Abstract
It is well known that phenotype of animals may be modified by the nutritional modulations through epigenetic mechanisms. As a key and central component of epigenetic network, DNA methylation is labile in response to nutritional influences. Alterations in DNA methylation profiles can lead to changes in gene expression, resulting in diverse phenotypes with the potential for decreased growth and health. Here, I reviewed the biological process of DNA methylation that results in the addition of methyl groups to DNA; the possible ways including methyl donors, DNA methyltransferase (DNMT) activity and other cofactors, the critical periods including prenatal, postnatal and dietary transition periods, and tissue specific of epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals.
Keywords: Critical period, DNA methylation, Epigenetics, Nutrition, Tissue specific
1. Introduction
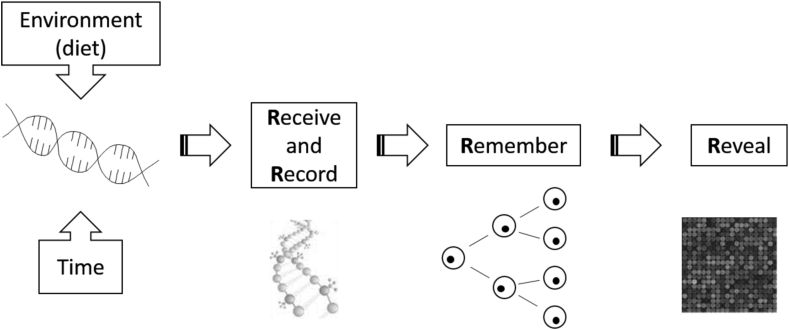
It is well known that many organisms may respond to different environmental/nutritional factors by exhibiting phenotypic plasticity. One epitome is that honeybees grow to be either queens or workers depending on whether they are fed royal jelly or beebread (Kucharski et al., 2008). Another paradigmatic example is that of the Agouti mouse model where the maternal methyl dietary content affects the coat color of the rodent offspring (Wolff et al., 1998, Waterland and Jirtle, 2003, Dolinoy et al., 2006). These observations demonstrate that phenotype of animals may be modified by the nutritional modulations through epigenetic mechanisms, meaning dietary exposures can have long-term consequences for growth and health (McKay and Mathers, 2011). Mathers (2008) developed a model of Four Rs to explain the mechanism of nutritional epigenomics (Fig. 1).
Fig. 1.
The conceptual model of the Four Rs of nutritional epigenomics. The changed epigenomics markings resulted from environmental (nutritional) exposures are Received, Recorded, Remembered and Revealed (Mathers, 2008).
Over recent years, there is increasing evidence that environmental (nutritional) stimuli can modify DNA methylation and therefore, affect phenotypic expression of genes (Lillycrop et al., 2005, van Straten et al., 2010, Farias et al., 2015, Farkas et al., 2015, Day et al., 2015). These work either empirical – possible changes in epigenetic marks were investigated in response to diet factor, or theoretical – hypothesized mechanisms were researched through which the nutrients could affect epigenetic markings. DNA methylation is a key component of an epigenetic network (Kucharski et al., 2008) and has long been considered as central to the field of epigenetics. DNA methylation process contributed the most significance of the "epi" prefix to "epigenetics". Hence, the mechanisms, critical periods, and the tissue specific of nutritional modulation of DNA methylation were reviewed here.
2. DNA methylation
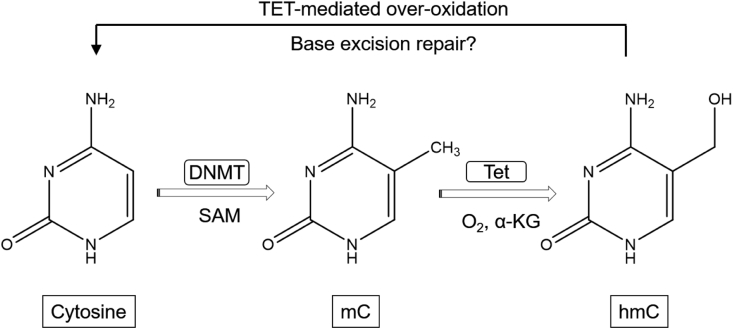
Epigenetic marks on the genome can be copied from one cell generation to the next, which may change gene expression, but not primary DNA sequence (Reik, 2001). DNA methylation is a biological process that results in the addition of methyl groups to DNA. It contributes to the epigenetic network that controls the gene expression. DNA methylation occurs in many key physiological processes that including X chromosome inactivation, imprinting and the silencing of germline-specific genes and repetitive elements. Methylation marks on DNA are focused on the 5׳ position of cytosine residues of a Cytidine-Guanine dinucleotide (CpG, where p stands for a phosphate group between the two nucleotides). The majority of cytosine residues within CpG dinucleotides are methylated, but some of them are normally unmethylated, e.g., those in CpG islands within the promoter regions of housekeeping genes. Both CpG-rich and CpG-poor regions exist but occur infrequently than expected in DNA (Jones and Liang, 2009). DNA methylation is mediated by de novo DNA methyltransferases (DNMT) to yield 5-methylcytosine (mC), primarily at cytosine-phosphate-guanine (CpG) dinucleoside sites (Fig. 2) (Day and Sweatt, 2010, Day and Sweatt, 2011, Day et al., 2015). There are three DNMT with different functions. DNMT3A and DNMT3B are responsible for addition of methyl group de novo, e.g., during embryogenesis, whereas DNMT1 is responsible for maintenance of DNA methylation patterns during cell replication. In addition, DNMT1 copying the methylation marks on the parental strand to the daughter strand after replication, DNMT3A and DNMT3B complete the methylation process and correct errors left by DNMT1 (Jones and Liang, 2009).
Fig. 2.
Cytosine is methylated in vivo by DNA methyltransferase (DNMT), which uses S-Adenosylmethionine (SAM) as an electrophilic methyl source, to produce 5-methylcytosine (mC) at cytosine-phosphate-guanine (CpG) sites in double-stranded DNA. Maintenance DNMT may then methylate the complimentary cytosine to produce double-stranded CpG methylation. An otherwise stable epigenetic mark, mC can be oxidized by the a-ketoglutarate (a-KG) -dependent ten-eleven translocation (Tet) family of dioxygenases to yield 5-hydroxymethylcytosine (hmC), which is the first step in removing the methyl as an epigenetic mark (Day et al., 2015).
Nevertheless, mC can be oxidized by the a-KG-dependent Tet family of dioxygenases to yield 5-hydroxymethylcytosine (hmC) (Fig. 2) (Tahiliani et al., 2009, Day et al., 2015). The hydroxymethyl mark itself is stable and exists in relatively elevated levels in the brain. However, it is also the first step in active demethylation, and either over-oxidation by the Tet family of proteins to aldehyde or carboxylate products or deamination mechanisms followed by base excision repair then erases cytosine alkylation (Ito et al., 2011, Song et al., 2013).
3. Mechanisms of nutritional modulation of DNA methylation
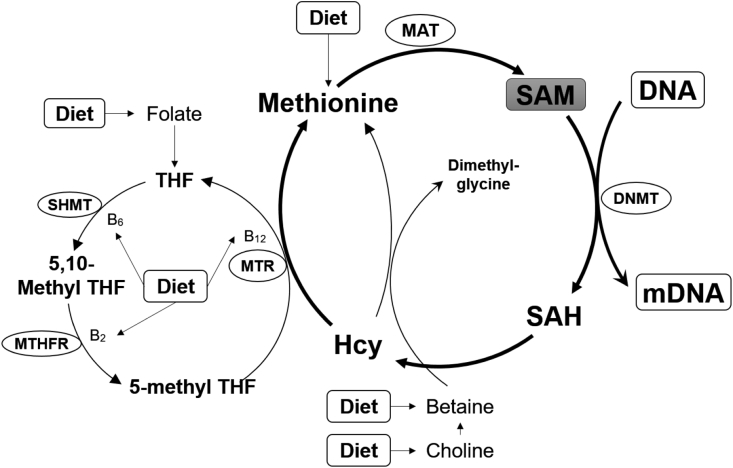
There are now mounting evidences supporting that nutrients may modify the pattern of DNA methylation, either at the global scale or at locus-specific sites (Vucetic et al., 2010, Bogdarina et al., 2010, Jousse et al., 2011, Dudley et al., 2011, Altmann et al., 2012). There are three possible ways that nutrition influences patterns of DNA methylation (Fig. 3): first, provision of substrates being necessary for proper DNA methylation; second, provision of cofactors modulating the enzymatic activity of DNMT; third, changing activity of the enzymes regulating the one-carbon cycle. Importantly, all three mechanisms are mutually compatible and may operate together in time.
Fig. 3.
Possible ways that nutrition influences patterns of DNA methylation (Revised from McKay and Mathers, 2011). MAT = methionine adenosyltransferase; SAM = S-adenosyl methionine; SHMT = serine hydroxymethyltransferase; THF = tetrahydrofolate; DNMT = DNA methyltransferase; MTR = 5-methyltetragydrofolate-homocysteine methyltransferase; MTHFR = methylentetrahydrofolate reductase; Hcy = homocysteine; SAH = S-adenosylhomocysteine; mDNA = methylated DNA.
3.1. Methyl donors from diet
As the universal methyl-donor for DNA and protein methyltransferases (Loenen, 2006), S-Adenosylmethionine (SAM) is synthesized in the methionine cycle from several precursors present in the diet (McKay and Mathers, 2011, Feil and Fraga, 2012). All these precursors, including methionine, folate, choline, betaine and vitamins B2, B6 and B12, enter at different sites in the methionine pathway and contribute to the net synthesis of SAM. Hence, reduced availability of methyl donors should result in low SAM synthesis and global DNA hypomethylation, and vice versa.
In fact, numerous information about the effects of methyl donors on DNA methylation is available from studies with animal models (Pogribny et al., 2008, Cordero et al., 2013, Cordero et al., 2014, Amarasekera et al., 2014, Llanos et al., 2015, Farkas et al., 2015). Accordingly, diets deficient in methyl donors result in global DNA hypomethylation in rodents (Pogribny et al., 2008, Pogribny et al., 2009, Mehedint et al., 2010, Craciunescu et al., 2010). Conversely, maternal diet supplemented with methyl donors increases DNA methylation in specific loci (Waterland, 2006, Waterland et al., 2008, de Vogel et al., 2011, Li et al., 2015, Farias et al., 2015).
Although methyl donors can alter DNA methylation patterns, little is known about the necessary doses and the exact durations of dietary exposure or depletion contributing changes in epigenetic marks. There are too many uncertainties about the effects of different doses and duration of dietary exposure on DNA methylation (Cravo et al., 1994, Cravo et al., 1998). Therefore, it merits more systematic studies to provide more unequivocal findings.
Besides, other studies showed a more complex scenario. Low protein or 50% global malnutrition during gestation in mice led to both hyper- and hypo-methylation at specific loci in the offspring (van Straten et al., 2010). Likewise, undernutrition in utero in humans resulted in both hypo- and hyper-methylation of different specific loci (Heijmans et al., 2008, Tobi et al., 2009, Waterland et al., 2010). It is not reported whether the amount of methyl-donors is reduced in these specific studies, but it is commonly accepted that undernutrition correlates with reduced methyl-donor availability. Therefore, there is not a simple correlation between methyl donor concentration and DNA methylation. Other mechanisms might contribute to set patterns of DNA methylation in cells.
3.2. DNA methyltransferase activity
It is well known that DNMT require SAM as a cofactor for their full activation. Methyl donors from the diet may contribute to modulate DNMT activity by changing the intracellular concentration of SAM. Besides an indirect regulation of DNA methylation patterns through modulation of SAM pools, several compounds can also directly influence the expression or activity of DNMT (Mukherjee et al., 2015). Evidence of a competitive inhibition of DNMT activity has been demonstrated for the (−)- epigallocatechin-3-gallate (EGCG), polyphenol in green tea, or the genistein present in soybean (Fang et al., 2007, Vanhees et al., 2011, Xie et al., 2014, Zampieri et al., 2015). Myricetin can also decrease DNA methylation by inhibiting SssI DNMT (Lee et al., 2005).
The EGCG can re-express many transcriptionally silenced genes through inhibition of DNMT1 enzymatic activity (Berletch et al., 2008, Kato et al., 2008), and was shown to decrease growth and induce apoptosis in renal cell carcinoma by re-expressing tissue factor pathway inhibitor-2 (TFPI-2) and decreasing its promoter hypermethylation (Gu et al., 2009). The EGCG also decreases the promoter methylation of other genes such as hTERT and CDX2, thereby helping in tumor suppression (Qi and Ohh, 2003, Hirata et al., 2009). Genistein was also found to decrease DNMT activity resulting in transcriptional activation of genes such as p16INK4a, RAR beta, MGMT, PTEN, and CYLD in prostate cancer and RAR beta 2 in cervical cancer (Fang et al., 2005, Kikuno et al., 2008). Genistein-mediated modulation of methylation of protumorigenic miRNA-1260b and its targets sFRP1 and Smad4 inhibits prostate cancer cell proliferation, invasion, and TCF reporter activity (Hirata et al., 2014). Other dietary phenolic compounds, including hesperetin, naringin, apigenin, and luteolin, can also modulate DNA methylation by indirectly regulate DNMT activity through regulating the ratio of SAM and SAH (Lee et al., 2005, Fang et al., 2007, Mukherjee et al., 2015), although these compounds are not as efficient as EGCG in directly inhibiting DNMT activity. Curcumin was found to bring about global hypomethylation in the MV4–11 leukemia cell line by inhibiting DNMT (Liu et al., 2009). It was also found to regulate DNMT1 negatively in ovarian cancer and melanoma (Abusnina et al., 2011, Parashar et al., 2012).
3.3. Activity of enzymes from methionine cycle
Vitamins B2, B6 and B12 are cofactors involved in the regulation of the catalytic activity of enzymes from the folate cycle, thus determining SAM bioavailability. Specifically, vitamin B6 is a cofactor to serine hydroxymethyltransferase (SHMT) in the conversion of tetrahydrofolate (THF) into 5,10-methylene THF. Vitamin B2 is a precursor to FAD, which is a cofactor to methylenetetrahyrofolate reductase (MTHFR) in the conversion of 5,10-methylene THF to 5-methyl THF. Vitamin B12 is a cofactor of the 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) that catalyzes the conversion of homocysteine (Hcy) into methionine, the direct precursor of SAM. Therefore, bioavailability of these cofactors may influence DNA methylation by modifying the activity of the one-carbon cycle and the production of SAM (Feil and Fraga, 2012).
Thus, it is conceivable that supplementing diets with these vitamins will contribute to the maintenance or establishment of DNA methyl marks. Farias et al. (2015) showed for the first time that HCT116, LS174T, and SW480 cells grown without adequate folate significantly impaired colonosphere forming ability. These differences were accompanied by concomitant changes to DNA methyltransferase (DNMT) enzyme expression and DNA methylation levels. Li et al. (2015) reported that folic acid can downregulate tau protein phosphorylation by inhibiting the demethylation reactions of PP2A. High folic acid concentrations (20 and 40 µmol/L) increased ratio of SAM to SAH and cell viability.
4. Critical periods of nutritional modulation of DNA methylation
4.1. Prenatal period
Early embryogenesis in mammals is the most critical period for the establishment of the epigenome. Failure to complete these programs in time might be irreversible and lead to permanent dysregulation of gene expression (Lumey et al., 1993, Gallou-Kabani and Junien, 2005). Importantly, this is a period especially vulnerable to environmental cues, such as nutrition, that can disrupt the correct establishment of epigenetic marks that, once established, remain highly stable (Burdge and Lillycrop, 2010). This is the reason why nutritional challenges during early development stage might have such long-term effects.
There is greater evidence about the impact of maternal nutrition on epigenetic marks in the progeny (Dominguez-Salas et al., 2014, Cannon et al., 2014, Mozhui et al., 2015). Protein restriction is frequently used model for maternal malnutrition. For example, Feeding a low protein diet to pregnant rats resulted in global or locus specific changes in DNA methylation (Rees et al., 2000, Altmann et al., 2013). Reported genes (or loci) affected by protein malnutrition include the glucocorticoid receptor (GR), peroxisome proliferator-activated receptor alpha (PPARa) and liver X receptor-alpha (Lxra) in liver (Lillycrop et al., 2007, Lillycrop et al., 2008, van Straten et al., 2010, van Straten et al., 2012, Altmann et al., 2013); the hepatocyte nuclear factor-4-alpha (Hnf4a) in islet cells (Sandovici et al., 2011); the AT(1b) angiotensin receptor in adrenal gland (Bogdarina et al., 2007, Bogdarina et al., 2010); the orexigenic/anorexigenic genes neuropeptide Y (Npy) and proopiomelanocortin C (Pomc) in hypothalamus (Coupé et al., 2010); and the leptin gene (Lep) in adipose tissue (Jousse et al., 2011).
In a similar rat model, 50% caloric restriction decreased the abundance of H3K4Me2 at the IGF1 locus of liver from the newborn offspring. This epigenetic modification alters IGF1 expression and contributes to post-natal catch-up growth (Fu et al., 2009, Tosh et al., 2010). Moderate caloric restriction (30%) decreased methylation in fetal kidney during early stages of gestation, whereas it increased DNA methylation by the end of gestation (Unterberger et al., 2009). Maternal high fat diet may also alter DNA methylation and gene expression in the offspring. For example, maternal high fat feeding during gestation changed methylation and gene expression of opioid and dopamine related genes in the brain of offsprings (Vucetic et al., 2010). Likewise, offspring from mothers fed a high fat diet showed reduced methylation, and increased expression, of the cyclin-dependent kinase inhibitor 1A (Cdkn1a) during neonatal liver development (Dudley et al., 2011). In another study, global and gene-specific (dopamine reuptake transporter, m-opioid receptor and preproenkephalin) promoter DNA hypomethylation were observed in the brains of offspring from dams that were fed a high-fat diet (Marco et al., 2014). In oocytes of obese mice that were induced by a high-fat diet, the leptin promoter DNA was significantly hypermethylated, but the PPARa was hypomethylated (Ge et al., 2014). More recently, maternal high-fat diet during gestation and lactation induced global DNA hypermethylation, including fatty acid and cholesterol metabolism-related genes (Yu et al., 2015).
Importantly, changes in DNA methylation correlate with altered gene expression. Therefore, such nutritionally-induced changes in DNA methylation may explain, at least in part, metabolic dysfunction in the adult. there is now sufficient evidence to support that maternal malnutrition may induce permanent alterations in gene expression through epigenetic modifications.
4.2. Postnatal period
It is now becoming clear that nutritional effects on the epigenome are not limited to the intrauterine life, but extend to early postnatal period. The early postnatal nutrition is a vital determinant of adult animal׳s health. Rat pups receiving high-protein diets via gastrostomy display enhanced short-term weight gain, insulin resistance, and modified expression of adipocyte glucose transporter type 4 and liver glucose transporter type 2 (des Robert et al., 2009). The high-carbohydrate rats have increased expression of pancreatic duodenal homeobox factor-1, protein kinase 2, and phosphatidylinositol 3-kinase (Srinivasan et al., 2000, Petrik et al., 2001, Srinivasan et al., 2001). Postnatal nutritional modulation altered the gene expression, suggesting that epigenetics may contribute to programming of animals. As expected, Neonatal overfeeding in rats led to rapid early weight gain, resulting in a metabolic syndrome phenotype. Accompanying, increased methylation of the promoter of the hypothalamic anorexigenic factor proopiomelanocortin (Pomc) was observed (Plagemann et al., 2009). It is the first time demonstrates a nutritionally acquired alteration of the methylation pattern and, consequently, the regulatory 'set point’ of a gene promoter that is critical for body weight regulation. Likewise, in a follow-up study, neonatal over nutrition increased mean methylation of the insulin receptor promoter in the hypothalamus (Plagemann et al., 2010). This alteration might additionally contribute to induce hypothalamic insulin resistance, thus contributing to the development of metabolic syndrome. Neonatal overfeeding in the mouse also provoked permanent modifications in DNA methylation in the liver from adult individuals, as assessed by CpG island microarrays (Pentinat et al., 2010). Furthermore, the obese phenotype from neonatal-overfeeding-mothers can be passed onto the subsequent generation, which is possibly associated with hypothalamic leptin resistance (Wang et al., 2015). Future epigenetic studies in animal models with postnatal dietary manipulation will be necessary to facilitate identification of dietary approaches that can be applied in the postnatal period.
4.3. Dietary transition period
Epigenetic variations are not restricted to pre- or post-natal period but may occur throughout an individual life-course (Grayson et al., 2014). Epigenetic research involving monozygotic twins, who are born with identical genomes, yet exhibit different phenotypes later in life, are an excellent example of how impactful environmental factors can be in the developmental plasticity of organisms. Such epigenetic variations accumulate over a long period and may ultimately influence phenotypic outcomes (growth and health). However, the amount of data linking adult dietary interventions with epigenetic modifications is much more limited than that for dietary interventions during pre- and post-natal development. Nevertheless, nutrition can still have long lasting effects, especially during long-term "Dietary Transitions" (Jiménez-Chillarón et al., 2012), which can be defined as a period in which animals are exposed over a prolonged period of time (ranging from weeks–months in animals) to a diet characterized by malnutrition. This type of transitions may cause subtle long-lasting (or permanent) changes in gene expression. Epigenetically associated changes in these gene expressions, although potentially reversible, tend to be stable and contribute to the age-dependent decrease of growth and health (Li et al., 2011a, Chalkiadaki and Guarente, 2012, Jiménez-Chillarón et al., 2012).
For example, Chronic high fat feeding in mice (from weaning to > 15 wk) altered patterns of DNA methylation within the promoter regions of the mu-opioid receptor in both the VTA and NAc in the brain (Vucetic et al., 2011, Pitman and Borgland, 2015). Likewise, post-weaning diet (high content of fat and carbohydrate) influence the hepatic intracisternal A particle (IAP) methylation patterns in mice (Warzak et al., 2015). Furthermore, Postweaning high-fat diet predisposes the mouse offspring for obesity, and hypomethylation of proopiomelanocortin (POMC) promoter in the hypothalamus occurred (Zheng et al., 2015). Likewise, male mice fed a low protein diet from weaning to age 9 to 12 wk induced numerous changes of DNA methylation, as assessed by microarray analysis, in livers from the offspring. Among positive loci, an enhancer of the lipid regulatory protein PPARa was identified (Carone et al., 2010). Again, a sustained dietary change on the epigenome of isogenic mice with methyl-supplemented diet over 6 generations increased DNA methylation variation in liver (Li et al., 2011b), suggesting that some of the induced changes are heritable. Moreover, caloric restriction influences expression of specific genes associated to age-related diseases and senescence through modulating the enrichment binding of HDAC1 to their promoter regions (Ferguson-Smith and Patti, 2011, Li et al., 2011b).
5. Tissue specific of nutritional modulation of DNA methylation
There is growing evidence that DNA methylation patterns are tissue specific (Ollikainen et al., 2010, Schneider et al., 2010, Herzog et al., 2013, Wan et al., 2015). Moreover, it is well documented that DNA methylation varies between tissue types (Illingworth et al., 2008, Christensen et al., 2009, Maegawa et al., 2010, Thompson et al., 2010). Recent technical advances have made it possible to monitor DNA methylation patterns on a genome-wide scale (Meissner et al., 2008). Unbiased analysis of genome wide methylation patterns reveals that DNA methylation is not always negatively correlated with gene expression, challenging the traditional view that DNA methylation represses gene expression. In addition, positive tissue-specific differentially methylated sites (T-DMRs) are likely to play a functional role in regulating tissue associated gene expression (Wan et al., 2015). It is evident that a clear tissue-specific dependence of methylation levels exists at the differentially-methylated-regions (DMR) in exon 9 of IGF2 (IGF2 ex9 DMR) for peripheral blood leucocytes and whole cord blood (Schneid et al., 1993, Reik et al., 1994, Guo et al., 2008, Ollikainen et al., 2010, Buckberry et al., 2012, Nordin et al., 2014, Ideraabdullah et al., 2014). The IGF2 ex9 DMR have highly variable tissue-specific methylation levels at imprinting associated DMR (Tierling et al., 2010). However, according to the current knowledge about the regulation of this locus and its postulated role in regulating fetal growth and development, factors contributing to tissue-specific DMR differences at birth are less clear (Guo et al., 2008, Dilworth et al., 2010). Schneider et al. (2010) studied the methylation patterns of seven neighboring CpG sites in the APC promoter region. They found the methylation patterns appeared to differ among tissues, indicating a true tissue-dependent differences in nucleosome positioning (Dodd et al., 2007) and/or binding of specific transcription factors (Pant et al., 2004).
For the effects of nutritional modulation on DNA methylation, a hepatic global DNA hypomethylation and brain hypermethylation was occur together in methyl deficient rats (Pogribny et al., 2006, Pogribny et al., 2008), suggesting differential tissue responsiveness to the same methyl deficient insult. At first, a long-term administration of a diet deficient in methionine, choline, and folic acid resulted in the progressive hypomethylation of DNA in livers associated with substantial alterations in one-carbon metabolism (Pogribny et al., 2006). Surprisingly, a significant increase in DNA methylation and unaffected SAM and SAH levels was found in the brains of folate/methyl-deficient rats (Pogribny et al., 2008). Furthermore, a partial change in the DNA methylation patterns of the prostate occur after genistein exposure, but not in the liver in male mice feeding a genistein supplemented diet (300 mg/kg diet) for 4 wk (Day et al., 2002). The methylation in the promoter region of the skeletal a-actin (Acta1) gene increased at some CpG sites within pancreas but not the liver in mice feeding a diet containing both daidzein and genistein (Guerrero-Bosagna et al., 2008). Whereas tissue specific differences in methylation profiles were also observed for estrogen receptor-a (ERa), which show a well-defined methylation profile in liver, contrasting with an absence of methylation seen in pancreas (Guerrero-Bosagna et al., 2008). Further, an interaction between folate depletion and tissue methylation was observed at the Igf2 DMR2 locus in which methylation was increased in the liver but decreased in the blood with folate depletion, whereas methylation within the kidney remained unchanged (McKay et al., 2011). It will be especially essential to specify the methylation in specific tissue of genes because it is basal for nutrition regulation.
6. Prospective of nutritional modulation of DNA methylation
It is unequivocal that nutrition is influencing the epigenome. This dynamic relationship between nutrition and genes throughout an organism׳s lifetime has now been recognized as a subfield called Nutritional Epigenomics or 'Nutri-epigenomics’ and provides promising insight for how to target growth and health from a nutritional standpoint (Reik, 2007). This process includes the modification of DNA by methylation at CpG sites (Jaenisch and Bird, 2003), one of the most widely studied form of epigenetic modification. This article has detailed the current knowledge regarding the biological mechanisms, the critical period, and the tissue specific of nutritional modulation of DNA methylation. The epigenetic mechanisms may explain the way by which dietary factors in several critical developmental steps modulate the growth and health of animals in adulthood. However, our knowledge regarding nutritional epigenomics is still limited. Further studies in animal subjects using the latest technologies are needed to better understand the roles of nutrition for maintaining health through modifiable epigenetic mechanisms. Future work is needed to systematically analyze the influence of methyl donors on the DNA methylation patterns in order to predict their usefulness as epigenetic modulators. Further, more attention should be focused on dietary bioactive ingredients when DNMT are being tested. Additionally, the interactions among micronutrients required in one-carbon metabolism and those that may indirectly affect their supply to maintain cycle efficiency merits more study. Totally, continued animal nutri-epigenomic research will strengthen our understanding in the biological pathways associated with diet and health.
Acknowledgments
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (201303143), and the Chinese scholarship council fund (201403250010).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abusnina A., Keravis T., Yougbaré I., Bronner C., Lugnier C. Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator uhrf1. Mol Nutr Food Res. 2011;55:1677–1689. doi: 10.1002/mnfr.201100307. [DOI] [PubMed] [Google Scholar]
- Altmann S., Murani E., Schwerin M., Metges C.C., Wimmers K., Ponsuksili S. Dietary protein restriction and excess of pregnant german landrace sows induce changes in hepatic gene expression and promoter methylation of key metabolic genes in the offspring. J Nutr Biochem. 2013;24:484–495. doi: 10.1016/j.jnutbio.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Altmann S., Murani E., Schwerin M., Metges C.C., Wimmers K., Ponsuksili S. Somatic cytochrome c (CYCS) gene expression and promoter-specific DNA methylation in a porcine model of prenatal exposure to maternal dietary protein excess and restriction. Brit J Nutr. 2012;107:791–799. doi: 10.1017/S0007114511003667. [DOI] [PubMed] [Google Scholar]
- Amarasekera M., Martino D., Ashley S., Harb H., Kesper D., Strickland D. Genome-wide DNA methylation profiling identifies a folate-sensitive region of differential methylation upstream of ZFP57-imprinting regulator in humans. FASEB J. 2014;28:4068–4076. doi: 10.1096/fj.13-249029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch J.B., Liu C., Love W.K., Andrews L.G., Katiyar S.K., Tollefsbol T.O. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103:509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdarina I., Haase A., Langley-Evans S., Clark A.J.L. Glucocorticoid effects on the programming of AT1B angiotensin receptor gene methylation and expression in the rat. Plos One. 2010;5:e9237. doi: 10.1371/journal.pone.0009237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdarina I., Welham S., King P.J., Burns S.P., Clark A.J.L. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckberry S., Bianco-Miotto T., Hiendleder S., Roberts C.T. Quantitative allele-specific expression and DNA methylation analysis of H19, IGF2 and IGF2R in the human placenta across gestation reveals H19 imprinting plasticity. Plos One. 2012;7:e51210. doi: 10.1371/journal.pone.0051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge G.C., Lillycrop K.A. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- Cannon M.V., Buchner D.A., Hester J., Miller H., Sehayek E., Nadeau J.H. Maternal nutrition induces pervasive gene expression changes but no detectable DNA methylation differences in the liver of adult offspring. Plos One. 2014;9:e90335. doi: 10.1371/journal.pone.0090335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone B.R., Fauquier L., Habib N., Shea J.M., Hart C.E., Li R. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A., Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol. 2012;8:287–296. doi: 10.1038/nrendo.2011.225. [DOI] [PubMed] [Google Scholar]
- Christensen B.C., Houseman E.A., Marsit C.J., Zheng S., Wrensch M.R., Wiemels J.L. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. Plos Genet. 2009;5:e1000602. doi: 10.1371/journal.pgen.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero P., Milagro F., Campion J., Martinez J. Maternal methyl donors supplementation during lactation prevents the hyperhomocysteinemia induced by a high-fat-sucrose intake by dams. Int J Mol Sci. 2013;14:24422–24437. doi: 10.3390/ijms141224422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero P., Milagro F.I., Campion J., Martinez J.A. Supplementation with methyl donors during lactation to high-fat-sucrose-fed dams protects offspring against liver fat accumulation when consuming an obesogenic diet. J Dev Orig Health Dis. 2014;5:385–395. doi: 10.1017/S204017441400035X. [DOI] [PubMed] [Google Scholar]
- Coupé B., Amarger V., Grit I., Benani A., Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151:702–713. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- Craciunescu C.N., Johnson A.R., Zeisel S.H. Dietary choline reverses some, but not all, effects of folate deficiency on neurogenesis and apoptosis in fetal mouse brain. J Nutr. 2010;140:1162–1166. doi: 10.3945/jn.110.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo M., Fidalgo P., Pereira A.D., Gouveia-Oliveira A., Chaves P., Selhub J. DNA methylation as an intermediate biomarker in colorectal cancer: modulation by folic acid supplementation. Eur J Cancer Prev. 1994;3:473–479. doi: 10.1097/00008469-199411000-00004. [DOI] [PubMed] [Google Scholar]
- Cravo M.L., Pinto A.G., Chaves P., Cruz J.A., Lage P., Nobre L.C. Effect of folate supplementation on DNA methylation of rectal mucosa in patients with colonic adenomas: correlation with nutrient intake. Clin Nutr. 1998;17:45–49. doi: 10.1016/s0261-5614(98)80304-x. [DOI] [PubMed] [Google Scholar]
- Day J.J., Kennedy A.J., Sweatt J.D. DNA methylation and its implications and accessibility for neuropsychiatric therapeutics. Annu Rev Pharmacol. 2015;55:591–611. doi: 10.1146/annurev-pharmtox-010814-124527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J.J., Sweatt J.D. DNA methylation and memory formation. Nat Neurosci. 2010;13:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J.J., Sweatt J.D. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J.K., Bauer A.M., DesBordes C., Zhuang Y., Kim B.E., Newton L.G. Genistein alters methylation patterns in mice. J Nutr. 2002;132:2419S–2423S. doi: 10.1093/jn/132.8.2419S. [DOI] [PubMed] [Google Scholar]
- de Vogel S., Wouters K.A.D., Gottschalk R.W.H., van Schooten F.J., de Goeij A.F.P.M., de Bruïne A.P. Dietary methyl donors, methyl metabolizing enzymes, and epigenetic regulators: diet–gene interactions and promoter CpG island hypermethylation in colorectal cancer. Cancer Cause Control. 2011;22:1–12. doi: 10.1007/s10552-010-9659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Robert C., Li N., Caicedo R., Frost S., Lane R., Hauser N. Metabolic effects of different protein intakes after short term undernutrition in artificially reared infant rats. Early Hum Dev. 2009;85:41–49. doi: 10.1016/j.earlhumdev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Dilworth M.R., Kusinski L.C., Cowley E., Ward B.S., Husain S.M., Constancia M. Placental-specific IGF2 knockout mice exhibit hypocalcemia and adaptive changes in placental calcium transport. Proc Natl Acad Sci U S A. 2010;107:3894–3899. doi: 10.1073/pnas.0911710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I.B., Micheelsen M.A., Sneppen K., Thon G. Theoretical analysis of epigenetic cell memory by nucleosome modification. Cell. 2007;129:813–822. doi: 10.1016/j.cell.2007.02.053. [DOI] [PubMed] [Google Scholar]
- Dolinoy D.C., Weidman J.R., Waterland R.A., Jirtle R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Persp. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Salas P., Moore S.E., Baker M.S., Bergen A.W., Cox S.E., Dyer R.A. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley K.J., Sloboda D.M., Connor K.L., Beltrand J., Vickers M.H. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. Plos One. 2011;6:e21662. doi: 10.1371/journal.pone.0021662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Chen D., Yang C.S. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- Fang M.Z., Chen D., Sun Y., Jin Z., Christman J.K., Yang C.S. Reversal of hypermethylation and reactivation of p16INK4a, RARβ, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–7041. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- Farias N., Ho N., Butler S., Delaney L., Morrison J., Shahrzad S. The effects of folic acid on global DNA methylation and colonosphere formation in colon cancer cell lines. J Nutr Biochem. 2015;26:818–826. doi: 10.1016/j.jnutbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Farkas S.A., Befekadu R., Hahn-Strömberg V., Nilsson T.K. DNA methylation and expression of the folate transporter genes in colorectal cancer. Tumor Biol. 2015;36:5581–5590. doi: 10.1007/s13277-015-3228-2. [DOI] [PubMed] [Google Scholar]
- Feil R., Fraga M.F. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Ferguson-Smith A.C., Patti M. You are what your dad ate. Cell Metab. 2011;13:115–117. doi: 10.1016/j.cmet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Fu Q., Yu X., Callaway C.W., Lane R.H., McKnight R.A. Epigenetics: intrauterine growth retardation (IUGR) modifies the histone code along the rat hepatic IGF-1 gene. FASEB J. 2009;23:2438–2449. doi: 10.1096/fj.08-124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallou-Kabani C., Junien C. Nutritional epigenomics of metabolic syndrome: new perspective against the epidemic. Diabetes. 2005;54:1899–1906. doi: 10.2337/diabetes.54.7.1899. [DOI] [PubMed] [Google Scholar]
- Ge Z., Luo S., Lin F., Liang Q., Huang L., Wei Y. DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity. Environ Health Perspect. 2014;122:159–164. doi: 10.1289/ehp.1307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson S.E., Ponce De Leon F.A., Muscoplat C.C. Epigenetics: understanding how our choices lead to our diseases. J Clin Case Rep. 2014;4:447. [Google Scholar]
- Gu B., Ding Q., Xia G., Fang Z. EGCG inhibits growth and induces apoptosis in renal cell carcinoma through TFPI-2 overexpression. Oncol Rep. 2009;21:635–640. [PubMed] [Google Scholar]
- Guerrero-Bosagna C.M., Sabat P., Valdovinos F.S., Valladares L.E., Clark S.J. Epigenetic and phenotypic changes result from a continuous pre and post natal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol. 2008;8:17. doi: 10.1186/1472-6793-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Choufani S., Ferreira J., Smith A., Chitayat D., Shuman C. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev Biol. 2008;320:79–91. doi: 10.1016/j.ydbio.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Heijmans B.T., Tobi E.W., Stein A.D., Putter H., Blauw G.J., Susser E.S. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E., Galvez J., Roks A., Stolk L., Verbiest M., Eilers P. Tissue-specific DNA methylation profiles in newborns. Clin Epigenetics. 2013;5:8. doi: 10.1186/1868-7083-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Hinoda Y., Nakajima K., Kawamoto K., Kikuno N., Kawakami K. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin Cancer Res. 2009;15:5678–5687. doi: 10.1158/1078-0432.CCR-09-0558. [DOI] [PubMed] [Google Scholar]
- Hirata H., Hinoda Y., Shahryari V., Deng G., Tanaka Y., Tabatabai Z.L. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Brit J Cancer. 2014;110:1645–1654. doi: 10.1038/bjc.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideraabdullah F.Y., Thorvaldsen J.L., Myers J.A., Bartolomei M.S. Tissue-specific insulator function at H19/igf2 revealed by deletions at the imprinting control region. Hum Mol Genet. 2014;23:6246–6259. doi: 10.1093/hmg/ddu344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth R., Kerr A., DeSousa D., Jørgensen H., Ellis P., Stalker J. A novel CpG island set identifies Tissue-Specific methylation at developmental gene loci. Plos Biol. 2008;6:e22. doi: 10.1371/journal.pbio.0060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Shen L., Dai Q., Wu S.C., Collins L.B., Swenberg J.A. Tet proteins can convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jiménez-Chillarón J.C., Dĺaz R., Martĺnez D., Pentinat T., Ramón-Krauel M., Ribó S. The role of nutrition on epigenetic modifications and their implications on health. Biochimie. 2012;94:2242–2263. doi: 10.1016/j.biochi.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Jones P.A., Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805–811. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C., Parry L., Lambert-Langlais S., Maurin A.C., Averous J., Bruhat A. Perinatal undernutrition affects the methylation and expression of the leptin gene in adults: implication for the understanding of metabolic syndrome. FASEB J. 2011;25:3271–3278. doi: 10.1096/fj.11-181792. [DOI] [PubMed] [Google Scholar]
- Kato K., Long N.K., Makita H., Toida M., Yamashita T., Hatakeyama D. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. Brit J Cancer. 2008;99:647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno N., Shiina H., Urakami S., Kawamoto K., Hirata H., Tanaka Y. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123:552–560. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- Kucharski R., Maleszka J., Foret S., Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319:1827–1830. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- Lee W.J., Shim J., Zhu B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005;68:1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- Li C.C., Cropley J.E., Cowley M.J., Preiss T., Martin D.I., Suter C.M. A sustained dietary change increases epigenetic variation in isogenic mice. Plos Genet. 2011;7:e1001380. doi: 10.1371/journal.pgen.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Jiang M., Xiao Y., Zhang X., Cui S., Huang G. Folic acid inhibits tau phosphorylation through regulation of PP2A methylation in SH-SY5Y cells. J Nutr Health Aging. 2015;19:123–129. doi: 10.1007/s12603-014-0514-4. [DOI] [PubMed] [Google Scholar]
- Li Y., Daniel M., Tollefsbol T.O. Epigenetic regulation of caloric restriction in aging. BMC Med. 2011;9:98. doi: 10.1186/1741-7015-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K.A., Phillips E.S., Jackson A.A., Hanson M.A., Burdge G.C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- Lillycrop K.A., Phillips E.S., Torrens C., Hanson M.A., Jackson A.A., Burdge G.C. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPARa promoter of the offspring. Brit J Nutr. 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K.A., Slater-Jefferies J.L., Hanson M.A., Godfrey K.M., Jackson A.A., Burdge G.C. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Brit J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xie Z., Jones W., Pavlovicz R.E., Liu S., Yu J. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009;19:706–709. doi: 10.1016/j.bmcl.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Llanos A.A., Dumitrescu R.G., Brasky T.M., Liu Z., Mason J.B., Marian C. Relationships among folate, alcohol consumption, gene variants in one-carbon metabolism and p16INK4a methylation and expression in healthy breast tissues. Carcinogenesis. 2015;36:60–67. doi: 10.1093/carcin/bgu219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W.A.M. S-adenosylmethionine: jack of all trades and master of everything? Biochem Soc Trans. 2006;34:330–333. doi: 10.1042/BST20060330. [DOI] [PubMed] [Google Scholar]
- Lumey L.H., Ravelli A.C., Wiessing L.G., Koppe J.G., Treffers P.E., Stein Z.A. The dutch famine birth cohort study: design, validation of exposure, and selected characteristics of subjects after 43 years follow-up. Paediatr Perinat Epidemiol. 1993;7:354–367. doi: 10.1111/j.1365-3016.1993.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Maegawa S., Hinkal G., Kim H.S., Shen L., Zhang L., Zhang J. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20:332–340. doi: 10.1101/gr.096826.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco A., Kisliouk T., Tabachnik T., Meiri N., Weller A. Overweight and CpG methylation of the pomc promoter in offspring of high-fat-diet-fed dams are not “reprogrammed” by regular chow diet in rats. FASEB J. 2014;28:4148–4157. doi: 10.1096/fj.14-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers J.C. Session 2: personalised nutrition. Epigenomics: a basis for understanding individual differences? Proc Nutr Soc. 2008;67:390–394. doi: 10.1017/S0029665108008744. [DOI] [PubMed] [Google Scholar]
- McKay J.A., Mathers J.C. Diet induced epigenetic changes and their implications for health. Acta Physiol. 2011;202:103–118. doi: 10.1111/j.1748-1716.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- McKay J.A., Xie L., Harris S., Wong Y.K., Ford D., Mathers J.C. Blood as a surrogate marker for tissue-specific DNA methylation and changes due to folate depletion in post-partum female mice. Mol Nutr Food Res. 2011;55:1026–1035. doi: 10.1002/mnfr.201100008. [DOI] [PubMed] [Google Scholar]
- Mehedint M.G., Craciunescu C.N., Zeisel S.H. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci U S A. 2010;107:12834–12839. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A., Mikkelsen T.S., Gu H., Wernig M., Hanna J., Sivachenko A. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K., Smith A.K., Tylavsky F.A. Ancestry dependent DNA methylation and influence of maternal nutrition. Plos one. 2015;10:e118466. doi: 10.1371/journal.pone.0118466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N., Kumar A.P., Ghosh R. DNA methylation and flavonoids in genitourinary cancers. Curr Pharmacol Rep. 2015;1:112–120. doi: 10.1007/s40495-014-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M., Bergman D., Halje M., Engstrom W., Ward A. Epigenetic regulation of the igf2/H19 gene cluster. Cell Prolif. 2014;47:189–199. doi: 10.1111/cpr.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollikainen M., Smith K.R., Joo E.J.H., Ng H.K., Andronikos R., Novakovic B. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Hum Mol Genet. 2010;19:4176–4188. doi: 10.1093/hmg/ddq336. [DOI] [PubMed] [Google Scholar]
- Pant V., Kurukuti S., Pugacheva E., Shamsuddin S., Mariano P., Renkawitz R. Mutation of a single CTCF target site within the H19 imprinting control region leads to loss of igf2 imprinting and complex patterns of de novo methylation upon maternal inheritance. Mol Cell Biol. 2004;24:3497–3504. doi: 10.1128/MCB.24.8.3497-3504.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar G., Parashar N.C., Capalash N. Curcumin causes promoter hypomethylation and increased expression of FANCF gene in SiHa cell line. Mol Cell Biochem. 2012;365:29–35. doi: 10.1007/s11010-012-1240-z. [DOI] [PubMed] [Google Scholar]
- Pentinat T., Ramon-Krauel M., Cebria J., Diaz R., Jimenez-Chillaron J.C. Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology. 2010;151:5617–5623. doi: 10.1210/en.2010-0684. [DOI] [PubMed] [Google Scholar]
- Petrik J., Srinivasan M., Aalinkeel R., Coukell S., Arany E., Patel M.S. A long-term high-carbohydrate diet causes an altered ontogeny of pancreatic islets of langerhans in the neonatal rat. Pediatr Res. 2001;49:84–92. doi: 10.1203/00006450-200101000-00019. [DOI] [PubMed] [Google Scholar]
- Pitman K.A., Borgland S.L. Changes in mu-opioid receptor expression and function in the mesolimbic system after long-term access to a palatable diet. Pharmacol Ther. 2015;154:110–119. doi: 10.1016/j.pharmthera.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Plagemann A., Harder T., Brunn M., Harder A., Roepke K., Wittrock-Staar M. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587:4963–4976. doi: 10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A., Roepke K., Harder T., Brunn M., Harder A., Wittrock-Staar M. Epigenetic malprogramming of the insulin receptor promoter due to developmental overfeeding. J Perinat Med. 2010;38:393–400. doi: 10.1515/jpm.2010.051. [DOI] [PubMed] [Google Scholar]
- Pogribny I.P., Karpf A.R., James S.R., Melnyk S., Han T., Tryndyak V.P. Epigenetic alterations in the brains of fisher 344 rats induced by long-term administration of folate/methyl-deficient diet. Brain Res. 2008;1237:25–34. doi: 10.1016/j.brainres.2008.07.077. [DOI] [PubMed] [Google Scholar]
- Pogribny I.P., Ross S.A., Wise C., Pogribna M., Jones E.A., Tryndyak V.P. Irreversible global DNA hypomethylation as a key step in hepatocarcinogenesis induced by dietary methyl deficiency. Mutat Res. 2006;593:80–87. doi: 10.1016/j.mrfmmm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Pogribny I.P., Tryndyak V.P., Bagnyukova T.V., Melnyk S., Montgomery B., Ross S.A. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 2009;51:176–186. doi: 10.1016/j.jhep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Ohh M. The von Hippel-Lindau tumor suppressor protein sensitizes renal cell carcinoma cells to tumor necrosis factor-induced cytotoxicity by suppressing the nuclear factor-κB-dependent antiapoptotic pathway. Cancer Res. 2003;63:7076–7080. [PubMed] [Google Scholar]
- Rees W.D., Hay S.M., Brown D.S., Antipatis C., Palmer R.M. Maternal protein deficiency causes hypermethylation of DNA in the livers of rat fetuses. J Nutr. 2000;130:1821–1826. doi: 10.1093/jn/130.7.1821. [DOI] [PubMed] [Google Scholar]
- Reik W. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Reik W., Brown K.W., Slatter R.E., Sartor P., Elliott M., Maher E.R. Allelic methylation of H19 and IGF2 in the beckwith – Wiedemann syndrome. Hum Mol Genet. 1994;3:1297–1301. doi: 10.1093/hmg/3.8.1297. [DOI] [PubMed] [Google Scholar]
- Sandovici I., Smith N.H., Nitert M.D., Ackers-Johnson M., Uribe-Lewis S., Ito Y. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci U S A. 2011;108:5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneid H., Seurin D., Vazquez M.P., Gourmelen M., Cabrol S., Le Bouc Y. Parental allele specific methylation of the human insulin-like growth factor || gene and Beckwith-Wiedemann syndrome. J Med Genet. 1993;30:353–362. doi: 10.1136/jmg.30.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Pliushch G., El Hajj N., Galetzka D., Puhl A., Schorsch M. Spatial, temporal and interindividual epigenetic variation of functionally important DNA methylation patterns. Nucleic Acids Res. 2010;38:3880–3890. doi: 10.1093/nar/gkq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Szulwach K.E., Dai Q., Fu Y., Mao S., Lin L. Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell. 2013;153:678–691. doi: 10.1016/j.cell.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Aalinkeel R., Song F., Lee B., Laychock S.G., Patel M.S. Adaptive changes in insulin secretion by islets from neonatal rats raised on a high-carbohydrate formula. Am J Physiol-Endoc M. 2000;279:E1347–E1357. doi: 10.1152/ajpendo.2000.279.6.E1347. [DOI] [PubMed] [Google Scholar]
- Srinivasan M., Song F., Aalinkeel R., Patel M.S. Molecular adaptations in islets from neonatal rats reared artificially on a high carbohydrate milk formula. J Nutr Biochem. 2001;12:575–584. doi: 10.1016/s0955-2863(01)00176-0. [DOI] [PubMed] [Google Scholar]
- Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.F., Atzmon G., Gheorghe C., Liang H.Q., Lowes C., Greally J.M. Tissue-specific dysregulation of DNA methylation in aging. Aging Cell. 2010;9:506–518. doi: 10.1111/j.1474-9726.2010.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierling S., Souren N.Y., Gries J., Loporto C., Groth M., Lutsik P. Assisted reproductive technologies do not enhance the variability of DNA methylation imprints in human. J Med Genet. 2010;47:371–376. doi: 10.1136/jmg.2009.073189. [DOI] [PubMed] [Google Scholar]
- Tobi E.W., Lumey L.H., Talens R.P., Kremer D., Putter H., Stein A.D. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh D.N., Fu Q., Callaway C.W., McKnight R.A., McMillen I.C., Ross M.G. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. Delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1023–G1029. doi: 10.1152/ajpgi.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberger A., Szyf M., Nathanielsz P.W., Cox L.A. Organ and gestational age effects of maternal nutrient restriction on global methylation in fetal baboons. J Med Primatol. 2009;38:219–227. doi: 10.1111/j.1600-0684.2008.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straten E.M.E., Bloks V.W., Huijkman N.C.A., Baller J.F.W., Meer H.V., Lutjohann D. The liver X-receptor gene promoter is hypermethylated in a mouse model of prenatal protein restriction. Am J Physiol Regul Integr Comp Physiol. 2010;298:R275–R282. doi: 10.1152/ajpregu.00413.2009. [DOI] [PubMed] [Google Scholar]
- van Straten E.M.E., Bloks V.W., van Dijk T.H., Baller J.F.W., Huijkman N.C.A., Kuipers I. Sex-dependent programming of glucose and fatty acid metabolism in mouse offspring by maternal protein restriction. Gend Med. 2012;9:166–179. doi: 10.1016/j.genm.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Vanhees K., Coort S., Ruijters E.J.B., Godschalk R.W.L., van Schooten F.J., van Doorn-Khosrovani S.B.V.W. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011;25:797–807. doi: 10.1096/fj.10-172155. [DOI] [PubMed] [Google Scholar]
- Vucetic Z., Kimmel J., Reyes T.M. Chronic high-fat diet drives postnatal epigenetic regulation of µ-opioid receptor in the brain. Neuropsychopharmacology. 2011;36:1199–1206. doi: 10.1038/npp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z., Kimmel J., Totoki K., Hollenbeck E., Reyes T.M. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J., Oliver V.F., Wang G., Zhu H., Zack D.J., Merbs S.L. Characterization of tissue-specific differential DNA methylation suggests distinct modes of positive and negative gene expression regulation. BMC Genom. 2015;16:49. doi: 10.1186/s12864-015-1271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ji J., Yu Y., Wei X., Chai S., Liu D. Neonatal overfeeding in female mice predisposes the development of obesity in their male offspring via altered central leptin signalling. J Neuroendocrinol. 2015;27:600–608. doi: 10.1111/jne.12281. [DOI] [PubMed] [Google Scholar]
- Warzak D.A., Johnson S.A., Ellersieck M.R., Roberts R.M., Zhang X., Ho S. Effects of post-weaning diet on metabolic parameters and DNA methylation status of the cryptic promoter in the A(vy) allele of viable yellow mice. J Nutr Biochem. 2015;26:667–674. doi: 10.1016/j.jnutbio.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland R.A. Assessing the effects of high methionine intake on DNA methylation. J Nutr. 2006;136:1706S–1710S. doi: 10.1093/jn/136.6.1706S. [DOI] [PubMed] [Google Scholar]
- Waterland R.A., Jirtle R.L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland R.A., Kellermayer R., Laritsky E., Rayco-Solon P., Harris R.A., Travisano M. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. Plos Genet. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland R.A., Travisano M., Tahiliani K.G., Rached M.T., Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes. 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G.L., Kodell R.L., Moore S.R., Cooney C.A. Maternal epigenetics and methyl supplements affect agouti gene expression in A(vy)/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- Xie Q., Bai Q., Zou L.Y., Zhang Q.Y., Zhou Y., Chang H. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosom Cancer. 2014;53:422–431. doi: 10.1002/gcc.22154. [DOI] [PubMed] [Google Scholar]
- Yu H., Dong S., Gao L., Li L., Xi Y., Ma W. Global DNA methylation was changed by a maternal high-lipid, high-energy diet during gestation and lactation in male adult mice liver. Brit J Nutr. 2015;113:1032–1039. doi: 10.1017/S0007114515000252. [DOI] [PubMed] [Google Scholar]
- Zampieri M., Ciccarone F., Calabrese R., Franceschi C., Bürkle A., Caiafa P. Reconfiguration of DNA methylation in aging. Mech Ageing Dev. 2015 doi: 10.1016/j.mad.2015.02.002. http://dx.doi.org/10.1016/j.mad.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Zheng J., Xiao X., Zhang Q., Yu M., Xu J., Wang Z. Maternal and post-weaning high-fat, high-sucrose diet modulates glucose homeostasis and hypothalamic pomc promoter methylation in mouse offspring. Metab Brain Dis. 2015;30:1129–1137. doi: 10.1007/s11011-015-9678-9. [DOI] [PubMed] [Google Scholar]