Abstract
Trace elements are essential dietary components for livestock species. However, they also exhibit a strong toxic potential. Therefore, their fluxes through the animal organism are tightly regulated by a complex molecular machinery that controls the rate of absorption from the gut lumen as well as the amount of excretion via faeces, urine and products (e.g., milk) in order to maintain an internal equilibrium. When supplemented in doses above the gross requirement trace elements accumulate in urine and faeces and, hence, manure. Thereby, trace element emissions represent a potential threat to the environment. This fact is of particular importance in regard to the widely distributed feeding practice of pharmacological zinc and copper doses for the purpose of performance enhancement. Adverse environmental effects have been described, like impairment of plant production, accumulation in edible animal products and the water supply chain as well as the correlation between increased trace element loads and antimicrobial resistance. In the light of discussions about reducing the allowed upper limits for trace element loads in feed and manure from livestock production in the European Union excessive dosing needs to be critically reconsidered. Moreover, the precision in trace element feeding has to be increased in order to avoid unnecessary supplementation and, thereby, heavy metal emissions from livestock production.
Keywords: Trace element, Livestock, Homeostasis, Pharmacological supplementation, Accumulation, Environment
1. Introduction
The increase in its sustainability is the key challenge for animal production in the 21st century. In this context animal nutrition is of considerable importance. The effectiveness by which the organism is able to transform feed biomass into edible animal products determines the total amount of feed which is necessary as well as the majority of emissions from animal production (Niemann et al., 2011, Gerber et al., 2013, Windisch et al., 2013). Trace elements play a special role in this context as they are not only highly essential dietary components but also heavy metals with strong toxic potential (Goldhaber, 2003). Their fluxes through the animal organism are tightly regulated and oversupplied amounts are excreted via faeces and urine (Windisch, 2002). Thereby, they have the potential to accumulate in manure and the environment, which demands great responsibility when using trace element supplements. This review focusses on trace elements in livestock feeding and environmental concerns arising thereof. The ambivalent nature of trace elements will be discussed as well as the mechanisms which regulate their fluxes within the animal organism, in order to highlight the connection between livestock feeding and element emissions. Furthermore, the consequences of an environmental accumulation of trace elements as a result of certain supplementation strategies are highlighted. Finally, potential approaches to reduce heavy metal emissions from animal production are discussed.
2. Between deficiency and toxicity – the ambivalent nature of essential trace elements
Most of the essential trace elements belong to the group of transition metals. Therefore, in nature they exist either in an ionic state or bound to certain molecular ligands (Weller et al., 2014). This physicochemical property is the base of their essentiality for the animal. Within the organism, trace elements interact with certain biomolecules, thereby maintaining their function (Fraga, 2005). Prominent examples include hemoglobin and alkaline phosphatase, which need to bind iron and zinc ions, respectively (Muginova et al., 2005, Camaschella, 2015). Therefore, under the terms of physiological deficiency of one or more trace elements metabolic imbalances arise, which foster the development of pathological deficiency diseases. These phenotypes are associated with unspecific symptoms like impaired growth development, feed refusal, impaired fertility etc., highlighting the ubiquitous importance of essential trace elements in maintaining metabolic function. Although, the more common phenotype in men and animals, especially livestock, is a latent deficiency which is characterized by a reduced trace element status as well as subsequent metabolic imbalance and, at the same time, the absence of visible symptoms of deficiency disease (Holt et al., 2012). It has been shown that even short periods of insufficient alimentary supply are able to promote significant physiological changes (e.g., Brugger et al., 2014). Such data clearly highlights the necessity to use trace element supplements in practical livestock husbandry in order to ensure animal wellbeing and productivity.
On the other hand, like various other transition metals (e.g., cadmium and mercury) essential trace elements also have a strong toxic potential as heavy metals. The mode of action of trace element toxicity equals that of trace element essentiality. In fact, in both cases it is due to their interaction with biomolecules (Goldhaber, 2003). Although under the impact of toxic overload, this interaction is no longer specific and not controlled by homeostatic counter regulation. Furthermore, increased trace element accumulation in animal tissues has been proven to promote oxidative stress which is another important aspect of their toxicity (Valko et al., 2005). The ambivalent nature of trace elements as essential nutrient compounds and toxic heavy metals becomes obvious by taking a closer look on the example of iron. On the one hand, iron is the most important cofactor for oxygen transport and transfer within the organism (Finch and Lenfant, 1972). Additionally, it is part of several other bio-factors like cytochromes and catalase, thereby, representing a key factor in essential pro- and anti-oxidative reactions within animal cells (Dlouhy and Outten, 2013). On the other hand, as a strong pro-oxidative agent, iron is involved in the production of reactive oxygen species through Fenton reactions. Iron overload is associated with a non-physiological increase in cellular stress (Puntarulo, 2005). Apart from this, iron is an important proliferative factor of pathological microorganisms like Escherichia coli (Schaible and Kaufmann, 2004). Therefore, iron fluxes have to be tightly regulated in health and disease in order to maintain a steady state of iron contents behind the gut barrier (Andrews and Schmidt, 2007).
3. Basic principles of trace element homeostasis in mammals
As a result of the ambivalent nature of trace elements, mammal evolution has evolved a complex network of regulative mechanisms to control trace element fluxes through the mammal organism (Lichten and Cousins, 2009, Colvin et al., 2010). This is particularly important as the homeostatic regulation machinery extends the range between satisfied trace element requirements and toxicity, thereby stabilizing trace element load behind the gut barrier under the terms of fluctuations in alimentary supply and physiological status. Furthermore, this has a great benefit for practical livestock feeding as it enables the usage of safety margins to compensate for such fluctuations.
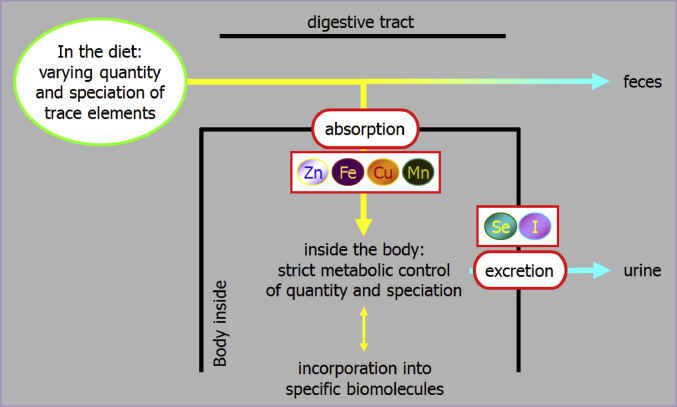
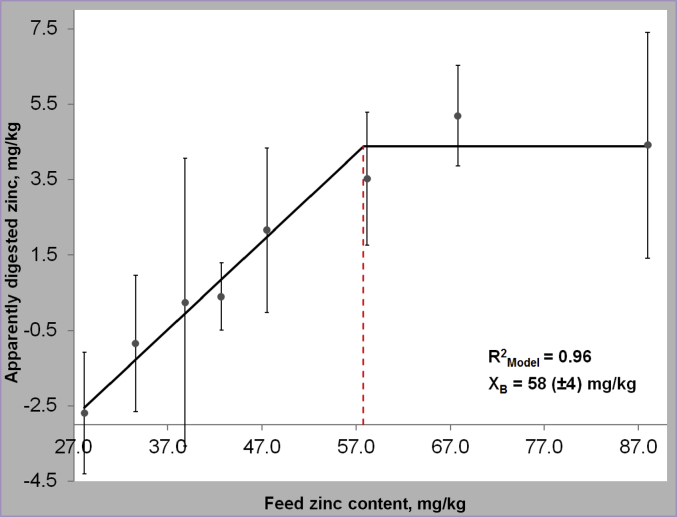
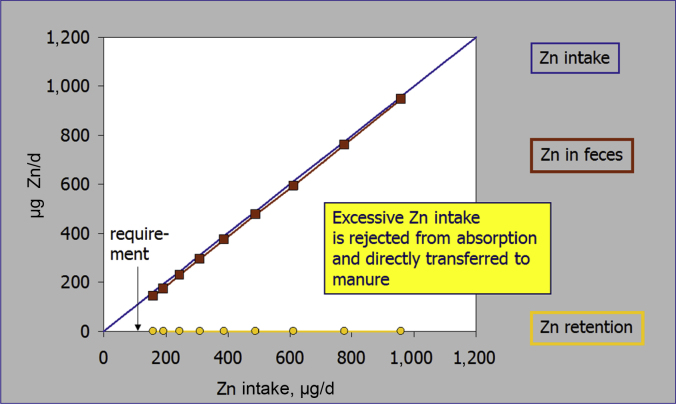
Homeostatic control of trace elements depends on two basic mechanisms – the control of absorption from the gastrointestinal lumen as well as the elimination of overload behind the gut barrier by endogenous excretion mainly via the kidney. Essential trace elements can be grouped by being either regulated via absorption (e.g., Zn, Cu, Mn, Fe) or excretion (e.g., Se, I) (Windisch, 2002) (Fig. 1). The behavior of homeostatic response to varying dietary supply (deficient, sufficient and oversupply) is very consistent. Under the impact of deficient alimentary supply, the response of homeostatic measures (bio-factors which are affected by either absorption or excretion) exhibit a direct dose–response to varying alimentary supply. In contrast, from the point of sufficient trace element supply on, these parameters exhibit a plateau in response with further rising alimentary supply (Kirchgessner et al., 1997, Brugger et al., 2014; Fig. 2). This becomes evident by monitoring trace element accumulation in animal products (e.g., egg, milk) which also exhibit a plateau in behavior above the level of satisfied requirements (Schwarz and Kirchgessner, 1975, Paulicks and Kirchgessner, 1994). Earlier published data on the response of zinc homeostasis dependent measures in weaned piglets clearly highlight this basic principle of trace element homeostasis. Below the gross zinc requirement threshold, the apparently digested amount of feed zinc linearly declined with further decreasing alimentary supply levels (Brugger et al., 2014). At the same time expression of the ZIP 4 (SLC39A4) gene, which represents the major transport pathway for luminal zinc into the enterocyte (Lichten and Cousins, 2009), showed an inverse relationship by increasing from the gross zinc requirement threshold with further declining alimentary Zn supply (Brugger et al., 2015). However, above the gross zinc requirement threshold (~60 mg Zn/kg feed; NRC, 2012) both factors exhibited a plateau in response with increasing alimentary supply. This nicely illustrates how the organism adapts to varying supply levels by increasing absorption efficiency in times of deficient supply and, on the contrary, by refusing active uptake of trace element amounts supplemented above the gross requirement threshold. Hence, every milligram (mg) trace element supply which exceeds the bodies׳ net demand accumulates in faeces or urine and subsequently in manure (Windisch and Kirchgessner, 1994, Kirchgessner et al., 1997; Fig. 3). Therefore, it can be concluded that excessive trace element supply for the purpose of demand coverage is useless from a physiological point of view.
Fig. 1.
Basic principles of trace element homeostasis in mammal organisms. The organism provides a steady state of trace element contents behind the gut barrier by regulation of absorption and excretion (Windisch, 2002).
Fig. 2.
Response of apparently digested feed zinc to varying levels of dietary zinc supply as an example for the response behavior of biomarkers of trace element homeostasis (Brugger et al., 2014). The red line marks the transition point from alimentary zinc deficiency to sufficient feeding at a brutto zinc requirement threshold (XB) of 58 mg Zn/kg feed.
Fig. 3.
Response of faecal zinc and zinc retention to varying zinc intake (Windisch and Kirchgessner, 1995).
4. The more, the better? – usefulness of excessive dietary trace element supplementation
The main feed sources for livestock nutrition are plant based. In case of monogastric species, this means that the bioavailable amount of trace elements is rather low. In an earlier study, we were able to show that short-term feeding of a practical corn-soybean diet without zinc supplementation (native Zn content: 28 mg/kg) to weaned piglets caused average daily body Zn losses via faecal excretion of 2.7 mg/kg feed intake (Brugger et al., 2014). Therefore it is inevitable to use trace element supplements in practical feeding. Due to an uncertainty in regard to the absolute bioavailability of trace element species, large safety margins above the gross requirement are used in order to compensate for fluctuations in alimentary supply and physiological status (NRC, 1994, NRC, 2000, NRC, 2001, NRC, 2005, NRC, 2006, NRC, 2011, NRC, 2012). For example, in the European Union a total zinc content of 150 mg/kg is allowed in mixed feed (European Union, 2005) which accounts for the 2.5-fold gross zinc requirement of growing piglets (60 mg/kg), as stated by the National Research Council of the United States of America (NRC, 2012). Although, looking at certain feeding practices in monogastric animals especially in regard to zinc and copper, the usage of much higher contents seems to be widely distributed. Such so-called ”pharmacological doses” have been shown to exhibit positive effects on the incidence of diarrhea in piglets (e.g., Poulsen, 1995, Højberg et al., 2005). Furthermore, growth promoting effects were evident (Hahn and Baker, 1993, Coffey et al., 1994, Apgar et al., 1995, Smith et al., 1997, Cromwell et al., 1998, Windisch et al., 1998, Windisch et al., 2001). The mode of action is largely due to the bactericidal nature of high doses of Zn and Cu. The increased zinc/copper loads within the gastrointestinal tract correlate with a decrease in the abundance of pathological bacteria and an increased availability of soluble nutrients at the gut barrier (Vahjen et al., 2011, Pieper et al., 2012, Starke et al., 2013). Although, such effects are only evident for a limited time frame (~2 weeks) in the early stages of weaning and disappear in the further course of piglet rearing. Moreover, in some cases the effects even turned into the negative. This has been explained with an overall weakening of the gut microflora which seems to exert some vital functions for the host organism (Shelton et al., 2009).
Apart from potential negative consequences in regard to production efficiency such feeding strategies have diverse undesirable effects on the environment. As has been highlighted above, the organism denies entrance of Zn and Cu in significant amounts above the requirement threshold by drastic decrease in absorption efficiency. This means that every mg that exceeds the bodies׳ net demand is transferred to manure. By spreading these organic fertilizers on agricultural land heavy metals potentially accumulate within the soil. There is clear evidence for livestock nutrition as a major promoter of Zn and Cu accumulation in agricultural areas (Wuana and Okieimen, 2011, UBA, 2004). Therefore, high Zn and Cu doses potentially express their bactericidal effects in the soil. A proper functioning soil microbiome is inevitable for normal plant development. Hence, toxic overload of Zn and Cu in soils impairs plant production (Rout and Das, 2003). Moreover, as the mobility of trace element pools can be rather high such increased amounts also represent a threat for the water supply chain and, hence, human health (Asada et al., 2010).
Another negative aspect of increased trace element loads in soil is the possibility of transfer into plants. It has been demonstrated that plants grown on soils with Cu contents between 32 and 640 mg/kg DM accumulate Cu in plant tissue between 8.1 and 82.6 mg/kg DM (Sauvé et al., 1996). Such plants are potentially inappropriate for animal and human nutrition in regard to published recommended upper limits of Cu contents in feed (pigs older than 12 weeks: 25 mg/kg, cattle and sheep: 15 mg/kg; European Union, 2005).
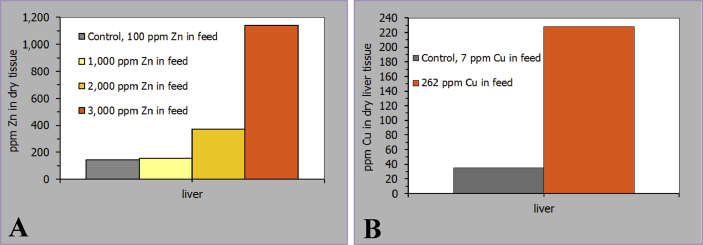
In terms of consumer safety the possibility of increased tissue accumulation of Zn and Cu when supplemented in pharmacological doses might be relevant. It is generally accepted that trace elements compete for binding motives at transport peptides. Taken the example of pharmacological Zn supplementation this means that although the main apical Zn importer is down-regulated there is still some unregulated influx of Zn using other cation transport mechanisms (Martin et al., 2013). Indeed, such influx is not as effective as via Zn specific peptides but is seems to be efficient enough to accumulate Zn in certain tissues (e.g., liver, kidney) (Schell and Kornegay, 1996, Carlson et al., 1999, Martin et al., 2013; Fig. 4A). The same effects were also evident for excessive Cu doses (Fry et al., 2012; Fig. 4B). As liver and kidney are potentially edible animal products such feeding practice might represent a threat for food safety. This becomes particularly evident by comparing the recommended tolerable upper limits for Zn and Cu uptake (25 to 45 mg Zn/d as suggested by SCF, 2003a, IOM, 2002, Food Standards Agency, 2003, FAO/WHO, 2002 and 5 to 10 mg Cu/d as suggested by FNB, 2001, EVM, 2003, SCF, 2003b) and reported amounts in liver and kidney of animals receiving pharmacological doses [400 and 1,500 mg Zn/kg dry liver tissue in animals receiving 2,000 and 3,000 mg Zn/kg, respectively (Schell and Kornegay, 1996); 228 mg Cu/kg dry liver tissue in animals receiving 262 mg Cu/kg; Fig. 4A and 4B (Fry et al., 2012)]. Including such Zn/Cu enriched animal products in human diets would obviously be critical in regard to maintaining daily uptake levels within tolerable limits. Therefore, in the light of possible risks for consumer safety pharmacological dosing in practical animal nutrition needs to be urgently reconsidered.
Fig. 4.
Response of dry tissue (A) zinc and (B) copper content to varying alimentary zinc and copper supply, respectively (Schell and Kornegay, 1996, Fry et al., 2012).
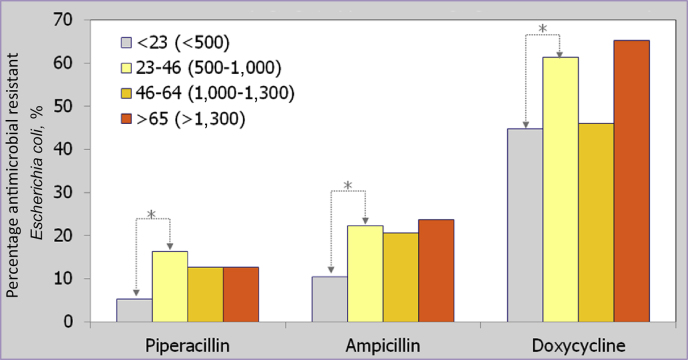
Another aspect that gained considerable attention during the last years is the interesting positive correlation between increased Zn and Cu loads in manure and the abundance of antimicrobial resistant bacteria therein. For example, Hölzel et al. (2012) explicitly demonstrated that Zn contents in manure above 23 ppm, which accounts for approximately ≥ 500 ppm in dry matter, significantly increased the percentage amount of resistant E. coli strains (relative to 100% of E. coli bacteria in manure; Fig. 5). This effect has also been shown to be evident within the gastro-intestinal tract of piglets where significantly increased copy numbers of tetracycline and sulphonamide resistant enterobacteria were evident (Vahjen et al., 2014). The mode of action behind these effects seems to be an upregulation of certain efflux pumps within the plasma membrane of bacteria. This response represents an effective defence strategy of bacteria against antimicrobial substances (Blair et al., 2014). The mechanism seems to be also effective against Zn and Cu toxification (Rosen, 1999). Therefore, it represents an unspecific, regulative adaption to increased abundance of antimicrobial substances. Moreover, it explains the earlier mentioned reports on the loss in health and growth promoting effects of pharmacological Zn and Cu doses. Obviously, the microbial community adapts after some time to the ongoing overload of the system with Zn and Cu by an increased excretion via efflux pumps. This is another hint towards the uselessness to positively modulate biological systems with trace element overload at least on a mid/long-term scale.
Fig. 5.
Relationship between zinc load in pig manure and the percentage abundance of phenotypic antimicrobial resistant Escherichia coli (Hölzel et al., 2012). Values in parentheses represent estimated dry matter zinc contents of manure.
5. The need for higher precision in trace element feeding
The negative aspects of excessive trace element loads raise the question whether there is an actual necessity for intervention in the animal production sector. The current situation in Europe might shed some light on this question. Excessive dietary Zn and Cu supplementation for the purpose of growth promotion is strictly prohibited within the European Union and pharmacological doses are only legal under the terms of veterinary intervention (European Union, 2005). Taking a closer look on Zn and Cu contents in manure for pig and cattle farms in Central Europe reveals up to 4-fold higher Zn contents (~1,200 vs. ~300 mg Zn/kg DM) and up to 8-fold higher Cu contents (~400 vs. ~50 mg Cu/kg DM) in pig manure relative to cattle manure (Hackenberg et al., 1996, Müller, 1997, Bannick et al., 2001, Müller and Ebert, 2002, Kühnen and Goldbach, 2002, UBA, 2004, Kickinger et al., 2008, Hölzel et al., 2012). This indicates that there might be a tendency for excessive dosing in monogastric feeding rather than ruminant feeding. Obviously, the intake of antimicrobial Zn and Cu doses by ruminants would have severe negative consequences for the integrity of the rumen microbiome. Therefore this practice makes more sense for monogastric species where most of the microbial fermentation takes place in the large intestine. The reported contents in pig slurry lie between the allowed upper limits defined for bio-waste (Zn: 400 mg/kg DM; Cu: 100 mg/kg DM; BMJV, 2012a) and sewage sludge (Zn: 2,500 mg/kg DM; Cu: 800 mg/kg DM; BMJV, 2012b). Hence, manure with considerable toxic potential might be produced in pig farms in central Europe and subsequently distributed on agricultural land. The fact that such data is even evident in a region with strict legal regulations in regard to excessive trace element dosing raises the question on the situation in countries without such regulations.
Currently, there is a passionate discussion within the European Union about reducing the allowed upper limits for trace element load in feed and manure of livestock as an attempt for intensified soil and water protection. In this context the European Food Safety Authority (EFSA) recently published a scientific opinion on the matter which recommends a significant reduction of zinc loads in feed (EFSA, 2014). This is supposed to somehow being transferred into European law on a mid-term scale. Therefore, it is foreseeable that in the near future there will be a reduction of the allowed upper limits in feed and manure, especially for Zn and Cu. The discussed new thresholds range for Zn between 200 and 450 mg/kg manure dry matter and for Cu between 60 and 90 mg/kg manure dry matter. A mere avoidance of pharmacological doses would not be enough to push the current contents below these thresholds. Data from Kickinger et al. (2010) clearly indicates that feeding according to the current legal regulations (European Union, 2005) still promotes Zn and Cu contents around 600 and 140 mg/kg manure dry matter, respectively. To further reduce the trace element load in manure from monogastric species׳ feed contents have to be adjusted much closer to the published gross requirement thresholds (NRC, 1994, NRC, 2011, NRC, 2012). Indeed, this will drastically reduce the possible range of safety margins in practical livestock feeding. Therefore, practical animal nutrition will be forced to use all available measures that stabilize feed trace element bioavailability. On the one hand, this accounts for the use of phytase supplements which already are of considerable importance for monogastric feeding (Pallauf and Rimbach, 1997). On the other hand, only trace element supplements with the highest bioavailability should be used. Although, this is currently very difficult as there is uncertainty in regard to the bioavailability of trace elements under varying feeding conditions. This is due to a lack in precise definition of the physical and chemical properties of native and supplemented trace element species which have an impact on their bioavailability. Precise feed table information is missing that specifies the amount of digestible trace element contents in feed and feed supplements. This information is already available for phosphorus (P). A closer look on German feed tables indicates precise values for the amounts of digestible P in various feedstuffs and supplements in the presence and without phytase supplementation (http://datenbank.futtermittel.net/). Animal nutrition research must extent these datasets in the near future on other minerals especially trace elements. This fosters the need for appropriate experimental models that can be used to measure the impact of dietary intervention on element availability under basal conditions (e.g., Brugger et al., 2014).
6. Conclusion
The animal organism actively counter-regulates excessive dietary supplementation of trace elements, leading to heavy metal enrichment in manure. This may promote environmental accumulation with various negative consequences arising thereof. In order to reduce heavy metal emissions from animal production, the use of pharmacological doses has to be avoided and the precision of trace element feeding has to be significantly increased. This can only be achieved by providing appropriate feed table information on trace element digestibility under varying feeding conditions.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgment
The authors like to express their gratitude to Peter Loibl, M.Sc., for valuable advice to the manuscript.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Andrews N.C., Schmidt P.J. Iron homeostasis. Ann Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- Apgar G.A., Kornegay E.T., Lindemann M.D., Notter D.R. Evaluation of copper sulfate and a copper lysine complex as growth promoters for weanling swine. J Animal Sci. 1995;73:2640–2646. doi: 10.2527/1995.7392640x. [DOI] [PubMed] [Google Scholar]
- Asada K., Toyota K., Nishimura T., Ikeda J., Hori K. Accumulation and mobility of zinc in soil amended with different levels of pig-manure compost. J Environ Sci Health B. 2010;45:285–292. doi: 10.1080/03601231003704580. [DOI] [PubMed] [Google Scholar]
- Bannick C.G., Bieber E., Böken H., Brach M., Brackemann H., Ehrmann H. Grundätze und Maßnahmen für eine vorsorgeorientierte Begrenzung von Schadstoffeinträgen in landbaulich genutzten Böden. UBA Texte. 2001;59 ISSN 0722–186x. [Google Scholar]
- BMJV . Bundesgesetzblatt; Jahrgang: 2012. Verordnung zur A¨nderung der Bioabfallverordnung, der Tierische Nebenprodukte- Beseitigungsverordnung und der Düngemittelverordnung. 2012; Teil I; Nr. 17. [Google Scholar]
- BMJV . Bundesgesetzblatt; Jahrgang: 2012. Gesetz zur Neuordnung des Kreislaufwirtschafts- und Abfallrechts. 2012 Teil I Nr. 10. [Google Scholar]
- Brugger D., Buffler M., Windisch W. Development of an experimental model to assess the bioavailability of zinc in practical piglet diets. Arch Animal Nutr. 2014;68:73–92. doi: 10.1080/1745039X.2014.898392. [DOI] [PubMed] [Google Scholar]
- Brugger D., Hanauer M., Windisch W. Using piglets as an animal model: preliminary results on the impact of short-term marginal zinc deficiency on zinc acquisition and storage dependent gene expression in jejunal and colonic tissue. Perspect Sci. 2015;3:30–31. [Google Scholar]
- Blair J.M., Richmond G.E., Piddock L.J. Multidrug efflux pumps in gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014;9:1165–1177. doi: 10.2217/fmb.14.66. [DOI] [PubMed] [Google Scholar]
- Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- Carlson M.S., Hill G.M., Link J.E. Early and traditional weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J Anim Sci. 1999;77:1199–1207. doi: 10.2527/1999.7751199x. [DOI] [PubMed] [Google Scholar]
- Coffey R.D., Cromwell G.L., Monegue H.J. Efficacy of a copper-lysine complex as a growth promotant for weanling pigs. J Anim Sci. 1994;72:2880–2886. doi: 10.2527/1994.72112880x. [DOI] [PubMed] [Google Scholar]
- Colvin R.A., Holmes W.R., Fontaine C.P., Maret W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- Cromwell G.L., Lindemann M.D., Monegue H.J., Hall D.D., Orr D.E. Tribasic copper chloride and copper sulfate as copper sources for weanling pigs. J Anim Sci. 1998;76:118–123. doi: 10.2527/1998.761118x. [DOI] [PubMed] [Google Scholar]
- Dlouhy A.C., Outten C.E. In: Metal ions in life sciences. Metallomics and the cell. Banci L., editor. Springer Publishing; Dordrecht (The Netherlands): 2013. pp. 241–278. [Google Scholar]
- EFSA Scientific opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. 2014;12:3668. [Google Scholar]
- European Union Regulation (EC) No. 1495/2005 of the European Parliament and of the Council of 8. Sept. 2005 amending the conditions for authorisation of a number of feed additives belonging to the group of trace elements. OJEU. 2005;48 L233/8–L233/10. [Google Scholar]
- EVM. Expert group on vitamins and minerals Safe upper levels for vitamins and minerals. Copper. May 2003:187–196. [Google Scholar]
- FAO/WHO . FAO; Bangkok (Thailand): 2002. Human vitamin and mineral requirements. Report of a joint FAO/WHO expert consultation. [Google Scholar]
- Finch C.A., Lenfant C. Oxygen transport in man. N Engl J Med. 1972;286:407–415. doi: 10.1056/NEJM197202242860806. [DOI] [PubMed] [Google Scholar]
- FNB . National Academy Press; Washington DC (USA): 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Food and Nutrition Board/Institute of Medicine (IOM) [PubMed] [Google Scholar]
- Food Standards Agency . 2003. Safe upper limits for vitamins and minerals.http://www.foodstandards.gov.uk/multimedia/pdfs/vitmin2003.pdf [accessed 08.08.15] [Google Scholar]
- Fraga C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol Asp Med. 2005;26:235–244. doi: 10.1016/j.mam.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Fry R.S., Ashwell S., Lloyd K.E., O׳Nan A.T., Flowers W.L., Stewart K.R. Level and source of dietary copper affects small intestine morphology, duodenal lipid peroxidation, hepatic oxidative stress and mRNA expression of hepatic copper regulatory proteins in weanling pigs. J Anim Sci. 2012 doi: 10.2527/jas.2011-4403. [DOI] [PubMed] [Google Scholar]
- Gerber P.J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J. Food and Agriculture Organization of the United Nations (FAO); Rome (Italy): 2013. Tackling climate change through livestock – a global assessment of emissions and mitigation. [Google Scholar]
- Goldhaber S.B. Trace element risk assessment: essentiality vs. toxicity. Reg Tox Pharmacol. 2003;38:232–242. doi: 10.1016/s0273-2300(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Hackenberg S., Wegener H.-R., Eurich-Menden B. Verlag Institut für Bodenkunde und Bodenerhaltung; 1996. Herkunft der Schadstoffe in Komposten: Schadstoffgehalte in Komposten und anderen Dünge- und Bodenverbesserungsmitteln; Vor- und Nachteile beim Einsatz von Komposten in der Land- und Forstwirtschaft sowie im Landschafts- und Weinbau; (Literaturstudie) [Google Scholar]
- Hahn J.D., Baker D.H. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci. 1993;71:3020–3024. doi: 10.2527/1993.71113020x. [DOI] [PubMed] [Google Scholar]
- Holt R.R., Uiu-Adams J.Y., Keen C.L. In: Present Knowledge in Nutrition. Erdman J.W., Macdonald I.A., Zeisel S.H., editors. Wiley-Blackwell; Hoboken, New Jersey: 2012. pp. 521–539. [Google Scholar]
- Højberg O., Canibe N., Poulsen H., Hedemann M.S., Jensen B.B. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol. 2005;71:2267–2277. doi: 10.1128/AEM.71.5.2267-2277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel C.C., Müller C., Harms K.S., Mikolajewski S., Schäfer S., Schwaiger K. Heavy metals in liquid pig manuer in light of bacterial antimicrobial resistance. J Envres Environ Res. 2012 doi: 10.1016/j.envres.2012.01.002. [DOI] [PubMed] [Google Scholar]
- IOM . National Academy Press; Washingtion DC (USA): 2002. Dietary reference intakes vitamin A, vitamin K, Arsenic, Borone, Chromium, Copper, Iodine, Iron, Mangenese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Institute of Medicine (IOM)/Food and Nutrition Board (FNB) [PubMed] [Google Scholar]
- Kickinger T., Humer J., Aichberger K.,H.W., Windisch W. Survey on zinc and copper contents in dung from Austrian livestock production. BODENKULTUR. 2008;59:101–110. [Google Scholar]
- Kickinger T., Würzner H., Windisch W. Zinc and copper in feeds, slurry and soils from Austrian pig fattening farms feeding commercial complete feed or feed mixtures produced on-farm. Bodenkultur. 2010;60:47–58. [Google Scholar]
- Kirchgessner M., Gabler S., Windisch W. Homeostatic adjustments of selenium metabolism and tissue selenium to widely varying selenium supply in 75Se labeled rats. J Anim Physiol Anim Nutr. 1997;78:20–30. [Google Scholar]
- Kühnen V., Goldbach H.E. Institut für Pflanzenernährung, Rheinische Friedrichs-Wilhelms-Universität Bonn (Germany); 2002. Schwermetallbilanzen verschiedener Betriebstypen: Eintragswege, Flüsse, Minderungspotential. Forschungsbericht Nr. 118. [Google Scholar]
- Lichten L.A., Cousins R.J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Martin L., Lodemann U., Bondzio A., Gefeller E.M., Vahjen W., Aschenbach J.R. A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J Nutr. 2013 doi: 10.3945/jn.113.177881. [DOI] [PubMed] [Google Scholar]
- Muginova S.V., Zhavoronkova A.M., Shekhovtsova T.N. Potentialities and prospects of the use of alkaline phosphatases for determining metal ions. J Anal Chem. 2005;60:218–233. [Google Scholar]
- Müller G. Nur noch geringer Eintrag anthropogener Schwermetalle in den Bodensee – neue Daten zur Entwicklung der Belastung der Sedimente. Naturwissenschaften. 1997;84:37–38. [Google Scholar]
- Müller C., Ebert T. Schwermetall-Einträge durch Wirtschaftsdünger von 1996 bis heute. Ergebnisse aus dem bayerischen Bodenbeobachtungsprogramm. Schriftenr. 2002;58:635–639. [Google Scholar]
- Niemann H., Kuhla B., Flachowsky G. Perspectives for feed-efficient animal production. J Anim Sci. 2011;89:4344–4363. doi: 10.2527/jas.2011-4235. [DOI] [PubMed] [Google Scholar]
- NRC . Nat. Acad. Press; Washington, D.C., USA: 1994. Nutrient requirements of poultry. [Google Scholar]
- NRC . Nat. Acad. Press; Washington, D.C., USA: 2000. Nutrient requirements of beef cattle. [Google Scholar]
- NRC . Nat. Acad. Press.; Washington D.C., USA: 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- NRC . The National Academics Press; Washington, D.C.: 2005. Mineral tolerance of animals. [Google Scholar]
- NRC . Nat. Acad. Press; Washington, D.C., USA: 2006. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. [Google Scholar]
- NRC . Nat. Acad. Press; Washington, D.C., USA: 2011. Nutrient requirements of fish and shrimp. [Google Scholar]
- NRC . 11th ed. Nat. Acad. Press; Washington, D.C., USA: 2012. Nutrient requirements of swine. [Google Scholar]
- Pallauf J., Rimbach G. Nutritional significance of phytic acid and phytase. Arch Anim Nutr. 1997;50:301–319. doi: 10.1080/17450399709386141. [DOI] [PubMed] [Google Scholar]
- Paulicks B.R., Kirchgessner M. Influence of zinc supply on feed intake and performance of layers. Arch fur Geflugelkd. 1994;58:186–191. [Google Scholar]
- Pieper R., Vahjen W., Neumann K., Van Kessel A.G., Zentek J. Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned piglets. J Anim Physiol Anim Nutr. 2012 doi: 10.1111/j.1439-0396.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- Poulsen H. Zinc oxide for weanling piglets. Act Agric Scand Sect A Anim Sci. 1995;45:159–167. [Google Scholar]
- Puntarulo S. Iron, oxidative stress and human health. Mol Asp Med. 2005;26:299–312. doi: 10.1016/j.mam.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Rosen B.P. The role of efflux in bacterial resistance to soft metals and metalloids. Essays Biochem. 1999;34:1–15. doi: 10.1042/bse0340001. [DOI] [PubMed] [Google Scholar]
- Rout G.R., Das P. Effect of metal toxicity on plant growth and metabolism: I. Zinc. Agronomie. 2003;23:3–11. [Google Scholar]
- Sauvé S., Cook N., Hendershot W.H., McBride M.B. Linking plant tissue concentrations and soil copper pools in urban contaminated soils. Environ Pollut. 1996;94:153–157. doi: 10.1016/s0269-7491(96)00081-4. [DOI] [PubMed] [Google Scholar]
- SCF . Scientific Committee on Food SCF/CS/NUT/UPPLEV/62; 2003. Opinion of the scientific committee on food on the tolerable upper intake level of zinc. Final, 19 March 2003 (expressed on 5 March 2003) [Google Scholar]
- SCF . Scientific Committee on Food SCF/CS/NUT/UPPLEV/57; 2003. Opinion of the Scientific Committee on Food on the tolerable upper intake level of copper. Final, 27 March 2003 (expressed on 5 March 2003) [Google Scholar]
- Schaible U.E., Kaufmann S.H.E. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Schwarz W.A., Kirchgessner M. Excretion of zinc in lactating cows receiving various supply of zinc. Arch fur Tierernahrung. 1975;25:597–608. doi: 10.1080/17450397509423228. [DOI] [PubMed] [Google Scholar]
- Schell T.C., Kornegay E.T. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-Methionine, Zn-Lysine or ZnSO4. J Anim Sci. 1996;74:1584–1593. doi: 10.2527/1996.7471584x. [DOI] [PubMed] [Google Scholar]
- Shelton N.W., Jacob M.E., Tokach M.D., Nelssen J.L., Goodband R.D., Dritz S.S. Effect of copper sulfate, zinc oxide and neoterramycin on weanling pig growth and antibiotic resistance rate for Escherichia coli. Kans Agric Exp Sta Prog Rep. 2009;1020:73–79. [Google Scholar]
- Smith J.W., Tokach M.D., Goodband R.D., Nelssen J.L., Richert B.T. Effects of the interrelationship between zinc oxide and copper sulfate on growth performance of early-weaned pigs. J Anim Sci. 1997;75:1861–1866. doi: 10.2527/1997.7571861x. [DOI] [PubMed] [Google Scholar]
- Starke I.C., Zentek J., Vahjen W. Ex vivo-growth response of porcine small intestinal bacterial communities to pharmacological doses of dietary zinc oxide. Plos ONE. 2013 doi: 10.1371/journal.pone.0056405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UBA . UBA Berling; Berlin (Germany): 2004. Erfassung von Schwermetallströmen in landwirtschaftlichen Tierproduktionsbetrieben. [Google Scholar]
- Vahjen W., Pieper R., Zentek J. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. J Anim Sci. 2011;89:2430–2439. doi: 10.2527/jas.2010-3270. [DOI] [PubMed] [Google Scholar]
- Vahjen W., Pietruszynska D., Zentek J. Pharmacological concentrations of dietary zinc oxide increase enterobacterial antibiotic resistance against tetracycline and sulfonamides in weaned piglets. Proc Soc Nutr Physiol. 2014;23:104. [Google Scholar]
- Valko M., Morris H., Cronin M.T.D. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12 doi: 10.2174/0929867053764635. 1161–1208(48) [DOI] [PubMed] [Google Scholar]
- Weller M., Overton T., Rourke J., Armstrong F. vol. 6. Oxford University Press; Oxoford (UK): 2014. (Inorganic chemistry). [Google Scholar]
- Windisch W. Interaction of chemical species with biological regulation of the metabolism of essential trace elements. Anal Bioanal Chem. 2002;372:421–425. doi: 10.1007/s00216-001-1117-6. [DOI] [PubMed] [Google Scholar]
- Windisch W., Kirchgessner M. Zur Messung der homöostatischen Anpassung des Zinkstoffwechsels an eine defizitäre und hohe Zinkversorgung nach alimentärer 65Zn-Markierung. 1. Mitteilung: Zum Effekt einer unterschiedlichen Zinkversorgung auf den quantitativen Zinkumsatz im Stoffwechsel adulter Ratten. J Anim Physiol Anim Nutr. 1994;71:98–107. [Google Scholar]
- Windisch W., Schwarz F.J., Gruber K., Kirchgessner M. Effect of pharmacological dietary doses of zinc oxide on performance and faecal characteristics of weanling piglets. Agribiol Res. 1998;51:277–285. [Google Scholar]
- Windisch W., Gotterbarm G.G., Roth F.X. Effect of potassium diformate (FormiTM LHS) in combination with high dietary doses of copper on production performance of weaning piglets. Arch Anim Nutr. 2001;54:87–100. doi: 10.1080/17450390109381969. [DOI] [PubMed] [Google Scholar]
- Windisch W., Fahn C., Brugger D., Deml M., Buffler M. Strategies for sustainable animal nutrition. Züchtungskunde. 2013;85:40–53. [Google Scholar]
- Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011;2011 Article ID 402647. [Google Scholar]