitudes | |
| Positive intentions | 61 (56) | 14 (13) | 49 (62) | 5 (6) | 110 (58) | 19 (10) |
| Negative intentions | 5 (4) | 3 (3) | 1 (1) | 0 (0) | 6 (3) | 3 (2) |
| Overall informed choice | 64 (59) | 49 (62) | 113 (60) | |||
| χ2 = 0.21; p = 0.65 | ||||||
Participant survey 3 data used for analysis
Sufficient (+) knowledge defined as a score of 75% (i.e., ≥0.75 on benefits subscale and ≥0.75 on limitations subscale)
Positive (+) attitude defined as a score ≥6
Positive (+) intentions to receive secondary variants defined as a score ≥9
Open-ended responses
Residual questions immediately following the consent process
Immediately following the consent process most participants (77%, n = 102) had no residual questions about participating in the genome sequencing study. For those who had questions, frequently asked questions related to data use, personal benefits from the study and secondary variants.
Concerns about participating in a genome sequencing study
Most participants (67%, n = 87) had no concerns about partaking in a genome sequencing study. However, some (37%, n = 48) noted worry/anxiety related to receiving results, and concerns about the privacy of results, particularly in relation to insurance implications. These concerns were raised more often from those who were randomized to the intervention encounter (46%, n = 39) compared to the standard encounter (17%, n = 9).
“I have a concern that I’ll find out that I have a variant that predisposes me to another chronic health condition. I will handle it, and be grateful for the info, but it still is a concern.” [Participant 12213, standard arm]
“I became a little nervous when talking about the risks and privacy and all of this information being stored, I guess I don’t know what anyone would do with it, but it made me anxious.” [Participant 21213, intervention arm]
Six-week follow-up survey
When asked whether they had any questions after six weeks, most participants (73%, n = 92) stated they had no further questions. Those who reported questions referred to study timelines and what to expect next, including when results would be communicated to them.
Similarly, the majority of participants (81%, n = 94) noted that nothing was missing from the consent encounters, with some adding they felt it was “very comprehensive”.
Discussion
To the best of our knowledge, this randomized controlled study presents the first efficacy data from real-time consent encounters for genome sequencing. We demonstrated no significant differences in primary outcomes using validated scales between an encounter using a lower literacy, less dense consent form and a standard (usual care) consent encounter. This suggests that a genetic counselor encounter using a lower literacy, less dense form may be as effective as standard care in consenting individuals to genome sequencing studies. Given that the time was similar, the most notable distinction between the encounters was interactivity. Use of the lower literacy, less dense and shorter consent form lead to more questions by the patient and exchange between the two parties. Participants given less information from the form had more opportunity to ask questions specific to their needs leading to more gratifying and less routinized interactions for the genetic counselor. Both encounters yielded significant increases in relevant knowledge and low decisional conflict about the decision to participate.
Prior studies have reviewed documents in use and provided recommendations for required minimal information to convey to participants [3, 4], though the efficacy of use of the documents largely has been unexplored. One recent randomized trial compared concise and standard consent forms to enroll participants in a trial investigating antiretroviral therapy in HIV-infected patients, rather than genome sequencing [7]. Similar to our study, they found no differences in outcomes between consent arms. These results, together with our findings, support efforts to make consent forms shorter and with lower literacy demand. Further, our results suggest such changes in forms may lead to more interactivity within the encounter.
Our study demonstrated that only 60% of our participants made an informed choice to learn secondary findings, with no differences between arms. Secondary variants have been previously reported as one of a group of complex topics that experienced genetic counselors found to be challenging in consenting participants to genome sequencing studies [17]. Compared to primary findings, secondary findings are more likely to be difficult for potential participants to grasp. Our informed choice data, and open responses from participants support this finding. Further, a qualitative interview study found that potential participants focus more on the aim of identifying a primary finding and may attend less to rare secondary findings [18].
Counselors and other professionals consenting participants to genome sequencing may need to emphasize the consequences of learning a secondary variant; while the chances are quite low that one will be found, their health implications are significant. The forms used in our study contained minimal information about secondary findings. Provision of information about secondary findings and elicitation of preferences are topics currently under investigation. For example, researchers from Canada have developed an interactive, online decision aid to help individuals undergoing sequencing to make choices about receiving secondary findings [19].
While the field of research ethics has traditionally endeavored to separate research and clinical care, the use of genomics may blur the boundary between the two [20]. Our consent form was developed for use in the context of gene discovery research, where the primary purpose of the research is to create generalizable knowledge. However, as the use of genomic sequencing advances into clinical care, our consent form may be adapted for use in translational genomics studies, and other more clinical-based settings.
The open responses from our study suggest that the majority of participants were satisfied with the consent encounters. Six weeks following the consent encounter, most women had no further questions about participating in the sequencing study. A minority of participants asked what they could expect to happen next, wanted to learn more about the timeline of the study and when they could expect to hear from the research team. This finding points to the importance of continuing communication between the research team and research participants. The study protocol of the POI genome sequencing study includes quarterly electronic newsletters to provide research participants with progress updates.
Our findings should be interpreted in light of several limitations. Our sample was well educated, reflecting the demographic characteristics of individuals participating in genome sequencing studies [21]. However, as our participants were well educated, scoring relatively highly on baseline knowledge, we detected small increases over time. Future studies with lower literacy individuals may see more variation in outcomes and greater differences between interventions. Particular efforts should be directed at including individuals with lower literacy in future studies which may require innovative methods for recruitment given that such individuals are often underrepresented in medical research. Our study was limited to a specific population and efficacy of the intervention encounters may differ among other cohorts. As such, our results may not be generalizable to a broader population consenting to genome sequencing studies and further investigation of consent interventions is needed with more diverse samples.
Despite these limitations, our study has a number of strengths including novel data supporting the efficacy of a lower literacy, shorter consent form. This form can be assessed in future studies to investigate its efficacy against other consent interventions such as multimedia and web-based platforms which may allow for more tailored interactivity with opportunities for participants to alter consent elements in real time [22]. Further, we suggest that question asking by participants resulting in interactivity in the consent encounter may help to reduce the potential monotony of standard consent encounters for those administering the consent and in addition, foster shared decision making [23].
Conclusion
As genome sequencing is increasingly implemented, there is a need for evidence-based materials and processes. We designed a lower literacy, less dense consent form that should be validated in diverse populations but in the meantime can be implemented. In the absence of evidence for the efficacy of most consent processes, evidence from our study is an important first step. We encourage the development of reduced complexity, evidence-based consent forms that promote interaction. These advancements are vital to ensure the ethical integration of genome sequencing technologies in research.
Electronic supplementary material
Acknowledgements
We thank the women with POI and the NIH genetic counselors who participated in this study. This trial has been registered with ClinicalTrials.gov, trial number NCT01927770. This research was reviewed and approved by the National Human Genome Research Institute (NHGRI) and the National Institute of Child Health and Human Development (NICHD) Institutional Review Boards (IRBs) at the National Institutes of Health.
Funding
Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health funded this study
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41431-018-0105-7) contains supplementary material, which is available to authorized users.
References
- 1.Biesecker LG, Green RC. Diagnostic clinical genome and exome sequencing. N Engl J Med. 2014;370:2418–25. doi: 10.1056/NEJMra1312543. [DOI] [PubMed] [Google Scholar]
- 2.Gutmann A, Wagner J, Ali Y, Allen AL, Arras JD, Atkinson BF, et al. Privacy and progress in whole genome sequencing. Presidential Commission for the Study of Bioethical Issues. 2012.
- 3.Ayuso C, Millan J, Mancheno M, Dal-Re R. Informed consent for whole-genome sequencing studies in the clinical setting. Proposed recommendations on essential content and process. Eur J Hum Genet. 2013;21:1054–9. doi: 10.1038/ejhg.2012.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson G, Wolf S, Kuczynski K, Joffe S, Sharp RR, Parsons DW, et al. The challenge of informed consent and return of results in translational genomics: empirical analysis and recommendations. J Law Med Ethics. 2014;42:344–55. doi: 10.1111/jlme.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect. 2001;4:99–108. doi: 10.1046/j.1369-6513.2001.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleiberg H, Decoster G, de Gramont A, Rougier P, Sobrero A, Benson A, et al. A need to simplify informed consent documents in cancer clinical trials. A position paper of the ARCAD Group. Ann Oncol. 2017;28:922–30. doi: 10.1093/annonc/mdx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grady C, Touloumi G, Walker AS, Smolskis M, Sharma S, Babiker AG, et al. A randomized trial comparing concise and standard consent forms in the START trial. PLoS One. 2017;12:e0172607. doi: 10.1371/journal.pone.0172607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348:721–6. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- 9.Albala I, Doyle M, Appelbaum PS. The evolution of consent forms for research: a quarter century of changes. IRB. 2010;32:7–11. [PubMed] [Google Scholar]
- 10.Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, et al. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470–5. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- 12.Kaphingst KA, Facio FM, Cheng MR, Brooks S, Eidem H, Linn A, et al. Effects of informed consent for individual genome sequencing on relevant knowledge. Clin Genet. 2012;82:408–15. doi: 10.1111/j.1399-0004.2012.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor AM. Validation of a decisional conflict scale. Med Decis Mak. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 14.Facio F, Eidem H, Fisher T, Brooks S, Linn A, Kaphingst KA, et al. Intentions to receive individual results from whole-genome sequencing among participants in the ClinSeq study. Eur J Hum Genet. 2013;21:261–5. doi: 10.1038/ejhg.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michie S, Dormandy E, Marteau TM. The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns. 2002;48:87–91. doi: 10.1016/S0738-3991(02)00089-7. [DOI] [PubMed] [Google Scholar]
- 16.Carey J, Morgan M, Oxtoby M. Intercoder agreement in analysis of responses to open-ended interview questions: examples from tuberculosis research. Cult Anthropol Methods. 1996;8:1–5. [Google Scholar]
- 17.Bernhardt B, Roche M, Perry D, Scollon S, Tomlinson A, Skinner D. Experiences with obtaining informed consent for genomic sequencing. Am J Med Genet A. 2015;167A:2635–46. doi: 10.1002/ajmg.a.37256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergner AL, Bollinger J, Raraigh KS, Tichnell C, Murray B, Blout CL, et al. Informed consent for exome sequencing research in families with genetic disease: the emerging issue of incidental findings. Am J Med Genet A. 2014;164:2745–52. doi: 10.1002/ajmg.a.36706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shickh S, Clausen M, Mighton C, Casalino S, Joshi E, Glogowski E, et al. Evaluation of a Decision Aid for Incidental Genomic Results, the Genomics ADvISER: Protocol for a Mixed Methods Randomized Controlled Trial. BMJ Open 2018. in press. [DOI] [PMC free article] [PubMed]
- 20.Wolf SM, Amendola LM, Berg JS, Chung WK, Clayton EW, Green RC, et al. Navigating the research-clinical interface in genomic medicine: analysis from the CSER Consortium. Genet Med. 2017. 10.1038/gim.2017.137. [DOI] [PMC free article] [PubMed]
- 21.Lewis KL, Han PK, Hooker GW, Klein WM. Characterizing participants in the ClinSeq genome sequencing cohort as early adopters of a new health technology. PLoS One. 2015;10:1–11. doi: 10.1371/journal.pone.0132690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaye J, Whitley EA, Lund D, Morrison M, Teare H, Melham K. Dynamic consent: a patient interface for twenty-first century research networks. Eur J Hum Genet. 2015;23:141–6. doi: 10.1038/ejhg.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krumholz HM. Informed consent to promote patient-centered care. JAMA. 2010;303:1190–1. doi: 10.1001/jama.2010.309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.