Abstract
The Liverpool Plains is a fertile agricultural region in New South Wales, Australia. Two sorghums from the 2009 Liverpool Plains harvest, sorghums #3 and #5, were extensively characterised which included concentrations of kafirin and phenolic compounds plus rapid visco-analysis (RVA) starch pasting profiles. Diets based on these two sorghums were formulated to be iso-nitrogenous and iso-energetic and were offered to male Ross 308 broiler chicks from 7 to 28 days post--hatch as either intact pellets or reground mash following steam-pelleting at conditioning temperatures of either 65 or 97°C. Thus the feeding study consisted of a 2 × 2 × 2 factorial array of dietary treatments: two sorghum varieties, two feed forms and two conditioning temperatures. Each of the eight treatments was replicated six times with six birds per replicate cage. Assessed parameters included growth performance, nutrient utilisation, apparent starch and protein (N) digestibility coefficients and disappearance rates from the distal jejunum and distal ileum. Intact pellets supported higher (P < 0.001) feed intakes and weight gains by 9.83 and 9.08%, respectively, than reground mash diets. Feed conversion ratios of broilers offered diets steam-conditioned at 97°C were 2.46% inferior (P < 0.001) in comparison to 65°C diets and both apparent metabolizable energy (AME) and N-corrected AME (AMEn) were compromised. Broilers offered sorghum #3-based diets significantly (P < 0.001) outperformed their sorghum #5 counterparts in terms of weight gain by 3.75% (1,334 versus 1,223 g/bird), FCR by 4.81% (1.524 versus 1.601), AME by 1.06 MJ (13.61 versus 12.55 MJ/kg), ME:GE ratio (ME:GE) by 4.81% (0.806 versus 0.769) and AMEn by 1.03 MJ (12.38 versus 11.35 MJ/kg). The inferiority of sorghum #5 appeared to be associated with higher concentrations of kafirin (61.5 versus 50.7 g/kg) and conjugated phenolic acids, including ferulic acid (31.1 versus 25.6 µg/g). There were no significant differences in jejunal and ileal starch and protein (N) digestibility coefficients between the two sorghums. However, starch to protein (N) disappearance rate ratios from the distal jejunum were significantly (P < 0.001) correlated with ME:GE and AME. The multiple linear regression equations indicated that energy utilisation was enhanced by coupling rapidly digestible protein with slowly digestible starch, which suggests that bilateral bioavailability of starch and protein is pivotal to efficient energy utilisation.
Keywords: Broiler chickens, Ferulic acid, Kafirin, Protein, Sorghum, Starch
1. Introduction
Sorghum has been described as an enigmatic grain for chicken-meat production because sorghum-based broiler diets have been associated with sub-optimal performance of chickens under Australian conditions (Selle et al., 2013). Six red 'tannin-free' grain sorghum varieties harvested on the Liverpool Plains of New South Wales in 2009 were extensively characterised and compared in broilers offered sorghum-casein diets (Khoddami et al., submitted for publication). On the basis of this comparison, two sorghums (sorghums #3 and #5) were selected and incorporated into conventional, iso-nitrogenous and iso-energetic diets that were steam-pelleted at conditioning temperatures of 65 or 97°C and were offered to broilers as either intact pellets or reground mash from 7 to 28 days post--hatch as a 2 × 2 × 2 factorial array of dietary treatments. It was anticipated from the initial comparison that broiler chickens offered diets based on sorghum #3 would outperform their sorghum #5 counterparts.
It is often considered that kafirin, the dominant sorghum protein fraction, is a poor source of protein and also negatively impacts on starch utilisation (Taylor, 2005). Both kafirin protein bodies and starch granules are embedded in close proximity in the glutelin protein matrix of sorghum endosperm (Selle et al., 2010). This close proximity would facilitate any biophysical and biochemical interactions between kafirin and starch in grain sorghum (Wong et al., 2010). A novel method to quantify kafirin in sorghum was developed during this investigation; therefore, the methodology is described in detail. Also, it has been suggested that 'non-tannin' phenolic compounds in sorghum may have negative effects on starch utilisation (Khoddami et al., submitted for publication). This infers that the deleterious effects of phenolic compounds are not solely the province of condensed tannin. Thus the objectives of this paper include assessments of the influence of kafirin and 'non-tannin' phenolic compounds on the performance of broiler chickens offered nutritionally equivalent diets based on sorghums #3 and #5. Another objective is to appraise starch and protein (N) digestive dynamics in tandem by coupling their digestibility coefficients and disappearance rates in the distal jejunum and distal ileum.
2. Materials and methods
The six red sorghums from the 2009 Liverpool Plains harvest were extensively characterised. The more relevant data specific to sorghums #3 and #5 are shown in Table 1. Neither sorghum contained a pigmented testa and, therefore do not contained condensed tannin which was confirmed by a vanillin assay (Khoddami et al., submitted for publication).
Table 1.
Selected characteristics of sorghum 3# and sorghum #5.
| Item | Sorghum #3 | Sorghum #5 | Item, µg/g | Sorghum #3 | Sorghum #5 |
|---|---|---|---|---|---|
| Kafirin | 50.7 | 61.5 | Total anthocyanin, Abs/(mL·g) | 11.55 | 7.27 |
| Protein | 99.4 | 116.3 | Flavan-4ols, Abs/(mL·g) | 4.05 | 4.12 |
| Kafirin proportion, % | 51.0 | 52.9 | Luteolinidin | 5.58 | 1.88 |
| Protein solubility, % | 49.5 | 41.2 | Apigeninidin | 16.84 | 6.06 |
| 5-methoxy-luteolinidin | 5.80 | 2.41 | |||
| Starch | 624 | 620 | 7-methoxy-apigeninidin | 25.60 | 11.45 |
| Amylose, % | 26.4 | 27.2 | Apigenin | 9.25 | 5.62 |
| Amylopectin, % | 73.6 | 72.8 | Luteolin | 8.30 | 4.75 |
| Peak RVA viscosity, cP | 4,202 | 3,750 | Eriodictyol | 57.29 | 62.27 |
| Holding RVA viscosity, cP | 3,088 | 3,086 | Naringenin | 86.51 | 85.68 |
| Final RVA viscosity, cP | 6,644 | 7,132 | |||
| Total phenolic acids | 545.4 | 538.1 | |||
| Total phosphorus | 2.95 | 3.35 | Bound phenolic acids | 458.0 | 448.0 |
| Phytate-P | 2.35 | 2.40 | Conjugated phenolic acids | 53.1 | 76.0 |
| Phytate-P proportion, % | 79.7 | 71.6 | Free conjugated acids | 34.3 | 14.1 |
| Phytate | 8.33 | 8.51 | |||
| Total ferulic acid | 421.7 | 407.9 | |||
| Symes PSI texture | 10 | 9 | Bound ferulic acid | 393.1 | 374.9 |
| Conjugated ferulic acid | 25.6 | 31.1 | |||
| Total phenolics, mg GAE/g | 3.52 | 3.59 | Free ferulic acid | 3.0 | 1.9 |
| Pigmented testa | 0 | 0 |
RVA = rapid visco-analysis; GAE = gallic acid equivalents.
2.1. Kafirin quantification
The kafirin concentrations were quantified by the following procedures. Kafirin was extracted from sorghum meal according to Wallace et al. (1990) and Hamaker et al. (1995). Sorghum meal (100 mg) was incubated with 1 mL total protein extraction buffer (12.5 mM sodium borate, pH 10.0, 1% SDS, 2% 2-mercaptoethanol) for 1 h with agitation at room temperature. Each sample was centrifuged for 15 min at 14,000 rpm at room temperature. Total protein was extracted twice more as above and the supernatants pooled. To isolate kafirin from the total protein extract, 100% t-butanol was added to the pooled supernatant at a final concentration of 60%, and incubated for 30 min at room temperature with occasional mixing. The extract was then centrifuged for 15 min at 9,500 rpm at room temperature, which resulted in the formation of a pellet (non-kafirin) and supernatant (alcohol-soluble kafirin). Kafirin was extracted from each sorghum sample in duplicate.
Both kafirin and total protein content were quantified by amino acid analysis. The amino acid analysis procedure employed pre-column derivatisation with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) and ultra-performance liquid chromatography (UPLC) analysis after first performing acid hydrolysis on the kafirin extract and whole sorghum grain samples to release the protein-bound amino acids. Each sample was quantified in duplicate. The method is based on Cohen (2001) but the amino acids were analysed on a UPLC system (Boogers et al., 2008). For quantification of total sorghum protein, 200 to 250 mg of sorghum meal was hydrolysed in duplicate by adding 5.0 mL of 20% HCl to the sorghum meal in a 10 mL hydrolysis vial, flushing with nitrogen for 1 min, followed by incubation at 110°C for 24 h. An internal standard (norvaline) was then added to the hydrolysate and it was diluted 1 part in 25 with MQ water prior to derivatisation. For quantification of kafirin, a 5 µL aliquot of the kafirin alcohol fraction was dried under vacuum in duplicate and hydrolysed by gas-phase acid (6 M HCl) hydrolysis at 110°C for 24 h following established protocols (Cohen, 2001).
Hydrolysed samples and amino acid standards (Standard H, Pierce, Rockford, IL, USA) were derivatised with AQC reagent using the AccQ-Tag Ultra derivatisation kit (Waters Corporation, Milford, MA, USA) according to the manufacturer׳s instructions. Briefly, for the hydrolysed sorghum meal, a 10 µL aliquot of diluted hydrolysate was mixed with 70 µL borate buffer (0.2 M, pH 8.8), then 20 µL of AQC reagent was added, then heated at 50°C for 10 min in a dry block. For the hydrolysed kafirin sample, it was dried under vacuum then reconstituted in 10 µL of an internal standard (norvaline) solution prior to derivatisation as above.
Chromatographic separation and quantitation of 16 acid hydrolysate amino acids was performed on an ACQUITY UPLC system (Waters Corporation) with UV detection at 260 nm. The column was a BEH RP C18 (2.1 × 100 mm, 1.7 µm, Waters Corporation) thermostated at 57°C. The flow rate was 0.7 mL/min over a 10.2 min runtime. The injection volume was 1 µL. The solvent system consisted of Waters AccQ-Tag Ultra eluent A, pH 2.8 (50 mL AccQ-Tag Ultra A concentrate + 950 mL MQ water) and Waters AccQ-Tag Ultra eluent B. The gradient conditions were similar to Boogers et al. (2008). Data were acquired and quantified using Empower 2 software (Waters Corporation) with 2.5, 10 and 50 pmol analytical standards and an internal standard calibration method.
2.2. Analyses of phenolic compounds
The complex methodologies to analyse sorghum for polyphenols, free, conjugated, bound phenolic acids, condensed tannin, detection of pigmented testas and rapid visco-analysis (RVA) of starch pasting profiles have been thoroughly documented by Khoddami et al. (submitted for publication). Amylose and amylopectin concentrations were determined using a megazyme kit (Megazyme International. Wicklow, Ireland). Total P levels were determined by ICP-MS and phytate-P concentrations by HPLC procedures.
2.3. Broiler feeding study
The feeding study was conducted so as to comply with specific guidelines approved by the Animal Ethics Committee of the University of Sydney. Nutritionally equivalent broiler diets based on either sorghum #3 or sorghum #5 were formulated to contain 215 g protein per kilogram diet with an energy density of 12.84 MJ/kg as shown in Table 2. These diets were offered to male Ross 308 chicks from 7 to 28 days post--hatch as a 2 × 2 × 2 factorial array of dietary treatments with two sorghum varieties, diets that were steam-pelleted at conditioning temperatures of 65 or 97°C and fed as either intact pellets or reground mash. Each of the eight treatments consisted of six replicate cages of six birds per cage or a total of 288 Cobb 500 male chicks.
Table 2.
Composition and nutrient specifications for diets based on sorghum #3 and sorghum #5, as-fed basis.
| Item, g/kg | Sorghum #3 | Sorghum #5 |
|---|---|---|
| Composition | ||
| Sorghum | 572.6 | 643.3 |
| Soyabean meal | 263.5 | 221.9 |
| Canola meal | 60.0 | 60.0 |
| Vegetable oil | 41.8 | 10.0 |
| Limestone | 9.9 | 10.0 |
| Dicalcium phosphate | 16.8 | 17.0 |
| Sodium chloride | 0.8 | 0.3 |
| Sodium bicarbonate | 4.0 | 4.5 |
| Lysine HCl | 3.5 | 4.9 |
| DL-methionine | 3.8 | 4.2 |
| L-threonine | 1.3 | 1.9 |
| Vitamin-trace mineral premix1 | 2.0 | 2.0 |
| Celite | 20.0 | 20.0 |
| Nutrient specifications | ||
| Metabolisable energy, MJ/kg | 12.84 | 12.84 |
| Crude protein | 215.0 | 215.0 |
| Calcium | 9.0 | 9.0 |
| Total phosphorus | 7.5 | 7.5 |
| Phytate phosphorus | 3.00 | 3.01 |
| Nonphytate phosphorus | 4.50 | 4.49 |
| Methionine + cystine | 10.2 | 10.2 |
| Lysine | 13.5 | 13.5 |
| Threonine | 9.0 | 9.0 |
| Arginine | 12.9 | 11.6 |
| Valine | 9.2 | 9.7 |
| Sodium | 1.8 | 1.8 |
| dEB, mEq/kg | 243 | 227 |
| Analysed | ||
| Starch | 358.3 | 388.5 |
| Protein (N) | 210.3 | 208.8 |
| Estimated | ||
| Phytate-P2 | 3.00 | 3.01 |
| Non-phytate P3 | 4.50 | 4.49 |
The vitamin-trace mineral premix supplied per tonne of feed: retinol 12MIU, cholecalciferol 5MIU, tocopherol 50g, menadione 3g, thiamine 3g, riboflavin 9g, pyridoxine 5g, cobalamin 0.025g, niacin 50g, pantothenate 18g, folate 2g, biotin 0.2g, copper 20g, iron 40g, manganese 110g, cobalt 0.25g, iodine 1g, molybdenum 2g, zinc 90g, selenium 0.3g.
Based on actual phytate-P sorghum values and recorded values for soyabean meal (4.53 g/kg) and canola meal (6.69) taken from Selle et al. (2003).
Non-phytate P = total P -- phytate-P.
The broiler chicks were initially fed a proprietary starter ration. At 7 days post--hatch they were allocated into 48 cages on the basis of body weight and offered experimental diets to 28 days post--hatch in an environmentally controlled facility. Weights were recorded at day 7 and day 28 and feed intakes monitored. FCR were calculated where the body weights of dead or culled birds were used to adjust calculations. Feed intakes over the final 8 days were recorded to deduce starch and protein (N) disappearance rates.
Total excreta were collected from 23 to 26 days post--hatch from each cage to determine apparent metabolisable energy (AME), ME:GE ratios, nitrogen (N) retention and N-corrected apparent metabolisable energy (AMEn). AME values (MJ/kg) of the diet were calculated using the following formula:
ME:GE ratios were calculated by dividing AME values of diets recorded for each cage by GE of the relevant diets. N content of the diets and excreta were obtained using an FP-428 determinator (Leco Corporation, St Joseph, MI USA) and N retention was calculated using the following formula:
N-corrected AME (MJ/kg) values were calculated by correcting to zero N retention by applying the factor of 36.54 kJ/g N retained in the body (Hill and Anderson, 1958).
Acid insoluble ash (Celite World Minerals, Lompoc, CA, USA) was included in diets at 20 g/kg as an inert marker to determine apparent digestibility coefficients of N and starch in two small intestinal sites. N content of digesta samples were obtained with a Leco FP-428 determinator and starch concentrations in diets and digesta were determined by a procedure based on dimethyl sulfoxide, a-amylase and amyloglucosidase, as described by Mahasukhonthachat et al. (2010). On day 28, digesta was collected from the distal halves of the jejunum and ileum to determine apparent digestibility coefficients by the following equation:
Starch and protein (N) disappearance rates (g/bird) were deduced from feed intakes over the final 8 days of the feeding period (d 7 to 28) using the following equation:
Distal ileal disappearance rates were obtained from the difference between total ileal disappearance rates and distal jejunal disappearance rates. Ratios of starch to protein disappearance rates in the distal jejunum and distal ileum were calculated.
2.4. Statistical analysis
Experimental data were analysed as a 2 × 2 × 2 factorial array of dietary treatments using the univariate linear models procedure of IBM SPSS Statistics 20 program (IBM Corporation. Somers, NY USA). A probability level of less than 5% was considered statistically significant. Pearson correlations and multiple linear regression equations for parameters of interest were obtained.
3. Results
The effects of sorghum variety, feed form and steam-pelleting conditioning temperature on weight gain, feed intake, feed conversion ratio (FCR), mortality/cull rates and relative gizzard weights are shown in Table 3. The overall mortality rate of 4.17% was satisfactory, although more mortalites occurred in sorghum #3-based diets subject to treatment interactions. This difference in mortalities between sorghums may not be meaningful. Birds offered intact pellets had greater feed intakes than those on reground mash diets by 9.83% (2,090 versus 1,903 g/d; P < 0.001), which translated into greater weight gains for birds on intact pellets than their counterparts by 9.08% (1,334 versus 1,223 g/bird; P < 0.001). Sorghum #3-based diets supported 3.75% higher weight gains than sorghum #5 (1,302 versus 1,255 g/bird; P < 0.005). There was a sorghum by feed form interaction (P < 0.01) for FCR; however, sorghum #3 was associated with a better feed conversion than sorghum #5-based diets by 4.81% (1.524 versus 1.601). The interaction stemmed from the fact that the transition from mash to pellets improved FCR of sorghum #3 diets but the reverse applied to sorghum #5-based diets. The higher conditioning temperature depressed FCR by 2.46% (1.582 versus 1.544; P < 0.001). Reground mash diets generated heavier relative gizzard weights by 4.45% (20.27 versus 19.76 g/kg; P < 0.05) in comparison to intact pellets. There was an interaction (P < 0.05) between sorghum and conditioning temperature for this parameter as the impact of higher temperatures generating havier gizzards was more pronounced with sorghum #3.
Table 3.
The effect of sorghum variety, feed form (reground mash versus intact pellet) and steam-pelleting conditioning temperature on growth performance, mortality/cull rates and relative gizzard weights from 7 to 28 days post--hatch.
| Treatment |
Weight gain, g/bird | Feed intake, g/bird | FCR, g/g | Mortalities, % | Gizzard wt, g/kg | ||
|---|---|---|---|---|---|---|---|
| Sorghum | Feed form | Temperature | |||||
| #3 | Mash | 65°C | 1,236 | 1,873 | 1.516 | 11.11 | 18.86 |
| 97°C | 1,241 | 1,922 | 1.549 | 2.78 | 20.47 | ||
| Pellet | 65°C | 1,364 | 2,040 | 1.497 | 2.78 | 18.08 | |
| 97°C | 1,366 | 2,097 | 1.535 | 11.11 | 20.53 | ||
| #5 | Mash | 65°C | 1,224 | 1,910 | 1.561 | 2.78 | 20.21 |
| 97°C | 1,190 | 1,906 | 1.602 | 0.00 | 21.55 | ||
| Pellet | 65°C | 1,295 | 2,071 | 1.600 | 0.00 | 19.81 | |
| 97°C | 1,311 | 2,151 | 1.641 | 2.78 | 20.60 | ||
| SEM | 20.936 | 32.533 | 0.0137 | 2.6353 | 0.2217 | ||
| Main effects: Sorghum | |||||||
| #3 | 1,302b | 1,983 | 1.524 | 6.95 | 19.49 | ||
| #5 | 1,255a | 2,009 | 1.601 | 1.39 | 20.54 | ||
| Feed form | |||||||
| Reground mash | 1,223a | 1,903a | 1.557 | 4.17 | 20.27b | ||
| Intact pellet | 1,334b | 2,090b | 1.568 | 4.17 | 19.76a | ||
| Temperature | |||||||
| 65°C | 1,279 | 1,974 | 1.544a | 4.17 | 19.24 | ||
| 97°C | 1,277 | 2,019 | 1.582b | 4.17 | 20.19 | ||
| Significance (P=) | |||||||
| Sorghum (S) | 0.003 | 0.258 | <0.001 | 0.005 | <0.001 | ||
| Feed form (FF) | <0.001 | <0.001 | 0.243 | 1.000 | 0.025 | ||
| Temperature (T) | 0.872 | 0.057 | <0.001 | 1.000 | <0.001 | ||
| Interactions: S × FF | 0.298 | 0.486 | 0.006 | 1.000 | 0.479 | ||
| S × T | 0.671 | 0.751 | 0.768 | 1.000 | 0.035 | ||
| FF × T | 0.441 | 0.319 | 0.894 | 0.005 | 0.754 | ||
| S × FF × T | 0.383 | 0.417 | 0.894 | 0.144 | 0.122 | ||
a,b Means within columns not sharing common superscripts are significantly different at the 5% level of probability.
The effects of dietary treatments on parameters of nutrient utilisation are shown in Table 4. Sorghum #3-based diets supported higher AME by 1.06 MJ (13.61 versus 12.55 MJ/kg; P < 0.001), AMEn by 1.03 MJ (12.38 versus 11.35 MJ/kg; P < 0.001) with 4.81% superior ME:GE ratios (0.806 versus 0.769; P < 0.001) in comparison to sorghum #5. Reground mash supported enhanced (P < 0.001) energy utilisation by 0.80 MJ AME, 0.83 MJ AMEn and by 6.10% for ME:GE ratios in comparison to intact pelleted diets. The higher 97°C conditioning temperature depressed (P < 0.05) AME by 0.28 MJ and AMEn by 0.26 MJ relative to the 65°C conditioning temperature. There was an interaction between sorghum and feed form (P < 0.05) for N retention in which better N retentions were associated with sorghum #3 and reground mash diets. The interaction was due to the fact that N retention of intact pelleted diets based on sorghum #5 were noticeably less than the balance of treatments.
Table 4.
The effect of sorghum variety, feed form (reground mash versus intact pellet) and steam-pelleting conditioning temperature on nutrient utilisation: apparent metabolisable energy (AME), ME to GE ratio (ME:GE), nitrogen (N) retention and N-corrected AME (AMEn).
| Treatment |
AME, MJ/kg DM | ME:GE | N retention, % | AMEn, MJ/kg DM | ||
|---|---|---|---|---|---|---|
| Sorghum | Feed form | Temperature | ||||
| #3 | Mash | 65°C | 14.12 | 0.820 | 66.5 | 12.91 |
| 97°C | 13.67 | 0.802 | 63.3 | 12.48 | ||
| Pellet | 65°C | 13.46 | 0.790 | 63.0 | 12.17 | |
| 97°C | 13.18 | 0.769 | 61.4 | 11.98 | ||
| #5 | Mash | 65°C | 13.11 | 0.789 | 61.0 | 11.94 |
| 97°C | 13.02 | 0.787 | 63.1 | 11.81 | ||
| Pellet | 65°C | 12.17 | 0.736 | 55.1 | 10.98 | |
| 97°C | 11.90 | 0.723 | 52.9 | 10.69 | ||
| SEM | 0.1775 | 0.0129 | 1.7441 | 0.1693 | ||
| Main effects: Sorghum | ||||||
| #3 | 13.61b | 0.806b | 63.6 | 12.38b | ||
| #5 | 12.55a | 0.769a | 58.0 | 11.35a | ||
| Feed form | ||||||
| Reground mash | 13.48b | 0.800b | 63.5 | 12.28b | ||
| Intact pellet | 12.68a | 0.754a | 58.1 | 11.45a | ||
| Temperature | ||||||
| 65°C | 13.22b | 0.784 | 61.4 | 12.00b | ||
| 97°C | 12.94a | 0.770 | 60.2 | 11.74a | ||
| Significance (P=) | ||||||
| Sorghum (S) | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Feed form (FF) | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Temperature (T) | 0.037 | 0.081 | 0.321 | 0.034 | ||
| Interactions S × FF | 0.079 | 0.087 | 0.036 | 0.091 | ||
| S × T | 0.443 | 0.444 | 0.352 | 0.670 | ||
| FF × T | 0.989 | 0.623 | 0.558 | 0.855 | ||
| S × FF × T | 0.496 | 0.753 | 0.241 | 0.406 | ||
a,b Means within columns not sharing common superscripts are significantly different at the 5% level of probability.
The effects of dietary treatments on apparent digestibility of starch and protein (N) in the distal jejunum (DJ) and distal ileum (DI) are shown in Table 5. The only significant response in starch digestibility was in the DI where reground mash diets supported 2.83% higher (0.871 versus 0.847; P < 0.05) digestibility coefficients than intact pelleted diets. Also, reground mash diets supported 10.5% higher (0.547 versus 0.495; P < 0.05) N digestibility coefficients in the DJ and 3.88% higher (0.749 versus 0.721; P < 0.05) N digestibility coefficients in the DI in comparison to intact pellets.
Table 5.
The effect of sorghum variety, feed form (reground mash versus intact pellet) and steam-pelleting conditioning temperature on apparent digestibility coefficients of starch and nitrogen in the distal jejunum (DJ) and distal ileum (DI).
| Treatment |
Starch digestibility |
Nitrogen digestibility |
||||
|---|---|---|---|---|---|---|
| Sorghum | Feed form | Temperature | DJ | DI | DJ | DI |
| #3 | Mash | 65°C | 0.717 | 0.901 | 0.563 | 0.777 |
| 97°C | 0.763 | 0.827 | 0.548 | 0.708 | ||
| Pellet | 65°C | 0.699 | 0.833 | 0.526 | 0.729 | |
| 97°C | 0.705 | 0.846 | 0.483 | 0.721 | ||
| #5 | Mash | 65°C | 0.735 | 0.881 | 0.515 | 0.753 |
| 97°C | 0.739 | 0.873 | 0.564 | 0.761 | ||
| Pellet | 65°C | 0.740 | 0.860 | 0.509 | 0.728 | |
| 97°C | 0.713 | 0.849 | 0.493 | 0.705 | ||
| SEM | 0.0224 | 0.0183 | 0.0316 | 0.0183 | ||
| Main effects: Sorghum | ||||||
| #3 | 0.721 | 0.852 | 0.530 | 0.734 | ||
| #5 | 0.731 | 0.866 | 0.512 | 0.736 | ||
| Feed form | ||||||
| Reground mash | 0.738 | 0.871b | 0.547b | 0.749b | ||
| Intact pellet | 0.714 | 0.847a | 0.495a | 0.721a | ||
| Temperature | ||||||
| 65°C | 0.723 | 0.869 | 0.528 | 0.747 | ||
| 97°C | 0.730 | 0.849 | 0.514 | 0.724 | ||
| Significance (P=) | ||||||
| Sorghum (S) | 0.510 | 0.235 | 0.419 | 0.841 | ||
| Feed form (FF) | 0.147 | 0.043 | 0.020 | 0.035 | ||
| Temperature (T) | 0.678 | 0.085 | 0.519 | 0.091 | ||
| Interactions S × FF | 0.401 | 0.954 | 0.954 | 0.378 | ||
| S × T | 0.257 | 0.355 | 0.481 | 0.246 | ||
| FF × T | 0.283 | 0.074 | 0.162 | 0.577 | ||
| S × FF × T | 0.881 | 0.057 | 0.430 | 0.092 | ||
a,b Means within columns not sharing common superscripts are significantly different at the 5% level of probability.
The effects of treatments on starch and protein (N) disappearance rates (g/bird) from 20 to 28 days post--hatch and the starch:N disappearance rate ratios are shown in Table 6. The distal jejunal starch disappearance rate was more rapid in sorghum #5-based diets by 9.09% (312 versus 286 g/bird; P < 0.005) than sorghum #3 and in intact pellets by 7.29% (309 versus 288 g/d; P < 0.05) in comparison to reground mash diets. There was a feed form by conditioning temperature interaction (P < 0.05) for distal ileal starch disappearance rates; however, the disappearance rate was more rapid in sorghum #5 by 9.50% (369 versus 337 g/bird; P < 0.001) than sorghum #3-based diets. The only significant effect for N disappearance rates was confined to the distal ileum where intact pellets were more rapid than reground mash diets by 6.71% (175 versus 164 g/d; P < 0.05). Sorghum #3-based diets generated `narrower' starch:N disappearance rate ratios in both the distal jejunum by 13.0% (2.35 versus 2.70; P < 0.001) and the distal ileum by 9.59% (1.98 versus 2.19; P < 0.001).
Table 6.
The effect of sorghum variety, feed form (reground mash versus intact pellet) and steam-pelleting conditioning temperature on starch and nitrogen disappearance rates (g/bird) from 20 to 28 days post--hatch and disappearance rate ratios in distal jejunum (DJ) and distal ileum (DI).
| Treatment |
Starch disappearance rate |
Nitrogen disappearance rate |
Disappearance rate ratio |
|||||
|---|---|---|---|---|---|---|---|---|
| Sorghum | Feed form | Temperature | DJ | DI | DJ | DI | DJ | DI |
| #3 | Mash | 65°C | 271 | 340 | 124 | 172 | 2.18 | 1.98 |
| 97°C | 292 | 316 | 123 | 159 | 2.45 | 1.99 | ||
| Pellet | 65°C | 280 | 334 | 124 | 172 | 2.27 | 1.95 | |
| 97°C | 300 | 359 | 121 | 180 | 2.49 | 2.00 | ||
| #5 | Mash | 65°C | 298 | 357 | 112 | 164 | 2.67 | 2.18 |
| 97°C | 294 | 347 | 120 | 163 | 2.48 | 2.14 | ||
| Pellet | 65°C | 324 | 376 | 120 | 171 | 2.74 | 2.21 | |
| 97°C | 333 | 397 | 116 | 177 | 2.92 | 2.24 | ||
| SEM | 12.323 | 12.742 | 8.009 | 7.148 | 0.126 | 0.039 | ||
| Main effects: Sorghum | ||||||||
| #3 | 286a | 337a | 123 | 171 | 2.35a | 1.98a | ||
| #5 | 312b | 369b | 117 | 169 | 2.70b | 2.19b | ||
| Feed form | ||||||||
| Reground mash | 288a | 340 | 120 | 164a | 2.45 | 2.07 | ||
| Intact pellet | 309b | 367 | 120 | 175b | 2.61 | 2.10 | ||
| Temperature | ||||||||
| 65°C | 293 | 352 | 120 | 170 | 2.47 | 2.08 | ||
| 97°C | 304 | 355 | 120 | 170 | 2.59 | 2.09 | ||
| Significance (P=) | ||||||||
| Sorghum (S) | 0.004 | <0.001 | 0.305 | 0.710 | <0.001 | <0.001 | ||
| Feed form (FF) | 0.022 | 0.005 | 0.936 | 0.040 | 0.079 | 0.322 | ||
| Temperature (T) | 0.208 | 0.718 | 0.988 | 0.985 | 0.188 | 0.660 | ||
| Interactions S × FF | 0.169 | 0.382 | 0.762 | 0.929 | 0.292 | 0.157 | ||
| S × T | 0.310 | 0.781 | 0.701 | 0.632 | 0.160 | 0.540 | ||
| FF × T | 0.754 | 0.034 | 0.544 | 0.166 | 0.382 | 0.307 | ||
| S × FF × T | 0.684 | 0.611 | 0.661 | 0.469 | 0.254 | 0.735 | ||
a,b Means within columns not sharing common superscripts are significantly different at the 5% level of probability.
Pearson correlations between feed intakes and starch and nitrogen disappearance rate ratios in the distal jejunum with parameters of nutrient utilisation are shown in Table 7. Distal jejunal disappearance rate ratios are negatively correlated with AME (r = −0.614), ME:GE (r = −0.572), N retention (r = −0.573) and AMEn (r = −0.586) to significant (P < 0.001) extents.
Table 7.
Pearson correlations between feed intakes and starch and nitrogen disappearance ratios in the distal jejunum (S:N-DJ) with parameters of nutrient utilisation (AME, ME : GE ratios, N retention and AMEn).
| Item | Feed intake | S:N-DJ | AME | ME:GE | N retention | AMEn |
|---|---|---|---|---|---|---|
| Feed intake | 1.000 | |||||
| S:N-J | r = 0.123 | 1.000 | ||||
| P = 0.406 | ||||||
| AME | r = −0.316 | r = −0.614 | 1.000 | |||
| P = 0.029 | P < 0.001 | |||||
| ME:GE | r = −0.376 | r = −0.572 | r = 0.971 | 1.000 | ||
| P = 0.008 | P < 0.001 | P < 0.001 | ||||
| N retention | r = −0.296 | r = −0.573 | r = 0.897 | r = 0.908 | 1.000 | |
| P = 0.041 | P < 0.001 | P < 0.001 | P < 0.001 | |||
| AMEn | r = −0.347 | r = −0.586 | r = 0.993 | r = 0.962 | r = 0.872 | 1.000 |
| P = 0.016 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
AME = apparent metabolizable energy; AMEn = N-corrected apparent metabolizable energy.
4. Discussion
Broilers offered intact pellets had greater feed intakes (9.88%) and weight gains (9.08%) but numerically poorer FCR in comparison to reground mash. It is established that compacted, intact pellets increases feed intake by facilitating prehension (Behnke, 1994, Behnke, 1996) but increases in feed intake and weight gain were pronounced in the present study. Furthermore, intact pelleted diets generated significantly inferior AME, AMEn, ME:GE and distal ileal starch digestibility coefficients (2.83%). Also, protein (N) digestibility coefficients in the jejunum (10.5%) and ileum (3.88%) were reduced by the transition from reground mash to intact pelleted diets. In the present study it appears that pronounced feed intake increases promoted by intact pellets resulted in 'overconsumption', thereby compromising nutrient utilisation. Svihus (2001) has considered the negative impacts of overconsumption and starch overload in broilers. This appears to have relevance in the present study because it is evident (Table 7) that feed intake was negatively correlated with AME (r = −0.316), ME:GE (r = −0.376), N retention (r = −0.296) and AMEn (r = −0.347) to significant extents which supports the overconsumption concept.
Raastad and Skrede (2003) drew attention to the likelihood that elevated steam-pelleting conditioning temperatures may compromise broiler performance and this proposal was subsequently supported by Creswell and Bedford (2006). The two conditioning temperatures used in the present study were quite extreme; nevertheless the outcomes are instructive. Increasing conditioning temperatures from 65 to 97°C significantly depressed FCR by 2.46% increased gizzard weights by 8.41%, depressed AME by 0.28 MJ and AMEn by 0.26 MJ. Also, the higher conditioning temperature tended to reduce distal ileal starch digestibility coefficients by 2.36% (P = 0.085) and distal ileal protein (N) digestibility coefficients by 3.18% (P = 0.091). The likelihood is that 97°C conditioning temperature generated a 'harder' pellet texture which is responsible for the heavier gizzards. However, the negative impact of increasing conditioning temperature on FCR and energy utilisation is noteworthy where reduced protein solubility probably was a factor. The nutritive value of sorghum is notoriously vulnerable to 'moist-heat' which is associated with the formation of disulphide bonds especially in the β- and γ-kafirin fractions located in the periphery of protein bodies in sorghum endosperm (Selle et al., 2010). The challenge in the feed-mill is to produce pellets of acceptable quality at moderate conditioning temperatures which is especially applicable to sorghum-based diets because sorghum has a relatively high starch gelatinisation temperature (Selle et al., 2013).
Broiler chickens offered sorghum #3-based diets outperformed their sorghum #5 counterparts. Sorghum #3 generated significant advantages in weight gain, FCR (subject to an interaction), AME, ME:GE and AMEn. In contrast, starch and protein (N) digestibility coefficients in both small intestinal sites were statistically similar. However, starch disappearance rates were higher with sorghum #5-based diets in both the jejunum and ileum but protein (N) disappearance rates were similar. Consequently, starch:protein disappearance rate ratios were compressed in sorghum #3 diets by 13.0% in the jejunum (2.35 versus 2.70; P < 0.001) and 9.59% in the ileum (1.98 versus 2.19; P < 0.001) in comparison to sorghum #5-based diets.
Sorghum #5 contained 21% more kafirin (61.5 versus 50.7 g/kg) than sorghum #3 and this difference was amplified by the higher dietary inclusion of sorghum #5. Thus sorghum #5 based diets contained 37% more kafirin (39.6 versus 29.0 g/kg) than those based on sorghum #3. Kafirin is a poor source of digestible amino acids due to its inherent hydrophobicity, the structure of the unique kafirin protein body and its amino acid profile (Selle, 2011). With the exception of leucine, kafirin contains low levels of essential amino acids, especially lysine (Hogan, 1918). However, of more concern is whether or not kafirin has a negative effect on starch and energy utilisation. The consensus appears to be that kafirin does have a negative impact (Rooney and Pflugfelder, 1986, Chandrashekar and Kirleis, 1998, Taylor, 2005, Wong et al., 2010), although dissenting opinions have been expressed (Gidley et al., 2011). Kafirin protein bodies and starch granules are located in close proximity in sorghum endosperm where they are both embedded in a glutelin protein matrix and this close proximity would facilitate any potential physical and chemical interactions between protein and starch in sorghum (De Mesa-Stonestreet et al., 2010).
Curiously, while the importance of starch--protein interactions is widely recognised (Rooney and Pflugfelder, 1986) but, as discussed by Truong et al. (2015), the responsible physico-chemical mechanisms have not been defined with any precision. However, with rice, Hamaker and Griffin (1993) found that proteins with disulfide bonds restrict granule swelling during gelatinisation in vitro and the swollen starch granules are not readily disrupted by shear forces. The implication is that kafirin may influence starch gelatinisation and pasting properties via disulphide bond formation in sorghum. It is not possible to be conclusive; however, it does appear that the higher kafirin content of sorghum #5 and sorghum #5-based diets contributed to its relative inferiority.
Phenolic compounds may also influence the properties of starch as Zhu (2015) concluded that non-covalent interactions involving starch and phenolic compounds influence physicochemical and nutritional properties of feedstuffs. However, total phenolics were similar in both sorghums (3.52 versus 3.59 mg GAE/g) in this study. Nevertheless, there are differences in concentrations of specific phenolic compounds as listed in Table 1. Phenolic compounds are a diverse group of phytochemicals ranging from highly-polymerised inert lignins to simple C7--C9 phenolic acids (Mangan, 1988) and their concentrations in sorghum are substantially more than maize and wheat (Bravo, 1998). Welsch et al. (1989) found phenolic compounds are capable of inhibiting Na+, K+, −ATPase or the 'sodium pump' which suggests that intestinal uptakes of nutrients including glucose via Na+-dependent transporters could be compromised. Barros et al. (2012) observed that phenolic acids interact with amylose and amylopectin and that amylose was more susceptible to such interactions. Kandil et al. (2012) reported that phenolic acids play an important role in the resistance of starch to hydrolysis in a study involving barley, maize, triticale and wheat but not sorghum. Ferulic, coumaric, gallic and syringic acids were the abundant phenolic acids in maize in this study.
In the Khoddami et al. (submitted for publication) study there were negative correlations between specific and total conjugated phenolic acids and parameters of energy utilisation in broilers offered sorghum-casein diets. Ferulic and p-coumaric acids were the most abundant conjugated phenolic acids. Total conjugated phenolic acids were negatively correlated with AME (r = −0.789; P = 0.062), ME:GE (r = −0.832; P = 0.040) and AMEn (r = −0.731; P = 0.099). Indeed, both ferulic (r = −0.831; P = 0.041), and p-coumaric (r = −0.826; P = 0.043) acids as well as total conjugated phenolic acids were negatively correlated with ME:GE to significant extents. Therefore, it is noteworthy that sorghum #5 contained 43% more (75.98 versus 53.10 µg/g) total conjugated phenolic acids than sorghum #3 in the present study. Conjugated phenolic acids are soluble and believed to be esterified to sugars and other low molecular mass compounds (Nicoletti et al., 2013). Thus there is the suggestion that these particular `non-tannin' phenolic compounds, which are not unique to sorghum, are deleterious to energy utilisation in poultry.
Sorghums #3 and #5 had similar starch levels (712 versus 700.0 g/kg) and proportions of amylose (26.4% versus 27.2%) and amylopectin (73.6% versus 72.8%). Nevertheless, RVA starch pasting profiles were different as sorghum #3 had higher peak (4,202 versus 3,750 cP) (1 cP = 0.01 g/cm/s) and final (7,132 versus 6,644 cP) RVA viscosities in comparison to sorghum #5. Protein is known to influence starch pasting profiles (Zhang and Hamaker, 1998, Ito et al., 2006) and one possibility is that the higher kafirin content in sorghum #5 may have depressed peak and final viscosities relative to sorghum #3 via starch--protein interactions.
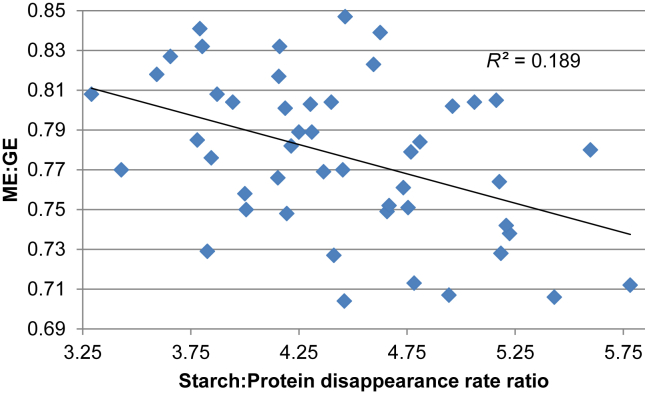
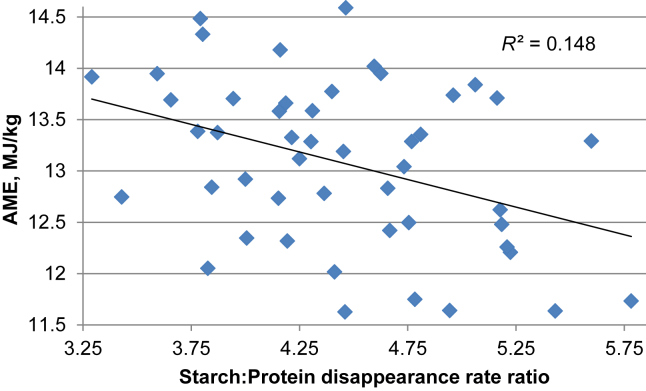
A consideration of starch and protein digestive dynamics in the present study is instructive and Liu et al. (2013) have demonstrated the importance of starch and protein kinetics in sorghum-based broiler diets. For example, there are significant negative relationships between distal jejunal starch and protein (N) disappearance rate ratios with ME:GE and AME as shown in Fig. 1 and Fig. 2. The two multiple linear regression equations derived from the disappearance rate ratios are as follows:
Fig. 1.
Linear relationship (r = 0.435; P = 0.002) between starch to protein disappearance rate ratios and ME:GE ratios in the distal jejunum.
Fig. 2.
Linear relationship (r = 0.385; P = 0.007) between starch:protein disappearance rate ratios and apparent metabolizable energy (AME) in the distal jejunum.
Both regression equations are significant with r = 0.623 (P < 0.001) for ME:GE ratios and r = 0.654 (P < 0.001) for AME. Thus there is the indication that energy utilisation will be enhanced when either protein is digested more rapidly or starch more slowly resulting in more compressed disappearance rate ratios. This intriguing topic is considered in more detail by Liu and Selle (2015) where an appropriately balanced supply of amino acids and glucose at sites of protein synthesis is given consideration. Collectively, the data generated by Liu et al. (2014) and Selle et al. (2014) from the inclusion of the sulfite reducing agent, sodium metabisulfite, in sorghum-based broiler diets supports the concept that slowly digestible starch is advantageous for broiler chickens. The present study suggests that the reciprocal, or rapidly digestible protein, is also advantageous.
The relative post-enteral availability of amino acids and glucose is dependent upon the digestion of protein and starch, intestinal uptakes of amino acids and glucose and the catabolism of amino acids and glucose in the enterocytes of the gut mucosa. Avian enterocytes are capable of catabolising either amino acids (specifically glutamate/glutamine) or glucose as energy substrates to drive gut function (Watford et al., 1979). Intuitively, extents of amino acid and glucose catabolism would be influenced by the relative availability of the substrates. Enting et al. (2005) suggested that slowly digestible starch might "prevent the use of amino acids as an energy source for the gut wall”; thereby, increasing their post-enteral availability for protein accretion. Rapidly digestible protein may have an analogous effect in that amino acids would be absorbed in more proximal small intestinal sites where more glucose is available as an alternative energy substrate. This is relevant as energy from glucose is probably more efficiently derived than from glutamic acid/glutamine in porcine gut mucosa (Fleming et al., 1997).
5. Conclusions
The superiority of sorghum #3 over sorghum #5 appeared to stem probably from lower concentrations of kafirin and possibly from lower concentrations conjugated phenolic acids in sorghum #3. It is perhaps instructive that peak and final RVA starch viscosities were higher in sorghum #3 than sorghum #5. In this study bilateral bioavailability of starch and protein appears to have had pivotal effects on energy utilisation which was enhanced by coupling rapidly digestible protein with slowly digestible starch as assessed in the distal jejunum.
Acknowledgements
The authors would like to acknowledge the Poultry CRC for providing Ms Ha Truong with a PhD scholarship and RIRDC Chicken-meat for funding the sorghum steam-pelleting temperatures project.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Barros F., Awika J.M., Rooney L.W. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J Agric Food Chem. 2012;60:11609–11617. doi: 10.1021/jf3034539. [DOI] [PubMed] [Google Scholar]
- Behnke K.C. Proceedings, Maryland Nutrition Conference for Feed Manufacturers. University of Maryland; College Park, MD: 1994. Factors affecting pellet quality; pp. 44–54. [Google Scholar]
- Behnke K.C. Feed manufacturing technology: current issues and challenges. Anim Feed Sci Technol. 1996;62:49–57. [Google Scholar]
- Boogers I., Plugge W., Stokkermans Y.Q., Duchateau A.L. Ultra-performance liquid hromatographic analysis of amino acids in protein hydrolyates using an automated pre-column derivatisation method. J Chromatogr. 2008;1189:406–409. doi: 10.1016/j.chroma.2007.11.052. [DOI] [PubMed] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Chandrashekar A., Kirleis A. Influence of protein on starch gelatinization in sorghum. Cereal Chem. 1998;65:457–462. [Google Scholar]
- Cohen S.A. Amino acid analysis using precolumn derivatisation with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. In: Cooper C., Packer N., Williams K., editors. vol. 159. Humana Press; Totowa, NJ, USA: 2001. pp. 39–47. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- Creswell D., Bedford M. High pelleting temperatures reduce broiler performance. Proc Aust Poult Sci Symp. 2006;18:1–6. [Google Scholar]
- De Mesa-Stonestreet N.J., Alavi S., Bean S.R. Sorghum proteins: the concentration, isolation, modification, and food applications of kafirins. J Food Sci. 2010;75:R90–R104. doi: 10.1111/j.1750-3841.2010.01623.x. [DOI] [PubMed] [Google Scholar]
- Enting H., Pos J., Weurding R.E., Veldman A. Starch digestion rate affects broiler performance. Proc Aus Poult Sci Symp. 2005;17:17–20. [Google Scholar]
- Fleming S.E., Zambell K.L., Fitch M.D. Glucose and glutamine provide similar proportions of energy to mucosal cells of rat small intestine. Am J Physiol Gastro Liver Physiol. 1997;273:G968–G978. doi: 10.1152/ajpgi.1997.273.4.G968. [DOI] [PubMed] [Google Scholar]
- Gidley M., Flanagan B., Shaepe K., Sopade P. Starch digestion in monogastrics--mechanisms and opportunities. Rec Adv Anim Nutr Aust. 2011;18:207–213. [Google Scholar]
- Hamaker B.R., Griffin V.K. Effect of disulfide bond-containing protein on rice starch gelatinization and pasting. Cereal Chem. 1993;70:377–380. [Google Scholar]
- Hamaker B.R., Mohamed A.A., Habben J.E., Huang C.P., Larkins B.A. Efficient procedure for extracting maize and sorghum kernel proteins reveals higher prolamin contents than the conventional method. Cereal Chem. 1995;72:583–588. [Google Scholar]
- Hill F.W., Anderson D.L. Comparison of metabolisable energy and productive energy determinations with growing chicks. J Nutr. 1958;64:587–603. doi: 10.1093/jn/64.4.587. [DOI] [PubMed] [Google Scholar]
- Hogan A.G. The nutritive properties of kafirin. J Biol Chem. 1918;33:151–159. [Google Scholar]
- Ito A., Hattori M., Yoshida T., Watanabe A., Sato R., Takahashi K. Regulatory effect of amino acids on the pasting behaviour of potato starch is attributable to its binding to the starch chain. J Agric Food Chem. 2006;54:10191–10196. doi: 10.1021/jf061823w. [DOI] [PubMed] [Google Scholar]
- Kandil A., Li J., Vasantan T., Bressler D.C. Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification. J Agric Food Chem. 2012;60:8444–8449. doi: 10.1021/jf3000482. [DOI] [PubMed] [Google Scholar]
- Khoddami A., Truong H.H., Liu S.Y., Roberts T.H., Selle P.H. Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilization in broiler chickens. Anim Feed Sci Technol. 2015 [accepted for publication] [Google Scholar]
- Liu S.Y., Selle P.H., Cowieson A.J. The kinetics of starch and nitrogen digestion regulate growth performance and nutrient utilisation of broilers fed coarsely ground, sorghum-based diets. Anim Prod Sci. 2013;53:1033–1040. [Google Scholar]
- Liu S.Y., Selle P.H., Khoddami A., Roberts T.H., Cowieson A.J. Graded inclusions of sodium metabisulphite in sorghum-based diets: II. Modification of starch pasting properties in vitro and beneficial impacts on starch digestion dynamics in broiler chickens. Anim Feed Sci Technol. 2014;190:68–78. [Google Scholar]
- Liu S.Y., Selle P.H. A consideration of starch and protein digestive dynamics in chicken-meat production. World׳s Poult Sci J. 2015;71:297–310. [Google Scholar]
- Mahasukhonthachat K., Sopade P.A., Gidley M.J. Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. J Cereal Sci. 2010;51:392–401. [Google Scholar]
- Mangan J. Nutritional effects of tannins in animal feeds. Nutr Res Rev. 1988;1:209–231. doi: 10.1079/NRR19880015. [DOI] [PubMed] [Google Scholar]
- Nicoletti I., Martini D., de Rossi A., Taddei F., D׳Egidio M.G., Corradini D. Identification and quantification of soluble free, soluble conjugated, and insoluble bound phenolic acids in durum wheat (Triticum turgidum L. var. durum) and derived products by RP-HPLC on a semimicro separation scale. J Agric Food Chem. 2013;61:11800–11807. doi: 10.1021/jf403568c. [DOI] [PubMed] [Google Scholar]
- Raastad N., Skrede A. Feed pelleting temperature influences growth performance of broiler chickens. Proc Eur Symp Poult Nutr. 2003:115–116. [Google Scholar]
- Rooney L., Pflugfelder R. Factors affecting starch digestibility with special emphasis on sorghum and corn. J Anim Sci. 1986;63:1607–1623. doi: 10.2527/jas1986.6351607x. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Walker A.R., Bryden W.L. Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry. Aust J Exp Agric. 2003;43:475–479. [Google Scholar]
- Selle P.H., Cadogan D.J., Li X., Bryden W.L. Implications of sorghum in broiler chicken nutrition. Anim Feed Sci Technol. 2010;156:57–74. [Google Scholar]
- Selle P.H. The protein quality of sorghum. Proc Aust Poult Sci Symp. 2011;22:147–160. [Google Scholar]
- Selle P.H., Liu S.Y., Cowieson A.J. Sorghum: an enigmatic grain for chicken-meat production. In: Parra Patricia C., editor. Sorghum: production, growth habits and health benefits. Nova Publishers Inc; Hauppauge, NY: 2013. pp. 1–44. [Google Scholar]
- Selle P.H., Liu S.Y., Cai J., Caldwell R.A., Cowieson A.J. Graded inclusions of sodium metabisulphite in sorghum-based diets: I. Reduction of disulphide cross-linkages in vitro and enhancement of energy utilisation and feed conversion efficiency in broiler chickens. Anim Feed Sci Technol. 2014;190:59–67. [Google Scholar]
- Svihus B. Research note: a consistent low starch digestibility observed in pelleted broiler chicken diets containing high levels of different wheat varieties. Anim Feed Sci Technol. 2001;92:45–49. [Google Scholar]
- Taylor J.R.N. Non-starch polysaccharides, protein and starch: form function and feed -- highlights on sorghum. Proc Aus Poult Sci Symp. 2005;17:9–16. [Google Scholar]
- Truong H.H., Liu S.Y., Selle P.H. Starch utilisation in chicken-meat production: the foremost influential factors. Anim Prod Sci. 2015 . 10.1071/AN15056. [Google Scholar]
- Wallace J.C., Lopes M.A., Palva E., Larkins B.A. New methods for extraction and quantitation of zeins reveal a high content of γ-zein in modified opaque-2 maize. Plant Physiol. 1990;92:191–196. doi: 10.1104/pp.92.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H.A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979;178:589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch C.A., Lachance P.A., Wasserman B.P. Dietary phenolic compounds: inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr. 1989;119:1698. doi: 10.1093/jn/119.11.1698. [DOI] [PubMed] [Google Scholar]
- Wong J.H., Marx D.B., Wilson J.D., Buchanan B.B., Lemaux P.G., Pedersen J.F. Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain. Plant Sci. 2010;179:598–611. [Google Scholar]
- Zhang G., Hamaker B.R. Low a-amylase starch digestibility of cooked sorghum flours and the effect of protein. Cereal Chem. 1998;75:710–713. [Google Scholar]
- Zhu F. Interactions between starch and phenolic compounds. Trends Food Sci Technol. 2015;43:129–143. [Google Scholar]