Abstract
Children and young adults with heart disease appear to be at increased risk of developing cancer, although the reasons for this are unclear. A cohort of 11,270 individuals, who underwent cardiac catheterizations while aged ≤ 22 years in the UK, was established from hospital records. Radiation doses from cardiac catheterizations and CT scans were estimated. The cohort was matched with the NHS Central Register and NHS Transplant Registry to determine cancer incidence and transplantation status. Standardized incidence ratios (SIR) with associated confidence intervals (CI) were calculated. The excess relative risk (ERR) of lymphohaematopoietic neoplasia was also calculated using Poisson regression. The SIR was raised for all malignancies (2.32, 95% CI 1.65, 3.17), lymphoma (8.34, 95% CI 5.22, 12.61) and leukaemia (2.11, 95% CI 0.82, 4.42). After censoring transplant recipients, post-transplant, the SIR was reduced to 0.90 (95% CI 0.49, 1.49) for all malignancies. All lymphomas developed post-transplant. The SIR for all malignancies developing 5 years from the first cardiac catheterization (2 years for leukaemia/lymphoma) remained raised (3.01, 95% CI 2.09, 4.19) but was again reduced after censoring transplant recipients (0.98, 95% CI 0.48, 1.77). The ERR per mGy bone marrow dose for lymphohaematopoietic neoplasia was reduced from 0.541 (95% CI 0.104, 1.807) to 0.018 (95% CI − 0.002, 0.096) where transplantation status was accounted for as a time-dependent background risk factor. In conclusion, transplantation appears to be a large contributor to elevated cancer rates in this patient group. This is likely to be mainly due to associated immunosuppression, however, radiation exposure may also be a contributing factor.
Keywords: Cardiac, Radiation exposure, Dose, Transplant, Cancer
Introduction
Information on cancer rates among children and young adults (< 22 years) with congenital or acquired heart conditions is limited. A small number of studies have been published [1–7], suggesting relatively high cancer incidence and mortality, compared to the general population. Potential explanations include shared genetic or environmental factors, immunosuppression, lifestyle, and radiation exposure [3, 5]. Children with heart disease are subjected to a number of forms of medical x-ray examinations, including computed tomography (CT), cardiac catheterizations and general radiography [8–11]. Although radiographs comprise the majority of procedures, the majority of the cumulative radiation dose in this patient group (around 80%) comes from CT and catheterizations [8, 12]. Diagnostic medical radiation exposure has increased substantially in recent decades among this patient group [9]. The long-term impact of this exposure is difficult to determine, however. In this study, we established a cohort of 11,270 patients who had undergone cardiac catheterizations before 22 years of age. We then conducted two analyses; (1) an overall assessment of the incidence of all malignant tumours in the cohort, and (2) an assessment of the potential contribution of radiation exposure and organ transplantation on cancer rates.
Materials and methods
The study received a favourable ethical opinion from the National Research Ethics Service Committee North East—Newcastle and North Tyneside 2 Ethics Committee, along with approval from the Confidentiality Advisory Group to use patient identifiable data.
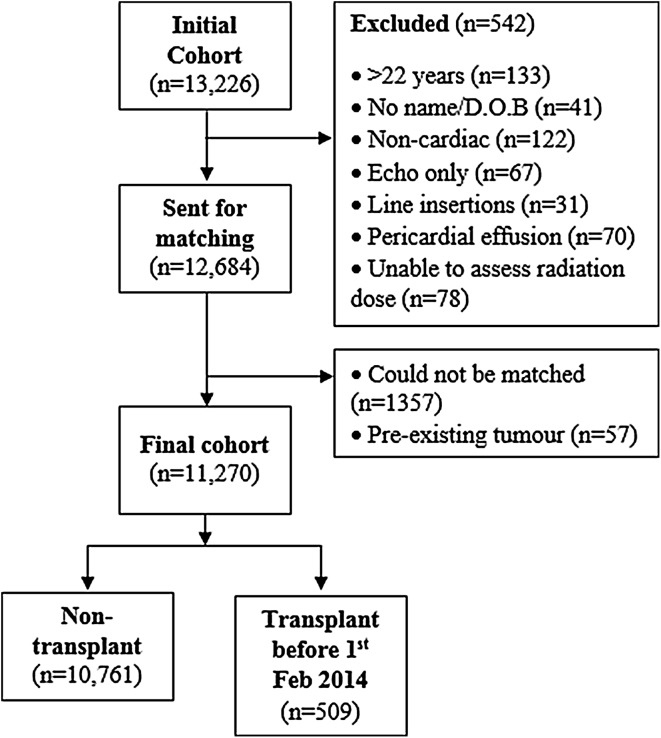
A retrospective cohort was created from hospital records of examinations carried out in catheterization laboratories at five participating English hospitals. Patients were eligible for inclusion if they had undergone at least one cardiac catheterization procedure while aged under 22 years. An initial cohort of 13,226 individuals was established. Patients lacking full name or date of birth (n = 41), those who were over 22 years old at the time of the first recorded procedure (n = 133) along with patients who had undergone only trans-oesophageal echocardiography (n = 67), non-cardiac fluoroscopy procedures (n = 122), Hickman or other line insertions without any other cardiac catheterization procedures (n = 31) were excluded, as were those for whom radiation doses could not be estimated (n = 78). Patients who had undergone isolated pericardial effusion drainage (n = 70) were also excluded. This left a cohort of 12,684 individuals which was matched with the National Health Service Central Register (NHSCR) to determine who had been diagnosed with a neoplasm (reported as ICD-0 codes), the date of diagnosis, and date and cause of death, where applicable. Fifty seven patients were diagnosed with a neoplasm before the date of their first recorded cardiac catheterization. These patients were excluded from the analysis to reduce the impact of reverse causality (i.e. malignancy or associated treatment causing heart disease). 1357 cohort members could not be matched by NHSCR. This left 11,270 patients who were included in the analysis (Fig. 1). Details of this final cohort are shown in Table 1.
Fig. 1.
Flow diagram for cohort
Table 1.
Details of cohort
| Characteristic | Whole cohort | Transplant recipients | Casesa |
|---|---|---|---|
| Sex | |||
| Male (% of whole cohort) | 5612 (50%) | 247 (49%) | 36 (49%) |
| Female | 5118 (45%) | 242 (48%) | 34 (47%) |
| Unknown | 540 (5%) | 20 (4%) | 3 (4%) |
| Total | 11,270 | 509 | 73 |
| Patient age/year of birth | |||
| Median age at 01/02/2014 [10th, 90th percentiles] | 13.5 y [3.0, 25.1] | 18.9 y [8.0, 28.7] | 24.0 y [8.5, 33.7] |
| Born < 1980 [% of total] | 217 [2%] | 27 [5%] | 9 [12%] |
| Born 1980–1989 | 1346 [12%] | 135 [27%] | 35 [48%] |
| Born 1990–1999 | 4156 [37%] | 209 [42%] | 17 [23%] |
| Born 2000–2009 | 4486 [40%] | 118 [24%] | 12 [16%] |
| Born > 2010 | 1065 [9%] | 11 [2%] | 0 |
| Cardiac catheterizations | |||
| Median age at first recorded procedure [10th, 90th percentiles] | 3.2 y [0.1, 15.5] | 9.0 y [1.1, 16.8] | 11.6 y [0.9, 17.9] |
| Mean number of procedures [median, 10th, 90th percentiles] | 1.5 [1, 1, 3] | 4.5 [1, 4, 10] | 3.4 [1, 2, 8] |
| 1 procedure [% of total] | 8489 [75%] | 124 [25%] | 36 [49%] |
| 2 procedures | 1555 [14%] | 67 [13%] | 9 [12%] |
| 3 procedures | 580 [5%] | 57 [11%] | 7 [10%] |
| 4 procedures | 229 [2%] | 44 [9%] | 2 [3%] |
| 5 procedures | 150 [1%] | 53 [11%] | 5 [7%] |
| > 5 procedures | 267 [2%] | 155 [31%] | 14 [19%] |
| Computed tomography | |||
| Median age at first recorded scan [10th, 90th percentiles] | 5.1 y [0.1, 17.8] | 8.7 y [1.2, 17.7] | 9.9 y [2.4, 20.6] |
| Mean number of procedures [median, 10th, 90th percentiles] | 0.4 [0, 0, 1] | 2.7 [1, 0, 8.2] | 4.9 [1, 0, 14] |
| No scans [% of total] | 9666 [86%] | 238 [47%] | 35 [48%] |
| 1 scan | 760 [7%] | 74 [15%] | 7 [10%] |
| 2 scans | 352 [3%] | 47 [9%] | 2 [3%] |
| 3 scans | 187 [2%] | 32 [6%] | 3 [4%] |
| 4 scans | 84 [1%] | 24 [5%] | 2 [3%] |
| 5 scans | 60 [1%] | 19 [4%] | 4 [5%] |
| > 5 scans | 161 [1%] | 75 [15%] | 20 [27%] |
SD standard deviation, y years
aMalignant, borderline malignant and benign tumours
Standardized incidence ratios (SIR) of malignancies, with associated confidence intervals (CI), were calculated as the ratio of observed to expected cases. Expected incidence, from the date of the first cardiac catheterization, up to the 1st of February 2014 was estimated from data published by Cancer Research UK [13], representing sex- and age-specific UK-wide rates. Observed/expected central nervous system tumours included benign or borderline malignant tumours (ICD codes D32-D33, D35.2-D35.4, D42-D43, and D44.3-D44.5). However, these non-malignant/borderline diseases, along with non-melanoma skin cancer (NMSC), were not included in the observed/expected figures for the analysis of all malignancies combined.
As organ transplantation, with associated immunosuppressant drug use, is also a known risk factor in cancer development [14], we matched our cohort with the NHS Transplant Registry, to determine which individuals had received a transplanted organ, the organ involved, and the date of transplant. We also examined notes fields in procedure logbooks (available for 55% of cohort members) and NHSCR death records for further information on transplantation.
To assess the possible role of radiation exposure on cancer incidence, expected cases were also calculated from 5 years following the first recorded cardiac catheterization (2 years for leukaemia and lymphoma) to 1st February 2014. These represent the apparent minimum latency periods for radiation induced tumours, based on previous epidemiological studies [15].
For cardiac catheterizations, active bone marrow (ABM) doses were estimated for 79% of examinations from dose indicators recorded at the time of each procedure (kerma area product in 71% and screening time for 8%), using a dosimetry system based on Monte Carlo computer simulations (PCXMC V2.0, STUK, Helsinki, Finland). This incorporated information on typical values of x-ray energy, beam projection angle and field size extracted from a review of clinical images and structured dose reports. For the remaining 21% of examinations for which no dose indicator was recorded, estimated doses were calculated based on the median doses for each procedure type at the same hospital, using the same equipment, in which dose indicators were recorded. Information on CT scans was obtained from a cohort of children and young adults scanned in Great Britain between 1983 and 2013 [16]. CT doses were estimated based on the methodology of Kim et al. [17], taking into account the body part scanned, patient age and year of scan (technological developments have led to a reduction in doses over time).
The excess relative risk (ERR) of lymphohaematopoietic neoplasia (leukaemia and lymphoma, including borderline malignancies) in relation to cumulative ABM dose was calculated using Poisson regression models, fitted by maximum likelihood estimation, using the maxLik function in R (R Foundation for Statistical Computing, Vienna, Austria). The expected number of cases in stratum i was assumed as:
where , ai and Di are the number of person-years of follow-up, the average attained age and the accumulated dose (in mGy) in stratum i, respectively. The covariate Ti represents transplantation status. This regression function is the product of the person-years as the offset, the exponential term which represents the baseline rate and the relative risk associated with the absorbed dose. The parameter α7 represents the risk factor for transplant status, while β1 represents the ERR per mGy and β2 represents the ERR transplantation variation effect. Person-years were calculated using the DATAB module of Epicure (HiroSoft International Corporation, Seattle, USA). Both exclusion and cumulative ABM dose lag periods were of 2 years.
Results
Across the whole cohort, 40 patients developed a malignant neoplasm following their first recorded cardiac catheterization, after 92,629 years of follow-up (mean = 8.4 years). One patient developed two distinct diseases on different occasions, giving a total of 41 malignancies, of which 30 (73%) were leukaemia (n = 7) or lymphoma (n = 23) (Table 2). There were no malignancies of the breasts, lungs, stomach or oesophagus. The SIR was raised, compared to UK-wide background rates (Table 3), for all malignancies combined (2.32, 95% CI 1.65, 3.17) and lymphoma (8.34, 95% CI 5.22, 12.61). The SIR was also raised for leukaemia, though with a wide confidence interval (2.11, 95% CI 0.82, 4.42).
Table 2.
Details of neoplasia (malignant or otherwise) diagnosed among cohort members
| Neoplasm type: | Whole cohort | Post-transplant | |||
|---|---|---|---|---|---|
| Total | Malignant | Borderline or benign | Malignant | Borderline or benign | |
| lymphohaematopoietic | 44 | 30 | 14 | 23 | 10 |
| Hodgkin’s lymphoma | 4 | 4 | 0 | 4 | 0 |
| Non-Hodgkin’s lymphoma | 19 | 19 | 0 | 19 | 0 |
| Lymphoproliferative disorder | 9 | 0 | 9 | 0 | 9 |
| Acute lymphoblastic leukaemia | 3 | 3 | 0 | 0 | 0 |
| Acute myeloid leukaemia | 3 | 3 | 0 | 0 | 0 |
| Other leukaemia | 1 | 1 | 0 | 0 | 0 |
| Other haematological neoplasia | 5 | 0 | 5a | 0 | 1 |
| Sarcoma | 3 | 2 | 1b | 0 | 0 |
| Central nervous system | 4 | 2 | 2 | 1 | 0 |
| Carcinoma/carcinoma in situ | 16 | 4 | 12 | 2 | 3 |
| Cervix/exocervix | 10 | 1 | 9 | 0 | 0 |
| Testes | 2 | 2 | 0 | 0 | 0 |
| Melanoma | 1 | 1 | 0 | 0 | 0 |
| Other | 4 | 0 | 4 | 0 | 0 |
| Total | 74c | 41 | 33 | 26 | 13 |
aIncludes polycythaemia vera
bUncertain diagnosis (dermatofibroma/fibrosarcoma)
cOne patient developed two diseases
Table 3.
Standardized incidence ratio (SIR) for malignancies developing between the date of each patient’s first recorded cardiac catheterization and 1st February 2014
| Disease | Observed | Expected | SIR [IQR] |
|---|---|---|---|
| All patients | |||
| All malignancies | 41 | 17.66 | 2.32 [1.65, 3.17] |
| Leukaemia | 7 | 3.31 | 2.11 [0.82, 4.42] |
| Lymphoma | 23 | 2.76 | 8.34 [5.22, 12.61] |
| Hodgkin lymphoma | 4 | 1.67 | 2.40 [0.60, 6.27] |
| Non-Hodgkin lymphoma | 19 | 1.09 | 17.45 [10.36, 27.50] |
| Central nervous system | 4 | 3.71 | 1.08 [0.27, 2.82] |
| After censoring post-transplant patients | |||
| All malignancies | 15 | 16.72 | 0.90 [0.49, 1.49] |
| Leukaemia | 7 | 3.19 | 2.19 [0.85, 4.59] |
| Lymphoma | 0 | 2.59 | – |
| Hodgkin lymphoma | 0 | 1.56 | – |
| Non-Hodgkin lymphoma | 0 | 1.03 | – |
| Central nervous system | 3 | 3.54 | 0.85 [0.15, 2.54] |
IQR interquartile range
In addition to the 41 malignancies, there were a further 33 diseases classified as benign or borderline malignancies, including nine lymphoproliferative disorders and nine intraepithelial neoplasia of the cervix/exocervix. In total, 73 patients developed a neoplasm, malignant or otherwise. The majority of the 25 tumours with clearly defined locations were in the abdomen/pelvis (n = 18) or head/neck (n = 6). There were no definite thoracic tumours (the location of one melanoma was listed as ‘trunk’).
Transplantation
Among the cohort, 509 individuals received a transplanted organ before February 2014. The majority of these transplantations involved the heart only (80%), or heart and lungs (5%). The mean age at transplantation was 9.2 years. Twenty six malignancies developed among this patient group (63% of malignancies in the cohort) including 23 lymphomas (all cases of this disease). The mean age at diagnosis was 16.9 years for post-transplant malignancies, compared to 15.1 among non-transplant patients. The potential impact of transplantation on SIR was investigated by censoring observations for transplant recipients at the date of transplantation. This resulted in a reduction in the SIR to 0.90 for all malignancies combined (95% CI 0.49, 1.49) (Table 3). The SIR for leukaemia was essentially unchanged, however (2.19, 95% CI 0.85, 4.59). Thirteen patients developed benign or borderline malignancies, post-transplant, including 9 with lymphoproliferative disorder (100% of cases in the cohort). Three patients developed NMSC. None of the patients developing cervical intraepithelial neoplasia were identified as transplant recipients.
Radiation exposure
Cohort members underwent a total of 17,154 recorded cardiac catheterizations and 4372 CT scans, up to February 2012 (i.e. 2 years before end of follow-up). The majority of patients had a single recorded catheterization (Table 1). Estimated organ doses are shown in Table 4. The mean ABM dose was 8.4 mGy. There were 384 patients with estimated cumulative bone marrow doses less than 0.1 mGy and 60 with doses over 100 mGy. The most common catheterizations were patent ductus arteriosus occlusion, coronary angiography, atrial septal defect occlusion and electrophysiology studies. The majority of CT scans in these patients were of the head, chest, or chest in combination with other body parts. A positive correlation was seen between the number of cardiac catheterizations and the number of CT scans (Spearman’s ρ = 0.252, 95% CI 0.233, 0.272). Transplant recipients underwent a total of 2395 recorded catheterization procedures and 1416 CT scans. The majority of these procedures (82%) and the majority of the associated dose (75%) (Table 4) occurred post-transplant, consistent with monitoring for rejection and coronary allograft vasculopathy.
Table 4.
Estimated cumulative organ doses, up to 1st February 2012 (2 years prior to end of follow-up), from cardiac catheterizations for cohort members
| Breastsa | Lungs | Oesophagus | Thyroid | ABM | ABM (CC + CT) | |
|---|---|---|---|---|---|---|
| Mean organ dose (mGy) [IQR] | ||||||
| Whole cohort | 33.1 [63.8] | 42.9 [78.8] | 26.2 [47.2] | 1.6 [3.3] | 6.6 [12.8] | 8.8 [16.5] |
| Transplant recipients | 49.4 [74.8] | 80.7 [109.1] | 48.3 [64.9] | 2.3 [3.5] | 15.8 [22.3] | 28.3 [32.4] |
| Pre-transplant | 16.5 [56.7] | 20.0 [62.4] | 11.5 [35.7] | 0.7 [2.2] | 3.1 [9.3] | 6.6 [14.8] |
| Post-transplant | 32.9 [53.6] | 60.7 [94.4] | 36.8 [56.8] | 1.6 [2.8] | 12.6 [20.6] | 21.7 [29.2] |
| Casesb,c | 63.7 [81.9] | 80.6 [86.0] | 43.3 [42.1] | 2.0 [1.7] | 14.2 [16.3] | 20.9 [22.2] |
| Transplant recipient casesb,c | 63.7 [81.9] | 81.7 [78.7] | 43.7 [38.9] | 1.9 [1.7] | 14.1 [13.3] | 22.2 [20.8] |
| Median organ dose (mGy) [IQR] | ||||||
| Whole cohort | 14.1 [5.1, 33.5] | 20.2 [8.2, 46.2] | 13.0 [5.4, 27.2] | 0.7 [0.3, 1.6] | 3.1 [1.2, 6.6] | 3.1 [1.3, 9.3] |
| Transplant recipients | 19.0 [5.7, 68.5] | 39.5 [14.5, 100.2] | 25.6 [9.6, 60.7] | 1.2 [0.4, 2.7] | 7.7 [2.7, 19] | 16.9 [6.4, 37.2] |
| Pre-transplant | 0.0 [0.0, 7.0] | 0.0 [0.0, 10.4] | 0.0 [0.0, 5.7] | 0.0 [0.0, 0.4] | 0.0 [0.0, 1.7] | 0.0 [0.0, 6.4] |
| Post-transplant | 9.3 [2.1, 37.5] | 23.3 [5.7, 72.1] | 15.9 [3.4, 44.4] | 0.7 [0.2, 1.8] | 4.7 [1.0, 14.5] | 11.1 [2.5, 28.7] |
| Casesb,c | 34.0 [7.3, 85.5] | 59.5 [22.5, 99.1] | 35.1 [15.9, 58.3] | 1.5 [0.7, 3.1] | 10.2 [3.0, 19.2] | 12.7 [3.8, 30.6] |
| Transplant recipient casesb,c | 34.0 [7.3, 85.5] | 69.8 [24.6, 102.9] | 36.9 [16.1, 58.4] | 1.4 [0.8, 2.8] | 11.9 [4.3, 19.5] | 14.6 [7.2, 31.8] |
ABM active bone marrow, ABM (CC + CT) cumulative doses from both cardiac catheterizations and computed tomography combined, IQR interquartile range
aFemale patients only
bCumulative dose up to 2 years prior to diagnosis
clymphohaematopoietic neoplasia, malignant and borderline, used in ERR models
The ‘post-latency’ SIR for malignancies developing at least 5 years following the first recorded cardiac catheterization (2 years for leukaemia or lymphoma), remained raised for all malignancies (3.01, 95% CI 2.09, 4.19) and lymphoma (9.15, 95% CI 5.66, 13.97) (Table 5) though with somewhat wider confidence intervals. After censoring observations for transplant recipients, the post-latency SIR for all malignancies was reduced to 0.98 (95% CI 0.48, 1.77). A sensitivity analysis was performed by calculating post-latency SIR after excluding all patients with cumulative ABM doses less than 0.1 mGy. This resulted in a slight increase in SIR for all malignancies to 3.04 (95% CI 2.11, 42.4) for the whole cohort, and 0.99 (95% CI 0.48, 1.79) after censoring transplant recipients.
Table 5.
Standardized incidence ratio (SIR) for malignancies developing at least 5 years following the first recorded cardiac catheterization (2 years for leukaemia and lymphoma)
| Disease | Observed | Expected3 | SIR [IQR] |
|---|---|---|---|
| All patients | |||
| All malignancies | 36 | 11.98 | 3.01 [2.09, 4.19] |
| Leukaemia | 4 | 2.31 | 1.73 [0.43, 4.53] |
| Lymphoma | 22 | 2.40 | 9.15 [5.66, 13.97] |
| Hodgkin lymphoma | 4 | 1.48 | 2.70 [0.68, 7.07] |
| Non-Hodgkin lymphoma | 18 | 0.92 | 19.49 [11.39, 31.10] |
| Central nervous system | 3 | 1.92 | 1.57 [0.28, 4.70] |
| After censoring post-transplant patients | |||
| All malignancies | 11 | 11.26 | 0.98 [0.48, 1.77] |
| Leukaemia | 4 | 2.22 | 1.80 [0.45, 4.71] |
| Lymphoma | 0 | 2.25 | – |
| Hodgkin lymphoma | 0 | 1.38 | – |
| Non-Hodgkin lymphoma | 0 | 0.87 | – |
| Central nervous system | 2 | 1.82 | 1.10 [0.09, 4.11] |
IQR interquartile range
The ERR was calculated based on 36 malignant and borderline malignant lymphohaematopoietic neoplasia (29 among transplant recipients) and a total of 74,405 person-years (3446 for transplant recipients). Ignoring the transplant status interaction term, i.e. β2 = 0, the ERR was mGy−1 (95% profile likelihood (PL) CI − 0.002, 0.096). The hat symbol over the beta parameter denotes the maximum likelihood estimator. Including the transplant status interaction term mGy−1 and . When transplant status information was not taken into account at all, i.e. where and β2 = 0, the ERR was (95% PL lower bound: 0.104, Wald based upper bound: 1.807). Where this latter model was modified by censoring transplant patients post-transplant, the ERR was 0.149 mGy−1 (95% PL CI 0.001, 0.564). Omitting the absorbed dose information, i.e. β1 = 0 and , the risk effect of transplant status estimate was , indicating that the risk of haematological neoplasia was almost 80 times larger for patients who underwent transplantation.
Discussion
This is the largest study to date investigating cancer incidence among young people who have undergone cardiac catheterizations, and the first to include detailed radiation dose estimation and transplant registry linkage. The most interesting finding was the large apparent impact of transplantation on cancer rates in this patient group. Transplant recipients are treated with immunosuppressive therapies, including cyclosporine and tacrolimus [18], and receive relatively high radiation doses, especially post-transplant. Immunosuppression [14] and ionising radiation [19] are both well-known risk factors for cancer development and cannot be disentangled by SIR analysis alone. Our dose response analysis modelled the impact of transplantation in a similar manner to sex in similar radiation epidemiology studies, albeit with transplantation status as a time-dependent variable. The results of this analysis suggest radiation exposure alone does not account for the high rates of lymphohaematopoietic tumours among post-transplant patients, which in turn suggests immunosuppression is the dominant underlying cause. Furthermore, the apparent impact of transplantation was greatest for lymphoma and lymphoproliferative disorders; conditions strongly associated with immunosuppression [18, 20, 21], though relatively weakly associated with radiation exposure (e.g. [19]). In contrast, rates for leukaemia, a disease strongly associated with radiation exposure [19, 22] but less so with immunosuppression [20], were almost unaffected by censoring transplant recipients.
The absence of any cancers of the lungs, breasts and oesophagus was expected, despite the high mean dose to these tissues (Table 4). These diseases are rare below age 35 years [23], even among individuals exposed to elevated radiation levels [24]. Only 135 cohort members had reached the age of 35 years by February 1st 2014. The lack of cancers of the breasts, lungs or oesophagus should not be regarded as evidence of no risk, at this stage of follow-up. The drive to keep radiation exposures as low as reasonably achievable must, therefore, be maintained.
We were only able to gather information on cardiac catheterizations and CT. What may be termed ‘dark dose’; exposure from other sources such as general radiography and nuclear medicine, could be considerable for certain individual patients, despite contributing only a small proportion of the total cumulative dose for the whole cohort. Uncertainties in dose estimates include measurement error (i.e. kerma area product) and uncertainty in the conversion factor from which organ doses are derived, due to variation in beam angle, field size and x-ray energy. A further source of uncertainty relates to the lack of dose indicator for 21% of examinations, meaning organ doses needed to be estimated based on era-specific median doses for which dose indicators were available. For bone marrow, this figure was 3.1 mGy for examinations prior to 2003. Doses vary considerably from one procedure to the next, however. For example, the interquartile range for ABM dose was 1.65–5.60 mSv, while the 95th percentile was 18.3 mGy. Future work will attempt to quantify these uncertainties using 2D Monte Carlo techniques, allowing them to be incorporated into risk estimates.
Other than transplantation, we had limited information on other potential tumour-predisposing syndromes, such as neurofibromatosis or ataxia telangiectasia. Around 55% of examination records included a notes field in which relevant history could be recorded, while other details could be obtained from the cause of death for patients who died. Four malignancies (all leukaemia) were found among patients identified as having Down syndrome, versus 0.14 expected. Very few cases were identified among children with the most serious congenital heart defects, including hypoplastic left/right ventricles (1 case) transposition of the great arteries or tetralogy of Fallot (no malignancies, one NMSC). This analysis is limited, however, by the small proportion of the cohort for whom examination notes were available. Future studies would benefit from linkage with congenital anomalies registries.
Comparison with previous studies
Three other studies have investigated cancer incidence or mortality among children undergoing cardiac catheterizations. A retrospective cohort study of 4861 children who underwent cardiac catheterisations between 1946 and 1968 in Ontario, Canada, reported 5 cancer deaths were observed, compared to 4.8 expected, after 13 years of follow-up [1]. A further study [2] using the same cohort (reduced to 3915 members due to exclusion of patients living outside the study area) reported a standardised mortality ratio (SMR) of 1.2 (90% CI 0.6, 2.3), based on 7 cancer deaths versus 5.7 expected. The SIR was 0.75 (0.3, 1.2), based on 13 cancer cases observed versus 17.3 expected. As with the current study, a number of cancers were reported in sites remote from the heart including the tongue, testis (two cases), prostate, ovary, cervix, colon and brain. Modan et al. [3] reviewed details of 674 Israeli children who underwent cardiac catheterizations between 1950 and 1970. Dose records were unavailable for 90% of cohort members. The SIR was 2.3 (95% CI 1.2, 4.1), based on 11 cases compared to 4.75 expected, including 4 lymphomas and 3 melanomas. At least six of the tumours occurred in locations remote from the heart (testis, prostate, bladder, inguinal lymph nodes and melanomas of the groin and lower limb). The location of the others was unclear. Interestingly, all cancers occurred in males, who represented 56% of the cohort.
Other studies have focused on cancer rates among people with CHD, irrespective of whether or not they underwent cardiac catheterizations. A recent study [5] focussed on 31,961 Taiwanese patients of all ages, identified from insurance records, diagnosed with CHD between 1998 and 2006. The SIR for all cancer sites was 1.45 (95% CI 1.25, 1.67). Around half of these patients had undergone a catheterization procedure (48.9%) while 18.9% underwent CT scanning. The most common cancers were haematological (SIR = 4.04). Lymphomas were not analysed separately. Heart transplants are carried out in Taiwan [25], though it is unclear how many patients had undergone this procedure. Other studies have reported increased rates of cancer among individuals with cardiovascular malformations, as part of wider studies investigating other forms of congenital diseases [4, 6, 7]. Although the results of our analysis are broadly consistent with previous studies, it should be noted that the current cohort was established from hospital records of patients undergoing cardiac catheterizations, rather than from registers of congenital malformations.
Our SIR figures can also be compared to more general studies of children exposed to diagnostic x-rays (i.e. not specifically cardiac patients). Hammer et al. [26] reported SIRs of 0.99 for all cancers (95% CI 0.79, 1.22), 1.08 for leukaemia (95% CI 0.74, 1.52) and 0.97 for lymphoma (95% CI 0.52, 1.66) for 92,957 German children postnatally exposed to diagnostic x-rays. Average estimated doses were very low (mean effective dose = 0.137 mSv, compared to 13.1 mSv in the current study). The SIR for all cancers was similar to that of the current study after censoring transplant patients. Our SIR for leukaemia was higher, though statistically compatible with that of Hammer et al. [26]. Focussing only on CT scans, Krille et al. [27, 28] reported raised SIRs for all cancers (1.82, 95% CI 1.29, 2.50), lymphoma (2.96, 95% CI 1.42, 5.45) and leukaemia (1.72, 95% CI 0.89, 3.01). After excluding patients at increased risk of cancer, SIR figures were reduced to 1.54 for all cancers (95%: CI 1.05, 2.19), 1.79 for leukaemia (95% CI 0.92, 3.12) and 1.85 for lymphoma (95% CI 0.68, 4.02). The impact of this change on lymphoma SIR was much smaller than found in the current study, although Krille et al. [27, 28] did not have the benefit of transplant registry linkage. Mean cumulative bone marrow doses (11.7 mGy) were a little higher than in the current study (8.8 mGy).
The average equivalent dose to bone marrow from natural sources, including gamma rays and radon has been estimated to be 1.5 mSv per year at age 1 year, falling to 1.3 mSv per year at age 15 years [29]. Thus, the mean cumulative ABM dose from recorded medical exposures in this group (8.3 mGy, equivalent to 8.3 mSv) is comparable to around 5–6 years of background radiation. Background gamma exposure has been associated with a relative risk (RR) of 1.08 mGy−1 (95% CI 1.02, 1.16) for leukaemia and non-Hodgkin lymphoma [22]; a figure higher than, but statistically compatible with the ERR for lymphohaematopoietic neoplasia in the current study.
Conclusion
Cancer rates among children and young adults who undergo x-ray guided cardiac catheterizations are high, compared to the general population. Despite transplant recipients making up less than 5% of the cohort, these individuals contributed more than 60% of observed malignancies. While immunosuppression may be the most likely explanation for this, the higher radiation doses received by transplant recipients, post-transplant, may also be a contributing factor. Further analysis is planned with an enlarged cohort, congenital anomaly registry linkage, extended follow-up and pooling with cohorts from other countries (e.g. [30]). We recommend that future studies of the cancer risks from cardiac x-ray procedures take steps to identify transplant recipients, ideally through registry linkage.
Acknowledgements
We are grateful for the help of Jane Salotti, Amy Berrington de González, Neige Journy, Hélène Baysson, Elly Castellano, Colin Muirhead, Katherine Kirton, Emma Thompson, Clare McLaren, Dawn Smith, Laurence Abernethy, Steve Charlton, John Crompton, Richard Hardy, Ian Honey, Kevin Robson, Sue Reed and Mike Dunn. The study received funding from the British Heart Foundation [Project Grant No. PG/15/1/31217) and the Newcastle-upon-Tyne Healthcare Charity. RWH and MSP are affiliated with the National Institute for Health Research Health Protection Research Unit (NIHR HPRU)] in Chemical and Radiation Threats and Hazards at Newcastle University in partnership with Public Health England (PHE). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest. Relevant source of funding have been disclosed.
References
- 1.Spengler R, Cook D, Clarke E, Olley P, Newman A. Cancer mortality following cardiac catheterization: a preliminary follow-up study on 4,891 irradiated children. Pediatrics. 1983;71:235–239. [PubMed] [Google Scholar]
- 2.McLaughlin JR, Kreiger N, Sloan MP, Benson LN, Hilditch S, Clarke EA. An historical cohort study of cardiac catheterization during childhood and the risk of cancer. Int J Epidemiol. 1993;22:584–591. doi: 10.1093/ije/22.4.584. [DOI] [PubMed] [Google Scholar]
- 3.Modan B, Keinan L, Blumstein T, Sadetzki S. Cancer following cardiac catheterization in childhood. Int J Epidemiol. 2000;29:424–428. doi: 10.1093/intjepid/29.3.424. [DOI] [PubMed] [Google Scholar]
- 4.Bjorge T, Cnattingius S, Lie RT, Tretli S, Engeland A. Cancer risk in children with birth defects and in their families: a population based cohort study of 5.2 million children from Norway and Sweden. Cancer Epidemiol Biomarkers Prev. 2008;17:500–506. doi: 10.1158/1055-9965.EPI-07-2630. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Chen YT, Jeng MJ, Tsao PC, Yen HJ, Lee PC, et al. The risk of cancer in patients with congenital heart disease: a nationwide population-based cohort study in Taiwan. PLoS ONE. 2015;10:e0116844. doi: 10.1371/journal.pone.0116844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carozza SE, Langlois PH, Miller EA, Canfield M. Are children with birth defects at higher risk of childhood cancers? Am J Epidemiol. 2012;175:1217–1224. doi: 10.1093/aje/kwr470. [DOI] [PubMed] [Google Scholar]
- 7.Fisher PG, Reynolds P, Von Behren J, Carmichael SL, Rasmussen SA, Shaw GM. Cancer in children with nonchromosomal birth defects. J Pediatr. 2012;160:978–983. doi: 10.1016/j.jpeds.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glatz AC, Purrington KS, Klinger A, King AR, Hellinger J, Zhu X, et al. Cumulative exposure to medical radiation for children requiring surgery for congenital heart disease. J Pediatr. 2014;164(789–94):e10. doi: 10.1016/j.jpeds.2013.10.074. [DOI] [PubMed] [Google Scholar]
- 9.Beausejour Ladouceur V, Lawler PR, Gurvitz M, Pilote L, Eisenberg MJ, Ionescu-Ittu R, et al. Exposure to low-dose ionizing radiation from cardiac procedures in patients with congenital heart disease: 15-year data from a population-based longitudinal cohort. Circulation. 2016;133:12–20. doi: 10.1161/CIRCULATIONAHA.115.019137. [DOI] [PubMed] [Google Scholar]
- 10.Hill KD, Frush DP, Han BK, Abbott BG, Armstrong AK, DeKemp RA, et al. Radiation safety in children with congenital and acquired heart disease: a scientific position statement on multimodality dose optimization from the image gently alliance. JACC Cardiovasc Imaging. 2017;10:797–818. doi: 10.1016/j.jcmg.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ait-Ali L, Andreassi M, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart (British Cardiac Society) 2010;96:269–274. doi: 10.1136/hrt.2008.160309. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JN, Hornik CP, Li JS, Benjamin DK, Jr, Yoshizumi TT, Reiman RE, et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation. 2014;130:161–167. doi: 10.1161/CIRCULATIONAHA.113.005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Research UK. Cancer incidence by age; 2013. http://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk. Accessed 20 May 2013.
- 14.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 15.Radiation Effects Research Foundation. Radiation Effects Research Foundation: a brief description; 2008. http://ns2.rerf.or.jp/shared/briefdescript/briefdescript_e.pdf.
- 16.Bosch de Basea M, Pearce MS, Kesminiene A, Bernier MO, Dabin J, Engels H, et al. EPI-CT: design, challenges and epidemiological methods of an international study on cancer risk after paediatric and young adult CT. J Radiol Prot Off J Soc Radiol Prot. 2015;35:611–628. doi: 10.1088/0952-4746/35/3/611. [DOI] [PubMed] [Google Scholar]
- 17.Kim KP, Berrington de Gonzalez A, Pearce MS, Salotti JA, Parker L, McHugh K, et al. Development of a database of organ doses for paediatric and young adult CT scans in the United Kingdom. Radiat Prot Dosim. 2012;150:415–426. doi: 10.1093/rpd/ncr429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dipchand AI, Kirk R, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, et al. The registry of the international society for heart and lung transplantation: sixteenth official pediatric heart transplantation report—2013; focus theme: age. J Heart Lung Transplant. 2013;32:979–988. doi: 10.1016/j.healun.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–243. doi: 10.1667/RR2629.1. [DOI] [PubMed] [Google Scholar]
- 20.Simard JF, Baecklund E, Kinch A, Brattstrom C, Ingvar A, Molin D, et al. Pediatric organ transplantation and risk of premalignant and malignant tumors in Sweden. Am J Transplant. 2011;11:146–151. doi: 10.1111/j.1600-6143.2010.03367.x. [DOI] [PubMed] [Google Scholar]
- 21.Euvrard S, Kanitakis J, Cochat P, Claudy A. Skin cancers following pediatric organ transplantation. Dermatol Surg. 2004;30:616–621. doi: 10.1111/j.1524-4725.2004.30146.x. [DOI] [PubMed] [Google Scholar]
- 22.Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JC, Vincent TJ, et al. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia. 2013;27:3–9. doi: 10.1038/leu.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curado M, Edwards B, Shin H, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. Lyon: IARC Scientific Publication No. 160; 2007. [Google Scholar]
- 24.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 25.Wang SS, Wang CH, Chou NK, Chi NH, Huang SC, Yu HY, et al. Current status of heart transplantation in Taiwan. Transplant Proc. 2014;46:911–913. doi: 10.1016/j.transproceed.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 26.Hammer GP, Seidenbusch MC, Schneider K, Regulla DF, Zeeb H, Spix C, et al. A cohort study of childhood cancer incidence after postnatal diagnostic X-ray exposure. Radiat Res. 2009;171:504–512. doi: 10.1667/RR1575.1. [DOI] [PubMed] [Google Scholar]
- 27.Krille L, Dreger S, Schindel R, Albrecht T, Asmussen M, Barkhausen J, et al. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys. 2015;54:1–12. doi: 10.1007/s00411-014-0580-3. [DOI] [PubMed] [Google Scholar]
- 28.Krille L, Dreger S, Schindel R, Albrecht T, Asmussen M, Barkhausen J, et al. Erratum to: risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys. 2017;56:293–297. doi: 10.1007/s00411-017-0694-5. [DOI] [PubMed] [Google Scholar]
- 29.Kendall GM, Fell TP. Doses to the red bone marrow of young people and adults from radiation of natural origin. J Radiol Prot Off J Soc Radiol Prot. 2011;31:329–335. doi: 10.1088/0952-4746/31/3/002. [DOI] [PubMed] [Google Scholar]
- 30.Baysson H, Réhel J, Boudjemline Y, Petit J, Girodon B, Aubert B, et al. Risk of cancer associated with cardiac catheterization procedures during childhood: a cohort study in France. BMC Public Health. 2013;13:266. doi: 10.1186/1471-2458-13-266. [DOI] [PMC free article] [PubMed] [Google Scholar]