Abstract
Objective
We conducted a retrospective, case–control study of neurocysticercosis patients to ascertain early markers that identify subjects likely to develop treatment‐resistant seizures.
Methods
Clinical histories and imaging studies from 38 neurocysticercosis patients who had been followed for 18 months after treatment were evaluated. Both pairwise and multifactorial analyses were conducted to identify factors associated with continued seizures.
Results
Eleven of 38 patients continued to have seizures during the follow‐up period. On univariate analysis, the number of neurocysticercosis lesions, number of bands on the baseline neurocysticercosis western blot, edema volumes on follow‐up MRI scans, edema volume changes between baseline and follow‐up images, and proportion of calcified lesions with perilesional edema were all significantly increased in subjects who had persistent seizures during the 18‐month follow‐up period. On multivariate analyses using recursive partition and random forest algorithms, variables associated with persistent seizures included: the number of total and calcified lesions, presence of perilesional edema, the rate of change in the lesion and edema volumes from baseline to follow‐up, and the number of bands on the neurocysticercosis western blot.
Interpretation
Measures of both inflammation and disease burden are key risk factors for persistent seizures despite anticonvulsant treatments in patients with neurocysticercosis. Inflammation is therefore a potentially modifiable risk factor for the frequently seen severe seizure disorders in patients with neurocysticercosis.
Introduction
The parasitic infection neurocysticercosis (NCC) frequently presents with seizures and lesions in the degenerating and calcified stages.1, 2, 3 These seizures are thought to be caused by perilesional gliosis, inflammation, and/or neurotoxicity from calcified lesions1, 4, 5, 6, 7 and may lead to epilepsy (a disease of chronic recurrent seizures). NCC is the most common cause of acquired adult epilepsy worldwide.4, 7, 8, 9, 10, 11, 12, 13 Epilepsy due to NCC can lead to severe health consequences as seizures are associated with an increased risk of sudden death14, 15, 16, 17 and 20–30% of NCC patients with epilepsy continue to have seizures despite treatment.9
Early identification of patients at high risk for persistent seizures is essential. This is because epilepsy involves the creation of abnormal electrical circuits that become increasingly well‐established with each seizure.16, 18, 19, 20, 21 Ideally, subjects would remain on anticonvulsant medications until their NCC lesions are no longer active, they have received an adequate treatment course, and they are no longer at high risk for seizure relapses. It is not optimal to keep subjects on anticonvulsants for longer than necessary as these medications can have severe side‐effects.22 Therefore, the ability to risk‐stratify NCC patients for persistent seizures early on would be beneficial to help identify subjects eligible for tapering of anti‐epileptic medications.
We retrospectively analyzed a subset of cases from a large clinical trial of NCC patients with seizures23 to identify clinical parameters associated with an increased risk for persistent seizures despite treatment. We found that disease burden and a strong and enduring immune inflammatory response to the parasite were the best predictors of persistent seizures.
Methods
Study design and population
Thirty‐eight patients from a blinded, placebo‐controlled phase 3 treatment trial for NCC23 were selected for retrospective study using an unmatched case–control design. The treatment trial had been conducted at the National Institute of Neurologic Sciences in Peru in conjunction with the National Institute of Neurological Disorders and Stroke at the US National Institutes of Health (NIH Project Number NS054805). The study randomly assigned subjects to receive dexamethasone, anticonvulsants, and one of three different antiparasitic treatment regimens (Table 1). Randomization was stratified by the number of viable lesions. All subjects had experienced a seizure in the preceding year, were ages 16–65, and had a positive NCC western blot. Close surveillance of anti‐inflammatory and anticonvulsant medications was performed to ensure compliance, including routine pill counts at each study visit and phone calls to assess compliance between visits. Patients were followed up for 18 months after treatment. MRI and CT scans were performed at baseline and 6‐months posttreatment.
Table 1.
Demographics and baseline presentation of 38 subjects with NCC and seizures
| Characteristic | Value (N = 38) |
|---|---|
| Treatment arm, N (%) | |
| Standard‐dose albendazole[Link] | 16 (42.1) |
| High‐dose albendazole[Link] | 13 (34.2) |
| Standard‐dose albendazole with praziquantel[Link] | 9 (23.7) |
| Age, median years (range, SD in years) | 32 (16–63, 13.3) |
| Gender, male/female, N (% male) | 22/16 (57.9) |
| Time between first seizure and study enrollment, mean months, (range, SD in months) | 15.7 (1–293, 64.7) |
| Time between most recent seizure and study enrollment, mean months, (range, SD in months) | 1.6 (1–15, 3) |
| Seizure type reported at baseline visit, N (%) | |
| Partial without generalization | 29 (76.3) |
| Partial secondarily generalized | 22 (57.9) |
| Generalized | 17 (44.7) |
| Additional symptoms reported at baseline, N (%) | |
| Headache | 35 (92.1) |
| Dizziness | 20 (52.6) |
| Paresthesia/paresis | 20 (52.6) |
| Decreased memory | 19 (50) |
| Number of NCC lesions per patient,[Link] median (IQR) | 4.0 (1–11.8) |
| Number of bands on baseline NCC western blot, mean (SD) | 4.3 (2) |
| Subjects with persistent seizures during follow‐up,[Link] N (%) | 11 (28.9) |
NCC, neurocysticercosis; N, Number of subjects; 1Standard dose albendazole = 15 mg/kg/day; 2High‐dose albendazole = 22.5 mg/kg/day; 3praziquantel dose = 50 mg/kg/day; SD, standard deviation; 4total number of NCC lesions seen on CT and MRI scans at baseline; IQR, interquartile range; 5defined as seizures which occurred greater than 60 days following the start of antiparasitic therapy.
There were no differences between the three treatment arms in the number of seizures during the follow‐up period. The 38 subjects selected for our retrospective evaluation comprised the stratified group with 1–2 viable cysts. This group was chosen in an effort to minimize bias caused by the treatment provided, because within this subgroup no differences were seen in efficacy between the three treatment arms.
Study procedures
Clinical data and imaging studies from 38 subjects were obtained. Calcified lesions were identified on CT scans, degenerating (colloidal and nodular stages) lesions were identified on T1 and T2 MRI images, and perilesional edema was assessed on T2 FLAIR images. Viable lesions were not evaluated as all subjects were from a stratified group with 1–2 viable cysts. Imaging scans were reviewed by JH, GG, and JK for the number of lesions and the location, size, and stage of each lesion. EEGs were evaluated by two trained epileptologists (AG and JL).
Identification of patients with persistent seizures
Persistent seizures were defined as those that occurred greater than 2 months following the start of antiparasitic therapy. This time frame was chosen to avoid confusing epileptic seizures with those provoked by treatment‐associated inflammation.24 As all enrolled subjects were treated with anticonvulsant medications, any persistent seizures were considered treatment resistant.
Edema volume
A reviewer blinded to the patients’ seizure histories and using digitized images taken of the original films calculated volumes of edema. On the T2 FLAIR MRI images, areas of edema were marked on each brain slice and the number of pixels within the marked area was determined using standard Adobe Photoshop tools. The number of pixels within a square area based on the 5‐ or 10‐cm scale printed on the films was counted and used as a reference to convert the edema regions in pixels to cm2. The slice thickness was multiplied and the volume measurements were then summed to get total volumes of edema.
Statistical analysis
Data were summarized using frequency with percentage for categorical variables and geometric mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables. Fisher's exact test was used to evaluate correlations between covariates and the main outcome, persistent seizures, for categorical variables and Mann–Whitney U test was used for continuous variables. Repeated measurements from the same subjects were compared using the Wilcoxon matched‐pairs signed‐rank test.
Multifactorial analysis
Data were further evaluated using machine‐learning algorithms (variable importance as well as model fit) to predict the primary outcome, persistent seizures. The consolidated data were processed using a recursive partition algorithm (rpart in R)25 and a random forest algorithm26 to classify patients with persistent seizures. Important variables contributing to continuing seizures were calculated using gene index and a classification model was built to predict the primary outcome.
Standard protocol approvals, registrations, and patient consents
The original treatment study was approved by the main institutional review board at the Universidad Peruana Cayetano Heredia (IRB Code 51070, FWA 00002541) and was registered with ClinicalTrials.gov, number NCT00441285. All subjects signed informed consent prior to enrollment. The retrospective analysis was granted an exemption from the University of Illinois at Chicago IRB as all data had previously been de‐identified. The original trial that provided these data was supported by NINDS grant R01 NS058405.
Results
Patient demographics
Thirty‐eight subjects from a stratified group with 1–2 viable cysts, and from all three of the study treatment arms (Table 1), were randomly selected. The median age was 32 years and 22 were male. Thirty‐one of 38 subjects (81.6%) had experienced generalized or secondarily generalized seizures prior to enrollment; 8 of 38 subjects (21%) reported having had more than one type of seizure. No significant abnormalities were seen on neurologic exam for any subjects.
On baseline imaging, the median number of NCC lesions per patient was 4 (Table 1). The mean number of bands on the baseline NCC western blot was 4.3 (of 7 possible bands),27 and 69.4% of patients (25 of the 36 subjects with documented western blot results) had a strong serologic reaction (≥4 bands).13 Eleven of the 38 subjects (28.9%) experienced persistent seizures during the follow‐up period.
Disease burden, continued edema on follow‐up imaging, and perilesional edema surrounding calcifications were all associated with persistent seizures
The median number of degenerating lesions was the same (1) in both groups (Fig. 1D). However, the median number of calcified (Fig. 1E) and total lesions on baseline imaging was increased in the persistent seizure group (median number of calcified lesions 11; median total number of lesions 12) compared to those who were seizure free (calcified lesions 1; total lesions 2, both P‐values = 0.006). The number of viable lesions was not compared since all subjects were from a stratified group with 1–2 viable cysts.
Figure 1.
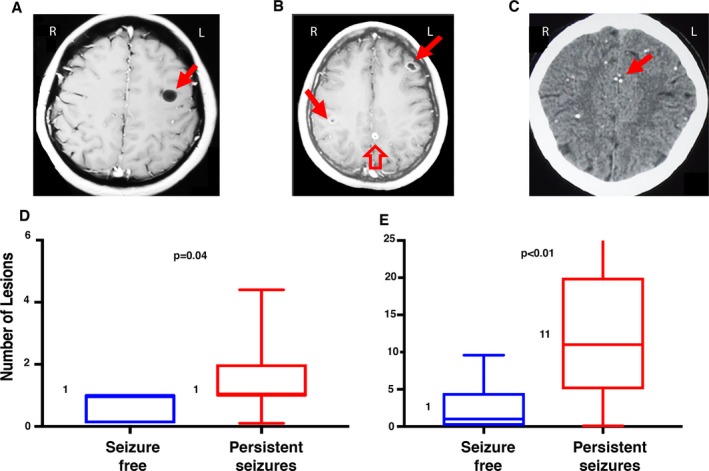
Number and stage of lesions on baseline imaging in those with and without persistent seizures. Representative baseline T1 MRI images that exemplify the four stages of NCC lesions are shown on the top row. Image A shows a vesicular lesion without a visible scolex (solid arrow). Image B shows lesions in the degenerating (colloidal and nodular) stages. The image has two colloidal lesions (solid arrows) and one nodular lesion (hollow arrow). All three lesions demonstrate perilesional enhancement. Image C shows multiple calcified lesions on a CT scan, with a solid arrow pointing to a cluster of calcifications. Images D and E (bottom row) show the median number of degenerating (Image D) and calcified (Image E) lesions at baseline in subjects from the persistent seizure group (in red) compared to those who were seizure free on follow‐up (blue). The box plots delineate the interquartile range and the whiskers span the 10th–90th percentile, with a horizontal line and number at the median. Viable lesions were not evaluated since all subjects in this retrospective study were from a stratified group with 1–2 viable cysts.
On the baseline imaging scans, nearly all subjects had edema. All areas of edema (at both baseline and follow‐up) surrounded an NCC lesion. For all evaluations of perilesional edema on follow‐up imaging studies, measurements were also compared with scans obtained within 60 days of a seizure excluded in order to avoid confusing inflammation for a postictal vasogenic response.28 Excluding these scans did not significantly alter any of the measured variables, so all data included in the results show the complete dataset with no scans excluded.
At baseline, no significant differences were seen between the groups in median edema volumes per scan or per lesion (Table 2). On the follow‐up imaging studies obtained 6 months after treatment, however, subjects with persistent seizures had markedly increased total and per lesion edema volumes compared to those who were seizure free. When evaluating edema volume changes from baseline to follow‐up within subjects, follow‐up MRI scans from the seizure‐free group showed a considerable reduction in both the total edema volume per scan and the edema volume per lesion compared to baseline values (median total edema 10.4 cm3 at baseline and 0.2 cm3 on follow‐up, Figure 2A; median edema volume per lesion 6.3 cm3 at baseline and 0.2 cm3 on follow‐up, both P ‐values < 0.001). In contrast, follow‐up imaging subjects in the persistent seizure group showed no statistically significant reductions in either of these volumes (median total edema per scan 7.7 cm3 at baseline and 3.1 on follow‐up, P = 0.6, Figure 2B; median volume per lesion at baseline 2.7 vs. 1.6 on follow‐up, P = 0.3). An increased volume of edema on the 6‐month follow‐up MRI scan was also seen in subjects with seizures during the time period between 6 and 18 months after treatment compared to those without (data not shown). Only one subject in the seizure‐free group demonstrated an increase in the total volume of perilesional edema per scan from the baseline to the follow‐up imaging (1/23, 4.3%, vs. 4/11, 36.4% of those with persistent seizures, odds ratio = 12.6, 95% CI 1.5–159, P = 0.03).
Table 2.
Imaging findings and clinical variables associated with persistent seizures
| Characteristic | Seizure free (N = 27) | Persistent seizure (N = 11) | P‐value | |
|---|---|---|---|---|
| Volume of edema on T2 FLAIR MRI scans | ||||
| Total edema volume per scan, median cm3 (IQR) | Baseline | 10.4 (1.9–20.5) | 7.7 (2–12.3) | 0.70 |
| Follow‐up | 0.2 (0–0.9) | 3.1 (0–15.8) | 0.01 | |
| Edema change within groups from baseline to follow‐up, P‐value[Link] | <0.001 | 0.6 | ||
| Edema volume per lesion,[Link] median cm3/lesion (IQR) | Baseline | 6.3 (1–13) | 2.7 (0.6–6.2) | 0.10 |
| Follow‐up | 0.2 (0–0.6) | 1.6 (0–2.2) | 0.04 | |
| Edema change within groups from baseline to follow‐up, P‐value[Link] | <0.001 | 0.3 | ||
| Proportion of calcified lesions with perilesional edema | ||||
| Number with edema/total number of calcifications per group, (%) | Baseline | 7/133 (5.3) | 18/121 (14.9) | 0.01 |
| Follow‐up | 0/123 (0) | 21/113 (18.6) | <0.001 | |
N, number; IQR, interquartile range; 1determined using Wilcoxon matched‐pairs signed‐rank test; 2defined as the total volume of edema on the scan divided by the number of lesions with perilesional edema seen on the scan.
The bold values in this table highlight the p‐values that were statistically significant (p<0.05).
Figure 2.
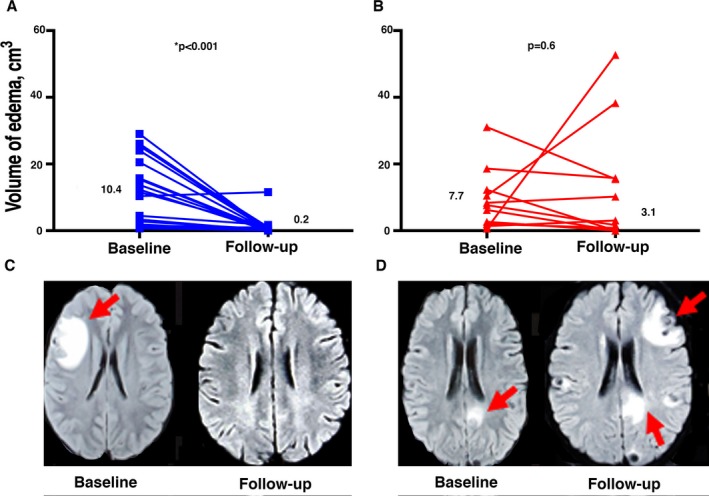
Edema volumes at baseline and follow‐up in subjects with and without persistent seizures. Images A and B show the total volume of edema seen on each T2 FLAIR MRI scan for subjects in the seizure‐free (Image A, denoted as blue squares) and persistent seizure (Image B, denoted as red triangles) groups at baseline and follow‐up. Numbers indicate the median values for each group. Images C and D show representative baseline and 6‐month follow‐up T2 FLAIR MRI scans from subjects in the seizure‐free (Image C) and persistent seizure (Image D) groups. Areas of edema are indicated by red arrows.
At both baseline and follow‐up, more subjects in the persistent seizure group compared to those who were seizure free had at least one calcified lesion with perilesional edema (both P ‐values <0.05, data not shown). The odds that an individual calcified lesion would have perilesional edema were higher at baseline for calcifications from the persistent seizure group (18/121 calcifications had perilesional edema, 14.9%) compared to the seizure‐free group (7/133, 5.3%, odds ratio = 3.2, 95% CI 1.3–8.2, P = 0.01), and this difference persisted on follow‐up imaging (Table 2).
An increased specific immunologic response to the parasite was seen in those with persistent seizures
The median number of bands on NCC western blot was increased in the persistent seizure group (7) compared to the seizure‐free group (4, P = 0.02, Fig. 3B), although for both groups this value was consistent with a strong serologic reaction (≥4 bands).13 Subjects with ≥4 bands on their baseline western blot who had edema at follow‐up had a larger median volume of edema (1.7 cm3) compared to those with 1–3 bands (median volume 0.3 cm3, P < 0.01, Fig. 3C). Subjects with a strong serologic reaction did not have an increased number of total, calcified, or degenerating NCC lesions compared to those without a strong reaction (all P ‐values >0.05). Interestingly, subjects with a strong serologic reaction had a greater number of seizures during the 18 months following treatment (median total number of seizures 2) compared to those with 1–3 bands (median number of seizures 0, P = 0.03).
Figure 3.
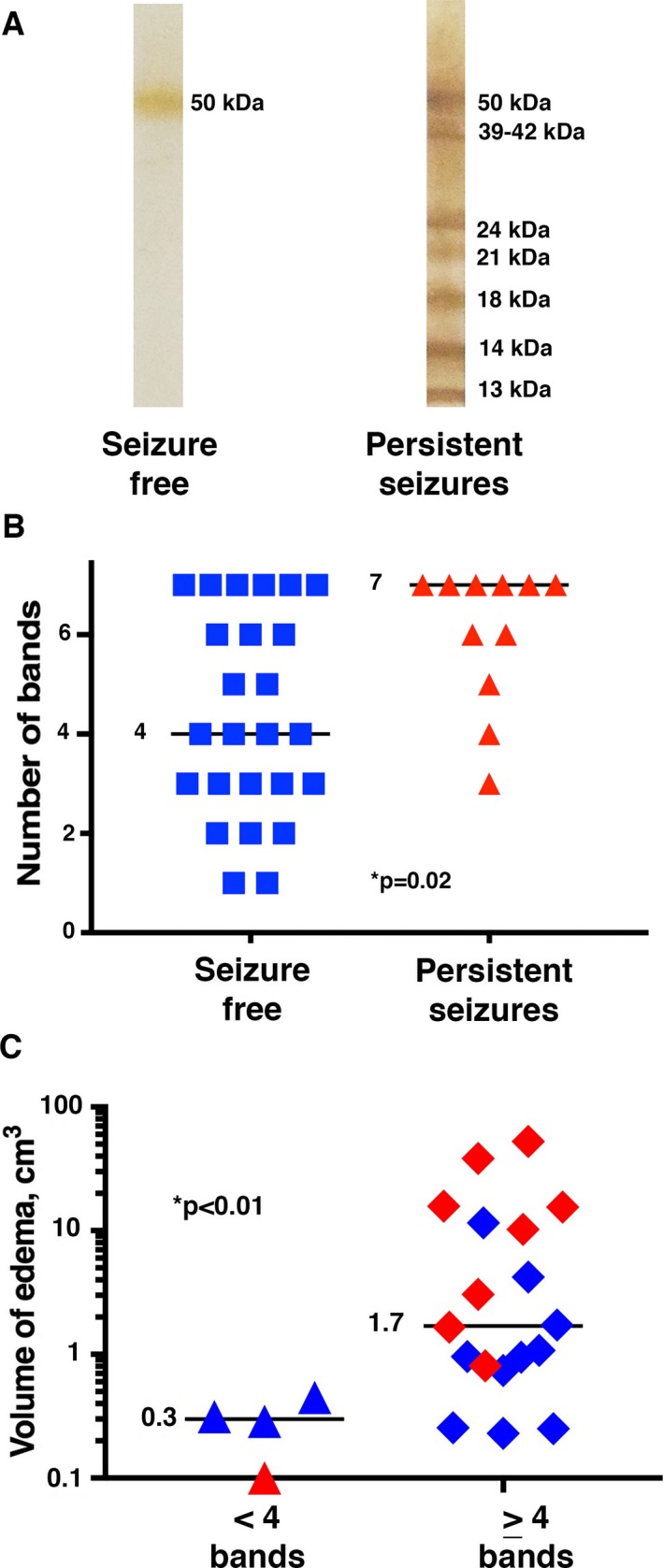
Association between persistent seizures and edema volume with western blot results. (A) shows representative NCC western blots from subjects in each group. The image on the left shows a western blot with 1 band from a subject in the seizure‐free group, while the one on the right has 7 bands (the maximum number possible) and was from a subject in the persistent seizure group. The diagnostic band groups are labeled on the right‐hand side of the western blots. (B) shows the number of bands on the baseline western blot for each subject in the seizure‐free (left, denoted by blue squares) and persistent seizure (right, denoted by red triangles) groups. The horizontal line and numbers show the median value for each group. (C) shows the volume of edema (of subjects with edema) on follow‐up T2 FLAIR images in subjects with (denoted as diamonds) and without (denoted as triangles) a strong serologic response (≥4 bands) on the baseline NCC western blot. The horizontal lines and numbers indicate the median values in each group. Subjects from the persistent seizure group are denoted in red, and those in the seizure‐free group are denoted in blue.
Multifactorial analysis confirmed that disease burden, chronic edema, and a heightened immunological response to the parasite were associated with persistent seizures
Significant variables associated with seizures on the recursive partition algorithm included: the number of calcified lesions, total number of NCC lesions, presence of perilesional edema, and the rate of change in the NCC lesion volume from baseline to follow‐up.
On the random forest algorithm, variables associated with continued seizures (arranged as decreasing value of gene index) included: the number of total and calcified NCC lesions, initial edema volume, rate of change in edema volume from baseline to follow‐up, number of bands on the NCC western blot, length of time from the initial seizure to study enrollment, and number of new NCC lesions at follow‐up. A 70/30 partition of the train and test datasets produced 100% accuracy prediction on the train dataset and 90% accuracy on the test dataset.
Neither clinical nor EEG variables differed between subjects with and without persistent seizures
There was no difference between groups in any evaluated EEG variables or in clinical symptoms, laboratory values, or anticonvulsant therapy given. A similar proportion of subjects with persistent seizures was seen among the three treatment arms (4/16, 25% had persistent seizures in the standard‐dose albendazole group; 3/13, 23% in the high‐dose albendazole group, and 4/9, 44% in the combined albendazole with praziquantel group, P = 0.5). There was no difference between the groups in the time elapsed from the first or most recent seizure to study enrollment (both P ‐values >0.7).
Discussion
Our study found that an increased immune inflammatory response in subjects with NCC, in addition to the burden of disease, was associated with continued seizures despite treatment with antiparasitic and anticonvulsant medications. This was demonstrated by the fact that subjects in the persistent seizure group had no reduction in the volume of perilesional edema per scan or per lesion on a 6‐month follow‐up MRI scan. In contrast, those who were seizure free had nearly complete resolution of edema following treatment.
It has been debated whether the perilesional edema frequently seen in NCC patients is due to a postictal vasogenic response or to inflammation.6, 28, 29, 30 In our study, we conducted a separate analysis with scans performed within 60 days of a seizure episode (those likely to contain vasogenic edema28) excluded. Even with these scans omitted from analysis, however, subjects in the persistent seizure group showed no significant reductions in perilesional edema from baseline to follow‐up images. It is possible that continued edema could result from a vasogenic response to subclinical seizures. Regardless of the underlying pathophysiology, however, continued perilesional edema on a posttreatment MRI scan could be a useful and novel biomarker predicting later treatment‐refractory seizures.
Our review found that an increased number of NCC lesions at baseline was seen in subjects with persistent seizures. Previous studies of the association between disease burden and seizure severity in NCC patients have had mixed results,4, 31 and thus it is possible that other factors can modify the strength of this association. Our study results suggest that the degree of the inflammatory response to the parasite may be one of these factors.
The proportion of NCC lesions that form calcifications rather than resolving has not been well‐established. In one study evaluating single enhancing lesions in young patients in India, approximately 10–20% of NCC granulomas formed calcified lesions.32 Many studies have shown an association between the formation of calcified lesions and seizures,1, 2, 6, 33, 34 both of which may be more likely in subjects with a strong inflammatory response. Although the process by which calcifications form is not completely understood, a breakdown in the blood–brain barrier due to inflammation could allow calcium from the bloodstream to be deposited into the brain parenchyma.35, 36
Seizures in subjects with calcified lesions could be caused by a neurotoxic effect of calcium or, as some authors have suggested, might be unrelated to calcifications.4 The role of inflammation as a contributing factor to seizures in subjects with calcified lesions has not been well studied, as these lesions have traditionally been thought to be immunologically inert. However, recent studies have provided evidence that calcifications may experience periodic remodeling that can expose parasite antigen and thus provoke a host immune response.1, 35, 37 Perilesional edema has been seen surrounding calcified lesions in symptomatic NCC patients,1, 2, 6 and PET scans evaluating this perilesional edema showed increased markers of neuroinflammation.29 Additionally, two case reports of excised calcified granulomas from NCC patients with recurrent seizures and perilesional edema demonstrated a marked mononuclear infiltrate surrounding these lesions,2, 37 suggesting that the perilesional edema was due to an immune inflammatory response.
Our results suggest that the seizure risk in subjects with calcifications may be associated with a subset of calcified lesions provoking an inflammatory response. As evidence for this, calcified lesions from subjects in the persistent seizure group were more likely than those in subjects without seizures to demonstrate perilesional edema. Why some calcifications provoke an inflammatory response while others do not is not well understood,35 and merits further study.
In population‐based studies performed in an endemic region, a strong serologic reaction (≥4 bands) on NCC western blot was shown to be associated with epilepsy.38 In our study, an increased number of bands was seen in subjects in the persistent seizure group, suggesting that the western blot results may also be associated with an increased risk for treatment‐refractory disease. Interestingly, the volume of perilesional edema, but not the number of NCC lesions, was increased in subjects with a strong serologic response. These results suggest that the immune reaction to the parasite, in conjunction with the burden of infection, determines the risk for treatment‐resistant seizures in subjects with NCC.
No differences were seen on any of the evaluated EEG parameters between the two groups. However, future evaluations using quantitative EEG analysis may prove to be helpful.
This study had several limitations. The sample size was small, and our review was retrospective. Additionally, as the clinical trial was not designed to evaluate imaging findings in subjects with persistent seizures, the MRI and CT scans were not always done at the same time. It is also possible that subjects with reactive calcified lesions (those showing perilesional edema) and an increased number of bands on the NCC western blot reflected an earlier stage of disease and these findings might not continue to be associated with persistent seizures at later disease stages. However, there was no difference between the two groups in the time between their first or most recent seizures and study enrollment, which argues against a difference in stages between the groups.
Despite our small sample size, we still identified factors that seemed to distinguish between subjects with and without persistent seizures. Our study results, including a multifactorial analysis, suggest that an inflammatory response to the parasite that does not resolve following treatment may be a key marker of an increased risk for persistent seizures in NCC patients. These findings raise intriguing questions about whether other measures of inflammation (such as erythrocyte sedimentation rate or c‐reactive protein) could be useful in identifying subjects with NCC at risk for persistent seizures and what effect treatment with intensive anti‐inflammatory regimens in high‐risk patients could have on seizure control. Because much of the research evaluating persistent seizures in NCC patients is retrospective, prospective and controlled studies using digital imaging and quantitative EEG analyses are urgently needed.
Author Contributions
Jesica A. Herrick was involved in acquisition of data, analysis and interpretation of data, evaluation of lesions on imaging data, statistical analyses, and writing of manuscript. Biswajit Maharathi performed volumetric measurements of edema on MRI scans and statistical analyses. Jin Suh Kim was the consulting radiologist responsible for evaluation of lesions on imaging data.
Gerardo Gomez Abundis also conducted evaluations of lesions on imaging data. Anjali Garg was responsible for evaluation of EEGs. Javier Bustos, Isidro Gonzales, and Herbert Saavedra were the clinicians providing care for patients of original clinical trial and therefore were involved in data collection; they also provided a critical review of the manuscript for intellectual content. H. Hugo Garcia was responsible for the study concept and design, acquisition of data, and study supervision of original clinical trial. Jeffrey A. Loeb performed a critical revision of manuscript for intellectual content and also helped with evaluation of EEG and imaging data, acquisition of data, and supervision of the concept and design of the retrospective review.
Conflict of Interest
Javier Bustos has received support from NIAID grant R01AI116456 and H. Hugo Garcia has received support from NIAID grant U19AI129909, NINDS grants U01NS086974 and R21NS094976, and FIC‐NIH training grant D43TW001140. The remaining authors have no conflicts of interest or disclosures and none of the authors have any financial associations with commercial entities that were involved with the project.
Acknowledgments
We acknowledge the funding support from the Chancellor's Global Health and Well‐Being Seed Grant Program of the UIC College of Medicine Center for Global Health. Dr. Herrick's research is supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050.
Funding Information
This study was funded in part using support from the Chancellor's Global Health and Well‐Being Seed Grant Program of the UIC College of Medicine Center for Global Health. Dr. Herrick's research is supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. HG, IG, and JB are supported by FIC‐NIH training grant D43 TW001140. The original trial that provided the data retrospectively assessed here was supported by NINDS grant R01 NS058405.
Funding Statement
This work was funded by Chancellor's Global Health grant ; UIC College of Medicine Center for Global Health grant ; National Center for Advancing Translational Sciences grant ; National Institutes of Health grant UL1TR000050; FIC‐NIH grant D43 TW001140; NINDS grant R01 NS058405.
References
- 1. Nash TE, Del Brutto OH, Butman JA, et al. Calcific neurocysticercosis and epileptogenesis. Neurology 2004;62:1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ooi WW, Wijemanne S, Thomas CB, et al. Short report: a calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: histopathological features. Am J Trop Med Hyg 2011;85:460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma LN, Garg RK, Verma R, et al. Seizure recurrence in patients with solitary cystic granuloma or single parenchymal cerebral calcification: a comparative evaluation. Seizure 2013;22:840–845. [DOI] [PubMed] [Google Scholar]
- 4. Carpio A, Romo ML. The relationship between neurocysticercosis and epilepsy: an endless debate. Arq Neuropsiquiatr 2014;72:383–390. [DOI] [PubMed] [Google Scholar]
- 5. de Souza A, Nalini A, Kovoor JM, et al. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long‐term seizure outcome: a prospective study using serial magnetization transfer imaging. Epilepsia 2011;52:1918–1927. [DOI] [PubMed] [Google Scholar]
- 6. Nash TE, Pretell EJ, Lescano AG, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case‐control study. Lancet Neurol 2008;7:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh G, Burneo JG, Sander JW. From seizures to epilepsy and its substrates: neurocysticercosis. Epilepsia 2013;54:783–792. [DOI] [PubMed] [Google Scholar]
- 8. Bruno E, Bartoloni A, Zammarchi L, et al. Epilepsy and neurocysticercosis in Latin America: a systematic review and meta‐analysis. PLoS Negl Trop Dis 2013;7:e2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burneo JG, Cavazos JE. Neurocysticercosis and epilepsy. Epilepsy Curr 2014;14:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Brutto OH. Neurocysticercosis: a review. ScientificWorldJournal 2012;2012:159821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahanty S, Garcia HH; Cysticercosis Working Group in Peru . Cysticercosis and neurocysticercosis as pathogens affecting the nervous system. Prog Neurobiol 2010;91:172–184. [DOI] [PubMed] [Google Scholar]
- 12. Millogo A, Nitiema P, Carabin H, et al. Prevalence of neurocysticercosis among people with epilepsy in rural areas of Burkina Faso. Epilepsia 2012;53:2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moyano LM, Saito M, Montano SM, et al. Neurocysticercosis as a cause of epilepsy and seizures in two community‐based studies in a cysticercosis‐endemic region in Peru. PLoS Negl Trop Dis 2014;8:e2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population‐based study. Epilepsia 1996;37:224–229. [DOI] [PubMed] [Google Scholar]
- 15. Shihabuddin BS, Herlopian AS, Greenfield LJ Jr. Ictal asystole in epilepsy patients undergoing inpatient video‐EEG monitoring. Neurosciences 2014;19:317–321. [PMC free article] [PubMed] [Google Scholar]
- 16. Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease‐modifying drugs. A comparison of the pharmacology of kindling and post‐status epilepticus models of temporal lobe epilepsy. Epilepsy Res 2002;50:105–123. [DOI] [PubMed] [Google Scholar]
- 17. O'Neal SE, Flecker RH. Hospitalization frequency and charges for neurocysticercosis, United States, 2003‐2012. Emerg Infect Dis 2015;21:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertram E. The relevance of kindling for human epilepsy. Epilepsia 2007;48(Suppl 2):65–74. [DOI] [PubMed] [Google Scholar]
- 19. Cela E, Sjostrom PJ. Optogenetic kindling of cortical circuits elicits epilepsy. Inter J Dev Neurosci 2015;47:122. [Google Scholar]
- 20. Langberg T, Dashek R, Mulvey B, et al. Distinct behavioral phenotypes in novel “fast” kindling‐susceptible and “slow” kindling‐resistant rat strains selected by stimulation of the hippocampal perforant path. Neurobiol Dis 2016;85:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sato M. Mesolimbic system and amygdaloid kindling. Electroencephalogr Clin Neurophysiol Suppl 1982;36:249–256. [PubMed] [Google Scholar]
- 22. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg 1999;91:593–600. [DOI] [PubMed] [Google Scholar]
- 23. Garcia HH, Gonzales I, Lescano AG, et al. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double‐blind, randomised controlled trial. Lancet Infect Dis 2014;14:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia HH, Lescano AG, Gonzales I, et al. Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin Infect Dis 2016;62:1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thernau T, Atkinson B, Ripley B. rpart: Recursive Partitioning and Regression Trees. R package version 4.1‐11. 2017. Available at: https://cran.r-project.org/package=rpart.
- 26. Liaw A, Wiener M. Classification and regression by randomForest. R News 2002;2:18–22. Available at: http://cran.r-project.org/doc/Rnews/. [Google Scholar]
- 27. Rodriguez S, Wilkins P, Dorny P. Immunological and molecular diagnosis of cysticercosis. Pathog Glob Health 2012;106:286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cianfoni A, Caulo M, Cerase A, et al. Seizure‐induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol 2013;82:1964–1972. [DOI] [PubMed] [Google Scholar]
- 29. Fujita M, Mahanty S, Zoghbi SS, et al. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS One 2013;8:e74052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JA, Chung JI, Yoon PH, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion‐weighted imaging. AJNR Am J Neuroradiol 2001;22:1149–1160. [PMC free article] [PubMed] [Google Scholar]
- 31. Bhattacharjee S, Biswas P, Mondal T. Clinical profile and follow‐up of 51 pediatric neurocysticercosis cases: a study from Eastern India. Ann Indian Acad Neurol 2013;16:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rathore C, Radhakrishnan K. What causes seizures in patients with calcified neurocysticercal lesions? Neurology 2012;78:612–613. [DOI] [PubMed] [Google Scholar]
- 33. Das K, Mondal GP, Banerjee M, et al. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. J Clin Neurosci 2007;14:1172–1177. [DOI] [PubMed] [Google Scholar]
- 34. Garcia‐Noval J, Moreno E, de Mata F, et al. An epidemiological study of epilepsy and epileptic seizures in two rural Guatemalan communities. Ann Trop Med Parasitol 2001;95:167–175. [DOI] [PubMed] [Google Scholar]
- 35. Nash T. Edema surrounding calcified intracranial cysticerci: clinical manifestations, natural history, and treatment. Pathog Glob Health 2012;106:275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verma A, Prasad KN, Nyati KK, et al. Association of MMP‐2 and MMP‐9 with clinical outcome of neurocysticercosis. Parasitology 2011;138:1423–1428. [DOI] [PubMed] [Google Scholar]
- 37. Nash TE, Bartelt LA, Korpe PS, et al. Calcified neurocysticercus, perilesional edema, and histologic inflammation. Am J Trop Med Hyg 2014;90:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia HH, Gilman RH, Catacora M, et al. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. Cysticercosis Working Group in Peru. J Infect Dis 1997;175:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]


