Abstract
Background: Recently, we demonstrated that in healthy newborn infants cerebral blood volume (CBV) was decreasing continuously after birth. We hypothesized that this was due to the increase in oxygen delivery to the brain during neonatal transition. Thus delayed cerebral oxygen delivery in infants in need for respiratory support (RS) during postnatal stabilization might influence changes in CBV.
Objective: Aim of the study was to evaluate transitional changes in CBV immediately after birth in term and preterm infants with and without need of RS.
Methods: We performed a post-hoc analysis of data collected as primary and secondary outcome parameters in prospective observational studies and randomized controlled trials at the Medical University of Graz (Austria). NIRS measurements by using “NIRO 200-NX” (Hamamatsu, Japan) were carried out over the first 15 min after birth in term and preterm infants delivered by cesarean section with and without requirement for RS.
Results: In 204 neonates, we observed a significant decrease in CBV within the first 15 min after birth (p < 0.001) with a trend toward smaller ΔCBV in neonates receiving RS (p = 0.097) compared to neonates without RS. Differences of ΔCBV between groups reached statistically significance (p < 0.05) at minutes 2, 6, and 7, and showed a trend (p < 0.1) at minutes 3, 4, and 5. After adjusting for gestational age, these differences became smaller and failed to reach significance.
Conclusions: We observed a significant decrease of CBV in term and preterm infants with and without RS. Interestingly, ΔCBV was smaller in the first 7 min in neonates with RS reaching statistically significance (p < 0.05) at minutes 2, 6, and 7. This study cannot differentiate, whether RS itself or the condition leading to requirement for RS is responsible for the observed CBV behavior.
Keywords: cerebral blood volume, near-infrared spectroscopy (NIRS), neonatal transition, ventilation induced brain injury, preterm infants, ventilation
Introduction
Hemodynamic disturbances have been shown to potentially result in alterations of cerebral perfusion and subsequent brain injury in newborn infants (1–3). This might be of particular relevance for preterm infants in which cerebral autoregulation may not be fully present immediately after birth (2–4).
The use of near-infrared spectroscopy (NIRS) during immediate neonatal transition enables a non-invasive method to evaluate cerebral hemodynamics. Most of the previously published NIRS studies in newborns during neonatal transition reported exclusively on cerebral tissue oxygenation (1, 5–15). However, since NIRS technology further provides measurements of changes in total hemoglobin (ΔHbT), the evaluation of changes in cerebral blood volume (ΔCBV) is feasible. Recently, our research group demonstrated that in healthy newborn infants CBV is continuously decreasing during the first 15 min after birth, hypothesizing that this was mainly due to the increase in oxygen delivery to the brain resulting in cerebral vasoconstriction (16).
Newborn infants receiving respiratory support (RS) during postnatal stabilization show a delayed increase in oxygen delivery (12), which potentially influences transitional CBV changes. The occurrence of hemodynamic disturbances in ventilated infants is discussed to be one pathway resulting in ventilation-induced brain injury, especially in newborn infants with impaired mechanisms of autoregulation (4). It has been shown, that significantly reduced cerebral tissue oxygen saturation during neonatal transition was associated with severe intraventricular hemorrhage (1). Not only pO2 and pCO2 may influence CBV, but compromised cerebral perfusion might further be caused by an increased pulmonary resistance and decreased cardiac output due to over-distension of alveoli and compression of pulmonary capillaries (2, 3). This indicates that monitoring of cerebral oxygenation and perfusion might be beneficial in infants with RS during postnatal stabilization. The aim of the present study was to analyze the potential influence of RS on the course of CBV during the transition of the newborn. We hypothesized that the physiological CBV decrease during neonatal transition would be diminished in infants receiving RS showing smaller ΔCBV compared to those infants with normal transition.
Materials and methods
Study design
We performed a post-hoc analysis of data collected as primary and secondary outcome parameters in three prospective observational studies and one randomized controlled trial at the Medical University of Graz; Austria (5, 16–18). The Regional Committee on Biomedical Research Ethics approved all of the included studies. Between October 2010 and January 2015 term and preterm infants delivered by cesarean section with and without the need of RS were included after a written informed consent was obtained from the parents prior to birth. Due to technical reasons the NIRS measurements couldn't be performed in the delivery room next to the mother. Therefore, vaginally born infants were excluded to avoid a delay or disturbance of immediate skin-to-skin contact with the mother. Further exclusion criteria were presence of congenital malformations, inherited disorders of metabolism, decision to not provide full life support, and application of sustained lung inflations during postnatal stabilization.
Procedure
After cord clamping, routinely performed after 30 s, infants were placed on the resuscitation table under an overhead heater. The newborn infants were dried and stimulated by using warm cotton diapers to induce effective breathing. In case of obvious or suspected upper airway obstruction immediate suction of the oropharynx was performed. If necessary, RS by using a “Neopuff Infant T-Piece Resuscitator” (Perivent, Fisher & Paykel Healthcare; New Zealand) was provided via face mask of appropriate size (LSR Silicon mask no. 0/0 or 0/1, Laerdal; Norway) according to the guidelines (19–21) either by applying PPV or CPAP depending on the breathing efforts of the patient. The pre-set FiO2 was 0.21 in term infants and 0.3 in preterm infants and was adapted to achieve defined oxygen saturation targets during immediate postnatal transition (22).
As soon as possible, scientific staff members attached the NIRS transducer on the newborn's right forehead by using gauze bandage without disturbing routine medical care. NIRS measurements were carried out with a “NIRO 200-NX” tissue oxygenation monitor (Hamamatsu; Japan). By using NIRS, cerebral tissue oxygenation index (cTOI) and changes in ΔHbT were measured with a sample rate of 2 Hertz. Additionally both, preductal arterial oxygen saturation (SpO2) and HR, were continuously monitored by using pulse-oximetry measured on the right wrist (M1193A Neonate Silicon Wrap, Philips; the Netherlands). The measurements were conducted over the first 15 min after birth. Singular measurements of blood pressure and rectal body temperature were recorded between minute 10 and 15 after birth and displayed on the “IntelliVue MP30/X2” monitor (Philips; the Netherlands).
For analysis all parameters and video recordings were stored using a multichannel system “alpha-trace digital MM” (BEST Medical Systems; Austria).
Statistics
In each neonate, ΔHbT values for each minute after birth were calculated by subtracting the mean ΔHbT value at minute 15 from the mean ΔHbT value of the related minute. The 15 min value was used as reference value, because at that time point NIRS signal quality was most stable and reliable. Next, ΔHbT values were converted to ΔCBV by the following equation, whereby Hb represents the hemoglobin concentration (g/dl) (23):
| (1) |
For the calculation either the actual Hb level of the individual (from routinely performed blood sampling within 30 min after birth) or the averaged group Hb of term or preterm infants (if the individual value was not available) was used.
Demographic variables are presented as absolute and relative counts, mean, and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Comparisons of categorical baseline characteristics between infants with and without RS and between preterm and term infants were made using chi-square test, t-test or Mann–Whitney U-test, as appropriate. Data of ΔCBV, cTOI, SpO2, and HR are presented as mean and 95% confidence interval (95%CI). We investigated the courses of ΔCBV, cTOI, SpO2, and HR within the first 15 min after birth using a linear mixed model with fixed effects for time, RS (with RS vs. without RS), and gestational age (preterm vs. term). Since most of preterm neonates (82.2%) required RS and most of term neonates (88.1%) did not receive RS, we tested a model including RS without considering gestational age (model 1) and a model including RS and gestational age (model 2). The decision which of these models to be used was based on a REML-based likelihood ratio test. In all models a first order autoregressive covariance structure was used. The autoregressive covariance structure assumes a systematically decreasing correlation with increasing distance between time points. Therefore, adjacent time points will have the highest correlations. Post-hoc analyses for differences between groups for each minute were performed for the comparison of RS groups (with RS vs. without RS) and if model 2 was chosen also for gestational age groups (term infants vs. preterm infants). If model 1 (without comparison: term infants vs. preterm infants) was chosen, the model 2 is given in the supplement together with the model 1. A p-value < 0.05 was considered statistical significant. Statistical analyses were performed using SPSS Statistics 20.0.0 (IBM; USA).
Results
In total, in 204 neonates measurements were performed during the study period including 45 preterm infants (37 with and 8 without RS) and 159 term infants (19 with and 140 without RS) born at a mean gestational age of 33+3 weeks (±15 days) and 38+6 weeks (±6 days), respectively. Thus, 56 newborn infants received RS during the transitional period, whereas 148 had a normal neonatal transition without the need of RS. As RS was according to individual needs of the infants, start points and duration of PPV and/or CPAP and extent of FiO2 were different in each subject. RS started at 2.5 (4.0) min, duration of RS within the first 15 min of life was 11.5 (9.0) min [median (interquartile range)].
Demographic and clinical data of newborn infants with and without RS during immediate postnatal transition are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of newborn infants with and without RS.
| With RS (n = 56) | Without RS (n = 148) | p-value | |
|---|---|---|---|
| Gestational age (wk), mean (±SD) | 35.0 (3.2) | 38.7 (1.2) | < 0.001 |
| Birth weight (g), mean (±SD) | 2,382 (916) | 3,232 (502) | < 0.001 |
| Head circumference (cm), mean (±SD) | 32.2 (3.3) | 34.6 (1.5) | < 0.001 |
| Female sex, n (%) | 31 (55) | 76 (51) | 0.609 |
| Apgar at 1 min, median (IQR) | 8 (8–8) | 9 (9–9) | < 0.001 |
| Apgar at 5 min, median (IQR) | 9 (9–9) | 10 (10–10) | < 0.001 |
| Apgar at 10 min, median (IQR) | 9 (9–10) | 10 (10–10) | < 0.001 |
| pH Umbilical artery, median (IQR) | 7.29 (7.27–7.31) | 7.29 (7.27–7.32) | 0.695 |
| Rectal body temperature (°C) at 15 min, mean (±SD) | 36.7 (0.5) | 36.7 (0.3) | 0.794 |
| Hemoglobin (g/dl) in umbilical cord blood, mean (±SD) | 17.4 (3.1) | 15.3 (1.6) | 0.002 |
| Systolic APB (mmHg) at 10 min, mean (±SD) | 60.2 (9.4) | 66.0 (9.4) | < 0.001 |
| Mean APB (mmHg) at 10 min, mean (±SD) | 41.2 (8.9) | 45.4 (7.8) | 0.003 |
| Diastolic APB (mmHg) at 10 min, mean (±SD) | 32.7 (9.4) | 35.0 (10.3) | 0.186 |
ABP, arterial blood pressure; RS, respiratory support.
Demographic and clinical data of preterm and term infants—irrespective of whether RS was applied or not—are summarized in Table 2.
Table 2.
Demographic and clinical characteristics of preterm and term infants.
| Preterm infants (n = 45) | Term infants (n = 159) | p-value | |
|---|---|---|---|
| Gestational age (wk), mean (±SD) | 33.5 (2.1) | 38.9 (0.9) | < 0.001 |
| Birth weight (g), mean (±SD) | 1,900 (1,442–2580) | 3,260 (2,954–3,480) | < 0.001 |
| Head circumference (cm), median (IQR) | 30.5 (29.0–38.0) | 35.0 (34.0–35.5) | < 0.001 |
| Female sex, n (%) | 26 (58) | 81 (51) | 0.418 |
| Apgar at 1 min, median (IQR) | 8 (8–8) | 9 (9–9) | < 0.001 |
| Apgar at 5 min, median (IQR) | 9 (8–9) | 10 (10–10) | < 0.001 |
| Apgar at 10 min, median (IQR) | 9 (9–10) | 10 (10–10) | < 0.001 |
| pH Umbilical artery, mean (±SD) | 7.30 (0.04) | 7.28 (0.05) | 0.015 |
| Rectal body temperature (°C) at 15 min, mean (±SD) | 36.7 (0.6) | 36.7 (0.3) | 0.546 |
| Hemoglobin (g/dl) in umbilical cord blood, mean (±SD) | 18.0 (3.0) | 15.2 (1.6) | < 0.001 |
| Systolic APB (mmHg) at 10 min, mean (±SD) | 59.1 (10.0) | 65.8 (9.2) | < 0.001 |
| Mean APB (mmHg) at 10 min, mean (±SD) | 40 (35–47) | 45 (40–50) | 0.002 |
| Diastolic APB (mmHg) at 10 min, median (IQR) | 32 (27–38) | 35 (28–42) | 0.090 |
ABP, arterial blood pressure.
Cerebral blood volume
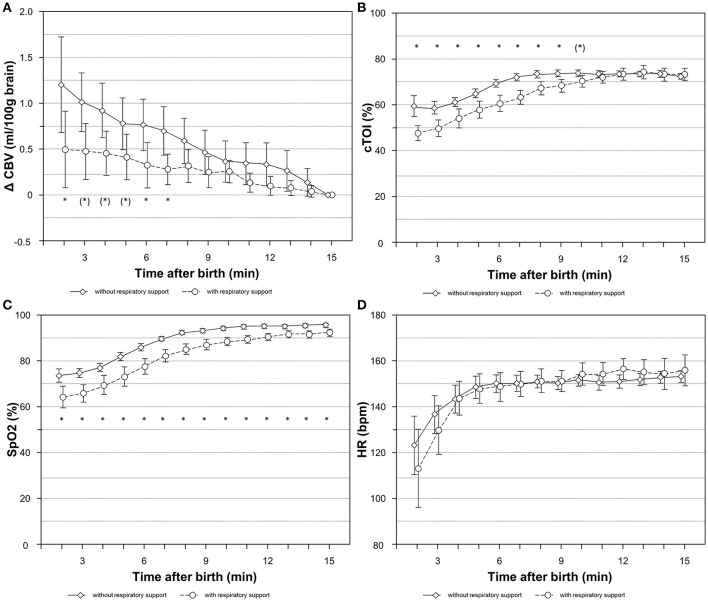
In the whole study population a significant decrease in CBV was observed within the first 15 min after birth (p < 0.001). Furthermore we observed a trend toward smaller ΔCBV in neonates with RS (p = 0.097), while the courses of ΔCBV were comparable between groups (p = 0.655). Differences of ΔCBV between groups reached statistically significance (p < 0.05) at minutes 2, 6, and 7. The ΔCBV values at minutes 3, 4, and 5 showed a trend (p < 0.10) toward a difference between groups (Figure 1A). The inclusion of the gestational age did not result in a significant better model fit (p = 0.172), meaning that gestational age could not explain further differences in ΔCBV between neonates. Nevertheless, gestational age was included in a sensitivity analysis, which resulted in comparable effects (Supplementary Tables 1, 2).
Figure 1.
Courses of ΔCBV (A), cTOI (B), SpO2 (C), and HR (D) during the first 15 min after birth in newborn infants with and without respiratory support. Values are mean (95%CI); *p < 0.05, (*)p < 0.1, significances are calculated for group comparisons for each minute. bpm, beats per minute; CBV, cerebral blood volume; cTOI, cerebral tissue oxygenation index; HR, heart rate; SpO2, arterial oxygen saturation.
cTOI, SpO2, and HR
In the whole study population cTOI (p < 0.001) and SpO2 values (p < 0.001) showed a significant increase during immediate postnatal transition. We found significant differences in cTOI and SpO2 levels between newborn infants with and without RS after birth showing significantly lower values in those infants who received RS (cTOI: p = 0.023; SpO2: p < 0.001). By comparing both groups, cTOI levels did not equalize before the 9th minute of age, whereas the differences of SpO2 continued until the end of the observational period of 15 min after birth (Figures 1B,C). cTOI and SpO2 values did not show significant differences comparing term to preterm infants (cTOI: p = 0.577; SpO2: p = 0.595). While courses of cTOI were comparable between term and preterm infants (cTOI: p = 0.379) SpO2 showed significant different courses between term and preterm infants (SpO2: p = 0.031) with a slightly steeper increase in term infants at the end of the observational period (Supplementary Table 2).
HR increased significantly (p < 0.001) in the study population. This significant increase was mainly due to an increase within the first 5 min after birth. HR values were higher in newborn infants with RS compared to newborn infants without RS (p = 0.007) (Figure 1D) and were higher in term compared to preterm infants (p = 0.003). Courses of HR were comparable between term and preterm infants (p = 0.218) (Supplementary Table 2).
Discussion
To the best of our knowledge this is the first study that incorporates detailed analysis of CBV in term and preterm infants with and without the need of RS during immediate postnatal transition. We observed a significant decrease in CBV within the first 15 min after birth in both groups, but the ΔCBV was smaller in the first 7 min in neonates with RS. Whether RS itself or the condition of the infant leading to requirement for RS is responsible for the observed CBV behavior cannot be explained in detail with the present analysis.
Our findings are in accordance with recently published data by our study group demonstrating a significant decrease in CBV in healthy term infants after birth (16). We hypothesized that the transitional CBV decrease reflected a physiological response to changing blood gases within the autoregulatory capacity of cerebral vessels as pO2 increases and pCO2 decreases postnatally (16). Blood gas changes may occur to a different extent in different individuals during postnatal immediate transition, but generally during neonatal transition the changes in pO2 are more distinct, compared to changes in pCO2. Furthermore, our study group demonstrated that preterm infants receiving RS during neonatal transition showed a significantly lower cerebral tissue oxygenation compared to infants without requirement of RS (6, 12). Thus, decreased cerebral oxygenation in infants receiving RS potentially may be accompanied by cerebral vasodilatation to increase cerebral blood flow and improve oxygen delivery (24). The extent of CBV decrease due to cerebral vasoconstriction might substantially depend on pO2 levels, which increase less rapidly in infants requiring RS immediately after birth (6, 12, 25).
Moreover, we recently published a randomized pilot study showing an influence of sustained lung inflation on CBV behavior in preterm infants. Whereas in preterm infants receiving RS standard care (PPV or CPAP) after birth CBV was decreasing, CBV basically remained unchanged in preterm infants receiving one to three sustained lung inflations followed by PPV or CPAP during immediate transition (17). Based on these observation we hypothesized that RS itself might influence postnatal CBV changes, most probably by increasing the intrathoracic pressure leading to cerebral venous stasis and an impairment of venous return to the heart (17). The significant differences in the postnatal CBV behavior justify the exclusion of infants receiving sustained lung inflations from the present post-hoc analysis.
However, it has been shown that initial RS immediately after birth is a considerable risk factor for cerebral injury and local brain inflammation in newborn neonates, particularly in preterm infants who already are at a high risk due to brain immaturity (3). Even though there is growing evidence that ventilation-induced injuries occur as early as ventilation is initiated in the delivery room, PPV immediately after birth still is one of the least controlled interventions in preterm infant (3). By using a respiratory function monitor, potentially harmful high tidal volumes were demonstrated during postnatal stabilization in more than 85% of preterm infants receiving PPV via face mask (26). These high tidal volumes might influence cerebral perfusion and might have hazardous effects on the brain (2, 27). The present study cannot add to that topic, as the number of infants was too small to draw a conclusion in regard to cerebral injury. Therefore, further research is needed to optimize initial ventilatory strategies to potentially achieve improved cerebral outcome in newborn infants.
Limitations
First, all the included infants were delivered by cesarean section and cord clamping was routinely performed 30 s after birth. Therefore it remains unclear, whether vaginal birth or delayed cord clamping would have resulted in a different CBV behavior.
NIRS assessment of the frontal cortical region by using a single transducer covers only a small part of the brain in supine positioned infants, although less likely, we cannot rule out that dynamics of CBV during transition might be different in different regions of the brain.
Furthermore, the conversion of NIRS derived ΔHbT to ΔCBV requires the individual Hb levels of every single patient. Unfortunately, it wasn't possible to obtain Hb levels in each patient, because the Regional Committee on Biomedical Research Ethics did not authorize blood sampling exclusively for study purposes. Therefore, in absence of the individual levels we used mean Hb values of the respective study group for calculating ΔCBV. Moreover, we cannot provide continuous pO2 or pCO2 values, which might have been beneficial for the interpretation of the CBV results.
Next, since groups differed in their gestational age, the influence of the gestational age on the primary could not be sufficient investigated. The influence of gestational age on ΔCBV should be a topic of further research.
Last, our results derived by post-hoc analysis need to be interpreted with caution and confirmation by other appropriately designed prospective studies is required.
Conclusion
We observed a significant decrease of CBV in both groups, in infants undergoing normal transition and in infants in need for RS (either PPV or CPAP). Interestingly, ΔCBV was smaller in the first 7 min in neonates with RS. This study did not yet determine, whether RS itself or the condition of the infant leading to requirement for RS is responsible for the observed differences in CBV compared to healthy newborn infants, but we would hypothesize, that differences in cerebral oxygen delivery may explain this best. Our results are of particularly great interest, since hemodynamic disturbances resulting in changes in cerebral perfusion are discussed to be an important pathway to ventilator-induced brain injury occurring as early as ventilation is initiated in the delivery room.
Author contributions
BS, GP, CB-H, NB-S, and BU: substantial contributions to the conception or design of the work; BS, GP, AA, and BU: the acquisition, analysis, or interpretation of data for the work; BS, GP, CB-H, NB-S, AA, and BU: drafting the work or revising it critically for important intellectual Content; BS, GP, CB-H, NB-S, AA, and BU: final approval of the version to be published; BS, GP, CB-H, NB-S, AA, and BU: agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to express our gratitude to the parents for putting us in position to investigate their infants, as well as all the midwives, nurses, and physicians involved in the treatment of these neonates. We also thank Evelyn Ziehenberger for her assistance in completing this study.
Glossary
Abbreviations
- ABP
arterial blood pressure
- CBV
cerebral blood volume
- CPAP
continuous positive airway pressure
- cTOI
cerebral tissue oxygenation index
- Hb
hemoglobin
- ΔHbT
changes in the concentration of total hemoglobin
- HR
heart rate
- NIRS
near-infrared spectroscopy
- PPV
positive pressure ventilation
- RS
respiratory support
- SpO2
arterial oxygen saturation.
Footnotes
Funding. Funding provided by Jubilaeumsfond, Oesterreichische Nationalbank (Grant No. 14312), BU received the funding. http://www.oenb.at/en/About-Us/Research-Promotion/The-OeNB-Anniversary-Fund.html. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2018.00132/full#supplementary-material
References
- 1.Baik N, Urlesberger B, Schwaberger B, Schmolzer GM, Avian A, Pichler G. Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Arch Dis Child Fetal Neonatal Ed. (2015) 100:F422–7. 10.1136/archdischild-2014-307590. [DOI] [PubMed] [Google Scholar]
- 2.Polglase GR, Miller SL, Barton SK, Baburamani AA, Wong FY, Aridas JD, et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS ONE (2012) 7:e39535. 10.1371/journal.pone.0039535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton SK, Tolcos M, Miller SL, Christoph-Roehr C, Schmolzer GM, Moss TJ, et al. Ventilation-induced brain injury in preterm neonates: a review of potential therapies. Neonatology (2016) 110:155–62. 10.1159/000444918 [DOI] [PubMed] [Google Scholar]
- 4.Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev. (2005) 81:423–8. 10.1016/j.earlhumdev.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Baik N, Urlesberger B, Schwaberger B, Schmolzer GM, Mileder L, Avian A, et al. Reference ranges for cerebral tissue oxygen saturation index in term neonates during immediate neonatal transition after birth. Neonatology (2015) 108:283–6. 10.1159/000438450 [DOI] [PubMed] [Google Scholar]
- 6.Binder C, Urlesberger B, Avian A, Pocivalnik M, Muller W, Pichler G. Cerebral and peripheral regional oxygen saturation during postnatal transition in preterm neonates. J Pediatr. (2013) 163:394–9. 10.1016/j.jpeds.2013.01.026 [DOI] [PubMed] [Google Scholar]
- 7.Fuchs H, Lindner W, Buschko A, Almazam M, Hummler HD, Schmid MB. Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J Perinatol. (2012) 32:356–62. 10.1038/jp.2011.110 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs H, Lindner W, Buschko A, Trischberger T, Schmid M, Hummler HD. Cerebral oxygenation in very low birth weight infants supported with sustained lung inflations after birth. Pediatr Res. (2011) 70:176–80. 10.1038/pr.2011.40110.1203/PDR.0b013e318220c1e0 [DOI] [PubMed] [Google Scholar]
- 9.Pichler G, Avian A, Binder C, Zotter H, Schmolzer GM, Morris N, et al. aEEG and NIRS during transition and resuscitation after birth: promising additional tools; an observational study. Resuscitation (2013) 84:974–8. 10.1016/j.resuscitation.2012.12.025 [DOI] [PubMed] [Google Scholar]
- 10.Pichler G, Binder C, Avian A, Beckenbach E, Schmolzer GM, Urlesberger B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr. (2013) 163:1558–63. 10.1016/j.jpeds.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 11.Pichler G, Urlesberger B, Baik N, Schwaberger B, Binder-Heschl C, Avian A, et al. Cerebral oxygen saturation to guide oxygen delivery in preterm neonates for the immediate transition after birth: a 2-center randomized controlled pilot feasibility trial. J Pediatr. (2016) 170:73-8.e1-4. 10.1016/j.jpeds.2015.11.053 [DOI] [PubMed] [Google Scholar]
- 12.Schwaberger B, Pichler G, Binder C, Avian A, Pocivalnik M, Urlesberger B. Even mild respiratory distress alters tissue oxygenation significantly in preterm infants during neonatal transition. Physiol Meas. (2014) 35:2085–99. 10.1088/0967-3334/35/10/2085 [DOI] [PubMed] [Google Scholar]
- 13.Urlesberger B, Grossauer K, Pocivalnik M, Avian A, Muller W, Pichler G. Regional oxygen saturation of the brain and peripheral tissue during birth transition of term infants. J Pediatr. (2010) 157:740–4. 10.1016/j.jpeds.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 14.Urlesberger B, Kratky E, Rehak T, Pocivalnik M, Avian A, Czihak J, et al. Regional oxygen saturation of the brain during birth transition of term infants: comparison between elective cesarean and vaginal deliveries. J Pediatr. (2011) 159:404–8. 10.1016/j.jpeds.2011.02.030 [DOI] [PubMed] [Google Scholar]
- 15.Fauchere JC, Schulz G, Haensse D, Keller E, Ersch J, Bucher HU, et al. Near-infrared spectroscopy measurements of cerebral oxygenation in newborns during immediate postnatal adaptation. J Pediatr. (2010) 156:372–6. 10.1016/j.jpeds.2009.09.050 [DOI] [PubMed] [Google Scholar]
- 16.Schwaberger B, Pichler G, Binder-Heschl C, Baik N, Avian A, Urlesberger B. Transitional changes in cerebral blood volume at birth. Neonatology (2015) 108:253–8. 10.1159/000437347 [DOI] [PubMed] [Google Scholar]
- 17.Schwaberger B, Pichler G, Avian A, Binder-Heschl C, Baik N, Urlesberger B. Do sustained lung inflations during neonatal resuscitation affect cerebral blood volume in preterm infants? A randomized controlled pilot study. PLoS ONE (2015) 10:e0138964. 10.1371/journal.pone.0138964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freidl T, Baik N, Pichler G, Schwaberger B, Zingerle B, Avian A, et al. Haemodynamic transition after birth: a new tool for non-invasive cardiac output monitoring. Neonatology (2016) 111:55–60. 10.1159/000446468 [DOI] [PubMed] [Google Scholar]
- 19.Richmond S, Wyllie J. European resuscitation council guidelines for resuscitation 2010 section 7. Resuscitation of babies at birth. Resuscitation (2010) 81:1389–99. 10.1016/j.resuscitation.2010.08.018 [DOI] [PubMed] [Google Scholar]
- 20.Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation (2010) 122(16 Suppl. 2):S516–38. 10.1161/CIRCULATIONAHA.110.971127 [DOI] [PubMed] [Google Scholar]
- 21.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology (2010) 97:402–17. 10.1159/000297773 [DOI] [PubMed] [Google Scholar]
- 22.Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics (2010) 125:e1340-7. 10.1542/peds.2009-1510 [DOI] [PubMed] [Google Scholar]
- 23.Wyatt JS, Edwards AD, Cope M, Delpy DT, McCormick DC, Potter A, et al. Response of cerebral blood volume to changes in arterial carbon dioxide tension in preterm and term infants. Pediatr Res. (1991) 29:553–7. 10.1203/00006450-199106010-00007 [DOI] [PubMed] [Google Scholar]
- 24.Kenosi M, O'Toole JM, Livingston V, Hawkes GA, Boylan GB, O'Halloran KD, et al. Effects of fractional inspired oxygen on cerebral oxygenation in preterm infants following delivery. J Pediatr. (2015) 167:1007-12.e1. 10.1016/j.jpeds.2015.07.063 [DOI] [PubMed] [Google Scholar]
- 25.Schwaberger B, Pichler G, Urlesberger B. Does cerebral vasoconstriction following delivery protect against hyperoxia? J Pediatr. (2016) 173:266. 10.1016/j.jpeds.2016.01.067 [DOI] [PubMed] [Google Scholar]
- 26.Schmolzer GM, Kamlin OC, O'Donnell CP, Dawson JA, Morley CJ, Davis PG. Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. (2010) 95:F393-7. 10.1136/adc.2009.174003 [DOI] [PubMed] [Google Scholar]
- 27.Barton SK, Melville JM, Tolcos M, Polglase GR, McDougall AR, Azhan A, et al. Human amnion epithelial cells modulate ventilation-induced white matter pathology in preterm lambs. Dev Neurosci. (2015) 37:338–48. 10.1159/000371415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.