Abstract
The pancreatic duct epithelium secretes the HCO3−-rich pancreatic juice. The HCO3− transport across the luminal membrane has been proposed to be mediated by SLC26A Cl−–HCO3− exchangers. To examine the electrophysiological properties of Cl−–HCO3− exchangers, we directly measured HCO3− conductance in the luminal membrane of the interlobular pancreatic duct cells from guinea pigs using an inside-out patch-clamp technique. Intracellular HCO3− increased the HCO3− conductance with a half-maximal effective concentration value of approximately 30 mM. The selectivity sequence based on permeability ratios was SCN− (1.4) > Cl− (1.2) = gluconate (1.1) = I− (1.1) = HCO3− (1.0) > methanesulfonate (0.6). The sequence of the relative conductance was HCO3− (1.0) > SCN− (0.7) = I− (0.7) > Cl− (0.5) = gluconate (0.4) > methanesulfonate (0.2). The current dependent on intracellular HCO3− was reduced by replacement of extracellular Cl− with gluconate or by H2DIDS, an inhibitor of Cl−–HCO3− exchangers. RT-PCR analysis revealed that the interlobular and main ducts expressed all SLC26A family members except Slc26a5 and Slc26a8. SLC26A1, SLC26A4, SLC26A6, and SLC26A10 were found to be localized to the luminal membrane of the guinea pig pancreatic duct by immunohistochemistry. These results demonstrate that these SLC26A Cl−–HCO3− exchangers may mediate the electrogenic HCO3− transport through the luminal membrane and may be involved in pancreatic secretion in guinea pig ducts.
Keywords: Bicarbonate, Duct, Exchanger, Pancreas, Patch-clamp, SLC26
Introduction
The pancreas plays a pivotal role in digestion. Pancreatic acini secrete digestive enzyme-rich neutral fluid that is not dependent on the presence of the CO2/HCO3−-buffer system. However, ducts secrete a HCO3−-rich fluid, which is dependent on the presence of CO2/HCO3−-buffer, and that neutralizes acid chyme in the duodenum [32]. The generally accepted model for HCO3− transport involves Cl−–HCO3− exchangers that operate in parallel with cAMP-activated Cl− channels [cystic fibrosis transmembrane conductance regulator (CFTR)] and Ca2+-activated Cl− channels, such as TMEM16A/ANO1, on the luminal membranes of duct cells [42, 49]. TMEM16A/ANO1 is also found specifically in the apical membranes of the acinar cells and is the critical channel for the control of acinar fluid secretion [33]. In addition, H+–K+ pumps and K+ channels are expressed on the luminal membrane of pancreatic ducts [11, 28, 45]. K+ channels are important for setting the resting membrane potential and for providing the driving force for anion transport, and may provide the transport partners for H+–K+ pumps [10].
Electrophysiological studies have found a luminal Cl− conductance in rat pancreatic ducts [6, 27]. Single-channel recordings revealed small-conductance Cl− channels on the luminal membrane of duct cells, which were identified as CFTR Cl− channels [6, 7]. The HCO3−/Cl− permeability ratios of CFTR Cl− channels have been reported as 0.1 to 0.4 [7, 29] and demonstrated to be increased to 1.0 by reducing the intracellular Cl− concentration in pancreatic duct cells [31]. Measurement of intracellular pH and membrane potential of guinea pig duct cells suggested that CFTR Cl− channels provide a significant pathway for HCO3− secretion [17].
Another pathway for HCO3− secretion across the luminal membrane is the Cl−–HCO3− exchanger, which has been identified as solute carrier family 26 member A6 (SLC26A6) [16, 20, 48]. SLC26A6 has been found to be electrogenic with a 1Cl−/2HCO3− exchange stoichiometry in Xenopus oocytes and HEK 293 cells [19, 38]. Consistently with this, deletion of Slc26a6 altered the overall stoichiometry of apical Cl−–HCO3− exchange in native mouse interlobular ducts, suggesting the upregulation of a Cl−–HCO3− exchanger with different stoichiometry [41]. Previous studies have demonstrated a functional coupling between CFTR Cl− channels and Cl−–HCO3− exchange activity in isolated pancreatic interlobular ducts [15, 43]. Furthermore, a computational model suggested that the HCO3−/Cl− permeability ratio of apical Cl− channels of 0.4 was able to support HCO3− secretion [50]. However, few studies have examined the electrophysiological properties and regulation of HCO3− conductance across the luminal membrane of native pancreatic duct cells.
The aim of the present study was to identify HCO3− conductance that is important for pancreatic secretion. For this purpose, we directly measured HCO3− currents through the luminal membrane of guinea pig pancreatic duct cells using the patch-clamp method in the inside-out configuration. We demonstrated that the inward conductance is dependent on intracellular HCO3− and extracellular Cl−, and is blocked by H2DIDS, an inhibitor of anion transporters, and thus conclude that such inward conductance is carried out via anion exchangers on the luminal membrane. Furthermore, we report the expression and localization of the SLC26A family in the interlobular and main pancreatic duct using molecular biological and immunohistochemical analyses.
Methods
Preparation of pancreatic duct cells from guinea pigs
Female Hartley guinea pigs (240–450 g, n = 35) were sacrificed by carbon dioxide stunning in accordance with protocols approved by the Animal Experimentation Committee, Kansai Medical University. Pancreatic ducts were isolated by enzymatic digestion and microdissection from the pancreas as previously described [12]. The pancreas was removed, and digested with collagenase (Type IV, 124 U/ml; Worthington) and trypsin inhibitor (0.01%; Sigma) in Tyrode solution at 37 °C for 1 h with vigorous shaking. Tyrode solution contained the following (in mM): 140 NaCl, 0.33 NaH2PO4, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 5 HEPES, and 5.5 D-glucose; pH was adjusted to 7.4 with NaOH. Interlobular and intralobular ducts (outside diameter of 30–60 μm) were microdissected under a stereomicroscope. The ducts were washed in Tyrode solution and then placed on coverslips pretreated with Cell-Tak (BD Biosciences). In order to allow patch-clamp access to the luminal membranes of the lining of epithelial cells, the ducts were split open by patch pipettes.
Patch-clamp recording
Standard patch-clamp techniques were used. Patch pipettes, pulled from capillaries of hard borosilicate glass (G-1.5; Narishige), had a resistance of 5–7 MΩ when filled with a standard N-methyl-D-glucamine (NMDG)-Cl solution. The standard NMDG-Cl solution contained the following (in mM): 130 NMDG, 130 HCl, 5 EGTA, and 10 HEPES; pH was adjusted to 7.4 with NMDG. The stripped duct was bathed in a standard bicarbonate solution consisting of the following (in mM): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 25 NaHCO3, 10 HEPES (pH 7.4, adjusted with NaOH), and 5.5 D-glucose. The standard bicarbonate solution was equilibrated with 5% CO2 in O2. The membrane potential was corrected for the liquid junction potential at the tip of the patch pipette in the bathing solution, and for that at the tip of the indifferent reference electrode filled with Tyrode solution and placed in the bath. Experiments were conducted at 23–30 °C. After the inside-out configuration was established, the solution in the perfusion chamber was switched to control bicarbonate solution. The control bicarbonate solution contained the following (in mM): 130 KHCO3, 5 EGTA, and 10 HEPES; pH was 7.8–8.0 after adding bicarbonate. A standard chloride solution contained the following (in mM): 130 KCl, 5 EGTA, and 10 HEPES; pH was adjusted to 7.8 with KOH. To record the HCO3− selective conductance, the control bicarbonate solution was mixed with the standard chloride solution to make different concentrations (0, 16, 33, 65, and 130 mM) of HCO3− around pH 7.8–8.0. To test the anion selectivity, KHCO3 in the control bicarbonate solution was replaced with anions such as KCl, K-gluconate, K-methanesulfonate, K-thiocyanate, or KI at pH 7.8. The concentration of free Ca2+ was calculated using the MaxChelator computer program. 4,4′-Diisothiocyano-2,2′-dihydrostilbenedisulfonic acid disodium salt (H2DIDS; Toronto Research Chemicals) was directly dissolved at 0.5 mM in control bicarbonate solution. 4-[[4-Oxo-2-thioxo-3-[3-trifluoromethyl)phenyl]-5-thiazolidinylidene]methyl]benzoic acid (CFTRinh-172; Santa Cruz Biotechnology) and 2-methyl-8-(phenylmethoxy)imidazo[1,2-a]pyridine-3-acetonitrile (Sch28080; Santa Cruz Biotechnology) were dissolved in DMSO at a 1000-fold concentration for application. The current was recorded in the inside-out configuration using the EPC 800 patch-clamp amplifier (HEKA). The amplifier was driven by Clampex 9 (Axon) in order to allow the delivery of a voltage-ramp protocol with concomitant digitization of the current. The membrane potential was generally held at 0 mV, and the command voltage was varied from − 80 to + 80 mV over a duration of 800 ms every 10 s.
RT-PCR analysis
RNA was extracted from the interlobular (outside diameter of 50–150 μm) and main ducts (outside diameter of around 500 μm) from three independent guinea pigs using the RNeasy Plus Micro kit with DNase I (Qiagen). RT-PCR analysis was performed using the OneStep RT-PCR kit (Qiagen) with primers designed to recognize different types of transporters (Table 1). For the negative control of reverse transcription, we used Taq DNA Polymerase (Promega). The amplification parameters used were as follows: 1 cycle at 50 °C for 30 min and 1 cycle at 95 °C for 15 min, followed by 40 cycles at 94 °C for 30 s, 57 °C for 30 s, 72 °C for 30 s, and 1 cycle at 72 °C for 10 min. The transcripts were subsequently verified by agarose gel electrophoresis.
Table 1.
Primer sets used for guinea pig pancreatic duct in RT-PCR analysis
| Gene (subunit) | Size (bp) | |
|---|---|---|
| Cftr | ||
| Forward: | 5′-GCTTAAAAGGACTATGGACACT -3′ | 623 |
| Reverse: | 5′-ACCTTCAGTGTTCAGCAGTCT -3′ | |
| Gapdh | ||
| Forward: | 5′-CAAAAGGGTCATCATCTCTGC -3′ | 610 |
| Reverse: | 5′-GCCGAACTCATTGTCATACCA -3′ | |
| CA2 | ||
| Forward: | 5′-AGCCTCTGCACCTTCACTATG -3′ | 535 |
| Reverse: | 5′-AACATCTGCTCACTGCTTACG -3′ | |
| Slc26a1 | ||
| Forward: | 5′-CTACTCTGTCCGTGCCAACCA -3′ | 914 |
| Reverse: | 5′-ACAGCTGCTCATCCTCCATTC -3′ | |
| Slc26a2 | ||
| Forward: | 5′-GGGGTTGGTTTTTCTATGTTTTG -3′ | 554 |
| Reverse: | 5′-AAACCCACCGCTTCATACACG -3′ | |
| Slc26a3 | ||
| Forward: | 5′-GTATGAGCCAGAAGGAGTGAA -3′ | 459 |
| Reverse: | 5′-TACACATCTACATTTATCCTTGC -3′ | |
| Slc26a4 | ||
| Forward: | 5′-AAACATCCCCACCACAGACAT -3′ | 558 |
| Reverse: | 5′-AAACCACATTGCTCCATCTGC -3′ | |
| Slc26a5 | ||
| Forward: | 5′-GTGACCTTGCTCTCGGGAAT -3′ | 621 |
| Reverse: | 5′-GAAGAGGGAGCCGATGGAAT-3′ | |
| Slc26a6 | ||
| Forward: | 5′-TCGGTCCTCAGCCACTTTGTA -3′ | 544 |
| Reverse: | 5′-ATGCTGCTTGGTGATAGATGC-3′ | |
| Slc26a7 | ||
| Forward: | 5′-CCCCAATGAACCTCCTGTCTG-3′ | 713 |
| Reverse: | 5′-AAGTAGGTGATTAGTGGCATTC-3′ | |
| Slc26a8 | ||
| Forward: | 5′-TCGGGGCTTGGTCGTCTTG-3′ | 646 |
| Reverse: | 5′-AGGTTGATAGATGGGCTGGTA-3′ | |
| Slc26a9 | ||
| Forward: | 5′-CTATCTGTACCCTCTCCCTAA -3′ | 721 |
| Reverse: | 5′-AACGAGGGTATGGAAGGTAAC -3′ | |
| Slc26a10 | ||
| Forward: | 5′-ACTTTGCTGTGTGGATGGTCA-3′ | 550 |
| Reverse: | 5′-GCATCCTGGACACTCACAAAC-3′ | |
| Slc26a11 | ||
| Forward: | 5′-CAGGCAGCTTTGGGCGGAC-3′ | 566 |
| Reverse: | 5′-AGAGAAAACCAGGGAGACACC-3′ | |
Immunolocalization
Immunolocalization was performed on the guinea pig pancreas. The pancreas was obtained from female Hartley guinea pigs (n = 3) in accordance with protocols approved by the Animal Experimentation Committee, Kansai Medical University. The guinea pigs were anesthetized with isoflurane and a mixture of medetomidine (0.5 mg/kg body weight), midazolam (5.0 mg/kg b.w.), and butorphanol (2.5 mg/kg b.w.), and perfused transcardially with 4% paraformaldehyde. The pancreas was fixed with 4% paraformaldehyde in PBS for 24 h, embedded in paraffin, and sectioned. Detailed methods for immunohistochemistry were described previously [12]. Briefly, autofluorescence was blocked by 0.1 M Tris-glycine. Non-specific binding was blocked with 2% normal donkey serum in PBS. Preparations were subsequently incubated with primary antibodies for SLC26A1, SLC26A3, SLC26A4, SLC26A6, or SLC26A10 (Table 2), along with Ezrin (1:400 to 1:800, clone 3C12, MS-661; Lab Vision) and PECAM-1 (platelet endothelial cell adhesion molecule-1, 1:800, sc-1506; Santa Cruz Biotechnology) in immunoreaction enhancer solution (Can Get Signal immunostain; Toyobo) overnight at 4 °C. Secondary antibodies conjugated to Alexa488 (SLC26A), Alexa568 (Ezrin), or Alexa647 (PECAM-1) (1:400; Molecular Probes) were added for 1 h. For the negative control, the primary antibodies were pre-absorbed with corresponding antigens for SLC26A1 (APrEST81987; Atlas), SLC26A10 (APrEST84901), SLC26A4 (synthesized peptide; Eurofins Genomics), or SLC26A6 (synthesized peptide) for 30 min at room temperature. In the controls, the primary antibodies were omitted and scanning was performed using the same settings. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml. Fluorescence was observed with a confocal laser scanning microscope (LSM510 META; Carl Zeiss).
Table 2.
Antibodies used for guinea pig pancreatic duct in immunohistochemistry (IHC) and western blotting (WB)
| Protein (accession) | Antigen | Correlation with guinea pig | Dilution | Catalogue number (manufacturer) | |
|---|---|---|---|---|---|
| IHC | WB | ||||
| SLC26A1 (NP_071325) | 518–587 | 77% | 1:100 | 1:500 | HPA041654 (Atlas) |
| SLC26A3 (NP_000102) | 617–733 | 85% | 1:100 | N/A | HPA036055 (Atlas) |
| SLC26A4 (NP_000432) | 317–344 | 93% | 1:200 | 1:1000 | bs-6787R (Bioss antibodies) |
| SLC26A6 (NP_075062) | 438–484 | 83% | 1:200 | 1:1000 | bs-20817R (Bioss antibodies) |
| SLC26A10 (NP_597996) | 427–487 | 75% | 1:200 | 1:100 | HPA044719 (Atlas) |
Western immunoblotting
The pancreatic duct was dissected from three independent guinea pigs as described above. The ducts were washed with cold PBS, treated with trichloroacetic acid (10%) on ice for 30 min, and then centrifuged. The pellet was solubilized in lysis buffer containing urea (9 M), Triton X-100 (2%), dithiothreitol (1%), and lithium dodecyl sulfate (2%). The samples (30 μg/lane protein) were fractionated on SDS polyacrylamide gel (7.5%), electroblotted to PVDF membranes (Merck Millipore), blocked with skim milk (1%), and reacted with anti-SLC26A4, anti-SLC26A6, or anti-SLC26A10 antibodies (Table 2). For anti-SLC26A1, we used signal enhancer Hikari solution (Nacalai Tesque, Kyoto, Japan). The reaction was visualized with a secondary antibody labeled with alkaline phosphatase (Promega).
Statistics
Data are shown as means ± SEM. A one-way analysis of variance (ANOVA) or Student’s paired t test was applied, and P < 0.05 was considered significant. Data were analyzed in Igor or Microsoft Excel.
Results
Bicarbonate conductance through the luminal membrane of the interlobular pancreatic duct cells
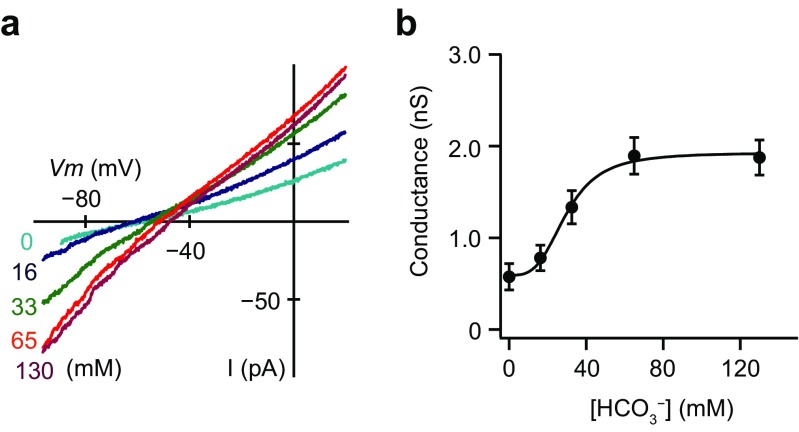
We recorded macroscopic currents from excised inside-out patches from the luminal membrane of the interlobular pancreatic duct cells of guinea pigs under unstimulated conditions. Figure 1a shows the macroscopic current–voltage (I–V) relationships in the presence of intracellular HCO3− at different concentrations (0, 16, 33, 65, and 130 mM). As we used the standard NMDG-Cl pipette solution and the bathing solution containing KHCO3, the inward current was due to HCO3− efflux through the luminal membrane. The inward conductance determined from the linear section of the I–V relationships (from − 80 to − 60 mV) increased with intracellular HCO3− concentration (Fig. 1a). The linear plot of conductance with the HCO3− concentration had a sigmoid relationship (Fig. 1b). The half-maximal effective concentration (Kd) value for the effects of HCO3− and Hill coefficient were 31.5 ± 5.1 mM and 3.5 ± 0.4 (n = 5), respectively. We also measured inward HCO3− currents in the bathing solution containing 130 mM NaHCO3. The inward conductance increased to 2.04 ± 0.95 nS in NaHCO3 from 0.34 ± 0.08 nS in NaCl (data not shown; n = 13). Thus, there was a minor contribution of K+ conductance under unstimulated conditions.
Fig. 1.
Bicarbonate conductance through the luminal membrane of the interlobular pancreatic duct cells. a Macroscopic current–voltage (I–V) relationships recorded from the luminal membrane of the pancreatic duct cells in the inside-out configuration with the standard NMDG-Cl pipette solution at different intracellular HCO3− concentrations. Inward conductance attributed to HCO3− efflux increased with HCO3− concentration from 0 to 130 mM. b Linear plot of conductance by the HCO3− concentration. The solid line is the fit by the Hill equation with the half-maximal effective concentration of 31.5 ± 5.1 mM and a Hill coefficient of 3.5 ± 0.4 (n = 5)
Ion selectivity of the bicarbonate conductance
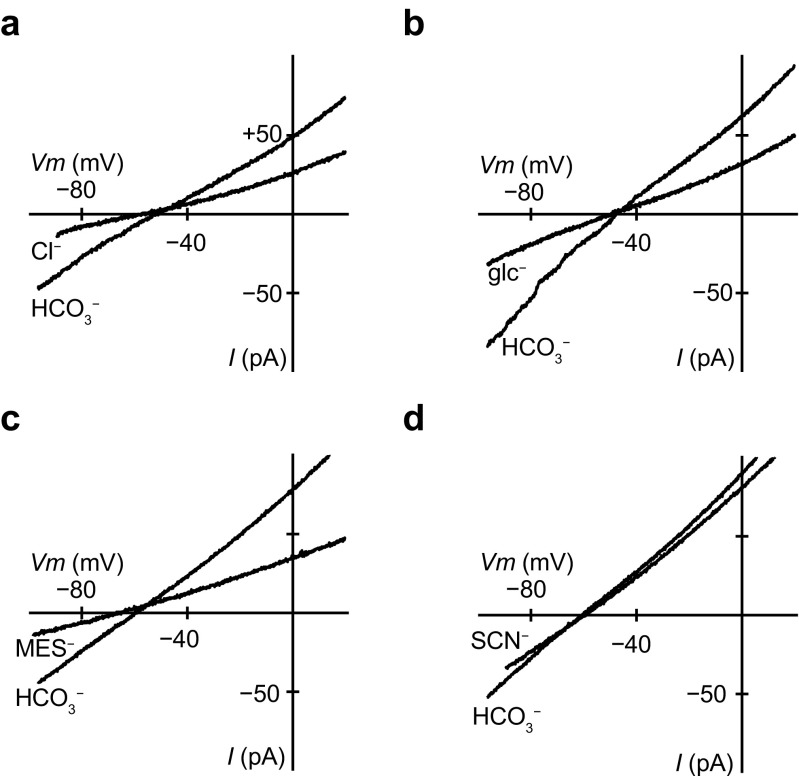
Ion selectivity of the bicarbonate conductance was examined by replacing 130 mM HCO3− in the intracellular bathing solution with other monovalent anions. Figure 2 shows macroscopic I–V relations recorded in the inside-out configuration with the standard NMDG-Cl pipette solution. In experiments where HCO3− in the bath was replaced with Cl− or gluconate (glc−), the reversal potential did not change, but inward conductance significantly decreased from 1.30 ± 0.09 nS in HCO3− to 0.64 ± 0.13 nS in Cl− (Figs. 2a and 3b (right); n = 5) and from 1.69 ± 0.08 nS in HCO3− to 0.72 ± 0.14 nS in glc− (Fig. 2b; n = 5). Replacement of HCO3− with methanesulfonate (MES−) shifted the reversal potential in a negative direction, indicating it was less permeable than HCO3−, and the inward conductance significantly decreased from 2.56 ± 0.73 nS in HCO3− to 0.73 ± 0.36 nS in MES− (Fig. 2c; n = 6). Replacement of HCO3− with thiocyanate (SCN−) slightly shifted the reversal potential in a positive direction, but the inward conductance had little change (Fig. 2d; n = 6). Finally, replacement of HCO3− with iodide (I−) did not cause a marked difference in the reversal potential or the inward conductance (data not shown; n = 6). We calculated the permeability ratio (PX/PHCO3) from the shift in the reversal potential (ΔVrev) when anion X is substituted for internal HCO3− [13]; that is, from:
where R, T, and F have their conventional thermodynamic meanings. The sequence of the permeability ratios was SCN− (1.41 ± 0.15) > Cl− (1.18 ± 0.14) = glc− (1.07 ± 0.03) = I− (1.06 ± 0.06) = HCO3− (1.00) > MES− (0.65 ± 0.11) (n = 5–6). The sequence of the relative inward conductance determined from − 80 to − 60 mV was HCO3− (1.00) > SCN− (0.69 ± 0.10) = I− (0.66 ± 0.09) > Cl− (0.48 ± 0.08) = glc− (0.43 ± 0.09) > MES− (0.26 ± 0.06) (n = 5–6).
Fig. 2.
Ion selectivity of bicarbonate conductance. Macroscopic I–V relationships recorded from different inside-out patches. The intracellular 130 mM HCO3− was substituted by equimolar chloride (Cl−), gluconate (glc−), methanesulfonate (MES−), or thiocyanate (SCN−) (n = 5–6). The inward conductance decreased significantly when HCO3− was substituted with Cl−, glc−, or MES−
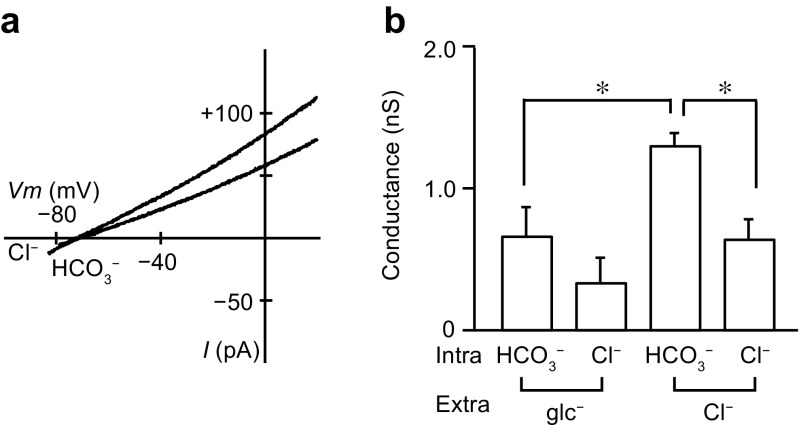
Fig. 3.
Effects of substitution of extracellular Cl− with gluconate. a Macroscopic I–V relationships recorded from the interlobular pancreatic duct cells with gluconate-rich extracellular and 130 mM HCO3− or 130 mM Cl− intracellular solutions. b The inward conductance attributed to efflux of HCO3− or Cl− with gluconate-rich and standard NMDG-Cl pipette solutions (n = 5, *P < 0.05, ANOVA). Intra and Extra, intracellular and extracellular, respectively
Bicarbonate conductance is dependent on luminal Cl−
To evaluate the activities of Cl−–HCO3− exchangers on the apical membrane of interlobular pancreatic ducts of the guinea pig, Ishiguro and colleagues replaced Cl− with gluconate in the lumen [14, 16, 43]. Similarly, we recorded macroscopic currents with extracellular solution containing 120 mM gluconate and 10 mM Cl−. With the control intracellular solution, Erev was − 46.6 ± 4.5 mV with a standard NMDG-Cl pipette solution (Fig. 2a; n = 5) and − 63.6 ± 3.9 mV with gluconate-rich pipette solution (Fig. 3a; n = 5), demonstrating a significant difference (ANOVA). We also compared the inward HCO3− conductance with gluconate-rich and standard NMDG-Cl pipette solutions (Fig. 3b). The HCO3− conductance was significantly lower with the gluconate-rich pipette solution (0.66 ± 0.20 nS) than with standard NMDG-Cl pipette solutions (1.30 ± 0.09 nS) (n = 5, ANOVA). Additionally, as described in the previous section, the inward conductance significantly decreased when HCO3− in the bath was replaced with Cl−, indicating that there was a minor contribution from Cl−-dependent current (Fig. 3b, right). However, the inward conductance was not significantly different with gluconate-rich pipette solution (Fig. 3b, left). The results described so far indicate that both intracellular HCO3− and luminal Cl− are essential for the HCO3− conductance, and that the HCO3− conductance is carried out through Cl−–HCO3− exchangers on the luminal membrane.
Effects of the anion exchanger inhibitor H2DIDS on bicarbonate conductance
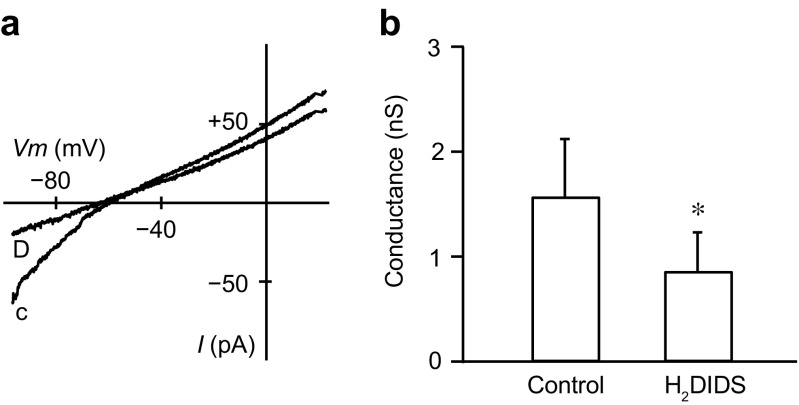
Previous studies reported that Cl−–HCO3− exchangers were inhibited by luminal H2DIDS, a disulfonic stilbene [14, 43]. For experimental ease, we applied H2DIDS (0.5 mM) intracellularly while recording macroscopic currents from excised inside-out patches with the control bicarbonate internal and the standard NMDG-Cl pipette solutions. To exclude the possibility of the contamination of CFTR Cl− conductance, we included 20 μM CFTRinh-172, an inhibitor of CFTR Cl− channels, in the pipette solution. H2DIDS applied intracellularly significantly decreased inward HCO3− conductance from 1.57 ± 0.55 to 0.86 ± 0.37 nS (Fig. 4; n = 6). We also tested 30 μM Sch28080, a H+–K+-pump inhibitor, but did not observe any inhibitory effects on the inward HCO3− conductance (n = 4; not shown). These results further support that the HCO3− conductance occurs through Cl−–HCO3− exchangers.
Fig. 4.
Effects of H2DIDS on bicarbonate conductance. a Macroscopic I–V relationships obtained in the absence or presence of 0.5 mM intracellular H2DIDS. c, control; D, H2DIDS. CFTRinh-172 at 20 μM was added to the standard NMDG-Cl pipette solution. b H2DIDS significantly decreased the average inward HCO3− conductance (n = 6, *P < 0.05)
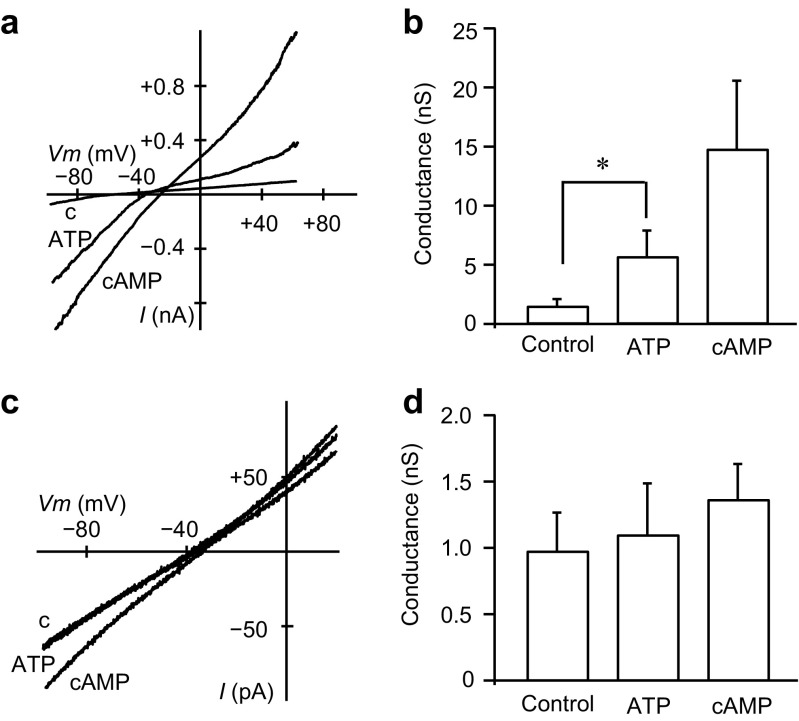
Regulation of bicarbonate conductance by intracellular ATP and cAMP
In pancreatic duct cells, cAMP and Ca2+ signaling pathways play a role in fluid secretion. As CFTR Cl− channels were regulated by intracellular cAMP [6, 8, 29] and ATP [40], we tested their effects on bicarbonate conductance. Application of intracellular 2 mM ATP-Mg significantly increased the inward conductance from 1.51 ± 0.59 to 5.70 ± 2.18 nS (Fig. 5a, b; n = 13). The addition of 1 mM cAMP further increased the inward conductance to 14.8 ± 5.57 nS (n = 4). cAMP also activated the marked outward conductance, which was attributed to Cl− influx, most likely through CFTR Cl− channels. Therefore, we tested the effects of intracellular ATP-Mg and cAMP with the pipette solution including CFTRinh-172 at 20 μM. In the presence of CFTRinh-172, application of intracellular 2 mM ATP-Mg and 1 mM cAMP had little effect on the conductance in either direction (Fig. 5c): the inward conductance was not significantly increased in the presence of ATP-Mg (1.11 ± 0.39 nS) or cAMP (1.37 ± 0.27 nS) as compared with the control (0.98 ± 0.29 nS) (Fig. 5d; n = 11). These results indicate that intracellular ATP and cAMP may not directly regulate Cl−–HCO3− exchangers, but instead regulate CFTR Cl− channels on the luminal membrane of duct cells. Additionally, 1 μM free Ca2+ added to the intracellular solution did not affect the inward HCO3− conductance (n = 3; not shown), suggesting that intracellular Ca2+ does not regulate Cl−–HCO3− exchangers directly.
Fig. 5.
Activation of bicarbonate conductance by intracellular ATP and cAMP. a Macroscopic I–V relationships from the interlobular pancreatic duct cells with the control bicarbonate internal solution (c), and with addition of ATP alone, or ATP and cAMP. The standard NMDG-Cl pipette solution was used. b Averaged HCO3− conductance with the control, ATP alone (n = 13, *P < 0.05), or ATP + cAMP (n = 4). c Macroscopic I–V relationships obtained in the presence of extracellular CFTRinh-172 at 20 μM along with the standard NMDG-Cl pipette solution. d Averaged HCO3− conductance with the control, ATP alone, or ATP + cAMP (n = 11)
Pancreatic duct epithelia expressed a variety of SLC26A family members
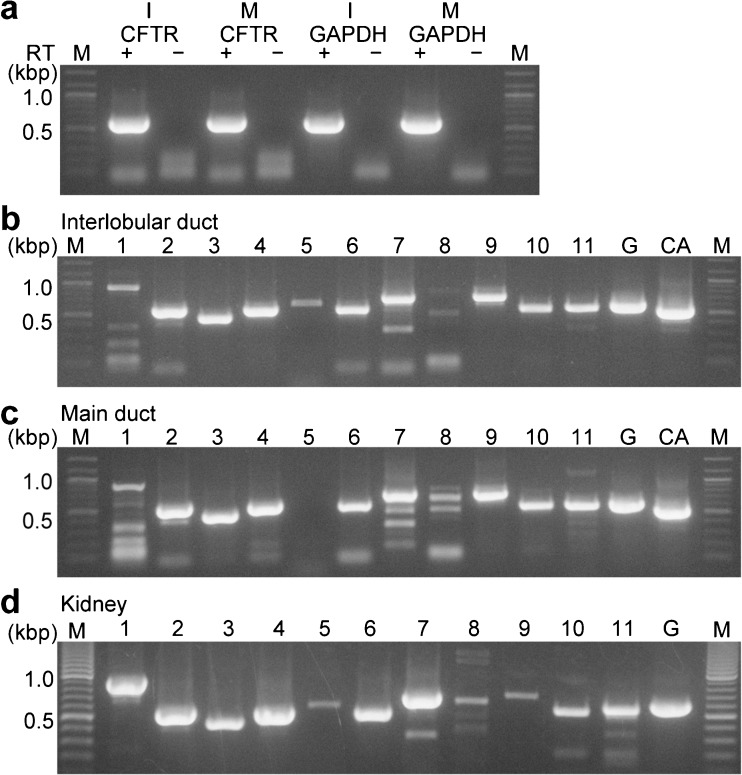
It is known that anion exchangers in pancreatic duct cells are members of the SLC26A family [26]. Two members of the family, SLC26A3 (DRA; downregulated in adenoma) [25] and SLC26A6 (PAT1; putative anion transporter-1) [24, 46], were reported to be expressed in the luminal membrane of pancreatic ducts and function as Cl−–HCO3− exchangers [9, 19, 20]. Interlobular ducts from guinea pigs expressed mRNAs encoding Slc26a3 and Slc26a6 [43]. In the present study, we evaluated the expression of all members of the SLC26A family using RT-PCR analysis on isolated interlobular and main ducts. Figure 6a shows the isolated interlobular and main pancreatic ducts expressing CFTR and GAPDH. Then, we screened all 11 members of the SLC26A family from the interlobular ducts (Fig. 6b; n = 3 animals) and main ducts (Fig. 6c; n = 3 animals), along with GAPDH and a duct marker of carbonic anhydrase II (CA2). We also screened all primer sets from the total RNA of the kidney as a positive control (Fig. 6d). RT-PCR analysis revealed that the interlobular and main ducts expressed Slc26a1, Slc26a2, Slc26a3, Slc26a4, Slc26a6, Slc26a7, Slc26a9, Slc26a10, and Slc26a11.
Fig. 6.
RT-PCR analysis of the SLC26A family. Ethidium bromide-stained agarose gels show RT-PCR products generated from total RNA isolated from the interlobular (I) and main (M) pancreatic ducts. a Control experiment shows the amplification of Cftr (623 bp) and Gapdh (610 bp). No DNA fragment was amplified with the template without reverse transcription (RT). The primers for the RT-PCR analysis from the interlobular (b) and main (c) ducts gave the expected fragment length for Slc26a1–11 (Table 1). d Positive control obtained from the kidney. A representative gel for at least three independent experiments is shown. M in a–d: molecular mass, G in b–d: GAPDH, CA in b and c: carbonic anhydrase II
Immunolocalization of the SLC26A family in pancreatic duct cells
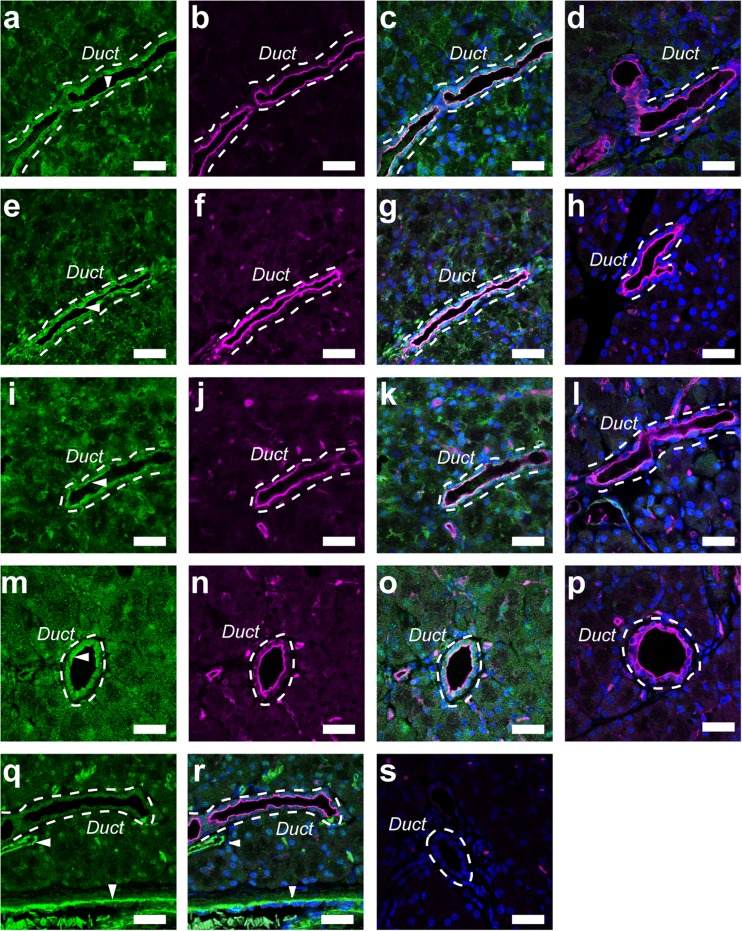
The immunolocalization of the SLC26A family was examined with paraffin sections of guinea pig pancreas. Immunofluorescence ascribed to the SLC26A exchanger was colocalized with Ezrin, an A-kinase anchoring protein, to the luminal membrane of the pancreatic duct (Fig. 7). In the guinea pig pancreas, immunofluorescence of SLC26A6 was detected on the luminal membranes of duct cells (Fig. 7a), as reported for the rat pancreas previously [20]. SLC26A6 were colocalized with Ezrin to the luminal membranes (Fig. 7b, c). The immunofluorescence on the luminal membranes was diminished with SLC26A6 antibody, which was pre-absorbed with the corresponding antigen for the negative control (Fig. 7d). Additionally, a strong signal ascribed to SLC26A1 was detected and colocalized with Ezrin to the luminal membrane (Fig. 7e–g). We also detected immunofluorescence of SLC26A4 and SLC26A10 on the luminal membrane of the duct cells (Fig. 7i–k and m–o, respectively). The immunofluorescence was reduced when antibodies were pre-absorbed with the corresponding antigens (Fig. 7h, l, p). We used HPA036055 (Atlas) as the anti-SLC26A3 antibody, but failed to immunostain SLC26A3 in the guinea pig pancreas. We stained with PECAM-1, a blood vessel marker, to distinguish between pancreatic ducts and blood vessels (Fig. 7q, r).
Fig. 7.
Immunolocalization of the SLC26A family in the interlobular pancreatic duct. a Fluorescence of SLC26A6 on the luminal membranes of duct cells. b Fluorescence image of ezrin. c Overlay image of a and b. d Overlay image of ezrin and green fluorescence with SLC26A6 antibody pre-absorbed with the corresponding antigen. The broken line indicates a duct. Arrowhead indicates the primary antibody signal on the luminal membrane. Fluorescence images of SLC26A1 (e), ezrin (f), overlay (g), and negative control with pre-absorbed SLC26A1 antibody (h). Fluorescence image of SLC26A4 (i), ezrin (j), overlay (k), and negative control with pre-absorbed SLC26A4 antibody (l). Fluorescence images of SLC26A10 (m), ezrin (n), overlay (o), and negative control with pre-absorbed SLC26A10 antibody (p). Fluorescence images of PECAM-1 (q), a blood vessel marker, and overlay with ezrin (r). Arrowheads show a blood vessel that does not overlap with the duct. s Control image of the guinea pig pancreas, in which primary antibodies were omitted. DAPI was used to stain nuclei (blue). Representative images for at least three independent experiments are shown. Bars = 20 μm
Expression of SLC26A protein in guinea pig pancreatic ducts
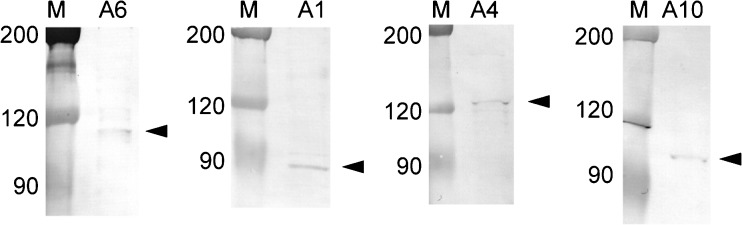
We next performed western blot analysis to examine the expression of SLC26A protein in the guinea pig pancreatic ducts. We detected SLC26A6 (~ 107 kDa), SLC26A1 (~ 78 kDa), SLC26A4 (~ 136 kDa), and SLC26A10 (~ 108 kDa) in the lysates of the isolated ducts (Fig. 8; n = 3 animals). The molecular mass values corresponded to those of human SLC26A proteins (~ 100 kDa), which were N-glycosylated, expressed in HEK-293 cells [22].
Fig. 8.
Immunoblot of the SLC26A family from the pancreatic duct. Protein samples were resolved by SDS-PAGE. Arrowheads indicate SLC26A proteins detected by immunoblotting using anti-SLC26A antibodies. Representative membranes for at least three independent experiments are shown. M, marker; A6, SLC26A6; A1, SLC26A1; A4, SLC26A4; A10, SLC26A10
Discussion
In the present study, we applied patch electrodes on the luminal membrane of guinea pig pancreatic duct cells and recorded macroscopic currents in the inside-out configuration. The inward conductance was dependent on the intracellular HCO3− concentration (Fig. 1) and was reduced when intracellular HCO3− was replaced with Cl−, glc−, or MES− (Fig. 2) or extracellular Cl− was replaced with glc− (Fig. 3). Furthermore, the inward conductance was decreased in the presence of H2DIDS, an inhibitor of Cl−–HCO3− exchangers (Fig. 4). These electrophysiological findings suggested that the inward conductance was ascribed to HCO3− efflux through Cl−–HCO3− exchangers on the luminal membrane. In addition, we found that SLC26A1, SLC26A4, SLC26A6, and SLC26A10 were localized to the luminal membrane of the pancreatic duct cells (Figs. 7 and 8).
HCO3− can flow outwardly not only through Cl−–HCO3− exchangers but also through CFTR Cl− channels on the luminal membrane [7]. The permeability ratio sequence of the Cl− channel in inside-out patches from the rat pancreatic duct cells was NO3− > Cl− > HCO3− > gluconate [7], and that in whole-cell patches from the guinea pig pancreatic duct cells was Br− > I− = Cl− > HCO3− > ClO4− > aspartate [29]. These were different from the permeability ratio sequence of the inward conductance obtained in the present study: SCN− > Cl− = gluconate = I− = HCO3− > MES− (Fig. 2). Similarly, a previous study reported that the anion selectivity of SLC26A6 in HEK 293 cells was SCN− > NO3− > Cl− [30]. Single-channel and whole-cell conductance through Cl− channels was reduced in the presence of HCO3− [7, 29], whereas the inward conductance was increased with increasing intracellular HCO3− in our experiments (Fig. 1). These results suggest that HCO3− efflux occurs by pathway independent from the Cl− channels. We followed previous studies that evaluated the activities of Cl−–HCO3− exchangers on the apical membrane of pancreatic ducts by replacing extracellular Cl− with gluconate [14, 16, 43], and observed that the reversal potential shifted to a negative direction and the inward HCO3− conductance decreased (Fig. 3). The dependency of inward HCO3− conductance on extracellular Cl− suggests that HCO3− is exchanged for Cl−. Our results demonstrated that intracellular HCO3− increased the conductance with a Kd value of approximately 30 mM (Fig. 1), corresponding to the physiological concentration of intracellular HCO3− in duct cells. Additionally, the Hill coefficient, which was estimated to be 3.5 for the effects of HCO3−, suggested that positive cooperative binding of HCO3− facilitated the binding of subsequent HCO3− at other sites on Cl−–HCO3− exchangers.
We detected SLC26A1, SLC26A4, SLC26A6, and SLC26A10 on the luminal membrane of the interlobular pancreatic duct (Fig. 7). SLC26A6 was localized to the luminal membrane of interlobular pancreatic ducts of humans [24] and rats [20], as well as to the intestine, kidney, parotid gland, and heart [1, 20, 21, 24, 47]. SLC26A6 cloned from guinea pig pancreatic ducts mediated Cl−–HCO3− exchange in HEK 293 cells [44]. SLC26A4 (pendrin) was localized to the apical membranes of the submandibular duct, type B and non-A, non-B intercalated cells in the cortical collecting duct of the kidney, and thyroid follicular cells, and was expressed in inner ear [3, 36, 37, 39]. SLC26A4 mediates HCO3− secretion across the apical membrane in Calu-3, a human airway epithelia cell line, monolayers [5] and in the cortical collecting ducts [37]. SLC26A1 identified as sulfate/bicarbonate/oxalate exchangers was expressed in the liver and kidney, and to a lesser extent, in the pancreas and testis [2, 35], and detected on the basolateral membrane of kidney and liver epithelial cells [18, 34]. SLC26A10 was found at the mRNA level in the heart and sarcoma [1, 4], but its function is unknown. Although the previous study demonstrated localization of SLC26A3 to the apical membrane of mouse pancreatic duct cells [9], we were unable to immunostain SLC26A3 in guinea pig pancreatic duct cells. The immunostaining signal in the guinea pig pancreas may be underestimated because we were only able to use antibodies against the human SLC26A family. Future studies are needed to establish the functional relevance of SLC26A molecules in pancreatic ducts.
We found that intracellular ATP and cAMP activated anion conductance on the luminal membrane in guinea pig pancreatic duct cells (Fig. 5a, b), as observed in rat pancreatic duct cells [6]. It was reported using HEK293 cells that CFTR stimulated by forskolin activated anion exchange of SLC26A3, SLC26A4, and SLC26A6 [19]. Thus, the increased conductance was attributed to activation of CFTR Cl− channels by intracellular ATP and cAMP [6, 8, 29, 40], and activation of Cl−–HCO3− exchangers by activated CFTR [19]. As anion conductance was not significantly increased in the presence of CFTRinh-172 in the pipette solution (Fig. 5c, d), we concluded that intracellular ATP and cAMP may not directly regulate Cl−–HCO3− exchangers.
We found that H2DIDS applied intracellularly inhibited inward HCO3− conductance by 50% in excised inside-out patches from the luminal membrane (Fig. 4). A previous study demonstrated that other disulfonic stilbenes, 4,4′-dinitrostilbene-2,2′-disulphonic acid and 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid, blocked CFTR Cl− channels when applied to the cytoplasmic face of membrane patches, with Kd values (at 0 mV) of 160 and 80 μM, respectively [23]. It is likely that disulfonic stilbenes are able to act on Cl−–HCO3− exchangers from not only the outside but also from the inside of the cell membrane.
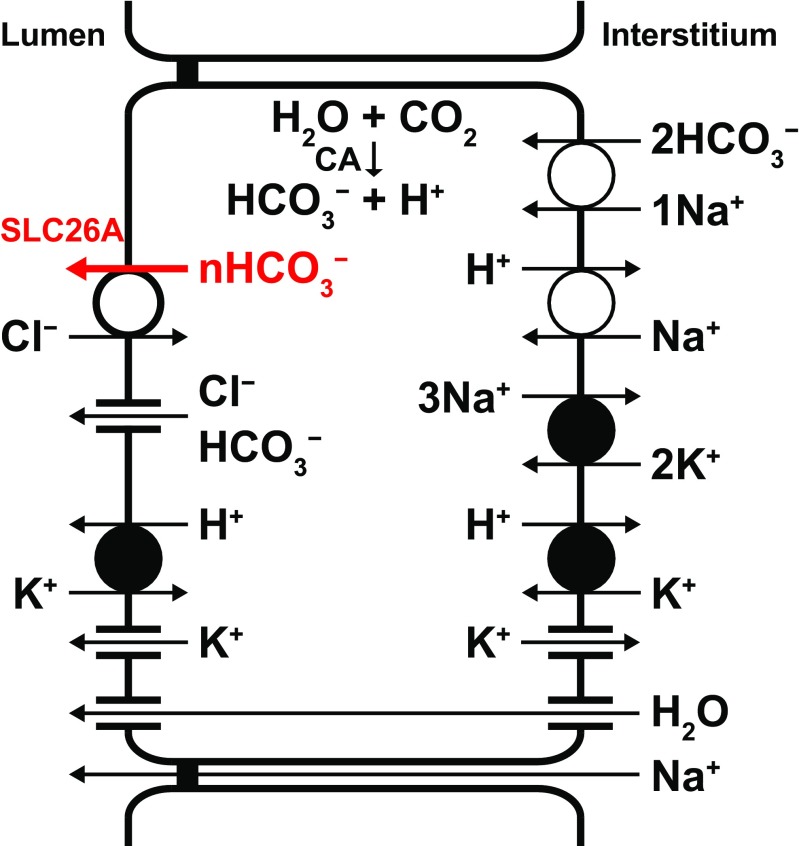
In conclusion, we used the patch-clamp technique in the inside-out configuration and demonstrated that the HCO3− conductance through the luminal membrane is mediated by Cl−–HCO3− exchangers under physiological HCO3− concentrations in pancreatic duct cells. Our findings suggest that SLC26A1, SLC26A4, SLC26A6, and SLC26A10 may be involved in the HCO3− transport through the luminal membrane. The SLC26A family may also play a role in pH homeostasis in the pancreatic lumen and duct cells. The direct measurement of the HCO3− current from the interlobular duct and its functional characterization helps to propose a useful model for HCO3− secretion from the pancreatic duct epithelia (Fig. 9).
Fig. 9.
Model of HCO3− transport in a pancreatic duct cell. Intracellular HCO3− is derived from CO2 through the action of carbonic anhydrase (CA) and from HCO3− uptake via the Na+–HCO3− cotransporter. H+ is extruded at the basolateral membrane by the Na+–H+ exchanger and H+–K+ pump. HCO3− efflux across the luminal membrane is mediated by Cl− channels (CFTR and TMEM16A/ANO1) and electrogenic Cl−–nHCO3− exchangers (SLC26A1, 4, 6, and/or 10; n > 1). K+ channels provide an exit pathway for K+ and play a vital role in maintaining the membrane potential, which is a crucial component of the driving force for anion secretion. Luminal H+–K+ pumps may provide a buffering/protection zone for the alkali-secreting epithelium. Primary active transport is indicated by filled circles
Funding information
This work was supported by the research grant D2 from Kansai Medical University, the Pancreas Research Foundation of Japan, and Japan Society for the Promotion of Science KAKENHI (24790226). N. Andharia was supported by the Rotary Yoneyama Memorial Foundation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.Alvarez BV, Kieller DM, Quon AL, Markovich D, Casey JR. Slc26a6: a cardiac chloride–hyroxyl exchanger and predominant chloride–bicarbonate exchanger of the mouse heart. J Physiol. 2004;561(3):721–734. doi: 10.1113/jphysiol.2004.077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem. 1994;269(4):3017–3021. [PubMed] [Google Scholar]
- 3.Everett LA, Morsli H, Wu DK, Green ED. Expression pattern of the mouse ortholog of the pendred’s syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci U S A. 1999;96(17):9727–9732. doi: 10.1073/pnas.96.17.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis P, Namløs HM, Müller C, Edén P, Fernebro J, Berner JM, Bjerkehagen B, Åkerman M, Bendahl PO, Isinger A, Rydholm A, Myklebost O, Nilbert M. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: hypoxia-induced transcription profile signifies metastatic potential. BMC Genomics. 2007;8(1):73. doi: 10.1186/1471-2164-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, Fong P, Gray MA. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem. 2011;286(47):41069–41082. doi: 10.1074/jbc.M111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray MA, Greenwell JR, Argent BE. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- 7.Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990;259(5):C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- 8.Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol. 1993;264(3):C591–C602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- 9.Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1301–G1308. doi: 10.1152/ajpgi.2001.281.5.G1301. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Novak I. Molecular basis of potassium channels in pancreatic duct epithelial cells. Channels (Austin) 2013;7(6):432–441. doi: 10.4161/chan.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi M, Wang J, Hede SE, Nova I. An intermediate-conductance Ca2+-activated K+ channel is important for secretion in pancreatic duct cells. Am J Physiol Cell Physiol. 2012;303(2):C151–C159. doi: 10.1152/ajpcell.00089.2012. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi M, Inagaki A, Novak I, Matsuda H. The adenosine A2B receptor is involved in anion secretion in human pancreatic duct Capan-1 epithelial cells. Pflügers Arch. 2016;468(7):1171–1181. doi: 10.1007/s00424-016-1806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hille B. Ionic channels of excitable membranes. 3. Sunderland: Sinauer Associates; 2001. [Google Scholar]
- 14.Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol. 1998;511(2):407–422. doi: 10.1111/j.1469-7793.1998.407bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro H, Naruse S, Kitagawa M, Mabuchi T, Kondo T, Hayakawa T, Case RM, Steward MC. Chloride transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol. 2002;539(1):175–189. doi: 10.1113/jphysiol.2001.012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M. Effect of Slc26a6 deletion on apical Cl−/HCO3− exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G447–G455. doi: 10.1152/ajpgi.00286.2006. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol. 2009;133(3):315–326. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karniski LP, Lӧtscher M, Fucentese M, Hilfiker H, Biber J, Murer H. Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol. 1998;275(1):F79–F87. doi: 10.1152/ajprenal.1998.275.1.F79. [DOI] [PubMed] [Google Scholar]
- 19.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 2002;21(21):5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6(4):343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kujala M, Tienari J, Lohi H, Elomaa O, Sariola H, Lehtonen E, Kere J. SLC26A6 and SLC26A7 anion exchangers have a distinct distribution in human kidney. Nephron Exp Nephrol. 2005;101(2):e50–e58. doi: 10.1159/000086345. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Xia F, Reithmeier RA. N-glycosylation and topology of the human SLC26 family of anion transport membrane proteins. Am J Physiol Cell Physiol. 2014;306(10):C943–C960. doi: 10.1152/ajpcell.00030.2014. [DOI] [PubMed] [Google Scholar]
- 23.Linsdell P, Hanrahan JW. Disulphonic stilbene block of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a mammalian cell line and its regulation by a critical pore residue. J Physiol. 1996;496(3):687–693. doi: 10.1113/jphysiol.1996.sp021719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohi H, Kujala M, Kerkelӓ E, Saarialho-Kere U, Kestilӓ M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70(1):102–112. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- 25.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl− /HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem. 1999;274(32):22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- 26.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch. 2004;447(5):710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 27.Novak I, Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflügers Arch. 1988;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- 28.Novak I, Wang J, Henriksen KL, Haanes KA, Krabbe S, Nitschke R, Hede SE. Pancreatic bicarbonate secretion involves two proton pumps. J Biol Chem. 2011;286(1):280–289. doi: 10.1074/jbc.M110.136382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Reilly CM, Winpenny JP, Argent BE, Gray MA. Cystic fibrosis transmembrane conductance regulator currents in guinea pig pancreatic duct cells: inhibition by bicarbonate ions. Gastroenterology. 2000;118(6):1187–1196. doi: 10.1016/S0016-5085(00)70372-6. [DOI] [PubMed] [Google Scholar]
- 30.Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol. 2011;137(2):239–251. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139(2):620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Petersen OH, Ueda N. Secretion of fluid and amylase in the perfused rat pancreas. J Physiol. 1977;264(3):819–835. doi: 10.1113/jphysiol.1977.sp011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen OH, Courjaret R, Machaca K. Ca2+ tunnelling through the ER lumen as a mechanism for delivering Ca2+ entering via store-operated Ca2+ channels to specific target sites. J Physiol. 2017;595(10):2999–3014. doi: 10.1113/JP272772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quondamatteo F, Krick W, Hagos Y, Kruger MH, Neubauer-saile K, Herken R, Ramadori G, Burckhardt G, Burckhardt BC. Localization of the sulfate/anion exchanger in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G1075–G1081. doi: 10.1152/ajpgi.00492.2005. [DOI] [PubMed] [Google Scholar]
- 35.Regeer RR, Lee A, Markovich D. Characterization of the human sulfate anion transporter (hsat-1) protein and gene (SAT1; SLC26A1) DNA Cell Biol. 2003;22(2):107–117. doi: 10.1089/104454903321515913. [DOI] [PubMed] [Google Scholar]
- 36.Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED. Pendrin, the protein encoded by the pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141(2):839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 37.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A. 2001;98(7):4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127(5):511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586(16):3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(1):S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 41.Song Y, Yamamoto A, Steward MC, Ko SB, Stewart AK, Soleimani M, Liu BC, Kondo T, Jin CX, Ishiguro H. Deletion of Slc26a6 alters the stoichiometry of apical Cl−/HCO3− exchange in mouse pancreatic duct. Am J Physiol Cell Physiol. 2012;303(8):C815–C824. doi: 10.1152/ajpcell.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67(1):377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 43.Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl−/HCO3− exchange with CFTR in stimulated HCO3− secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1307–G1317. doi: 10.1152/ajpgi.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol. 2011;301(2):C289–C303. doi: 10.1152/ajpcell.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venglovecz V, Hegyi P, Rakonczay Z, Jr, Tiszlavicz L, Nardi A, Grunnet M, Gray MA. Pathophysiological relevance of apical large conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut. 2011;60(3):361–369. doi: 10.1136/gut.2010.214213. [DOI] [PubMed] [Google Scholar]
- 46.Waldegger S, Moschen I, Ramirez A, Smith RJ, Ayadi H, Lang F, Kubisch C. Cloning and characterization of SLC26A6, a novel member of the solute carrier 26 gene family. Genomics. 2001;72(1):43–50. doi: 10.1006/geno.2000.6445. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282(3):G573–G579. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J. 2006;25(21):5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilschanski M, Novak I. The cystic fibrosis of exocrine pancreas. Cold Spring Harb Perspect Med. 2013;3(5):a009746. doi: 10.1101/cshperspect.a009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi M, Steward MC, Smallbone K, Sohma Y, Yamamoto A, Ko SB, Kondo T, Ishiguro H. Bicarbonate-rich fluid secretion predicted by a computational model of guinea-pig pancreatic duct epithelium. J Physiol. 2017;595(6):1947–1972. doi: 10.1113/JP273306. [DOI] [PMC free article] [PubMed] [Google Scholar]