Abstract
Four delivery routes, via, feed, water, litter and oral gavage, were examined for their efficacy in delivering a novel probiotic of poultry origin, Lactobacillus johnsonii, to broilers. Seven treatments of 6 replicates each were allocated using 336 one-day-old Cobb broiler chicks. The treatments consisted of a basal diet with the probiotic candidate, L. johnsonii, added to the feed, and three treatments with L. johnsonii added to the drinking water, sprayed on the litter, or gavaged orally. In addition, a positive control treatment received the basal diet supplemented with zinc-bacitracin (ZnB, 50 mg/kg). The probiotic strain of L. johnsonii was detected in the ileum of the chicks for all four delivery routes. However, the addition of L. johnsonii as a probiotic candidate did not improve body weight gain, feed intake and feed conversion ratio of broiler chickens raised on litter during the 5-week experimental period regardless of the route of administration. The probiotic treatments, regardless of the routes of delivery, affected (P < 0.05) the pH of the caecal digesta and tended (P = 0.06) to affect the pH of the ileal digesta on d 7, but the effect disappeared as the birds grew older. All probiotic treatments reduced the number of Enterobacteria in the caeca on d 21, and tended (P < 0.054) to reduce it in the ileum and caeca on d 7 and in the ileum on d 21 compared with the controls. The probiotic also tended to increase the number of lactic acid bacteria and lactobacilli in the ileum and caeca on d 7, but this trend was not evident on d 21. The trend appeared most pronounced when the probiotic was delivered orally or via litter. The probiotic also decreased (P < 0.05) the population of Clostridium perfringens rapidly from an early age to d 21 in the caeca, leading to a 3-fold decrease in the number of C. perfringens between d 7 and 21. It also showed that the probiotic treatment presented the lowest number of C. perfringens in the caeca. Delivery of the probiotic through feed, water and litter increased (P < 0.01) the weight of the pancreas on d 21, but the probiotic did not affect other morphometric parameters of the gut. Furthermore, the probiotic did not affect the pH and the concentrations of short chain fatty acids and lactic acid in either the ileum or caeca.
Keywords: Probiotics, Delivery routes, Broiler, Performance, Intestinal morphology
1. Introduction
Probiotics display numerous health benefits beyond providing basic nutritional advantages. Probiotic products consisting of beneficial microflora can help to establish and maintain the balance of the intestinal microflora in commercial broilers. However, selecting a probiotic microorganism that has beneficial effects in broiler chickens requires an extensive search for the optimum candidate, and one which will perform under practical conditions. Inoculating one-day-old chicks with competitive exclusion (CE) cultures or more classical probiotics serves as an effective model for determining the modes of action and efficacy of these microorganisms. Because of the susceptibility of one-day-old chicks to infection, this practice is also of commercial importance. By using this model, a number of probiotics have been shown to reduce colonization and shedding of Salmonella and Campylobacter (Netherwood et al., 1999, Fritts et al., 2000). However, one of the key factors determining their efficacy in practical use is stability during storage, delivery and feed processing.
There are many different methods for administering probiotic preparations to broiler chickens: through feed, water, gavage (including droplet or inoculations), spray or litter, but adding to feed is the most commonly used method in poultry production.
Introducing probiotics through drinking water, into the crop by tube and syringe, with crumbles, or by spraying on bird environment and litter had no effects on the survival rate of bacteria (Gardiner et al., 2000, Morelli, 2000, Corcoran et al., 2004). The feed-type probiotic products rarely produce optimum results in pelletized diets usually fed to broilers (Nguyen et al., 1988, Scheuerman, 1993). Kozasa (1986) found that two probiotic bacteria incorporated into crumbles, successfully survived the duration of the experiment. Also, Gould and Hurst (1969) reported that spores of bacillus are well known for being able to survive high temperatures. Thus, the best natural solution to the challenge of stability in direct-fed microbial products is to use spore-forming beneficial strains of microbes or fed as crumbles (Crawford, 1979). However, Seuna et al. (1978) showed that the viability of the organisms rapidly declined, especially in chlorinated water when bacteria via the drinking water rather than gavage compared.
The literature suggests that spray application of probiotic cultures, either on the environment of the birds or on the litter material seems to be an effective way of administering probiotic cultures (Blankenship, 1992), whilst according to Nurmi and Rantala (1973) intubation into the crop is perhaps the most satisfactory method for delivering a precise dose of probiotics to the animal.
The aim of this study was to determine the efficacy of administering a probiotic strain of Lactobacillus johnsonii which chosen by antimicrobial activities showed the best resistant in promoting growth performance, intestinal morphology and gut microflora in broiler chickens.
2. Materials and methods
2.1. Probiotic strains
The bacterial strain used in this experiment was selected using the antagonistic activity assay described by Teo and Tan (2005).
A pure L. Johnsonii isolate was grown in MRS broth overnight (at 39°C) and harvested by centrifugation at 4,420 × g for 15 min (Induction Drive Centrifugation, Beckman Model J2-21M, Beckman Instruments Inc., Palo Alto, California, USA). It was re-suspended in phosphate-buffered solution (PBS, pH 7.4) and mixed by constant mechanical stirring (Heidolph MR 3001K stirrer, Heidolph Instruments GmbH & Co., Schwabach, Germany) for 10 min. This pre-mixture of PBS probiotic solution was added to feed, drinking water, or was gavaged orally. The quantities of MRS broth and pre-mix phosphate-buffered solution (PBS, solution used were calculated by determining the bacterial concentration needed for the experiment. In this study, the concentration of the probiotic candidate, L. johnsonii, supplied via different routes was: feed delivery >106 cfu/gram of feed samples; oral delivery >108 cfu/mL of BPS solution; litter delivery >108 cfu/mL of PBS spray solution and water delivery >106 cfu/mL of water sample.
Representative feed, water, and litter samples of each treatment batch were tested for bacterial concentrations weekly on d 1 and 7. Ten grams (or millilitres) of samples were dissolved in 90 mL of peptone water (Oxoid, CM0009) and 10-fold dilutions were performed in Hungate tubes with 9mL of peptone water. The numbers of lactic acid bacteria in the samples were determined on MRS agar (Oxoid, CM0361) inoculated with 0.1 mL of diluted sample and after anaerobic incubation at 39°C for 48 h.
2.2. Bird husbandry
A total of 336 one-day-old male Cobb broiler chicks, which were vaccinated against Marek׳s disease, infectious bronchitis, and Newcastle disease, were obtained from a local hatchery (Baiada hatchery, Kootingal, NSW, Australia) and randomly allocated to 42 cages in four-tier floor pens (600 × 600 × 300 mm dimension, with a floor space of 0.36 m2/cage) sit on sawdust litter in climate-controlled rooms. Each of the 7 dietary treatments was randomly assigned to 6 cages with 8 birds per cage (except for the water treatment group which needed to be in line in order to be serviced by the same water pipe that supplied the water containing the probiotics). At d 21, birds were transferred to slide-in cages (800 × 740 × 460 mm) in an environmentally controlled room.
The room temperature was gradually decreased from 33°C on d 1 to 24°C on d 21. Eighteen hours of light was provided per day throughout the trial, excluding d 1 to 7 during which 23 h of light was provided. Relative humidity was between 65 and 70%. Each cage was equipped with a feeding trough placed outside and had water pipes providing drinking nipples inside. Feed and water were provided ad libitum.
2.3. Experimental treatments
2.3.1. The diet and treatments
The basal diets (starter and finisher) were based on corn, wheat and soybean meal as shown in (Table 1), and fed as a one-phase mash feed to avoid inactivation of the probiotic. Seven treatments were provided as three diet batches during the first three weeks for starter as follows: 1) the negative control, litter delivery, negative oral gavage and probiotic oral gavage treatment groups were provided with the basal diet; 2) the positive control treatment was provided with the antibiotic, zinc-bacitracin (ZnB, 50 mg/kg) added; and 3) the feed supplementary treatments groups (starter feed) included an overnight culture of L. johnsonii. Four strains of Lactobacillus (No. 1286 tentatively identified as L. johnsonii, No. 709 tentatively identified as L. crispatus, No. 697 tentatively identified as L. salivarius and No. 461 unidentified Lactobacillus sp.) were selected as probiotic candidates and added to the feed to make up the different treatments. The experimental diet with the probiotic candidate was mixed weekly and supplied for the first three weeks.
Table 1.
Ingredient composition and calculated chemical composition of basal diets (as-fed basis).
| Item | 1 to 3 weeks (Starter) | 4 to 6 weeks (Finisher) |
|---|---|---|
| Ingredient, g/kg | ||
| Wheat | 262.0 | 214.0 |
| Sorghum | 350.25 | 400.2 |
| Mung beans | 100.0 | 100.0 |
| Tallow in mixer | 32.5 | 34.0 |
| Sunflower meal | 25.0 | |
| Canola meal | 60.0 | 60.0 |
| Cottonseed meal | 50.0 | |
| Soybean meal | 157.0 | 81.5 |
| Limestone B10 | 15.5 | 16.0 |
| Kynofos/biofos MDCP | 11.5 | 11.0 |
| Salt | 1.75 | 1.5 |
| Sodium bicarbonate | 2.0 | 2.0 |
| Choline chloride 75% | 0.6 | 0.6 |
| DL-Methionine | 2.1 | 1.3 |
| L-Lysine scale 3 | 2.1 | 0.4 |
| L-Threonine | 0.2 | |
| Vitamin and mineral premix1 | 2.5 | 2.5 |
| Calculated chemical composition, g/kg | ||
| ME, MJ/kg | 12.26 | 12.39 |
| Crude protein | 200.02 | 190.00 |
| Crude fibre | 35.17 | 43.14 |
| Crude fat | 52.16 | 54.47 |
| Lys | 11.49 | 8.98 |
| Met + Cys | 8.32 | 7.37 |
| Ca | 9.73 | 9.79 |
| Available phosphorous | 6.50 | 6.71 |
| Na | 1.62 | 1.65 |
| Cl | 2.19 | 1.75 |
Vitamin and mineral premix (Ridley Agriproducts Pty Ltd., Tamworth, NSW) contained the following minerals in milligrams per kilogram of diet: vitamin A (as all-trans retinol), 12,000 IU; cholecalciferol, 3,500 IU; vitamin E (as d-a-tocopherol), 44.7 IU; vitamin B12, 0.2 mg; biotin, 0.1 mg; niacin, 50 mg; vitamin K3, 2 mg; pantothenic acid, 12 mg; folic acid, 2 mg; thiamine, 2 mg; riboflavin, 6 mg; pyridoxine hydrochloride, 5 mg; D-calcium pantothenate, 12 mg; Mn, 80 mg; Fe, 60 mg; Cu, 8 mg; I, 1 mg; Co, 0.3 mg; and Mo, 1 mg.
All treatments received the same basal finisher diet once the birds were transferred to slide-in cages, and growth performance was measured weekly. Feed was provided ad libitum. The delivery routes of experimental treatments are shown in (Table 2).
Table 2.
Experimental treatments via different delivery routes.1
| Treatment & routes | NC | PC | Feed | Water | Litter | Oral-NC | Oral-Pro |
|---|---|---|---|---|---|---|---|
| Feed | Basal | Basal + Antibiotic | Basal + Pro | Basal | Basal | Basal | Basal |
| Water | Tap | Tap | Tap | Tap + Pro | Tap | Tap | Tap |
| Litter | Sawdust | Sawdust | Sawdust | Sawdust | Sawdust + Pro | Sawdust | Sawdust |
| Antibiotic | Non | ZnB, 50 mg/kg | Non | Non | Non | Non | Non |
| Oral gavage | Non | Non | Non | Non | Non | PBS | Pro-L. Johnsonii |
Dietary treatments: NC, negative control, with no additives added to the basal feed, water and litter; PC, positive control, with the antibiotic, zinc-bacitracin (ZnB, 50 mg/kg) added in feed; Oral-NC, negative control, with no additives added to the basal feed, water and litter, orally inoculated with phosphate-buffered solution (PBS) solution. Other treatments, with probiotic (Pro) L. johnsonii delivery by oral gavage, feed, water and litter, respectively.
2.3.2. Delivery via feed
The experimental diets with the probiotic candidates were mixed weekly. The individual strains were grown in MRS broth contained 5 g/L of yeast extract (powder, Oxoid, LP0021) and 20 g/L of glucose, for overnight (at 39°C) and harvested by centrifugation at 4,420 × g for 15 min (Induction Drive Centrifugation, Beckman Model J2-21M, Beckman Instruments Inc., Palo Alto, California, USA), resuspended in PBS (pH 7.4) and mixed into a premix with the basal diet for 10 min using a miniature mixer. This pre-mixture of product with feed (1 kg) was then transferred into a larger mixer (total capacity 300 kg) where the final volume of the weekly feed batch was prepared. The mixer equipment was thoroughly cleaned between the mixing of different treatments by using a vacuum cleaner and a wash diet (basal feed).
2.3.3. Delivery via drinking water
For the first three weeks, drinking water was supplied through pipes (nipples drinker installed) connected to a 20-L drum. A small pump (low power, Aqua One maxi series power head, Kongs International Co., Ltd, China) was installed to constantly agitate the water. The water containing the probiotic was prepared daily and supplied for the first three weeks in probiotic water treatment groups. After three weeks the birds were transferred to slide-in cages and drinking water was supplied in troughs placed outside the cages. Water was provided ad libitum.
2.3.4. Litter application
The sawdust used as litter for this experiment was selected from commercial products produced by Bellsouth Pty. Ltd., Australia. The lactic acid bacterial concentration was determined using an MRS agar plate display. The sawdust contained a low number of lactic acid bacteria before use (<102 cfu/g of sawdust). The probiotic solution (PBS, pH 7.4 containing >106 cfu/mL of L. johnsonii) was sprayed on litter daily for the first three weeks for the litter treatment groups.
2.3.5. Oral gavage
L. johnsonii cultures were resuspended into PBS solution (pH 7.4) which contained approximately 108 cfu/mL. Each bird received 1 mL of PBS mixed solution on d 1, 2, 4, 6 and 14; the birds in the negative control group received 1 mL of PBS solution (pH 7.4) on the same days.
2.3.6. Sample collection and processing
Feed leftovers and birds were weighed on a weekly basis for calculation of average feed intake (FI) and body weight. Mortality was recorded when it occurred and feed conversion ratio (FCR; feed intake/weight gain) was corrected for mortality. Three birds on d 7 and two birds on d 21, from each cage were randomly selected and killed by cervical dislocation. The abdominal cavity was opened and visceral organs were weighed.
The weights of the empty gizzard, the duodenum, jejunum and ileum were recorded individually. The weights of the pancreas, liver, spleen, and bursa were also measured and recorded individually. The contents of the gizzard, ileum and caeca were collected in plastic containers, and stored at −20°C until volatile fatty acids (VFA) analysis was performed. A 2-cm piece of the proximal ileum was flushed with ice-cold phosphate-buffered saline (PBS saline) at pH 7.4 and fixed in 10% formalin for gut morphological measurements. One gram (approximately) each of ileal and caecal fresh digesta was transferred individually into 15 mL MacCartney bottles containing 10 mL of anaerobic broth for bacterial enumeration using the methods described in Section 2.3.8
2.3.7. Digesta pH, VFA analysis and gut morphology
Intestinal pH was measured immediately after death and excision of viscera. The pH of ileal and caecal contents was determined by the modified procedure of Corrier et al. (1990). After thawing at room temperature, the concentrations of short-chain fatty acids (SCFA) and lactic acid of each digesta sample from the ileum and caeca were measured using gas chromatography (Varian CP-3800. Netherlands) according to the method described by Jensen et al. (1995).
Tissue samples were collected from the proximal ileum and flushed with buffered saline and fixed in 10% neutral buffered formalin for histomorphological analysis. Samples were embedded in paraffin wax, sectioned and stained with haematoxylin and eosin. Sample sections were captured at 10× magnification using a Leica DM LB microscope (Leica Microscope GmbH, Wetzlar, Germany) and morphometric indices were determined as described by Iji et al. (2001). Each sample was measured in 15 vertically, well-oriented, intact villi, muscle depth and crypts photomicrographs of a stage micrometer recorded at 5× magnification.
2.3.8. Enumeration of intestinal bacteria and isolation of lactobacilli
A 10 mL aliquot of anaerobic broth was homogenized for 2 min in CO2-flushed plastic bags using a bag mixer (Interscience, St. Norm, France) immediately after sample collection. The 10-fold increment serial dilution technique was conducted according to Miller and Wolin (1974). One millilitre of the homogenized suspension was then transferred into 9 mL of anaerobic broth and serially diluted from 10−1 to 10−5 (for the ileal samples) or 10−1 to 10−6 (for the caecal samples). From the last three diluted samples, 0.1 mL each was plated on the appropriate medium (10 mL) for enumeration of microbial populations.
Total anaerobic bacteria were determined using anaerobic roll tubes containing 3 mL of Wilkins-Chalgren anaerobe agar (Oxoid, CM0619) incubated at 39°C for 7 d. Lactic acid bacteria were enumerated on MRS agar (Oxoid, CM0361) incubated in anaerobic conditions at 39°C for 48 h. Coliforms and lactose-negative Enterobacteria were counted on MacConkey agar (Oxoid, CM 0007) incubated aerobically at 39°C for 24 h as red and colourless colonies, respectively. Lactobacilli were enumerated on Rogosa agar (Oxoid, CM 0627) after anaerobic incubation at 39°C for 48 h. Numbers of Clostridium perfringens (Cp) were counted on Tryptose–Sulfite–Cycloserine and Shahidi-Ferguson Perfringens agar base (TSC & SFP) (Oxoid, CM0587 OPSP) mixed with egg yolk emulsion (Oxoid, SR0047) and Perfringens (TSC) selective supplement (Oxoid, SR0088E) according to the pour-plate technique, where plates were overlaid with the same agar after spreading the inoculums and incubated anaerobically at 39°C for 24 h. All plates were incubated in the anaerobic cabinet (Model SJ-3, Kalter Pty. Ltd., Edwardstown, SA, Australia) and bacterial number counted using colony counter (Selby, Model SCC100, Biolab Australia, Sydney, NSW, Australia).
Twenty pure colonies were randomly collected from the highest dilution Rogosa agar plates from the oral gavage treatment groups (negative and probiotic). The bacterial isolates were transferred to MRS broth individually and aerobically incubated at 39°C for 24 h. The amplification of bacterial colonies was collected in Eppendorf tubes (2.5 mL) and stored at −20°C for further DNA analysis.
2.3.9. Extraction of genomic DNA
Forty bacterial colonies, 20 colonies from each treatment were randomly picked from Rogosa agar plates (ileum, most of colonies from the highest dilution and some from different dilutions) from the oral inoculation treatment and negative control oral inoculation treatment on d 7. Using a sterile toothpick, cells from a single (pure) colony were used to individually inoculate 10 mL of MRS broth in screw cap tubes. The cells were grown at 39°C for 24 h. The supernatant (about 8 mL) was discarded and 1.5 mL of broth containing the bacterial cells were transferred into Eppendorf tubes. The bacterial cells were harvested by centrifugation (5,000 × g, 5 min) in an Eppendorf centrifuge (Eppendorf 5,415D, Eppendorf AG, Hamburg, Germany). The supernatant was removed and the cells were re-suspended in 1.0 mL of TES buffer (0.05 M Tris, 0.05 M NaCI, 0.005 M EDTA, pH 8.0), before being centrifuged again (5,000 × g, 5 min) and the supernatant discarded. After washing the pellet cells were stored at −20°C for 24 h to improve lysis. The pellet was then again resuspended into 0.5 mL of TES buffer (same as above) with 5 µL of lysozyme (10 mg/mL, freshly prepared) added and incubated at 37°C for 30 min. Subsequently, 5 µL each of proteinase K (10 mg/mL) and RNase (10 mg/mL) were added and mixed by vortex (VM1 vortex mixer, Stansens, Mt. Waverley VIC, 3149, Australia) and incubated at 65°C for 1 h. After the above steps lysis was finally achieved by the addition of 50 µL 24% (wt/vol) sodium dodecyl sulphate (SDS), followed by incubation for another 10 min at 65°C, with the suspension clearing as the cells lyse. The lysed suspension was then cooled and the cells were subjected to bead beating with 0.5 g of glass-beads (0.5 mm of diameter) cell disruption media in a mini bead-beater (Disruptor Genie, Scientific Industries Inc., New York, USA) at 5,000 × g for 5 min. The precipitation and purification of DNA were carried out using the DNeasy Tissue kit (Qiagen Pty. Ltd., Doncaster, VIC, Australia) according to manufacturer instructions after recovering the supernatants.
2.3.10. PCR amplification of 16-23S rDNA
The primers used in this experiment for PCR amplification are listed in Table 3. The method was according to Guan et al., 2003, Mikkelsen et al., 2003, Vidanarachchi, 2006) and as reported as lactobacillus 16-23S rDNA (16S rRNA gene and the entire 16S-23S rRNA intergenic region) analysis with modifications. The reaction mixture (50 µL) contained a 0.01 mM deoxynucloside triphosphate (dNTP), 1.5 nM MgCI2, 1.1 Unit Taq (Thermus aquaticus) DNA polymerase supplied with the 10× PCR buffer (all from Fisher Biotec, West Perth, WA, Australia), 10 pmol both forward and reverse primers (Proligo Australia Pty. Ltd., Lismore, NSW, Australia) and 2.0 µL purified template DNA. The reaction mixtures were amplified in an Eppendorf PCR Thermal Cycler (MasterCycler, Eppendorf AG, Hamburg, Germany) under the following conditions: initial cycle of 1 min denaturation at 95°C, followed by 30 cycles of 30 s denaturation at 95°C, 30 s of annealing at 57°C and 45 s elongation at 72°C with a final extension of 10 min at 72°C. Amplified PCR products were electrophoresed on a 1% agarose gel containing 5 µL of GelStar nucleic acid gel stain (BioWhittaker Molecular Application, Rochland, ME, USA), viewed by UV transillumination and digitized on an Infinity CN – 3000 Gel Documentation System (Vilber Lourmat, Cedex, France). The formulation of the master mixture is listed in Table 4.
Table 3.
Primers used for amplification for 16-23S r DNA (from Guan et al., 2003, Mikkelsen et al., 2003, Vidanarachchi, 2006).
| Primer | Direction | Nucleotide sequence (5′ to 3′) |
|---|---|---|
| Lb 16a | Forward | GTG CCT AAT ACA TGC AAG TCG |
| 23-1B | Reverse | GGG TTC CCC CAT TCG GA |
Table 4.
Formulation of reaction mixture for PCR amplification of 16-23S rDNA (from Mikkelsen et al., 2003).
| Composition | Concentration | Volume, µL |
|---|---|---|
| Master mixture | ||
| Deoxynucleoside triphosphate (dNTP) | 2.0 nmol/µL | 5.0 |
| Taq DNA polymerase | 5.5 U/µL | 0.2 |
| MgCI2 | 25 nM | 6.0 |
| PCR buffer | 10× | 5.0 |
| Forward primer – Lb16a | 5 pmol/µL | 2.0 |
| Reverse primer – 23-1B | 5 pmol/µL | 2.0 |
| PCR grade water | – | 27.8 |
| Total volume of master mixture for each sample | 48 | |
| DNA crude extracts | 2.0 | |
| Total reaction mixture for each sample | 50 | |
2.3.11. Amplified Ribosomal DNA Restriction Analysis (ARDRA) of 16-23S rDNA
The amplified 16-23S rDNA intergenic spacer regions of lactobacillus isolates were digested with the restriction endonuclease HaeIII enzymes (restriction enzyme isolated from Haemophilus aegptius) according to the manufacturer׳s instructions (New England BioLabs, Brisbane, QLD, Australia). HaeIII restriction enzyme recognizes and cleaves directly the centre of the 5′ … GG/CC … 3′, 3′ … CC/GG … 5′ DNA sequence. Restriction digestion was carried out for 2 h at 37°C in 40 µL final volume containing 4 µL 10× buffer, 15 µL PCR grade water, 1 µL enzyme (10 U/µL) and 20 µL of amplified PCR product. Restriction digestion products were electrophoretically resolved in a 2% agarose gel containing 5 µL of GelStar nucleic acid gel stain (BioWhittaker Molecular Applications, Rockland, ME, USA) for 4 h at 90 V and band patterns were viewed by UV transillumination and digitized on Infinity CN – 3000 Gel Documentation System (Vilber Lourmat, Cedex, France). Infinity Capture version 12.6 for Windows software was used for image analysis.
2.4. Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) (StatGraphics Plus version 5.1 – Professional Edition, Manugistics Inc., Rockville, Maryland, USA) with diet as the factor. The differences between mean values were identified by the least significant difference (LSD). Differences among treatments were deemed to be significant only if the P-value <0.05. Regression analysis was carried out only with control diets and different routes of delivery administration. All results were expressed as means. Bacterial counts were transformed to log10 values before analysis.
2.5. Animal ethics
Health and animal husbandry practices complied with the 'Australian code of the care of animal for scientific purposes’ (NHMRC, 2004). The Animal Ethic Committee of the University of New England approved the experiments in this study (authority number: AEC07/016).
3. Results
3.1. Growth performance
Body weight gain (BWG), FI and FCR were not affected by different delivery methods of probiotic supplementation (Table 5). The oral gavage tended (P = 0.3) to give higher BWG than the negative control groups.
Table 5.
The effects of delivering L. johnsonii via different routes on the performance of broilers.1
| Item | Treatments2 |
SE | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | Oral – NC | Feed | Water | Litter | Oral – Pro | |||
| Day 1 to 7 | |||||||||
| BWG, g/bird | 157 | 158 | 156 | 155 | 156 | 157 | 158 | 1.57 | 0.87 |
| FI, g/bird | 156 | 157 | 154 | 157 | 155 | 160 | 160 | 2.88 | 0.67 |
| FCR | 0.995 | 0.998 | 0.988 | 1.010 | 0.995 | 1.017 | 1.015 | 0.02 | 0.94 |
| Day 1 to 21 | |||||||||
| BWG, g/bird | 854 | 874 | 854 | 851 | 856 | 867 | 862 | 9.22 | 0.54 |
| FI, g/bird | 1,201 | 1,222 | 1,201 | 1,198 | 1,202 | 1,216 | 1,210 | 9.27 | 0.48 |
| FCR | 1.407 | 1.398 | 1.407 | 1.410 | 1.403 | 1.403 | 1.405 | 0.01 | 0.67 |
| Day 1 to 35 | |||||||||
| BWG, g/bird | 1,797 | 1,816 | 1,794 | 1,800 | 1,792 | 1,792 | 1,824 | 11.44 | 0.31 |
| FI, g/bird | 2,899 | 2,935 | 2,891 | 2,908 | 2,883 | 2,899 | 2,952 | 27.05 | 0.55 |
| FCR | 1.623 | 1.617 | 1.622 | 1.636 | 1.619 | 1.637 | 1.634 | 0.02 | 0.99 |
| Mortality, % | 6.25 | 4.17 | 4.17 | 2.08 | 6.26 | 4.17 | 8.33 | – | – |
BWG = body weight gain; FI = feed intake; FCR = feed conversion ratio
Values are means (n = 6) and standard error of means (SE).
Dietary treatments: NC, negative control, with no additives added to the basal feed, water and litter; PC, positive control, with the antibiotic, zinc-bacitracin (ZnB, 50 mg/kg) added in feed; Oral-NC, negative control, with no additives added to the basal feed, water and litter, orally inoculated with PBS solution; Other treatments, with probiotic (Pro) L. johnsonii delivery by oral gavage, feed, water and litter, respectively.
3.2. Organ weights, intestinal pH and SCFA concentrations
The relative weight of the pancreas was significantly increased (P < 0.01) at d 21 with oral gavage giving the heaviest pancreas (Table 6). There were no effects of diet on the relative weights of visceral organs, including the small intestine.
Table 6.
Relative weights (% BW) of organs from broilers given a probiotic via different routes.1
| Item | Treatment2 |
SE | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | Oral – NC | Feed | Water | Litter | Oral – Pro | |||
| Day 7 | |||||||||
| Liver | 5.33 | 4.59 | 5.62 | 5.09 | 4.74 | 5.62 | 5.37 | 0.43 | 0.5071 |
| Spleen | 0.09 | 0.08 | 0.10 | 0.08 | 0.06 | 0.08 | 0.09 | 0.01 | 0.3327 |
| Pancreas | 0.35 | 0.34 | 0.45 | 0.40 | 0.40 | 0.38 | 0.37 | 0.04 | 0.6937 |
| Bursa | 0.15 | 0.12 | 0.13 | 0.12 | 0.16 | 0.17 | 0.13 | 0.02 | 0.2055 |
| Gizzard | 4.76 | 4.25 | 4.79 | 4.49 | 4.84 | 4.30 | 4.19 | 0.24 | 0.2467 |
| Duodenum | 2.07 | 1.69 | 1.79 | 2.00 | 2.09 | 1.90 | 2.03 | 0.16 | 0.5204 |
| Jejunum | 2.71 | 2.43 | 2.77 | 2.72 | 2.63 | 2.68 | 2.87 | 0.21 | 0.8536 |
| Ileum | 2.02 | 1.72 | 1.74 | 1.80 | 1.84 | 2.02 | 2.01 | 0.17 | 0.6834 |
| Day 21 | |||||||||
| Liver | 3.23 | 3.35 | 3.34 | 3.28 | 2.98 | 3.28 | 3.43 | 0.36 | 0.1328 |
| Spleen | 0.09 | 0.08 | 0.07 | 0.09 | 0.09 | 0.07 | 0.08 | 0.01 | 0.4059 |
| Pancreas | 0.30a | 0.27a | 0.25b | 0.24b | 0.32c | 0.30a | 0.37d | 0.02 | 0.0077 |
| Bursa | 0.16 | 0.16 | 0.12 | 0.17 | 0.19 | 0.15 | 0.16 | 0.02 | 0.3899 |
| Gizzard | 2.48 | 2.54 | 2.81 | 2.36 | 2.44 | 2.53 | 2.22 | 0.13 | 0.1144 |
| Duodenum | 1.17 | 1.04 | 1.27 | 1.27 | 1.24 | 1.17 | 1.22 | 0.08 | 0.4325 |
| Jejunum | 1.90 | 1.62 | 1.74 | 1.86 | 1.78 | 1.64 | 1.78 | 0.08 | 0.1842 |
| Ileum | 1.23 | 1.01 | 1.13 | 1.07 | 1.06 | 1.14 | 1.15 | 0.08 | 0.6000 |
a,b,c,d Means within the same row with no common superscripts differ significantly (P < 0.05).
Values are means (n = 6) and standard error of means (SE).
Dietary treatments: NC, negative control, with no additives added to the basal feed, water and litter; PC, positive control, with the antibiotic, zinc-bacitracin (ZnB, 50 mg/kg) added in feed; Oral-NC, negative control, with no additives added to the basal feed, water and litter, orally inoculated with PBS solution; Other treatments, with probiotic (Pro) L. johnsonii delivery by oral gavage, feed, water and litter, respectively.
The probiotic treatments, regardless of the routes of delivery, affected (P < 0.05) the pH of the caecal digesta and tended (P = 0.06) to affect the pH of the ileal digesta on d 7, but the effect disappeared as the birds grew older (Table 6). Although there were numerically higher concentrations of lactic acid in the ileal digesta and succinic acid in the caecal digesta compared with the negative controls, these were not statistically significant. Furthermore, the trend diminished as the birds grew older (Table 7).
Table 7.
Digesta pH and organic acids concentrations (µmol/g) on d 7 and 21.1
| Item | Treatments2 |
SE | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | Oral – NC | Feed | Water | Litter | Oral – Pro | |||
| Day 7 | |||||||||
| Gizzard | |||||||||
| pH | 3.06 | 3.01 | 3.08 | 2.95 | 3.15 | 3.09 | 3.02 | 0.09 | 0.7866 |
| Ileum | |||||||||
| pH | 6.71 | 6.64 | 6.67 | 6.84 | 6.97 | 6.79 | 6.51 | 0.10 | 0.0600 |
| Formic acid | 0.34 | 0.29 | 0.31 | 0.46 | 0.39 | 0.36 | 0.58 | 0.31 | 0.9451 |
| Acetic acid | 1.68 | 1.35 | 1.54 | 1.73 | 1.61 | 1.59 | 1.67 | 0.52 | 0.7956 |
| Lactic acid | 3.03 | 3.46 | 4.37 | 4.32 | 5.41 | 3.49 | 3.87 | 2.57 | 0.8351 |
| Caeca | |||||||||
| pH | 6.19b | 6.08c | 6.13b | 6.13b | 6.56a | 5.71a | 6.11c | 0.14 | 0.0158 |
| Acetic acid | 57.51 | 52.32 | 58.53 | 47.97 | 61.27 | 55.69 | 52.27 | 6.79 | 0.8769 |
| Propionic acid | 2.83 | 2.45 | 2.26 | 3.11 | 2.49 | 3.91 | 2.89 | 0.34 | 0.1021 |
| Butyric acid | 14.11 | 14.41 | 13.43 | 13.02 | 13.87 | 14.19 | 14.54 | 0.87 | 0.8801 |
| Succinic acid | 2.12 | 2.25 | 2.68 | 3.41 | 2.69 | 2.76 | 2.91 | 0.59 | 0.7708 |
| Day 21 | |||||||||
| Gizzard | |||||||||
| pH | 2.75 | 2.67 | 2.48 | 3.04 | 2.64 | 2.94 | 2.69 | 0.19 | 0.4784 |
| Ileum | |||||||||
| pH | 6.96 | 7.04 | 6.72 | 6.91 | 6.70 | 6.82 | 6.98 | 0.15 | 0.5746 |
| Formic acid | 0.48 | 0.39 | 0.53 | 0.53 | 0.32 | 0.51 | 0.45 | 0.24 | 0.5671 |
| Acetic acid | 2.41 | 2.57 | 2.49 | 2.76 | 2.34 | 2.55 | 2.70 | 0.67 | 0.8317 |
| Lactic acid | 7.24 | 6.77 | 9.41 | 6.91 | 7.18 | 8.51 | 8.76 | 3.21 | 0.6270 |
| Caeca | |||||||||
| pH | 5.77 | 5.86 | 5.62 | 5.87 | 5.77 | 5.89 | 5.86 | 0.15 | 0.8511 |
| Acetic acid | 57.41 | 69.24 | 49.71 | 64.28 | 61.49 | 55.06 | 58.12 | 12.34 | 0.3745 |
| Propionic acid | 4.57 | 4.49 | 3.89 | 3.76 | 4.72 | 4.28 | 4.51 | 0.89 | 0.6841 |
| Butyric acid | 12.64 | 11.47 | 12.38 | 13.16 | 11.78 | 12.68 | 12.97 | 3.54 | 0.7680 |
| Succinic acid | 1.08 | 1.24 | 1.29 | 1.31 | 1.27 | 1.09 | 1.11 | 0.38 | 0.8620 |
a,b,c Means within the same row with no common superscripts differ significantly (P < 0.05).
Values are means (n = 6) and standard error of means (SE).
Dietary treatments: NC, negative control, with no additives added to the basal feed, water and litter; PC, positive control, with the antibiotic, zinc-bacitracin (ZnB, 50 mg/kg) added in feed; Oral-NC, negative control, with no additives added to the basal feed, water and litter, orally inoculated with PBS solution; Other treatments, with probiotic (Pro) L. johnsonii delivery by oral gavage, feed, water and litter, respectively.
3.3. Bacterial populations in intestinal digesta
The probiotic treatment groups had significant effects on the bacterial count in the caecal digesta with the number of Enterobacteria decreasing (P < 0.05) on d 7 and 21. The probiotic treatments tended (P = 0.08) to reduce the number of Enterobacteria in the ileum on d 7. However, it did not affect the counts of total anaerobic bacteria, LAB, lactobacilli and C. perfringens in the digesta of the ileum and caeca either at d 7 or at d 21. Furthermore, the number of Enterobacteria in the ileal digesta at d 21 was not affected (Table 8).
Table 8.
Bacterial counts (lg cfu/g) in the digesta of birds on d 7 and 21.1
| Item | Treatments2 |
SE | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | Oral –NC | Feed | Water | Litter | Oral – Pro | |||
| Day 7 | |||||||||
| Ileum | |||||||||
| Total anaerobes | 8.28 | 8.08 | 8.49 | 7.69 | 8.16 | 8.26 | 8.10 | 0.23 | 0.383 |
| LAB | 8.07 | 8.18 | 8.71 | 8.16 | 8.27 | 8.24 | 8.23 | 0.29 | 0.801 |
| Lactobacilli | 7.72 | 8.03 | 8.05 | 8.00 | 7.80 | 7.85 | 7.97 | 0.28 | 0.967 |
| Enterobacteria3 | 6.27 | 6.14 | 6.17 | 5.69 | 5.45 | 6.72 | 5.94 | 0.28 | 0.084 |
| C. perfringens | 3.87 | 3.71 | 3.85 | 3.73 | 3.96 | 3.96 | 3.50 | 0.25 | 0.856 |
| Caeca | |||||||||
| Total anaerobes | 10.26 | 10.14 | 10.02 | 10.33 | 10.43 | 10.00 | 10.32 | 0.16 | 0.385 |
| LAB | 9.69 | 9.50 | 9.54 | 9.54 | 9.61 | 9.41 | 9.58 | 0.17 | 0.947 |
| Lactobacilli | 8.82 | 8.52 | 9.22 | 8.96 | 9.22 | 8.96 | 9.30 | 0.28 | 0.457 |
| Enterobacteria3 | 9.33 | 9.25 | 9.51 | 9.13 | 9.14 | 9.31 | 8.76 | 0.15 | 0.054 |
| C. perfringens | 8.14 | 7.41 | 8.11 | 7.68 | 7.75 | 7.76 | 7.76 | 0.22 | 0.250 |
| Day 21 | |||||||||
| Ileum | |||||||||
| Total anaerobes | 6.78 | 6.93 | 6.52 | 7.39 | 7.52 | 7.24 | 7.55 | 0.35 | 0.291 |
| LAB | 7.47 | 7.01 | 7.36 | 7.37 | 7.21 | 7.58 | 7.52 | 0.17 | 0.232 |
| Lactobacilli | 7.30 | 6.86 | 7.16 | 7.41 | 7.36 | 6.96 | 7.61 | 0.23 | 0.106 |
| Enterobacteria3 | 6.19 | 5.68 | 5.97 | 5.58 | 5.83 | 5.78 | 5.33 | 0.26 | 0.380 |
| C. perfringens | 4.42 | 4.55 | 4.35 | 4.19 | 4.15 | 4.82 | 4.63 | 0.34 | 0.791 |
| Caeca | |||||||||
| Total anaerobes | 8.92 | 8.70 | 8.80 | 8.80 | 9.01 | 8.78 | 9.15 | 0.17 | 0.548 |
| LAB | 8.45 | 8.29 | 8.61 | 8.75 | 8.63 | 8.50 | 8.91 | 0.19 | 0.370 |
| Lactobacilli | 8.31 | 8.17 | 7.79 | 8.35 | 8.31 | 8.21 | 8.81 | 0.26 | 0.223 |
| Enterobacteria3 | 8.16a | 8.02a | 8.08a | 7.60c | 7.82b | 7.93b | 7.59c | 0.14 | 0.040 |
| C. perfringens | 5.36 | 4.83 | 5.26 | 4.66 | 4.44 | 4.83 | 4.83 | 0.41 | 0.708 |
a,b,c Means within the same row with no common superscripts differ significantly (P < 0.05).
Values are means (n = 6) and standard error of means (SE).
Treatments: NC, negative control, with no additives added to the basal feed, water and litter; PC, positive control, with the antibiotic, zinc-bacitracin (ZnB, 50 mg/kg) added in feed; Oral-NC, negative control, with no additives added to the basal feed, water and litter, orally inoculated with PBS solution; Other treatments, with probiotic (Pro) L. johnsonii delivery by oral gavage, feed, water and litter, respectively.
Enterobacteria are coliform and lactose negative enterobacteria.
The number of the LAB was the highest in the ileal digesta in the oral gavage treatment (8.23; 7.52) and litter treatment (8.24; 7.58) on d 7 and 21, respectively. They were also highest in the caecal digesta for the oral gavage treatment (8.91) on d 21. The lactobacillus population was greatest in the caecal digesta for the oral gavage treatment on d 7 (9.30) and d 21 (8.81), and in the ileal digesta for the oral gavage treatment the lactobacillus population reached its peak (7.61) at d 21.
3.4. Intestinal histomorphology
The effects of different treatments on villus height, crypt depth and villi:crypt ratio of ileum on d 7 and 21 are shown in Table 9. Results show that the probiotic candidate L. johnsonii did not significantly influence ileal morphology of broiler chickens when administered by different delivery routes, compared with the positive and negative control treatments.
Table 9.
Ileal morphormetry of broilers on d 21 and 35.1
| Item | Treatments2 |
SE | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | Oral – NC | Feed | Water | Litter | Oral – Pro | |||
| Day 7 | |||||||||
| Villus height, µm | 603 | 593 | 589 | 574 | 583 | 605 | 579 | 37.29 | 0.532 |
| Crypt depth, µm | 110 | 98 | 103 | 117 | 106 | 107 | 103 | 6.25 | 0.741 |
| Villi:crypt ratio | 5.48 | 6.05 | 5.72 | 4.91 | 5.50 | 5.65 | 5.62 | 0.57 | 0.312 |
| Muscle depth, µm | 278 | 256 | 268 | 267 | 255 | 259 | 283 | 14.24 | 0.231 |
| Day 21 | |||||||||
| Villus height, µm | 795 | 803 | 827 | 793 | 759 | 782 | 798 | 47.38 | 0.178 |
| Crypt depth, µm | 122 | 135 | 132 | 127 | 130 | 129 | 136 | 8.92 | 0.615 |
| Villi:crypt ratio | 6.52 | 5.95 | 6.27 | 6.24 | 5.84 | 6.06 | 5.87 | 0.74 | 0.236 |
| Muscle depth, µm | 311 | 302 | 291 | 285 | 272 | 298 | 307 | 16.36 | 0.347 |
Values are means (n = 6) and standard error of means (SE).
Dietary treatments: NC, negative control, with no additives added to the basal feed, water and litter; PC, positive control, with antibiotic, zinc-bacitracin (ZnB, 50 mg/kg) added in feed; Oral-NC, negative control, with no additives added to the basal feed, water and litter, orally inoculated with PBS solution; Other treatments, with probiotic (Pro) L. johnsonii delivery by oral gavage, feed, water and litter, respectively.
3.5. Amplified Ribosomal DNA Restriction Analysis of 16–23s rDNA
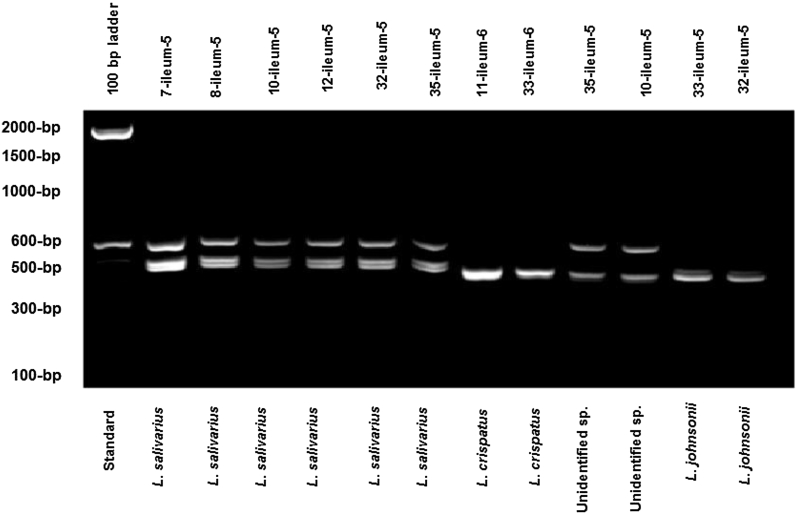
Forty isolates tentatively assigned to different groups of Lactobacillus spp. are listed in Table 10. The isolates were tentatively identified as L. crispatus and L. salivarius by Vidanarachchi (2006) who used the Amplified Ribosomal DNA Restriction Analysis (ARDRA) method for Lactobacillus spp. analysis. The L. johnsonii group was tentatively identified by comparing patterns from a pure culture used for oral inoculation. This pure culture was identified by Vidanarachchi (2006) using the sequences of 16S rRNA gene (Gen Bank accession No. AE017198) (Fig. 1). The result showed that L. johnsonii was detected from the oral inoculation treatment and also showed high numbers (8/20) of probiotic candidate colonies in the oral gavage groups in 20 randomly selected isolates. However, no L. johnsonii strains were found in the negative control group.
Table 10.
Distribution of major genotypic groups of lactobacilli isolates from ileum of broiler on d 7.1
| Isolates ID | Treatment2 | DT | ARDRA patterns | Tentative distribution |
|---|---|---|---|---|
| L. johnsonii | Origin | 150-bp, 300-bp, 500-bp | L. johnsonii | |
| 7-Ileum-5 | Oral – NC | 5 | 250-bp, 500 bp, 700 bp | Unidentified Lactobacillus sp. |
| 7-Ileum-5 | Oral – NC | 5 | 300-bp, 400-bp, 500-bp, 700-bp | Unidentified Lactobacillus sp. |
| 7-Ileum-5 | Oral – NC | 5 | 150-bp, 200 bp, 500 bp | Possibly L. crispatus |
| 7-Ileum-5 | Oral – NC | 5 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 8-Ileum-5 | Oral – NC | 5 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 8-Ileum-5 | Oral – NC | 5 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 9-Ileum-6 | Oral – NC | 6 | 350-bp | Unidentified Lactobacillus sp. |
| 9-Ileum-6 | Oral – NC | 6 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 10-Ileum-5 | Oral – NC | 5 | 150-bp, 200 bp, 500 bp | Possibly L. crispatus |
| 10-Ileum-5 | Oral – NC | 5 | 350-bp | Unidentified Lactobacillus sp. |
| 10-Ileum-5 | Oral – NC | 5 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 10-Ileum-5 | Oral – NC | 5 | 200-bp, 500-bp, 600-bp | Unidentified Lactobacillus sp. |
| 11-Ileum-6 | Oral – NC | 6 | 150-bp, 200 bp, 500 bp | Possibly L. crispatus |
| 11-Ileum-5 | Oral – NC | 5 | 350-bp | Unidentified Lactobacillus sp. |
| 11-Ileum-5 | Oral – NC | 5 | 150-bp, 200 bp, 500 bp | Possibly L. crispatus |
| 12-Ileum-6 | Oral – NC | 6 | 300-bp, 400-bp, 500-bp, 700-bp | Unidentified Lactobacillus sp. |
| 12-Ileum-6 | Oral – NC | 6 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 12-Ileum-5 | Oral – NC | 5 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 12-Ileum-5 | Oral – NC | 5 | 150-bp, 200 bp, 500 bp | Possibly L. crispatus |
| 12-Ileum-5 | Oral – NC | 5 | 300-bp, 400-bp, 500-bp, 700-bp | Unidentified Lactobacillus sp. |
| 31-Ileum-5 | Oral – Pro | 5 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 31-Ileum-5 | Oral – Pro | 5 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 31-Ileum-5 | Oral – Pro | 5 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 31-Ileum-4 | Oral – Pro | 4 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 31-Ileum-4 | Oral – Pro | 4 | 300-bp, 400-bp, 500-bp, 700-bp | Unidentified Lactobacillus sp. |
| 32-Ileum-6 | Oral – Pro | 6 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 32-Ileum-5 | Oral – Pro | 5 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 32-Ileum-5 | Oral – Pro | 5 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 33-Ileum-6 | Oral – Pro | 6 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 33-Ileum-6 | Oral – Pro | 6 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 33-Ileum-6 | Oral – Pro | 6 | 150-bp, 200 bp, 500 bp | Possibly L. crispatus |
| 34-Ileum-5 | Oral – Pro | 5 | 350-bp | Unidentified Lactobacillus sp. |
| 34-Ileum-5 | Oral – Pro | 5 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 34-Ileum-5 | Oral – Pro | 5 | 150-bp, 200 bp, 500 bp | Possibly L. crispatus |
| 35-Ileum-5 | Oral – Pro | 5 | 200-bp, 500-bp, 600-bp | Unidentified Lactobacillus sp. |
| 35-Ileum-5 | Oral – Pro | 5 | 250-bp, 350-bp, 500-bp, 600-bp | Possibly L. salivarius |
| 36-Ileum-6 | Oral – Pro | 6 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 36-Ileum-5 | Oral – Pro | 5 | 150-bp, 300-bp, 500-bp | Possibly L. johnsonii |
| 36-Ileum-5 | Oral – Pro | 5 | 350-bp | Unidentified Lactobacillus sp. |
| 36-Ileum-5 | Oral – Pro | 5 | 350-bp | Unidentified Lactobacillus sp. |
DT = dietary treatment; ARDRA = amplified ribosomal DNA restriction analysis.
Pure isolates were randomly selected from the ileum.
Oral-NC, negative control, with no additives added to the basal feed, water and litter, orally inoculated with PBS solution; Oral - Pro, with probiotic (Pro) L. johnsonii delivery by oral gavage.
Fig. 1.
Results for ARDRA analysis for 40 isolates from ileum of broiler chicken on d 7 (paret).
The results showed that two genotypic L. johnsonii patterns (300-bp, 500-bp) were present in the ARDRA test (Fig. 1). They are clearly differentiated from other patterns on the test. There are three patterns with L. crispatus (250-bp, 500-bp and 700-bp), two patterns with L. salivarius (200-bp, 500-bp), and one or four patterns with the unidentified strains (350-bp, 300-bp, 400-bp, 500-bp and 700-bp).
4. Discussion
4.1. Delivery routes and growth performance
A well-accepted method to quickly introduce a commensal microflora in chicks is through the administration of probiotics. Probiotic strains have been administrated in feed (Jin et al., 2000, Kalavethy et al., 2003) and water (Timmerman et al., 2006). Many reports have demonstrated that probiotics improve the growth performance and feed efficiency, and are potentially able to enhance nutrient absorption in broiler chickens. However, spraying of litter with probiotics is a method that has not been widely reported in poultry management. On the other hand, administering probiotics in drinking water is generally reported to result in a smaller increase in average daily gain compared with administering them via feed (Jin et al., 2000, Kalavethy et al., 2003). Compared to probiotics delivered via drinking water or compared with a negative control treatment, L. johnsonii, delivered as a feed supplement, did not significantly affect growth performance or feed conversion between d 1 and 21 in broiler chickens (Pelicano et al., 2004). They also observed that FI was slightly higher when a probiotic containing L. reuteri and L. johnsonii had been administered, but giving via feed or drinking water did not present different effects on growth performance and gut microbial composition in broilers.
The results of this study showed that different routes for administering L. johnsonii did not significantly influence the parameters of growth performance. The probiotic, when given via oral inoculation, achieved the highest weight gain (1,824 g) and FI (2,952 g) during the 35 d of the experiment, but these were not statistically significant. It is not uncommon that the use of L. johnsonii as a probiotic does not markedly improve bird performance (Maiorka et al., 2001, Murry et al., 2006). It is evident that probiotics such as L. johnsonii are effective in controlling pathogens (Cho et al., 2000, La Ragione et al., 2004) although growth enhancement by probiotics has also been reported (Schneitz, 2005).
4.2. Effects of delivery routes on organ weights and gut development
The probiotic did not affect the relative weights of intestinal tracts of broilers after 21 d of feeding. Jin et al. (1998) demonstrated that the probiotic supplement lactobacillus does not have an effect on organ weights and intestinal weight. Similar results were observed by Huang et al. (2004) who supplemented either L. casei or L. acidophilus with or without cobalt in the diets of broiler chickens.
The relative (to body weight) weights of the liver, spleen, and bursa of broilers were not affected by the probiotic L. johnsonii administrated by different delivery routes. However, delivery of the probiotic through feed, water and litter increased the pancreas weight on d 21. The reason(s) for this increase is not known.
The relative weights of the key organs can often be used as an indicator of changes in the morphology of the gut. The results of ileal morphology from the current study show that probiotic supplementation did not influence villus height, crypt depth and villi:crypt ratio compared with control treatments on d 7 and 21. Additives such as probiotics are regarded as modifying agents of the intestinal wall thickness due to the elimination of prejudicial bacteria (Rosen, 1995), thus germ-free birds have lighter intestinal tracts than birds originating from commercial farms (Coates et al., 1981). In an investigation on the impact of antibiotics on the organs of broilers Jong et al. (1985) reported physical alterations in the structure of the intestine, leading to a reduction in the intestinal weight. Henry et al. (1987) speculated that a decrease in the intestinal mass may result in less utilization of nutrients by the mucosa, sparing nutrients for the birds. However, neither antimicrobials (Loddi et al., 2004) nor probiotics (Pedroso, 1999) produced significant changes in the micro-structure of the intestine of birds.
4.3. Bacterial populations, intestinal pH and SCFA concentrations
The present results show that Enterobacteria and Lactobacilli are the most important groups of bacteria in the ileum and caeca during the early life of the chicks. The number of Enterobacteria starts to decrease from d 7 to 21 whereas that of lactobacilli decreases progressively from d 7 to 21. This result is supported by Van der Wielen et al. (2000) who reported that, after a decline in the early life of broilers, the number of Enterobacteria and Lactobacilli stabilized after 3 weeks of age.
Direct-fed microbials are known to benefit the host animal by improving its intestinal microflora balance (Fuller, 1998). The current study showed that the number of Enterobacteria decreased in the caeca and ileum significantly in probiotic treatment groups compared with control treatments. This is may indicate that the Enterobacteria group was inhibited by the dominant probiotic group. Thus, with the establishment of L. johnsonii in the gastrointestinal tract (GIT) of the birds, the enterobacterial population was outcompeted and the equilibrium of the gut microflora in the ileum and caeca was restored. This result, supported by those of Salminen and Wright (1993), demonstrates that Lactobacillus spp. exert a direct influence on enterobacterial colonization and it is tempting to describe the observed effects in such a manner. Vahjen et al. (1998) also indicated that a high lactobacillus population competitively excluded other members of the intestinal microflora of broilers, which displayed a slow rise in numbers in the ileum on d 21 followed by a rather sharp decline (up to tenfold) on d 28. The number of enterobacteria in the ileum followed the same declining trend.
One of the mechanisms by which CE occurs is through the production of SCFA by the dominating microflora. This study shows the presence of high concentration of acetic and lactic acids in the ileum, and butyric and succinic acids in the caeca in the probiotic treatment groups compared with control groups on d 7 and 21. This may mean that Enterobacteria are more susceptible to SCFA than lactobacilli. Indeed, Van der Wielen et al. (2000) demonstrated that an increasing concentration of SCFA caused a gradual decrease in the proliferation rate of Enterobacteria, but not that of the lactobacilli.
C. perfringens is a ubiquitous bacterium present in the chicken gut that causes necrotic enteritis when the conditions are right for the organism (Kocher, 2003). Necrotic enteritis is estimated to cost the global broiler industry US$2 billion per annum (Keyburn et al., 2006). The current study examined the effect of supplemental L. johnsonii on the number of C. perfringens in the ileum and the caeca. The population of C. perfringens decreased rapidly from an early age to d 21 in the caeca, leading to a 3-fold decrease in the number of C. perfringens between d 7 and 21. It also showed that the probiotic treatment presented the lowest number of C. perfringens in the caeca (7.76 vs. 8.14 on d 7; and 4.83 vs. 5.36 on d 21). This finding is consistent with previous research (Olnood et al., 2015a or b or c) showing that L. johnsonii, used as a feed supplement, resulted in lower populations of C. perfringens in the caeca compared to the negative control on d 35 (3.67 vs. 4.24). This seems to suggest that the probiotic used in the current study may be used to alleviate the risk associated with the proliferation of C. perfringens in the gut, which predisposes broiler flocks to economically devastating disease of necrotic enteritis.
4.4. Probiotic candidates dominant in the gut
The microbial community of the GIT ultimately reflects the coevolution of microorganisms with their animal host and the diet adopted by the host (Drasar and Barrow, 1985). In chickens, the diet and the environment affect the microbial status of the GIT. Dirty litter and other management parameters affect the microbial composition of the chickens both directly by providing a continuous source of bacteria and indirectly by influencing the physical condition and defence of the birds (Apajalahti et al., 2003). Changes in the composition of the animal׳s microflora can have beneficial or detrimental effects on the health, growth, and maturation of the host animal (Hill, 1982). Lu et al. (2003) analysed the composition of the bacterial flora in the ileum and caeca of broilers by the percent G + C profiling sequencing of 1,230 clones from a 16S rDNA community DNA library. Their results showed that Lactobacillus species were most abundant at 68.5% of the total sequences and L. acidophilus, L. salivarius, L. crispatus, L. delbrueckii, L. reuteri and L. aviarius were the dominant strains of lactobacilli in the ileum and caeca of chickens. Their results also indicated that L. johnsonii was not a dominant bacterial species in the intestinal tract of a normal chicken. Also (Dumonceaux et al., 2006) analysed the microbiota in the caeca of broilers on d 47. Their results demonstrated that the most commonly recovered sequences were lactobacilli that accounted for more than 65% of the total isolates. L. salivarius, and L. crispatus were the predominant lactobacilli in the caecal microflora and only three sequences (L. salivarius, L. buchneri and L. crispatus) were found in both the small intestine and the caeca.
A single dose of bacteria inoculated to newly hatched chicks can change digestal communities (Apajalahti et al., 1998). The results of this study show that L. johnsonii colonies were not detected in 20 of the ileal isolates in the negative control groups. This may indicate that L. johnsonii isolates (8/20), which were found in the oral inoculation treatment, had become dominant strains in the composition of lactobacilli in the ileum of broilers.
5. Conclusions
The novel probiotic candidate L. johnsonii was dominant in the intestinal tract of broiler chickens in the treatment groups. This was detected by 16-23S rDNA ARDRA patterns which also confirmed the influence of L. johnsonii on the gastrointestinal microfloral composition and notably the associated decrease in enterobacterial colonization in the ileum of broiler chicken between 1 and 21 d of age.
The delivery of the probiotic via drinking water, in feed, by litter application or oral gavage did not improve bird performance during the experimental period. Furthermore, there were no statistically significant differences between the various methods of delivery on the gut microflora, but individual oral application showed best regarding the reduction of Enterobacteria numbers in trial. The probiotic decreased the number of Enterobacteria and C. perfringens, a finding which may be regarded as a key attribute of probiotic application in poultry diets.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Apajalahti J.H., Paivi A.K., Nurminen H., Hanna Jatila I., Holben W.E. Selective plating underestimates abundance and shows differential recovery of Bifidobacterial species from human faces. J Appl Environ Microbiol. 2003;69:5731–5735. doi: 10.1128/AEM.69.9.5731-5735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J.H., Sarkilahti L.K., Maki B.R., Heikkinen J.P., Nurminen P.H., Holben W.E. Effective recovery of bacterial DNA and percent-guanine-plus-cytosine-based analysis of community structure in the gastrointestinal tract of broiler chickens. J Appl Environ Microbiol. 1998;64:4084–4088. doi: 10.1128/aem.64.10.4084-4088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship L.C. 1992. Report at international poultry exposition in Atlanta; pp. 22–24. January. [Google Scholar]
- Cho J.S., Choi Y.J., Chung D.K. Expression of Clostridium thermocelluum endoglucanase gene in Lactobacillus gasseri and Lactobacillus johnsonii and characterization of the genetically modified probiotic. Curr Microbiol. 2000;40:257–263. doi: 10.1007/s002849910051. [DOI] [PubMed] [Google Scholar]
- Coates M.E., Cole C.B., Fuller R., Houghton S.B., Yorota H. The gut microflora and the uptake of glucose from the small intestine of the chick. Br Poult Sci. 1981;22:289–294. doi: 10.1080/00071688108447888. [DOI] [PubMed] [Google Scholar]
- Corcoran B.M., Ross R.P., Fitzgerald G.F., Stanton C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J Appl Microbiol. 2004;96:1024–1039. doi: 10.1111/j.1365-2672.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- Corrier D.E., Hinton A.J., Ziprin R.L., Beier R.C., DeLoach J.R. Effect of dietary lactose on cecal pH, bacteriostatic volatile fatty acids and Salmonella typhimurium colonisation of broiler chicks. Avian Dis. 1990;34:617–625. [PubMed] [Google Scholar]
- Crawford J.S. Proceedings of 1979 Arkansas Conference, Fayetteville, AR, USA. 1979. Probiotic in animal nutrition; pp. 45–55. [Google Scholar]
- Drasar B.S., Barrow P.A. Benefits and mischief from the intestinal microflora. In: Drasar B.S., Barrow P.A., editors. Intestinal microbiology. American Society for Microbiology; Washington, D. C: 1985. pp. 19–42. [Google Scholar]
- Dumonceaux T.J., Hill J.E., Hemmingsen S.M., Van Kessel A.G. Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chickens. J Appl Environ Microbiol. 2006;72:2815–2823. doi: 10.1128/AEM.72.4.2815-2823.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritts C.A., Kersey J.H., Motl M.A., Kroger E.C., Yan F., Si J. Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens. J Appl Poult Res. 2000;9:149–155. [Google Scholar]
- Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1998;66:365–378. [PubMed] [Google Scholar]
- Gardiner G.E., O׳sullivan E., Kelly J., Auty M.A., Fitzgerald G.F., Collins J.K. Comparative survival rates of human-derived probiotic Lactobacillus paracesei and L. Salivarius strains during heat treatment and spray drying. J Appl Environ Microbiol. 2000;66:2605–2612. doi: 10.1128/aem.66.6.2605-2612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G.W., Hurst A. Academic Press; London and New York: 1969. The bacterial spore. [Google Scholar]
- Guan L.L., Hagen K.E., Tannock W.G., Korver D.R., Fasenko G.M., Allison G.E. Detection and identification of Lactobacillus species in crops of broiler chickens of different ages by using PCR- denaturing gradient gel electrophoresis and amplified ribosomal DNA restriction analysis. J Appl Environ Microbiol. 2003;69:6750–6757. doi: 10.1128/AEM.69.11.6750-6757.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P., Ammerman C.B., Campbell D.R., Miles R.D. Effect of antibiotics on tissue trace mineral concentration and intestinal tract weight of broiler chicks. Poult Sci. 1987;66:1014–1018. doi: 10.3382/ps.0661014. [DOI] [PubMed] [Google Scholar]
- Hill M.J. Gut flora associated diseases in man. J Vet Med. 1982;33(Suppl):32–36. [Google Scholar]
- Huang M.K., Choi Y.J., Houde R., Lee J.W., Lee B., Zhao X. Effects of lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poult Sci. 2004;83:788–795. doi: 10.1093/ps/83.5.788. [DOI] [PubMed] [Google Scholar]
- Iji P.A., Saki A.A., Tivev D.R. Intestinal development and body growth of broiler chicks on diets supplemented with non-starch polysaccharides. Anim Feed Sci Technol. 2001;89:175–188. [Google Scholar]
- Jensen M., Cox R., Jensen B.B. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. J Anim Sci. 1995;61:293–304. [Google Scholar]
- Jin L.Z., Ho Y.W., Abdullah N., Jalaludin S. Digestive and bacterial enzyme activities in broiler fed diets supplemented with Lactobacillus cultures. Poult Sci. 2000;79:866–891. doi: 10.1093/ps/79.6.886. [DOI] [PubMed] [Google Scholar]
- Jin L.Z., Ho Y.W., Abdullah N., Jalaludin S. Effects of adherent Lactobacillus cultures on growth, weight of organs and intestinal microflora and VFAs in broilers. Anim Feed Sci Technol. 1998;70:197–209. [Google Scholar]
- Jong E.U., Leboute E.M., Ciocca M.L., Penz Júnior A.M. Uso de avoparcina e virginiamicina como promotores de crescimento em rações de frangos de corte. 2. Efeito sobre a flora intestinal e estrutura física do intestino. Rev Soc Bras Zootecn. 1985;14:536–542. [Google Scholar]
- Kalavethy R., Abdullah N., Jalaludin S., Ho Y.W. Effects of lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br Poult Sci. 2003;44:139–144. doi: 10.1080/0007166031000085445. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher A. Nutritional strategies to minimise necrotic enteritis outbreaks in poultry. Rec Adv Anim Nutr Aus. 2003;14:111–116. [Google Scholar]
- Kozasa M. Toyocerin (Bacillus toyoi) as growth promoter for animal feeding. Microbiol Alim Nutr. 1986;4:121–135. [Google Scholar]
- La Ragione R.M., Narbad A., Gasson M.J., Woodward M.J. In vivo characterization of Lactobacillus johnsonii F19785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett Appl Microbiol. 2004;38:197–205. doi: 10.1111/j.1472-765x.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- Loddi M.M., Sato R.N., Ariki J., Pedroso A.A., Moraes V.M., Kishibe R. Reunião Anual da Sociedade Brasileira de Zootecnia. 2004. Ação isolada ou combinada de antibiótico ou probiótico como promotores de crescimento em rações iniciais de frangos de corte; p. 254. [Viçosa, Anais] [Google Scholar]
- Lu J.R., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. J Appl Environ Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorka A., Santin E., Sugeta S.M., Almeida J.C., Macari M. Utilization of prebiotics, probiotics and symbiotic in diets parameters. Rev Bras Cienc Avic. 2001;3:75–82. [Google Scholar]
- Mikkelsen L.L., Bendixen C., Jakobsen K., Jensen B.B. Enumeration of bifidobacteria in intestinal samples from pigs. J Appl Environ Microbiol. 2003;69:654–658. doi: 10.1128/AEM.69.1.654-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T.L., Wolin M.J.A. Serum bottle modification of the hungate technique for cultivating obligate anaerobes. J Appl Environ Microbiol. 1974;27:985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli L. In vitro selection of probiotic Lactobacilli: a critical appraisal. Curr Issues Intest Microbiol. 2000;1:59–67. [PubMed] [Google Scholar]
- Murry J.A.C., Hinton J.A., Buhr R.J. Effect of botanical probiotic containing Lactobacilli on growth performance and populations of bacteria in the ceca, cloaca and carcass rinse of broiler chickens. Int J Poult Sci. 2006;5:344–350. [Google Scholar]
- Netherwood T., Gilbert H.J., Parker D.S., O׳Donnell A.G. Probiotics shown to change bacterial community structure in the avian gastrointestinal tract. J Appl Environ Microbiol. 1999;65:5134–5138. doi: 10.1128/aem.65.11.5134-5138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H., Eckenfelder B., Levesque A. Growth promoting efficiency of two probiotics, Toyocerin and Paciflor, in broiler diets. Arch Geflugelkd. 1988;56:240–245. [Google Scholar]
- Nurmi E., Rantala M. New aspects of Salmonella infection in broiler production. Nature. 1973;241:210–211. doi: 10.1038/241210a0. [DOI] [PubMed] [Google Scholar]
- Pedroso A.A. Faculdade de Ciências Agrárias e Veterinárias; Jaboticabal: 1999. Efeito de probiótico dietético sobre o desempenho, qualidade dos ovos e alguns aspectos morfológicos do trato intestinal e tecido ósseo de poedeiras. [Google Scholar]
- Pelicano E.R.L., Souza P.A., Souza H.B.A., Oba A., Zeola N.M., Boiago M.M. Conferência Apinco de Ciência e Tecnologia Avícolas. 2004. Efeito do uso de probióticos e/ou prebióticos sobre o rendimento de carcaça de frangos de corte; p. 8. [Santos, São Paulo, Brasil] [Google Scholar]
- Rosen G.D. Antibacterials in poultry and pig nutrition. In: Wallace R.J., Chesson A., editors. Biotechnology in animal feeds and animal feeding. VCH Verlagsgesellschaft mbH; 1995. pp. 143–172. [Google Scholar]
- Salminen S., Wright A. Lactic acid bacteria. In: Salminen S., Deighton M.A., Gorbach S.L., editors. Lactic acid bacteria in health and disease. Marcel Dekker Inc; New York: 1993. pp. 199–225. [Google Scholar]
- Scheuerman S.E. Effect of probiotic Paciflor (CIP5832) on energy and protein metabolism in growing pigs. Anim Feed Sci Technol. 1993;41:181–189. [Google Scholar]
- Schneitz C. Competitive exclusion in poultry – 30 years of research. Food Control. 2005;16:657–667. [Google Scholar]
- Seuna E., Raevuori M., Nurmi E. An epizootic of Salmonella typhimurium var. copenhagen in broiler and the use of cultured chicken intestinal flora for its control. Br Poult Sci. 1978;19:309–314. doi: 10.1080/00071667808416481. [DOI] [PubMed] [Google Scholar]
- Teo A.Y., Tan H. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. J Appl Environ Microbiol. 2005;71:4185–4190. doi: 10.1128/AEM.71.8.4185-4190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman H.M., Veldman A., van den Elsen E., Rombouts F.M., Beynen A.C. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult Sci. 2006;85:1383–1388. doi: 10.1093/ps/85.8.1383. [DOI] [PubMed] [Google Scholar]
- Vahjen W., Glaser K., Schafer K., Simon O. Influence of xylanase-supplemented feed on the development of selected bacterial group in the intestinal tract of broiler chicks. J Agric Sci Cambrige. 1998;130:489–500. [Google Scholar]
- Van der Wielen P.W., Biesterveld S., Notermans S., Hofstra H., Urlings B.A.P., van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. J Appl Environ Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidanarachchi J.K. The University of New England; Australia: 2006. Regulation of intestinal microflora and productivity of broiler chickens by prebiotic and bioactive plant extracts. (PhD thesis) [Google Scholar]