Abstract
This study investigated the effect of dietary supplementation of yeast nucleotides on the growth, non-specific immunity, intestine growth and intestinal microbiota of juvenile hybrid tilapia. Tilapia (initial average weight of 8.02 g) was fed test diets supplemented with a yeast-originated nucleotide mixture (0, 0.15, 0.30, 0.60, and 1.20 g/100 g diet) for 8 weeks. Fish fed the diet with 0.60% nucleotide had significantly higher weight gain than the control group (P < 0.05). Feed efficiency was improved in the fish fed 0.60 and 1.20% nucleotide compared with that in the control group. The optimal doses of nucleotides supplementation for growth and feed efficiency of fish were determined as 0.63 and 0.81%, respectively. Intestinal growth was improved in the 0.30 and 0.60% groups, as indicated by significant increase in intestine length. The fish fed 0.60 and 1.20% nucleotide showed higher super oxide dismutase (SOD) activity and lower malondialdehyde (MDA) level in the liver than the control fish, indicating enhancement of the anti-oxidant status. Serum lysozyme activity was significantly increased in the 0.15 and 0.3% nucleotide supplementation groups, suggesting an enhancement effect on the non-specific immune response. Lastly, dietary nucleotides supplementation exerted moderate influence on the intestinal microbiota of hybrid tilapia. A reduction in the cumulative abundance of putative butyrate-producing species was observed in the intestinal microbiota of fish fed diets with 0.60% nucleotide compared with the control, implying an interaction between dietary nucleotides and butyrate production. Briefly, dietary supplementation with 0.60% nucleotide improve the growth performance, immune activity and intestine growth in tilapia.
Keywords: Nucleotides, Growth performance, Hybrid tilapia, Intestinal microbiota, Denaturing gradient gel electrophoresis
1. Introduction
Nucleotides are low molecular weight biological compounds that play important roles in essential physiological and biochemical functions (Carver and Walker, 1995). Nucleotides are synthesized de novo in most tissues, but some immune and intestinal cells lack this process and depend on exogenous supply (Quan, 1992). Hence, administration of exogenous nucleotides guarantees increased availability to the body at the time of high demand for various physiological activities (Whitehead et al., 2006). Nucleotide supplementation has been one important aspect of research on clinical nutrition and functional food development for humans (Li and Gatlin, 2006), and the requirement for nucleotides in infants has been well accepted (Carver, 2003).
Research into potential growth and health benefits of dietary nucleotides in aquaculture species began in early1990׳s (Ramadan and Atef, 1991, Ramadan et al., 1994), and most of the further studies on nucleotide supplementation for fish started after the reports of Burrells et al., 2001a, Burrells et al., 2001b. Till now, dietary supplementation of nucleotides has been tested in different aquatic species, and various beneficial effects of dietary nucleotides on fish have been reported, including improved growth, enhanced immune response (Burrells et al., 2001a, Burrells et al., 2001b, Leonardi et al., 2003, Li et al., 2004), increased tolerance to stresses (Burrells et al., 2001a, Burrells et al., 2001b, Leonardi et al., 2003, Lin et al., 2009, Tahmasebi-Kohyani et al., 2011, Xiang et al., 2011, Welker et al., 2011, Huu et al., 2012), and improved intestinal morphology (Borda et al., 2003, Cheng et al., 2011, Peng et al., 2013). Dietary nucleotide supplementation has been reported to improve the balance of infant intestinal microbiota (Singhal et al., 2008, Uauy et al., 1994). The effect of nucleotide supplementation of infant formula on intestinal microbiota was proposed as potential mediator of the benefits of nucleotides supplementation for immune function, as neonatal gut microbiotas have long-term effects on immunity (Singhal et al., 2008). In addition, dose-response effects of dietary nucleotides inclusion were investigated in a few studies (Huu et al., 2012, Lin et al., 2009, Tahmasebi-Kohyani et al., 2011, Xiang et al., 2011), and doses of nucleotides products at 0.5 g/kg (Xiang et al., 2011), 1.5 g/kg (Lin et al., 2009), 2 g/kg (Tahmasebi-Kohyani et al., 2011) and 8 g/kg (Huu et al., 2012) were registered as optimal for growth or immunity. Therefore, the optimal dosage of nucleotide addition was necessary to study depend on fish species. In addition, the influence of intestinal microbiota by dietary nucleotides in fish species has never been studied.
Hybrid tilapia (Oreochromis niloticus ♀ × Oreochromis aureus ♂) are cultured worldwide for its fast growth rate, easy adaptation to different aquaculture conditions, and tender flesh (Zhou et al., 2007). Effects of nucleotides supplementation on tilapia were studied earlier; improved growth and specific immune responses to vaccination were reported (Ramadan and Atef, 1991, Ramadan et al., 1994). However, only one or two ‘recommended’ inclusion doses were tested in these studies. The potential dose-response effect of nucleotide supplementation on growth and immune response, as well as the optimal supplementation rate, which have been investigated in other aquatic species, have never been studied in hybrid tilapia. Also, the influence of dietary nucleotide supplementation on non-specific immune responses, which is more directly relevant to regular disease resistance of fish, was not tested for hybrid tilapia in the above-mentioned studies. In this research, a series of nucleotides supplementation rates were incorporated to detect the dose effect of dietary nucleotides on growth and non-specific immune responses of hybrid tilapia. The impact of nucleotide supplementation on intestine growth and intestinal microbiota of hybrid tilapia was also investigated, as an initial trial to uncover the associated mechanisms of the beneficial effects of nucleotide supplementation on fish.
2. Materials and methods
2.1. Animals and feeding
All animal research was approved by the animal ethics committee of Feed Research Institute, Chinese Academy of Agricultural Sciences (permit number 2012-ZZG-ZF-001). Three hundred hybrid tilapias (O. niloticus ♀ × O. aureus ♂) were obtained from a commercial aquaculture farm in Jiangmen City, Guangdong province, China. After disinfection with 2‰ salt solution within 30 min, the fish were acclimatized to the laboratory condition and fed the basal experimental diet without exogenous nucleotides for 2 weeks. After acclimation, fish with an average weight of approximately 8 g were randomly distributed into fifteen tanks (60 × 60 × 65 cm, 3 tanks per treatment and 20 fish per tank). The aquaria were supplied with recirculated de-chlorinated water at a rate of 0.5 L/min. During the feeding trial, the dissolved oxygen concentrations in the aquaria were higher than 6.0 mg O/L, and total nitrate/nitrite (, ) levels were less than 0.092 mg N/L with water temperature between 25.2 and 28.5°C. Aquaria were illuminated from 0800 to 2100 h every day.
The basal dietary formulation and proximate composition are given in Table 1 (Council NR, 1993). The experimental diet was supplemented with a Saccharomyces cerevisiae-originated nucleotide mixture from Biotogether (Nanjing, China) at the dosage of (0, 0.15, 0.30, 0.60, and 1.20 g/100 g diet). The nucleotide mixture contained 49% nucleotides (12 ± 1% of 5′-AMP, 10 ± 1% of 5′-CMP, 16 ± 1% of 5′-GMP·Na2, and 11 ± 1% of 5′-UMP·Na2), 35% oligonucleotides, and 10% water, with the remainder comprised of proteins and polysaccharides. Dry and powdered ingredients were thoroughly mixed with water to make soft dough and then processed into 3 mm-diameter pellets. The pellets were dried in a hot air oven at 60°C until their moisture content was reduced to less than 10%. The dried pellets were then stored at 4°C until use. During the 8-week feeding trial, fish were hand-fed to apparent satiation twice daily (0900 and 1500).
Table 1.
Feed formulation and proximate composition of test diets used in the experiment.1
| Item | Control | 0.15% nucleotides | 0.30% nucleotides | 0.60% nucleotides | 1.20% nucleotides |
|---|---|---|---|---|---|
| Ingredients, % DM | |||||
| White fishmeal | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 |
| Fly maggot meal | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Soybean meal | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Wheat | 42.00 | 42.00 | 42.00 | 42.00 | 42.00 |
| Vitamin C | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix2 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Mineral premix3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| α- starch (%) | 2.85 | 2.85 | 2.85 | 2.85 | 2.85 |
| Zeolite powder | 4.25 | 4.10 | 3.95 | 3.65 | 3.05 |
| Yeast-originated NT | 0 | 0.15 | 0.30 | 0.60 | 1.20 |
| Chemical composition, % DM | |||||
| Dry matter | 92.1 | 92.1 | 92.1 | 92.1 | 92.1 |
| Crude protein | 31.2 | 31.2 | 31.2 | 31.2 | 31.2 |
| Crude lipid | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 |
| Ash | 6.5 | 6.4 | 6.2 | 5.9 | 5.3 |
| Gross energy, MJ/100 g | 1.72 | 1.72 | 1.72 | 1.72 | 1.72 |
Ingredient source: white fishmeal (Siberia, Russia), fly maggot meal (Shangdong, China), soybean meal (Jiangsu, China), wheat (Henan, China), vitamin C (Hebei, China), choline chloride (Hebei, China), a-starch (Jiangsu, China), zeolite powder (Hebei, China).
Contains (per kg premix): thiamin, 20 mg; riboflavin 20 mg; pyridoxine 20 mg; cyanocobalamine 0.020 mg; phylloquinone 10 mg; folic acid 5 mg; calcium patotheniate 50 mg; inositol 100 mg; niacin 100 mg; tocopherol 50 mg; biotin 0.1 mg; retinol 55,000 IU; cholecalciferol 10,000 IU; carrier up to 1 kg.
Contains (as g/kg premix): NaCl, 1 g; MgSO4·7H2O, 15 g; KH2PO4, 32 g; FeC6H5O7·5H2O, 0.25 g; C6H10CaO6·5H2O, 3.5 g; ZnSO4·7H2O, 0.353 g; MnSO4·4H2O, 0.162 g; CuSO4·5H2O, 0.031 g; CoCl2·6H2O, 0.001 g; KIO3, 0.003 g; carrier up to 1 kg.
2.2. Growth measurements and sampling
The fish in the experimental groups were pooled in a tank to weigh prior to the initial feeding. After the feeding trial was finished, tilapia were fasted for 24 h and weighed by tank. To determine feed stability in water, a known amount of feed was placed into a tank filled with water. The feed residue was collected 1 h later, dried at 70 °C and weighed. For calculation of the feeding rate and feed usage efficiency, any residual feed in the aquarium was collected one hour after feeding and then dried at 70°C. The measured weight of residue feed was adjusted by dissolving rate of feed to calculate the final feed residue weight. Weight gain (WG), feeding rate (FR) and feed efficiency (FE) were calculated as follows:
After final weighing of fish for each tank, seven fish were randomly selected from each tank for measuring body weight (BW) and body length (BL). Intestine growth was assessed by ratio of intestine length to weight (as percent body length to weight). Blood was collected from the caudal vasculature of each fish and equally pooled based on each tank. One part of the blood was added to heparin-containing tubes, stored at 4°C for 1 h, followed by tests for haematological parameters. Another part of the blood was allowed to clot at room temperature for 1 h. The clot was removed and serum was collected by centrifugation (836 × g, 10 min, 4°C) and stored at −70°C until use. Equal portion of liver sample from each fish pooled for each tank, and stored at −70°C for biochemical index analysis. The intestines without the mesenteric adipose were weighed, and measured for total length. Dorsal muscles from fish back were dissected and dried at 105°C for analysis of biochemical components.
2.3. Biochemical analysis and nutritional composition
The total protein and albumin contents in the blood were assessed based on clinical methods (Walker et al., 1990) using the appropriate NJBRI kits (Nanjing, China) according to the manufacturer׳s instructions. Blood hemocrit levels were measured using the capillary tube method. The haemoglobin levels were measured by the haemiglobincyanide (HiCN) method. The erythrocytes and leucocytes were counted using blood-cell counting plates. The tilapia livers were analysed for malondialdehyde levels and activities of superoxide dismutase (SOD) by NJBRI kits (Nanjing, China).
The proximate compositions of the dried muscles were determined. Protein level was assayed by the Kjeldahl method (Bradstreet, 1954); fat content was measured via Soxhlet extraction (Luque-Garcιa and Luque de, 2004); and ash weight was measured after burning at 550°C in a muffle furnace.
2.4. Non-specific immunological assays
The levels of complement proteins C3 and C4 and lysozyme activity in the serum were assayed. The complement proteins C3 and C4 were tittered using an ELISA kit (Shanghai Jianglai Biotechnology Co., Ltd., Shanghai, China). This kit was fully validated for Nile tilapia tissue extracts. Lysozyme activity was determined following the previous method (Rawles and Gatlin, 2000) based on the lysis of the lysozyme sensitive Gram positive bacterium, Micrococcus lysodeikticus (Sigma, St. Louis, MO USA).
2.5. Intestinal microbial analyses by denaturing gradient gel
Three fish per tank were selected for intestinal microbial analysis according to the method of Zhou et al. (2009). The full intestines of the hybrid tilapias were aseptically removed, opened, and gently washed three times with sterile phosphate-buffered saline (PBS, pH 7.2) to remove the contents and non-adhesive bacteria. Then the genomic DNA of intestine and adhesive bacteria was extracted, followed by PCR- (denaturing gradient gel electrophoresis) DGGE analysis. The target sequence (the V3 region of the gene ssr) was amplified by PCR using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 519R (5′-ATTACCGCGGCTGCTGG -3′). The PCR amplification consisted of an initial denaturing step at 95°C for 5 min, followed by 30 cycles (94°C for 30 s, 56°C for 30 s and 72°C for 30 s), and an additional final extension at 72°C for 10 min. The PCR products were analysed by DGGE with a constant voltage of 60 V at 60°C for about 16 h. Gels were stained with ethidium bromide (5 µg/mL) for 20 min and photographed with UV transillumination. The richness of the detected microorganism was evaluated by the relative intensity of the band. Computer-assisted comparison of DGGE patterns was performed with NTsys v2.10. The representative bands were excised, reamplified with primers 338F and 519R, and sequenced at Beijing Calculation Centre (Beijing, China). The sequences of the partial 16S rRNA gene were input as the query sequence in BLASTn and the top hit species was recorded.
2.6. Data analysis
Data were subjected to one-way ANOVA to test the effects of dietary nucleotide supplementation. Duncan׳s multiple range test was used to compare the means among the different groups. Broken-line or polynomial regression analysis was used to determine the optimum doses for growth and feed efficiency. Differences in the relative abundance of phylotypes were analysed by Student׳s t-test. Differences with a P value lower than 0.05 were considered as significant. All the statistical analysis was conducted on SPSS 17.0.
3. Results
3.1. Growth performance and intestine growth
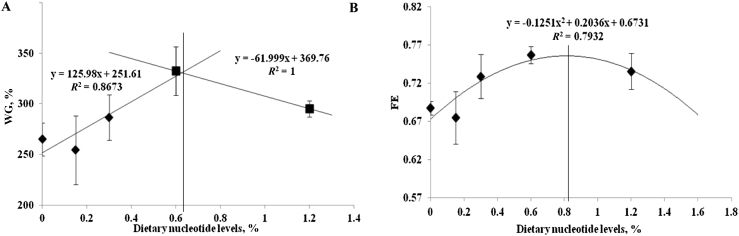
The initial tilapia weights in all treatments were similar. Nucleotide supplementation did not affect the feeding intake, indicating no effect of nucleotides on palatability (Table 2). Significantly improved WG was recorded in the 0.60% group compared with the control (P < 0.05). In 0.30 and 1.20% groups, the WG was marginally increased but was not significantly different from the WG of control group. Significantly higher FE was observed in 0.60 and 1.20% supplement groups compared with the control, with the highest efficiency recorded in 0.60% group. Based on the broken line and polynominal regression analysis, the optimum doses corresponding to the highest WG and FE were calculated as 0.63 and 0.81%, respectively (Fig. 1).
Table 2.
Growth and developmental performance of hybrid tilapia after feeding nucleotide supplemented diets for eight weeks.
| Treatment | IBW, g | WG, % | FR | FE | IL:BL | IW:BW |
|---|---|---|---|---|---|---|
| Control | 7.85 ± 0.23a | 265.46 ± 9.50a | 2.95 ± 0.04a | 0.69 ± 0.01ab | 4.34 ± 0.09a | 1.52 ± 0.13a |
| 0.15% nucleotides | 8.07 ± 0.12a | 254.36 ± 19.60a | 2.87 ± 0.07a | 0.67 ± 0.02a | 4.81 ± 0.17ab | 1.58 ± 0.21a |
| 0.30% nucleotides | 8.03 ± 0.15a | 286.76 ± 13.01ab | 2.89 ± 0.11a | 0.73 ± 0.02bc | 4.87 ± 0.12b | 1.61 ± 0.21a |
| 0.60% nucleotides | 8.03 ± 0.12a | 332.62 ± 14.00b | 2.94 ± 0.06a | 0.76 ± 0.01c | 4.87 ± 0.08b | 1.73 ± 0.12a |
| 1.20% nucleotides | 8.13 ± 0.09a | 295.36 ± 4.65ab | 2.91 ± 0.07a | 0.74 ± 0.01c | 4.56 ± 0.14ab | 1.62 ± 0.13a |
a, b, c Data are means of triplicate, and presented as mean ± SEM Means in each column with a common superscript are not significantly different (P > 0.05.)
IBW = initial body weight; WG = weight gain; FR = feeding rate; FE = feed efficiency; IL:BL = ratio of intestine length to body length; IW:BW = the ratio of intestine weight to body weight.
Fig. 1.
Regression analysis of dose-response effect of nucleotides supplementation on weight gain (A) and feed efficiency (B) of hybrid tilapia. Broken-line and polynomial models were fitted for the two cases, respectively. The vertical line indicates doses corresponding to the optimal performance. WG = weight gain; FE = feed efficiency.
No significant changes were observed between treatment groups and the control in the content of dry matter, protein, lipid and ash in fish muscle, suggesting a negligible effect of nucleotide supplementation on the storage of such components in muscle (Table 3).
Table 3.
The chemical compositions of fish flesh (% as wet) after feeding nucleotide supplemented diets for eight weeks.
| Treatment | Dry matter, % | Protein, % as wet | Lipid, % as wet | Ash, % as wet |
|---|---|---|---|---|
| Control | 24.97 ± 0.89a | 15.93 ± 0.58a | 3.87 ± 0.09ab | 2.21 ± 0.06a |
| 0.15% nucleotides | 24.36 ± 0.6a | 15.20 ± 1.46a | 4.18 ± 0.02a | 2.09 ± 0.20a |
| 0.30% nucleotides | 24.43 ± 0.5a | 15.23 ± 0.21a | 3.53 ± 0.16b | 1.96 ± 0.06a |
| 0.60% nucleotides | 25.17 ± 0.68a | 16.51 ± 0.82a | 3.73 ± 0.19ab | 1.90 ± 0.05a |
| 1.20% nucleotides | 25.82 ± 0.66a | 16.24 ± 0.70a | 3.99 ± 0.20ab | 2.03 ± 0.10a |
a, b Data are means of triplicate, and presented as means ± SEM. Means in each column with a common superscript are not significantly different (P > 0.05).
Fish, which were fed diets with 0.30 and 0.60% nucleotides, had increased intestine length (P < 0.05). However, the intestine weight was not significantly different between treatment groups and the control, although the nucleotide supplementation groups showed numerically higher weight (Table 2).
3.2. Haematological parameters
Dietary nucleotide supplementation had no significant influence on hemocrit. However, the haemoglobin content was significantly improved in 0.30 and 0.60% groups compared with the control, and the 0.60% group exhibiting the highest content. Serum total protein and globulin content were not influenced by nucleotide supplementation. Albumin content decreased significantly in the 0.15, 0.30 and 0.60% groups compared with the control. Significantly reduced albumin-to-globulin ratio (A:G) was observed in the 0.60% group (Table 4).
Table 4.
Effect of dietary nucleotides on haematological parameters.
| Treatment | Total protein, g/L | Albumin, g/L | Globulin, g/L | A:G ratio |
|---|---|---|---|---|
| Control | 46.05 ± 1.47b | 13.37 ± 0.53b | 32.69 ± 1.35a | 0.41 ± 0.02a |
| 0.15% nucleotides | 42.52 ± 2.4b | 11.28 ± 0.27a | 31.24 ± 2.22a | 0.36 ± 0.02ab |
| 0.30% nucleotides | 43.89 ± 1.08b | 11.15 ± 0.29a | 32.73 ± 0.90a | 0.34 ± 0.01ab |
| 0.60% nucleotides | 45.82 ± 1.87b | 11.05 ± 0.49a | 34.78 ± 1.40a | 0.32 ± 0.01b |
| 1.20% nucleotides | 43.64 ± 1.45b | 12.24 ± 0.56ab | 31.40 ± 1.62a | 0.39 ± 0.03a |
a, b Data are means of triplicate, and presented as means ± SEM. Means in each column with a common superscript are not significantly different (P > 0.05).
A:G = ratio of albumin to globulin.
3.3. Anti-oxidant status and non-specific immunity
Super oxide dismutase activity in the liver was significantly improved in the 0.15, 0.60 and 1.20% nucleotide groups than in the control, with the highest SOD activity recorded in the 0.60% group. Accordingly, the MDA concentration in the liver decreased significantly in the 0.6 and 1.20% groups compared to the control (Table 5). The lysozyme activity was significantly increased in the 0.15 and 0.30% groups compared with the control (P < 0.05). Both C3 and C4 concentrations were not significantly influenced by dietary nucleotides (Table 5).
Table 5.
Effects of dietary nucleotides on anti-oxidant activity in the liver and non-specific immunological parameters in serum.
| Treatment | SOD, U/mgprot | MDA, nmol/mgprot | LYZ, U/mL | C3, g/L | C4, g/L |
|---|---|---|---|---|---|
| Control | 387.06 ± 9.71a | 2.03 ± 0.11a | 1400.00 ± 43.26a | 0.49 ± 0.03ab | 0.45 ± 0.04a |
| 0.15% nucleotides | 478.59 ± 19.43b | 2.08 ± 0.17a | 1620.05 ± 60.41b | 0.53 ± 0.03ab | 0.45 ± 0.06a |
| 0.30% nucleotides | 434.76 ± 32.18ab | 1.87 ± 0.09b | 1671.43 ± 44.38b | 0.48 ± 0.09ab | 0.48 ± 0.01a |
| 0.60% nucleotides | 488.58 ± 27.91b | 1.72 ± 0.12c | 1557.14 ± 32.72ab | 0.60 ± 0.02a | 0.45 ± 0.05a |
| 1.20% nucleotides | 467.89 ± 12.37b | 1.73 ± 0.13c | 1541.27 ± 72.86ab | 0.44 ± 0.05b | 0.40 ± 0.01a |
a, b, c Data are means of triplicate, and presented as means ± SEM. Means in each column with a common superscript are not significantly different (P > 0.05).
SOD = super oxide dismutase; MDA = malondialdehyde; LYZ = lysozyme; C3 and C4 are complement proteins C3 and C4.
3.4. Effects of nucleotide supplements on adhesive intestinal microbiota
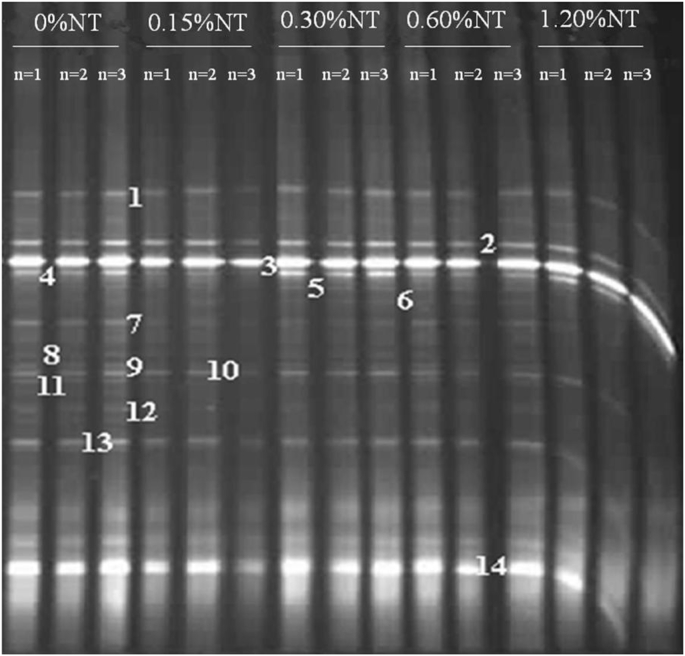
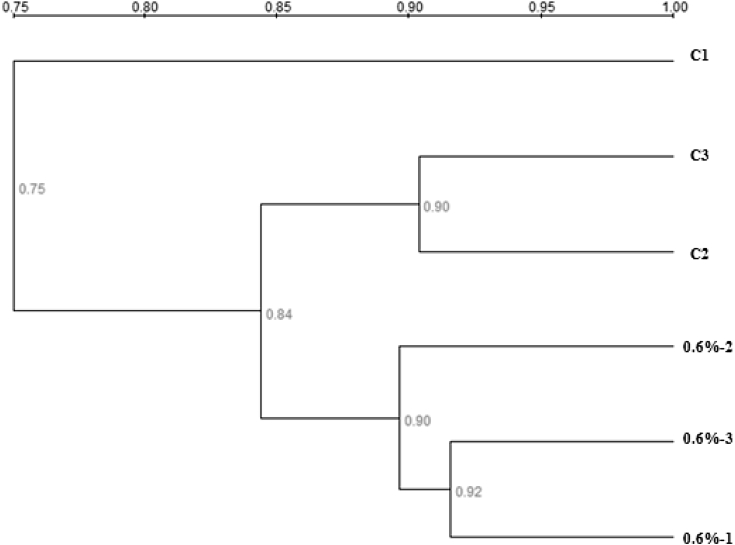
The PCR-DGGE fingerprints of the 16S rRNA gene (V3 region) from the adhesive intestinal microbiota are shown in Fig. 2. Band excision and sequencing produced sequences for 14 representative bands. BLASTn analysis of the band sequences revealed species belonging to Firmicutes, Enterobacteriaceae, and Bacteroides (Table 6). Firmicutes represented the largest group of bacteria, accounting for 9 out of the 14 sequenced bands and around 70% in relative abundance. The microbial composition profile of the 0.60% nucleotide group was compared with that of the control in detail, for its optimum effect on growth and other parameters. Overall, nucleotide supplementation did not exert significant influence on the intestinal microbiota, as indicated by cluster analysis, where Cs over 0.75 was observed for all the intestinal samples (Fig. 3). Relative abundance of species related to Clostridium acidurici (Band 4) and Plesiomonas shigelloides (Band 7) was significantly reduced in the 0.6% group compared with the control. Richness of a species related to Lachnospiraceae (Band 12) decreased to zero in the 0.6% group compared with the control, while a Plesiomonas species (Band 6) was significantly enriched in the 0.60% supplemented group, as compared to zero abundance in the control (Table 6). Notably, species closely related to Anaerostipes hadrus (Band 11) and Faecalibacterium prausnitzii (Band 10), which are both predominant butyrate-producing bacteria in human colon (Allen-Vercoe et al., 2012, Lopez-Siles et al., 2012), were revealed in both the control and nucleotide supplement groups. On top of that, another two phylotypes (Band 1 & Band 12) were considered as putative butyrate-producing bacteria, as they were identified as Lachnospiraceae, a family of bacteria with many of its members associated with butyrate production (Meehan and Beiko, 2014). The cumulative abundance of Band 10 and Band 11 was 7.41 and 5.36%, in the control and 0.6% groups, respectively, while the cumulative abundance of Bands 1, 10, 11 and 12, representing all the putative butyrate-producing bacteria, were 19.09 and 11.77%, in the control and the 0.60% group, respectively (Table 6). Notably, in both cases, the cumulative abundance of putative butyrate-producing bacteria was reduced in the nucleotide supplementation group compared with the control (P = 0.08 and P < 0.0001, for the two bands and four bands, respectively) (Table 6).
Fig. 2.
The PCR-DGGE fingerprints of the16S rRNA gene (V3 region) from the adhesive intestinal microbiota of hybrid tilapia after the eight weeks of feeding. Three fish were randomly selected to isolate gut content for gut bacteria detection by PCR-DGGE (n = 3). NT = nucleotides.
Table 6.
Closest relatives and relative abundance of bands from 16S rRNA gene V3 PCR-DGGE fingerprint of adhesive microbiota for the control and 0.6% groups.
| Phylum | Band No. | Closest relative (BLAST search) | Identity, % | Relative abundance1, % |
|
|---|---|---|---|---|---|
| Control | 0.60% nucleotides | ||||
| Firmicutes | 1 | Lachnospiraceae bacterium (GU723317.1) | 99 | 6.4 ± 1.0 | 6.0.4 ± 0.5 |
| 3 | Streptococcus pleomorphus (NR_044660.1) | 100 | 18.1 ± 0.7 | 21.1 ± 2.0 | |
| 4⁎ | Clostridium acidurici (HE582772.1) | 98 | 9.9 ± 0.3 | 7.9 ± 0.6 | |
| 8 | Clostridium beijerinckii (AB600543.1) | 100 | 3.5 ± 0.8 | 1.4 ± 0.7 | |
| 9 | Robinsoniella peoriensis (JN642224.1) | 98 | 3.3 ± 0.5 | 2.6 ± 0.3 | |
| 10 | Faecalibacterium prausnitzii (HQ457030.1) | 100 | 4.1 ± 0.4 | 4.4 ± 0.6 | |
| 11 | Anaerostipes hadrus (NR_104799.1) | 100 | 3.3 ± 0.3 | 1.0 ± 1.0 | |
| 12⁎⁎⁎ | Lachnospiraceae bacterium (EU728751.1) | 100 | 5.3 ± 0.5 | 0 | |
| 14 | Streptococcus sp. (FJ611790.1) | 100 | 24.0 ± 4.0 | 28.1 ± 0.7 | |
| 10 + 112 | – | – | 7.4 ± 0.2 | 5.4 ± 0.9 | |
| 1 + 10+11 + 12⁎⁎⁎2 | – | – | 19.1 ± 0.5 | 11.8 ± 0.4 | |
| Proteobacteria | 5 | Escherichia coli (KC138767.1) | 100 | 1.4 ± 1.4 | 3.9 ± 0.2 |
| 6⁎⁎⁎ | Plesiomonas sp. (FJ405284.1) | 100 | 0 | 3.6 ± 1.0 | |
| 7⁎ | Plesiomonas shigelloides (JQ582978.1) | 99 | 6.3 ± 0.6 | 3.9 ± 0.5 | |
| 13 | Plesiomonas sp. (AB192384.1) | 100 | 7.4 ± 1.0 | 8.0 ± 0.6 | |
| Bacteroidetes | 2 | Bacteroides propionicifaciens (AB264624.2) | 100 | 7.2 ± 0.2 | 7.7 ± 0.5 |
⁎ and ⁎⁎⁎ represent significant differences P < 0.05 and P < 0.001, respectively, in relative abundance of the band(s) between the control and 0.60% groups.
Relative abundance data are means of triplicate and presented as means ± SEM, n = 3.
Cumulative relative abundance of putative butyrate-producing species in the adhesive intestinal microbiota.
Fig. 3.
Cluster analysis of the intestinal microbiota of hybrid tilapia in the control and 0.6% nucleotides inclusion group based on 16S rRNA gene V3 denaturing gradient gel electrophoresis (DGGE) fingerprints. C1, C2, C3 = triplicates of control group; 0.6%-1, 0.6%-2, 0.6%-3 = triplicates of 0.6% group.
4. Discussion
One of main contributions was to uncover the optimal dosage of nucleotide supplement for the aquaculture of juvenile hybrid tilapia. Combined with growth performance, feed efficiency, immunity and intestine growth, 0.60% addition of nucleotide in diets was optimal. Dose-response effects of dietary nucleotides inclusion were investigated in a few studies (Huu et al., 2012, Lin et al., 2009, Tahmasebi-Kohyani et al., 2011, Xiang et al., 2011) and the large variation of optimal doses in these studies may be due to differences among species. Another important confounder may come from the difference in actual levels of nucleotides in the commercial products. Moreover, some ingredients in the basal diet, such as fish meal, are high in nucleotides, and the reported optimal doses of nucleotides actually reflect levels that was additional to nucleotides already present in the basal diet (Huu et al., 2012). The commercial nucleotide products extracted from yeast commonly contain impure components such as trace element and polysaccharides (Lin et al., 2009). The compromised performance of fish fed the nucleotide at a level higher than 0.60% confirmed nucleotides as the active component for the observed improvements, as the effects of other potential ingredients would not likely peak at such a low inclusion dose, considering their low concentrations in the yeast nucleotides product.
Dietary nucleotides supplementation has been reported to improve intestinal microstructure morphology in terms of increased fold height, enterocyte height and microvilli length in different enteric sections of fish (Burrells et al., 2001a, Cheng et al., 2011). The resultant increase in the mucosal surface area was suggested as a contributing factor to the improved growth of fish (Burrells et al., 2001a). In our study, longer intestine length were observed in 0.30 and 0.60% nucleotide groups, indicating improvement of intestine growth by dietary nucleotide supplementation. Notably, the intestine length also peaked at 0.6% nucleotide supplementation, with reduced length observed at the 1.2% group. This pattern was consistent with that for the growth performance and feed utilization, implying that the improved growth of the intestine may contribute to nutrient digestion and absorption and therefore the growth of fish.
Tissue MDA content is widely used as biomarker for oxidative damage of lipids, which may result from generation of excess reactive oxygen species (ROS) (Zhao et al., 2014). The ROS can be removed by antioxidant enzymes, with SOD as a key component in this system. In this study, the MDA content and SOD activity in the liver tissue were tested. Significantly improved SOD activity and lower MDA were observed in the 0.60 and 1.20% groups compared with the control, indicating that nucleotides supplementation may enhance the anti-oxidant status of the liver in hybrid tilapia. Further research is warranted to investigate if similar effect exists in other tissues.
Serum complement and lysozyme activity are important components of the humoural innate immune system, protecting fish from potentially invasive organisms (Tahmasebi-Kohyani et al., 2011). In the present study, serum lysozyme activity was improved in the 0.15 and 0.30% groups, indicating a stimulating effect of nucleotides on the innate immune response of hybrid tilapia. However, the complement activity, as assayed by C3 and C4 levels which plays a central role in the complement system and contributes to innate immunity, was not influenced, which doesn׳t agree with the positive effects reported in other species (Sakai et al., 2001, Tahmasebi-Kohyani et al., 2011). Marginally higher levels of serum globulin and significantly lower A:G ratio were observed in the 0.60% group compared with the control, indicating the presence of more globulin. A higher concentration of globulin and lower A:G ratio have been associated with enhanced antibody response in fish (Choudhury et al., 2005). Therefore, dietary nucleotides supplementation has the potential to lead to enhanced antibody response of tilapia under vaccination or challenges. Nevertheless, further research is warranted to investigate whether the improved immunity may translate to enhanced disease resistance of tilapia, as reported in many other aquatic species (Burrells et al., 2001b, Li et al., 2004, Sakai et al., 2001).
Only the microbial pattern of the 0.60% group was compared with the control in detail, due to its optimal effect on growth and other parameters. The nucleotide supplementation exerted a moderate influence on the intestinal microbiota of tilapia, with only four low-abundance phylotypes significantly affected in the 0.60% group compared with the control. In humans, a similar scale of influence on gut microbiota was observed with nucleotide supplementation, which was associated with considerable differences in microbiota balance and function (Singhal et al., 2008). Therefore, the change of the intestinal microbiota of hybrid tilapia induced by nucleotide supplementation should not be considered negligible.
Species of bacteria associated with butyrate production were found in the microbiota of tilapia, including Anaerostipes hadrus, Faecalibacterium prausnitzii, and species belonging to Lachnospiraceae. Anaerostipes hadrus represents more than 2% of the total microbiota in the healthy colon of humans and may produce butyrate from lactic acid at mildly acidic conditions (Allen-Vercoe et al., 2012). In both cases, the cumulative abundance of putative butyrate-producing species decreased in the nucleotide supplementation group. Although the information needs to be confirmed in fish, butyrate is the primary energy source for the epithelial enterocytes (Lopez-Siles et al., 2012). The decreased abundance of butyrate-producing bacteria in the intestinal microbiota of fish might be due to a competing or substituting effect between butyrate and nucleotides as energy-providers for the enterocytes of fish, as nucleotides may also provide energy for cells, besides acting as base units for nucleic acid synthesis and a series of other physiological and biochemical functions (Vanburen et al., 1994). It is also known that intestinal mucosal tissues have limited capacity for the de novo synthesis of nucleotides and depend on supply by the salvage pathway (Ramadan and Atef, 1991, Yamauchi et al., 2002). In this regard, dietary nucleotides may save energy for the intestinal mucosal tissues, as the salvage pathway requires high energy (Barness, 1994, Cosgrove, 1998). The consequent decreased demand for energy of the intestinal mucosal tissues might also contribute to the reduction in the abundance of butyrate-producers, as butyrate is putative energy source for the enterocytes. Alternatively, the observed change in the abundance of butyrate-producing bacteria may be a direct inhibition effect of nucleotides. In human study, the improved richness of bifidobacteria was attributed to a direct nutritional or prebiotic effect of nucleotides (Singhal et al., 2008). Due to the limited knowledge about dietary supplemented nucleotides in fish, especially on their metabolism and influence on various physiological processes, it is hard to determine the mechanisms of the effect of dietary nucleotides on intestinal microbiota and in particular the potential interaction of nucleotides supplementation with butyrate production, metabolism and associated butyrate producing bacteria. Further investigations on this topic are meaningful, especially considering that butyrate has been used as functional dietary supplements in fish feed (Liu et al., 2014).
5. Conclusion
Dietary nucleotide supplementation may improve the growth, feed utilization, intestinal growth, anti-oxidant status and non-specific immune response of hybrid tilapia. Regression analysis results for weight gain and feed efficiency, as well as the dose-response results of other parameters, suggest a dietary level between 0.6 and 0.8% to give optimal performance of juvenile hybrid tilapia. Nucleotide supplementation exerted moderate influence on the intestinal microbiota of tilapia, and the cumulative abundance of putative butyrate-producing species in the intestinal microbiota was reduced by nucleotide supplementation, suggesting interaction between dietary nucleotides and butyrate production, which deserves further investigation.
Acknowledgements
This work was supported by grants from the National Science and Technology Support Program Project of China (2014CB138600, 2012BAD25B02, 2015CB150605), the National Natural Science Foundation of China (31272672 to ZGZ) and the Beijing Earmarked Fund for Modern Agro-industry Technology Research System (SCGWZJ20141104-4). We thank Dr. T Wang at University of Aberdeen for helpful discussion and manuscript editing.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Allen-Vercoe E., Daigneault M., White A., Panaccione R., Duncan S.H., Flint H.J. Anaerostipes hadrus comb. nov., a dominant species within the human colonic microbiota; reclassification of Eubacterium hadrum Moore et al. 1976. Anaerobe. 2012;18:523–529. doi: 10.1016/j.anaerobe.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Barness L.A. Dietary sources of nucleotides-from breast-milk to weaning. J Nutr. 1994;124 doi: 10.1093/jn/124.suppl_1.128S. 128S--30S. [DOI] [PubMed] [Google Scholar]
- Borda E., Martinez-Puig D., Cordoba X. A balanced nucleotide supply makes sense. Feed Mix. 2003;11:24–26. [Google Scholar]
- Bradstreet R.B. The Kjeldahl method for organic nitrogen. Anal Chem. 1954;26:185–187. [Google Scholar]
- Burrells C., Williams P.D., Southgate P.J., Wadsworth S.L. Dietary nucleotides: a novel supplement in fish feeds: 2. Effects on vaccination, salt water transfer, growth rate and physiology of Atlantic salmon (Salmo salar L.) Aquaculture. 2001;199:171–184. [Google Scholar]
- Burrells C., Williams P.D., Forno P.F. Dietary nucleotides: a novel supplement in fish feeds: 1. Effects on resistance to disease in salmonids. Aquaculture. 2001;199:159–169. [Google Scholar]
- Carver J.D. Advances in nutritional modifications of infant formulas. Am J Clin Nutr. 2003;77 doi: 10.1093/ajcn/77.6.1550S. 1550S--4S. [DOI] [PubMed] [Google Scholar]
- Carver J.D., Walker W.A. The role of nucleotides in human nutrition. J Nutr Biochem. 1995;6:58e72. [Google Scholar]
- Council N.R. National Academy Press; Washington, DC: 1993. Nutrient requirements of fish; p. 114. [Google Scholar]
- Cheng Z., Buentello A., Gatlin D.M., 3rd Dietary nucleotides influence immune responses and intestinal morphology of red drum Sciaenops ocellatus. Fish Shellfish Immunol. 2011;30:143–147. doi: 10.1016/j.fsi.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Choudhury D., Pal A.K., Sahu N.P., Kumar S., Das S.S., Mukherjee S.C. Dietary yeast RNA supplementation reduces mortality by Aeromonas hydrophila in rohu (Labeo rohita L.) juveniles. Fish Shellfish Immunol. 2005;19:281–291. doi: 10.1016/j.fsi.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Cosgrove M. Nucleotides. Nutrition. 1998;14:748–751. doi: 10.1016/s0899-9007(98)00075-6. [DOI] [PubMed] [Google Scholar]
- Huu H.D., Tabrett S., Hoffmann K., Köppel P., Lucas J.S., Barnes A.C. Dietary nucleotides are semi-essential nutrients for optimal growth of black tiger shrimp (Penaeus monodon) Aquaculture. 2012;366--367:115–121. [Google Scholar]
- Leonardi M., Sandino A., Klempau A. Effect of a nucleotide-enriched diet on the immune system, plasma cortisol levels and resistance to infectious pancreatic necrosis (IPN) in juvenile rainbow trout (Oncorhynchus mykiss) Bull Eur Assoc Fish Pathol. 2003;23:52–59. [Google Scholar]
- Li P., Gatlin D.M., III Nucleotide nutrition in fish: current knowledge and future applications. Aquaculture. 2006;251:141–152. [Google Scholar]
- Li P., Lewis D.H., Gatlin D.M., 3rd Dietary oligonucleotides from yeast RNA influence immune responses and resistance of hybrid striped bass (Moronechrysopsx Moronesaxatilis) to Streptococcus iniae infection. Fish Shellfish Immunol. 2004;16:561–569. doi: 10.1016/j.fsi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Wang H., Shiau S.Y. Dietary nucleotide supplementation enhances growth and immune responses of grouper, Epinephelus malabaricus. Aquacult Nutr. 2009;15:117–122. [Google Scholar]
- Liu W., Yang Y., Zhang J., Gatlin D.M., Ringø E., Zhou Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br J Nutr. 2014;112:15–29. doi: 10.1017/S0007114514000610. [DOI] [PubMed] [Google Scholar]
- Lopez-Siles M., Khan T.M., Duncan S.H., Harmsen H.J., Garcia-Gil L.J., Flint H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl Environ Microbiol. 2012;78:420–428. doi: 10.1128/AEM.06858-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Garcιa J.L., Luque de M.D. Ultrasound-assisted Soxhlet extraction: an expeditive approach for solid sample treatment: Application to the extraction of total fat from oleaginous seeds. J Chromatogr A. 2004;1034:237–242. doi: 10.1016/j.chroma.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Meehan C.J., Beiko R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 2014;6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Xu W., Ai Q., Mai K., Liufu Z., Zhang K. Effects of nucleotide supplementation on growth, immune responses and intestinal morphology in juvenile turbot fed diets with graded levels of soybean meal (Scophthalmus maximus L.) Aquaculture. 2013;392--395:51–58. [Google Scholar]
- Quan R. Dietary nucleotides: potential for immune enhancement. In: Paubert-Braquet M., Dupont C., Paoletti R., editors. Foods, nutrition and immunity. Dyn Nutr Res Basel Karger; New York: 1992. pp. 13–21. [Google Scholar]
- Ramadan A., Atef M. Effect of the biogenic performance enhancer (Ascogen ”S”) on growth rate of tilapia fish. Acta Vet Scand. 1991;87:S304–S306. [Google Scholar]
- Ramadan A., Afifi N.A., Moustafa M., Samy A.M. The effect of ascogen on the immune response of tilapia fish to Aeromonas hydrophila vaccine. Fish Shellfish Immunol. 1994;5:159–165. [Google Scholar]
- Rawles S.D., Gatlin D.M., III Nutrient digestibility of common feedstuffs in extruded diets for sunshine bass (M. chrysops ♀ × M. saxatilis ♂) J World Aquac Soc. 2000;31:570–579. [Google Scholar]
- Sakai M., Taniguchi K., Mamoto K., Ogawa H., Tabata M. Immunostimulant effects of nucleotide isolated from yeast RNA on carp, Cyprinus carpio L. J Fish Dis. 2001;24:433–438. [Google Scholar]
- Singhal A., Macfarlane G., Macfarlane S., Lanigan J., Kennedy K., Elias-Jones A. Dietary nucleotides and fecal microbiota in formula-fed infants: a randomized controlled trial. Am J Clin Nutr. 2008;87:1785–1792. doi: 10.1093/ajcn/87.6.1785. [DOI] [PubMed] [Google Scholar]
- Tahmasebi-Kohyani A., Keyvanshokooh S., Nematollahi A., Mahmoudi N., Pasha-Zanoosi H. Dietary administration of nucleotides to enhance growth, humoral immune responses, and disease resistance of the rainbow trout (Oncorhynchus mykiss) fingerlings. Fish Shellfish Immunol. 2011;30:189–193. doi: 10.1016/j.fsi.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Uauy R., Quan R., Gil A. Role of nucleotides in intestinal development and repair: implications for infant nutrition. J Nutr. 1994;124 doi: 10.1093/jn/124.suppl_8.1436S. 1436S--41S. [DOI] [PubMed] [Google Scholar]
- Vanburen C.T., Kulkarni A.D., Rudolph F.B. The role of nucleotides in adult nutrition. J Nutr. 1994;124:S160–S164. doi: 10.1093/jn/124.suppl_1.160S. [DOI] [PubMed] [Google Scholar]
- Walker H.K., Hall W.D., Hurst J.W. 3rd ed. Butterworth Publishers; Boston: 1990. Clinical methods: the history, physical, and laboratory examinations. [PubMed] [Google Scholar]
- Welker T.L., Lim C., Yildirim-Aksoy M., Klesius P.H. Effects of dietary supplementation of a purified nucleotide mixture on immune function and disease and stress resistance in channel catfish, Ictalurus punctatus. Aquac Res. 2011;42:1878–1889. [Google Scholar]
- Whitehead J., Wadsworth S., Carr I. The power of purified nucleotides. Aquac Health Int. 2006;4:14–16. [Google Scholar]
- Xiang X., Zhou X., Chen J., Zheng Z. Effects of yeast nucleotide on growth performance, body composition and immune indices of common carp (Cyprinus carpio) Chin J Anim Nutr. 2011;23:171–178. [Google Scholar]
- Yamauchi K., Hales N.W., Robinson S.M., Niehoff M.L., Ramesh V., Pellis N.R. Dietary nucleotides prevent decrease in cellular immunity in ground-based microgravity analog. J Appl Physiol. 2002;93:161–166. doi: 10.1152/japplphysiol.01084.2001. [DOI] [PubMed] [Google Scholar]
- Zhao J., Feng L., Liu Y., Jiang W., Wu P., Jiang J. Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol. 2014;41:663–673. doi: 10.1016/j.fsi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Zhou Z.G., Wang P.B., Lv H.Y., Wang H.K., Lemme A. L-lysine sulphate in diets improves FCR and morphological parameters of hybrid tilapia. Asia Pac Aquac. 2007;3:21–23. [Google Scholar]
- Zhou Z., Liu Y., Shi P., He S., Yao B., Ringø E. Molecular characterization of the autochthonous microbiota in the gastrointestinal tract of adult yellow grouper (Epinephelus awoara) cultured in cages. Aquaculture. 2009;286:184–189. [Google Scholar]