Abstract
To determine whether CSF leucine‐rich glioma‐inactivated 1(LGI1)‐IgG titer, index or IgG subclass has prognostic significance, we tested serum and CSF specimens collected concomitantly from 39 seropositive patients. LGI1‐IgG index was elevated (>1) in 21 patients (54%), suggesting intrathecal synthesis. Patients with worse outcome at last follow‐up (modified Rankin Scale >2) had significantly higher index (median 6.57 vs. 0.5, P = 0.048) compared to those with better outcome. Higher CSF LGI1‐IgG4 subclass‐specific titer and index correlated with worse outcome (P < 0.005 for both). These data suggest that evidence of intrathecal LGI1‐IgG synthesis may correlate with neuronal injury and warrant consideration of aggressive immunotherapy.
Introduction
Autoantibodies targeting the leucine‐rich glioma inactivated 1(LGI1) protein, an extracellular component of the voltage‐gated Kv1 potassium channel‐complex (VGKC), have potential to cause neurological autoimmunity with both central and peripheral nervous system manifestations.1, 2 Most cases are immunotherapy‐responsive.3, 4, 5 Studies have suggested that delayed initiation of therapy, lack of response to initial immunotherapy and presence of relapses correlated with increased morbidity but there is no early serological marker to identify patients who might have a worse outcome and may warrant aggressive immunotherapy.4, 6
LGI1 autoantibodies are often detectable both in serum and CSF; serum testing is generally more sensitive, with few exceptions reported.3, 4, 5 Data regarding intrathecal synthesis are lacking for most pathogenic neural autoantibodies. Aquaporin‐4‐IgG, the effector of neuromyelitis optica spectrum disorders, is detected in serum more readily than in cerebrospinal fluid,7 but some intrathecal production of aquaporin‐4‐IgG occurs.8 In contrast, NMDAR‐IgG is detected more readily in the CSF,9 despite at least some NMDAR‐IgG production in peripheral immune responses.10 Preliminary data by Irani et al. in two patients with LGI1‐IgG encephalitis suggest that part of the affinity maturation occurs in the CNS compartment.11
In our recently published systematic analysis of patients with LGI1‐IgG, we observed that some patients with refractory symptoms had high CSF titers of LGI1‐IgG.3 In this report, we describe the correlation between CSF LGI1‐IgG levels and index, IgG subclasses and clinical outcome in 39 patients.
Methods
Patients
The Mayo Clinic Institutional Review Board approved this study. We identified 39 patients with concomitant serum and CSF specimens (collected within 7 days of each other). Clinical information was reviewed (electronic records for 12 Mayo Clinic patients; outside records or physician‐provided information). Outcomes were calculated using the modified Rankin Scale (mRS) at last follow‐up (favorable outcome was defined as mRS ≤ 2). In two cases, the mRS score differed by 1 point and a third review was used for the final score.
Detection and titration of LGI1 IgG
LGI1‐IgG was identified by a CLIA‐approved clinically validated indirect immunofluorescence assay using HEK293 cells overexpressing recombinant human LGI1 (EUROIMMUN, Lϋbeck Germany). CSF was tested at 1:2.5 dilution, serum at 1:10 and samples were titrated with doubling dilutions to determine positivity endpoint. LGI1‐IgG subclasses were identified using subclass‐specific secondary antibodies (mouse anti‐human IgG1‐Fc‐specific Alexa Fluor 488‐conjugated [Invitrogen]; mouse anti‐human IgG2, IgG3, and IgG4‐Fc‐specific FITC‐conjugated [Southern Biotech]).
LGI1‐IgG‐specific CSF index calculation
CSF index for LGI1‐IgG and subclasses as compared to serum was calculated according to the Link formula,12 modified for specific antibodies13, 14:
Values exceeding 1, support intrathecal synthesis of designated IgG.13, 14
Statistical analysis
Data were summarized with frequencies and percentages or medians and ranges, as appropriate. Continuous measures (age, LGI1‐IgG index etc) were compared between patients with favorable versus unfavorable outcomes using Wilcoxon rank‐sum tests. Dichotomous variables (sex, LG1‐IgG index>1, etc) were compared with Fisher's exact tests. Receiver‐operating characteristic (ROC) curve analysis was used to identify a cut‐off to discriminate patients with favorable or unfavorable outcomes based on the LG1‐IgG and LGI1‐IgG4 subclass‐specific index; sensitivity and specificity were reported. P‐values < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Clinical findings
Demographics, clinical presentation, and ancillary testing findings are summarized in Table 1. Among 39 patients, 36 (92%) had a central nervous system presentation (seizures, spells, encephalopathy, cognitive impairment), 2 (5%) had a solely peripheral nervous system presentation (neuropathy, pain with or without autonomic manifestations, peripheral nerve hyperexcitability), and 1 (3%) had both. Thirty patients were treated with steroids and 17 with IVIg (10 with both) with median time to treatment of 4 months (range 0.6–24). Median follow‐up period did not differ significantly for groups with favorable and unfavorable outcomes (28 months [range 4–85] vs. 16 months [range 6–46], P = 0.5). Patients with a worse outcome (mRS > 2) were more frequently treated with both IVIg and steroids (56% vs. 17% of patients, P = 0.04) suggesting an escalating treatment choice by the treating physician. Second‐line immunotherapies as maintenance treatments included mycophenolate mofetil (11), rituximab (5), azathioprine (2), methotrexate (2), and cyclophosphamide (1).
Table 1.
Demographics, clinical presentation, and ancillary testing
| Median age (range), years | 65 (27–80) |
| Proportion male | 26/39 (67%) |
| Median serum LGI1‐IgG titer (range) | 640 (40–1280) |
| Median CSF LGI1‐IgG titer (range) | 5 (0–320) |
| Median serum VGKC value (range), nmol/L | 0.33 (0.14–4.63) |
| Median CSF VGKC value (range), nmol/L | 0.02 (0–0.4) |
| Manifestations | |
| Central nervous system | 37/39 (95%) |
| Seizures | 35/39 (90%) |
| Cognitive decline | 36/39 (92%) |
| Peripheral nervous system | 3/39 (8%) |
| Cancers diagnosed on follow‐up | 2/39 – 1 prostate and 1 ovary |
| Ancillary Testing | |
| Mesio‐temporal hyperintensity on MRI | 16/31 (52%) |
| Epileptic activity on EEG | 13/30 (43%) |
| Abnormal CSF | 23/33, 70% |
| Elevated protein | 22/33, 67% |
| Median protein (range), mg/dL | 56, (24–94) |
| Median WBC number (range) | 2 (0–55) |
| WBC > 5 | 6/39 (15%) |
| Oligoclonal IgG bands exceeding 4 | 3/27, 11% |
Serological and CSF findings
Twenty‐one patients (54%) had elevated LGI1‐IgG‐specific CSF index (>1), supportive of intrathecal synthesis. The two patients with isolated peripheral presentation had low LGI1‐IgG index. Of the 23 patients with available remaining sera all had LGI1‐IgG4 subclass specificities (12 had LGI1‐IgG4 in the CSF; for the rest no subclass specificities were identified, presumably due to low titer and assay‐detection limitations). Only six had co‐existing LGI1‐IgG1 (none detected in the CSF). No LGI1‐IgG2 and LGI1‐IgG3 subclasses were detected in the patients’ samples. There was no difference in age, sex, relapse rate, and MRI findings between patients with serum LGI1‐IgG4 detected alone versus in coexistence with LGI1‐IgG1.
Outcome
At last follow‐up, 30 patients had favorable outcome (77%) which is similar to what has been reported previously.3, 4 Eight of nine patients with mRS > 2 had evidence of intrathecal synthesis of LGI1‐IgG (index >1) compared to 13/30 patients with mRS≤2 (P = 0.02). The difference was still significant when continuous LGI1‐IgG index values were compared between patients with favorable and unfavorable outcome (median 0.5 vs. 6.57, P = 0.048) (Fig. 1). There was no statistical difference for age, gender, VGKC‐IgG values (nmol/L, by radioimmunoprecipitation assay) in serum or CSF, mRS at nadir, time to CSF evaluation, inflammatory CSF findings, time to treatment, or use of second‐line immunotherapy between the groups with favorable and unfavorable outcome. Patients with unfavorable outcome were just as likely to receive steroids as those with favorable outcome (78% vs. 79%), however, those with unfavorable outcome were more likely to have received IVIg (78% vs. 35%). To adjust, we stratified by IVIg treatment. Among patients who did not receive IVIg, there was no significant association (but only two had an unfavorable outcome). Among those who received IVIg, the association of LGI1‐IgG index and outcome was still significant (7/7 patients with unfavorable outcome had index>1 as compared to 5/10 with favorable outcome, P = 0.04).
Figure 1.
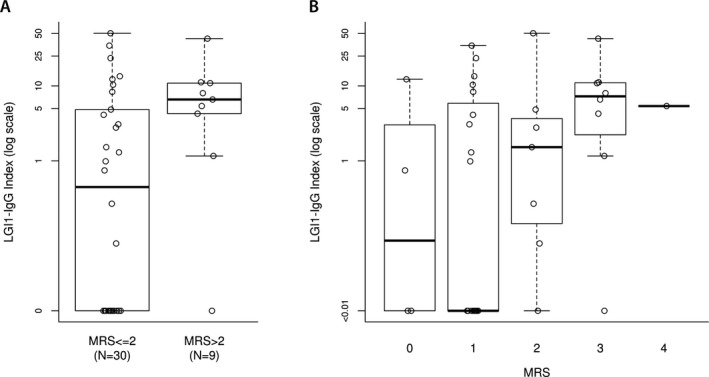
(A) Patients with unfavorable outcome (mRS>2) had higher LGI1‐IgG index by comparison with patients with a favorable outcome (P = 0.048). (B) Individual patients’ LGI1‐IgG index per mRS score at last follow‐up.
In a univariate analysis (not enough patients to perform a multivariate analysis), higher CSF LGI1‐IgG4 titers and LGI1‐IgG4 index were associated with worse outcome (Fig. 2). A cut‐off for IgG4 index at ≥3.4 yielded 100% sensitivity for unfavorable outcome and 88% specificity (15/17 patients with favorable outcome had an index < 3.4).
Figure 2.
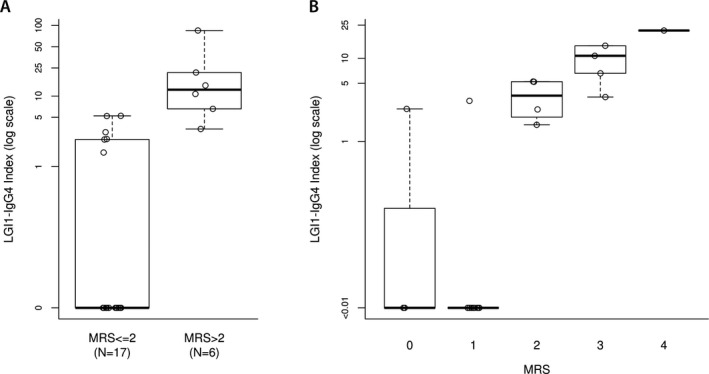
(A) Patients with unfavorable outcome (mRS>2) had higher LGI1‐IgG4 subclass‐specific CSF index by comparison with patients with a favorable outcome (P < 0.005 for both) (B) Individual patients’ LGI1‐IgG4 index per final mRS score at last follow‐up.
Discussion
This study suggests that higher LGI1‐IgG‐specific CSF index, indicative of intrathecal antibody synthesis, correlates with worse outcome, irrespective of age, initial presentation, treatment modalities, or time to treatment. As suggested for antineuronal‐nuclear antibody 1 (ANNA1/anti‐Hu)‐related autoimmunity, no patient with an isolated peripheral nervous system presentation had evidence of intrathecal synthesis.15 Intrathecal synthesis reflects the maturation of antigen‐specific plasmablasts/plasma cells residing in the CNS. Our observation that patients whose central manifestations are not accompanied by evidence of intrathecal synthesis have better outcomes, suggests that treatments targeting peripheral B‐cell populations are efficient in these cases. However, the unfavorable outcome of patients with evidence of intrathecal LGI1‐IgG synthesis, is plausibly explained by the limited accessibility of these treatments across a relatively intact blood–brain barrier.3
We confirmed the presence of IgG4 as the major subclass of LGI1‐IgG. Higher CSF IgG4 subclass‐specific titers in the CSF and index strongly correlated with worse outcome. While it has been suggested by other groups, that LGI1‐seropositive patients with faciobrachial dystonic seizures associated with cognitive decline have a higher proportion of LGI1‐IgG1 subclass autoantibodies in comparison to the patients without cognitive decline, we did not have enough LGI1‐IgG1 seropositive patients in our cohort to confirm this finding.16
Limitations of our study include the relatively small number of patients, the use of mRS for outcome calculations, the broad range of duration of follow‐up, and the retrospective design. Future studies are required to positively identify the existence of uniquely CNS‐resident LGI1‐specific B‐cell populations to account for intrathecal production of LGI1‐IgG‐specific autoantibodies.
LGI1‐IgG and LGI1‐IgG4 subclass‐specific CSF indices warrant further evaluation as a prognostic factor in LGI1 autoimmunity. A higher index may indicate the need for more aggressive initial immunotherapy. In addition, the presence of LGI1‐specific plasmablasts/plasma cells in the CSF would be a reason to consider intrathecal delivery of anti‐CD20‐specific, or anti‐CD19‐specific therapeutic monoclonal antibodies as targeted immunotherapy in selected refractory cases.
Author Contributions
Gadoth, A: conception and design of the study, acquisition and analysis of data, drafting of the manuscript. Zekeridou, A: design of the study, acquisition and analysis of data, drafting of the manuscript. Klein, C.J.: acquisition and analysis of data, revising manuscript for intellectual content. Thoreson, C.J.: acquisition and analysis of data, revising manuscript for intellectual content. Majed, M.: acquisition and analysis of data, revising manuscript for intellectual content. Dubey, D.: acquisition and analysis of data, revising manuscript for intellectual content. Flanagan E.: acquisition and analysis of data, revising manuscript for intellectual content. McKeon, A.: acquisition and analysis of data, revising manuscript for intellectual content. Jenkins, M.S.: statistical analysis of the data, drafting of manuscript. Lennon, V.A.: acquisition and analysis of data, revising manuscript for intellectual content. Pittock, S.J.: conception and design of the study, drafting of the manuscript, revising manuscript for intellectual content.
Conflict of Interest
The authors report no conflict of interest.
Funding Information
No funding information provided.
References
- 1. Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel‐complex proteins leucine‐rich, glioma inactivated 1 protein and contactin‐associated protein‐2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia Sarosh. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai M, Huijbers MGM, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2‐IgG positive patients. Ann Neurol 2017;82:79–82. [DOI] [PubMed] [Google Scholar]
- 4. Arino H, Armangue T, Petit‐Pedrol M, et al. Anti‐LGI1‐associated cognitive impairment: presentation and long‐term outcome. Neurology 2016;87:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Sonderen A, Thijs RD, Coenders EC, et al. Anti‐LGI1 encephalitis clinical syndrome and long‐term follow‐up. Neurology 2016;87:1–8. [DOI] [PubMed] [Google Scholar]
- 6. Finke C, Prüss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine‐rich, glioma‐inactivated 1 antibodies. JAMA Neurol. 2017;74:50–59. [DOI] [PubMed] [Google Scholar]
- 7. Fryer JP, Lennon VA, Pittock SJ, et al. AQP4 autoantibody assay performance in clinical laboratory service. Neurol Neuroimmunol Neuroinflamm 2014;1:e11–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowarik MC, Dzieciatkowska M, Wemlinger S, et al. The cerebrospinal fluid immunoglobulin transcriptome and proteome in neuromyelitis optica reveals central nervous system‐specific B cell populations. J Neuroinflammation 2015;28:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gresa‐Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow‐up of anti‐NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreye J, Wenke NK, Chayka M, et al. Human cerebrospinal fluid monoclonal N‐methyl‐D‐aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain 2016;139:2641–2652. [DOI] [PubMed] [Google Scholar]
- 11. Irani SR, Lehmann‐Horn K, Geschwind M, et al. The active intrathecal B‐cell response in LGI1‐antibody encephalitis. Lancet 2015;385:S46. [DOI] [PubMed] [Google Scholar]
- 12. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within th ecentral nervous system in multi[le sclerosis. Scand J Clin Lab Invest 1977;37:397–401. [DOI] [PubMed] [Google Scholar]
- 13. Dalakas M, Stone G, Elder G, et al. Tropical spastic paraparesis : clinical, immunological, and virological Studies in two patients from martinique. Ann Neurol 1988;23:136–142. [DOI] [PubMed] [Google Scholar]
- 14. Dalakas MC, Li M, Fujii M, Jacobowitz DM. Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology 2001;57:780–784. [DOI] [PubMed] [Google Scholar]
- 15. Schwenkenbecher P, Chacko LP, Wurster U, et al. Intrathecal synthesis of anti‐Hu antibodies distinguishes patients with paraneoplastic peripheral neuropathy and encephalitis. BMC Neurol 2016;16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson J, Bi M, Murchison A, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2017;. https://doi.org/10.109:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


