Abstract
Glutamine and glutamate are not considered essential amino acids but they play important roles in maintaining growth and health in both neonates and adults. Although glutamine and glutamate are highly abundant in most feedstuffs there is increasing evidence that they may be limiting during pregnancy, lactation and neonatal growth, particularly when relatively low protein diets are fed. Supplementation of diets with glutamine, glutamate or both at 0.5 to 1.0% to both suckling and recently weaned piglets improves intestinal and immune function and results in better growth. In addition such supplementation to the sow prevents some of the loss of lean body mass during lactation, and increases milk glutamine content. However, a number of important questions related to physiological condition, species under study and the form and amount of the supplements need to be addressed before the full benefits of glutamine and glutamate supplementation in domestic animal production can be realized.
Keywords: Amino acid, Glutamate, Glutamine, Lactation, Pregnancy, Growth
1. Introduction
Glutamate and glutamine are highly abundant amino acids found in most foodstuffs and, in total, comprise somewhere between 5 and 15% of dietary protein (Lenders et al., 2009, Li et al., 2011). Similarly, these two amino acids comprise a large proportion of the body pool of amino acids, both in the free form and incorporated into protein. Traditionally glutamate and glutamine have been considered to be non-essential (dispensable) and, due to the almost complete catabolism of dietary glutamate and glutamine in the intestine, essentially all of the large body pool is synthesized endogenously (Curthoys and Watford, 1995). The definition of what is, and what is not, an essential amino acid however, is not unequivocal. Rose et al., 1948, Rose et al., 1949 defined an essential amino acid as one that the body cannot make in sufficient amounts to maintain growth or nitrogen balance. This definition is noteworthy since it does not say that the body cannot make the amino acid, rather that it cannot make sufficient amounts for a key purpose. Recently there has been a reconsideration of additional functions of amino acids and some, including glutamine, are now considered as conditionally essential at key times (Wernerman, 2008, Wu, 2010). In this paper the nutritional aspects of glutamate and glutamine will be described and the requirements, if any, during gestation and lactation, and for optimal growth in neonates, will be considered particularly with reference to feeding lower protein diets.
2. Glutamate and glutamine
2.1. Metabolism
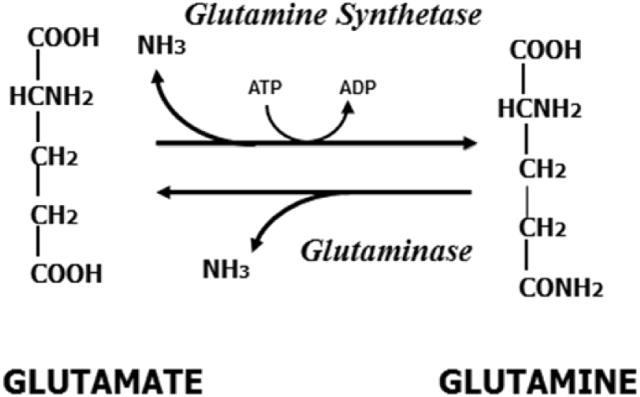
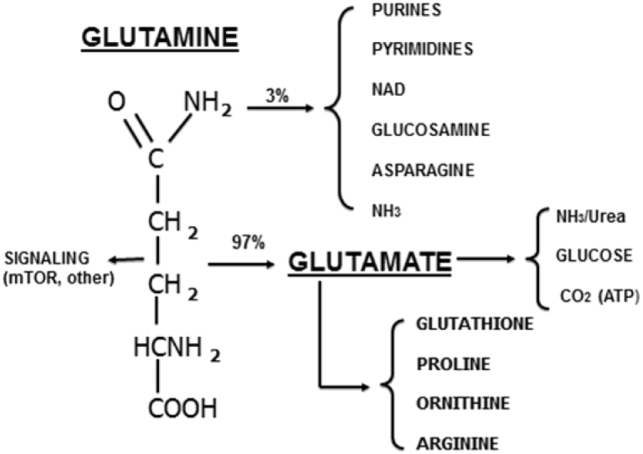
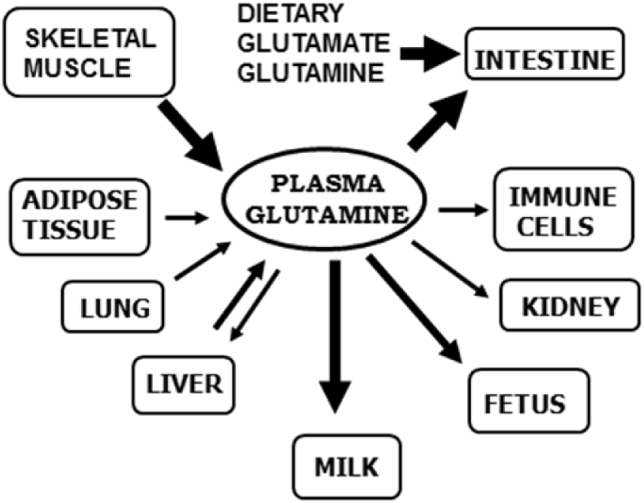
The healthy adult human contains over 80 g of free glutamine, with >98% of it inside skeletal muscle cells at concentrations of 20 mM and above. In addition, plasma glutamine is turning over at very high rates (60 to 80 g per day in healthy subjects) (Curthoys and Watford, 1995, Watford, 2008, Wernerman, 2008). Glutamine is made via the action of glutamine synthetase from glutamate and ammonia (Fig. 1), primarily in skeletal muscle, lungs, adipose tissue and liver. Glutamine is a precursor for a number of biosynthetic pathways required for growth and cell division (Fig. 2). The bulk of glutamine however, is hydrolyzed by glutaminase Fig. 1 and 2 and ultimately serves as substrate for hepatic gluconeogenesis and urea synthesis, renal ammoniagenesis, and is the major respiratory fuel for enterocytes and cells of the immune system (Curthoys and Watford, 1995). Glutamine is also an important signaling molecule, often acting by activation of the mammalian target of rapamycin (mTOR) (Fig. 2), stimulating anabolic functions such as protein synthesis, cell growth and differentiation, and inhibiting catabolic functions such as protein degradation and apoptosis (Curi et al., 2007). In addition, during gestation there is a large uptake of glutamine across the placenta, together with placental glutamine synthesis, resulting in large amounts of glutamine available for fetal growth (Fig. 3). Likewise in lactation, free and protein bound glutamate and glutamine, derived from both the circulation and mammary gland synthesis (Fig. 3), are the most abundant amino acids in milk (Wu and Knabe, 1994, Davis et al., 1994, Wang et al., 2008). Thus the maintenance of glutamate and glutamine homeostasis is important for the well-being of a number of tissues in the body, particularly during gestation, lactation and growth.
Fig. 1.
Glutamine synthetase and glutaminase.
Fig. 2.
Pathways of glutamine and glutamate metabolism. The majority of glutamine is degraded to glutamate and then used as a substrate for urea and glucose synthesis or as a fuel (ATP production). Although glutamine is an essential precursor for many important compounds but these represent a small (<3%) fraction of glutamine metabolism. Glutamine also acts as an anabolic signal, often via activation of mammalian target of rapamycin (mTOR).
Fig. 3.
Glutamine metabolism during pregnancy and lactation. Increased dietary intake provides glutamine and glutamate for the small intestine, but skeletal muscle provides most of the circulating glutamine that is derived from other amino acids, including those from both the diet and muscle proteolysis. The size of the arrows indicates a rough estimate of the magnitude of glutamine flux.
2.2. Glutamine becomes conditionally essential in hyper-catabolic states
In healthy animals the body is able to synthesize considerable quantities of glutamine and there is no apparent evidence of a shortage. About 30 years ago however, it became apparent that in hyper-catabolic patients glutamine requirements (for the immune system, wound healing, acid–base balance and gluconeogenesis) could increase beyond the endogenous capacity for glutamine synthesis (Wernerman, 2008). An early response to such stress is a rapid release of muscle glutamine and a resultant drop in both muscle and plasma free glutamine concentrations. Subsequently there is an up-regulation of muscle glutamine synthesis from amino acids derived from a net increase in muscle proteolysis. In clinical studies it has been proposed that a plasma glutamine concentration <0.42 mM (normal 0.6 to 0.8 mM) indicates glutamine insufficiently and exogenous glutamine should be provided (Wernerman, 2008). Although beyond the scope of this text, a number of studies have shown benefits for supplemental glutamine given to such patients (Wang et al., 2010).
2.3. Glutamine and glutamate are conditionally essential during pregnancy, lactation and growth
In the domestic animal industry the treatment of hyper-catabolic conditions is of little interest and thus the important question is ”is glutamine conditionally essential at other times”? We observed that plasma and muscle glutamine concentrations fell steadily throughout lactation in the horse and this was accompanied by a loss of skeletal muscle, representing a mildly catabolic state (Manso Filho et al., 2008) as seen in a number of other species (Manso et al., 2012, Clowes et al., 2005, Pine et al., 1994). Thus the question arises if glutamate or glutamine supplementation would be beneficial during gestation and lactation. As mentioned above these conditions are associated with increased glutamine needs, for fetal growth and milk production, and it was traditionally assumed that such needs were met by catabolism of extra dietary amino acids (Fig. 3). Thus it was surprising to find that such substrates were insufficient and that additional endogenous substrates for glutamine synthesis were drawn from the mother׳s lean body mass. We proposed that supplementation with glutamine and/or glutamate may provide the extra glutamine required during lactation and thus spare the lean body mass of the mother. Similarly, it is well established that the neonatal gut is particularly sensitive to stress and that weaning, particularly abrupt early weaning, is often associated with negative growth and pathological outcomes that are clearly related to intestinal and immunologicadysfunction. Given that glutamate and glutamine are the preferred fuels of this tissue, and of cells of the immune system, (Curthoys and Watford, 1995, Curi et al., 2007) then supplements may aid to maintain intestinal function throughout weaning.
2.4. Glutamine and glutamate supplements are beneficial during pregnancy, lactation and growth
A number of studies over the past 10 years have shown that glutamine and/or glutamate supplementation can be beneficial to recently weaned piglets (Jiang et al., 2009, López-Pedrosa et al., 2007, Wang et al., 2008, Wu et al., 1996, Yi et al., 2005, Yoo et al., 1997). Such studies have indicated benefits in preventing intestinal atrophy, maintaining anti-oxidant status and lessening the incidence of diarrhea, and result in increased weight gain and improved food efficiency. One study (Haynes et al., 2009) has looked at oral glutamine supplementation to suckling piglets and reported increased weight gain and protection of the intestine from oxidative damage, particularly in piglets that were treated with bacterial endotoxin as a model of inflammatory disease. Similarly, a recent study has shown that supplementing creep feeders with glutamine and glutamate results in improvements in intestinal and immunological health, particularly at abrupt weaning (Cabrera et al., 2013). In recent work (Manso et al., 2012, Santos de Aquino et al., 2014) we showed that supplemental glutamine and glutamate to the lactating sow increased the glutamine content of the milk and so delivered more glutamine to the piglets. Such an approach may offer a very attractive mechanism to increase the availability of glutamine to the piglet at this very vulnerable time. In addition, we found that glutamine, and glutamine with glutamate, supplementation also attenuated some of the loss of lean body mass in the sow during lactation.
2.5. The future of glutamine supplementation
There is therefore extensive evidence that glutamine and glutamate supplementation may have considerable benefits both to the sow and the piglet when given orally to the sow during the transition period and to the piglet throughout the post-weaning period. Thus future questions regarding glutamine and glutamate supplementation are related to when to give it, how much to give, what outcomes are important, and what mechanisms are involved? The evidence from published trials (Cabrera et al., 2013, Haynes et al., 2009, Jiang et al., 2009, López-Pedrosa et al., 2007, Manso Filho et al., 2008, Santos de Aquino et al., 2014, Wang et al., 2008, Wu et al., 1996, Yi et al., 2005, Yoo et al., 1997) using pure glutamine or a mixture of glutamine and glutamate, show beneficial effects between 0.5 and 1.5% (by weight). There is limited evidence of benefits at lower levels (0.25%) and no evidence of any increased benefits at levels above 1.0%. Grain based diets are relatively deficient in glutamine (Li et al., 2011) and supplementation can correct this deficiency without the need to increase total protein. Since glutamate/glutamine are not essential in the traditional sense, any future studies should focus on requirement trials using models where there is evidence that they are conditionally essential, e.g., the sow during gestation and lactation, and the neonatal piglet. However, it is also important to decide on the outcomes that will be used to set the requirement. Will this be weight gain, feed intake, or more mechanistic variables such as intestinal morphology and biochemistry, or even include detailed examination of muscle growth and quality? To date such variables have received very little attention and neither the mechanisms involved nor the physiological significance have been adequately addressed.
3. Conclusions
In the original work of Rose et al., 1948, Rose et al., 1949), that defined our classification of dietary amino acids into essential and non-essential, it was noted that animals fed a mixture of essential amino acids did not grow as well as animals fed these amino acids plus an additional source of nitrogen. Such early studies clearly illustrate that a certain amount of dietary non-essential amino acids are required and that glutamate may be the ideal source (Featherston et al., 1962). A number of studies were carried out that claimed dietary glutamate could be essential for growth in chickens (Maruyama et al., 1975, Maruyama et al., 1976), and it has been proposed that glutamine is limiting for milk production in the cow (Meijer et al., 1993, Meijer et al., 1995), but overall, the use of dietary glutamate/glutamine has received very little attention over the past 50 years. But, despite having been classified as non-essential, there is increasing evidence that supplemental glutamate/glutamine may be beneficial, not only in hypercatabolic states, but also in the maintenance of optimal health and maximal rates of growth in healthy animals. Currently a number of important questions relating to physiological condition, species under study and the form and amount of the supplements, remain to be addressed before the full benefits to domestic animal production can be realized.
Conflict of interest
The author has received research funding from Ajinomoto do Brasil.
Acknowledgments
Work done in the author׳s laboratory was supported, in part, by the New Jersey Agricultural Experiment Station, the New Jersey Institute of Food, Nutrition and Health, and Ajinomoto do Brasil.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Cabrera R.A., Usry J.L., Arrellano C., Nogueira E.T., Kutschenko M., Moeser A.J. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J Anim Sci Biotech. 2013;4:29. doi: 10.1186/2049-1891-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes E.J., Aherne F.X., Baracos V.E. Skeletal muscle protein mobilization during the progression of lactation. Am J Physiol Endocrinol Metab. 2005;288:E564–E572. doi: 10.1152/ajpendo.00198.2004. [DOI] [PubMed] [Google Scholar]
- Curthoys N.P., Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- Curi R., Newsholme P., Procopio J., Lagranha C., Gorjão R., Pithon-Curi T.C. Glutamine, gene expression, and cell function. Front Biosci. 2007 Jan 1;12:344–357. doi: 10.2741/2068. [DOI] [PubMed] [Google Scholar]
- Davis T.A., Nguyen H.V., Garcia-Bravo R., Fiorotto M.L., Jackson E.M., Reeds P.J. Amino acid composition of the milk of some mammalian species changes with stage of lactation. Br J Nutr. 1994;72:845–853. doi: 10.1079/bjn19940089. [DOI] [PubMed] [Google Scholar]
- Featherston W.R., Bird H.R., Harper A.E. Effectiveness of urea and ammonium nitrogen for the synthesis of dispensable amino acids by the chick. J Nutr. 1962;78:198–206. doi: 10.1093/jn/78.2.198. [DOI] [PubMed] [Google Scholar]
- Haynes T.E., Li P., Li X., Shimotori K., Sato H., Flynn N.E. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids. 2009;37:131–142. doi: 10.1007/s00726-009-0243-x. [DOI] [PubMed] [Google Scholar]
- Jiang Z.Y., Sun L.H., Lin Y.C., Ma X.Y., Zheng C.T., Zhou G.L. Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J Anim Sci. 2009;87:4050–4056. doi: 10.2527/jas.2008-1120. [DOI] [PubMed] [Google Scholar]
- López-Pedrosa J.M., Manzano M., Baxter J.H., Rueda R. N-acetyl-L-glutamine, a liquid-stable source of glutamine, partially prevents changes in body weight and on intestinal immunity induced by protein energy malnutrition in pigs. Dig Dis Sci. 2007;52:650–658. doi: 10.1007/s10620-006-9500-y. [DOI] [PubMed] [Google Scholar]
- Lenders C.M., Liu S., Wilmore D.W., Sampson L., Dougherty L.W., Spiegelman D. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur J Clin Nutr. 2009;63:1433–1439. doi: 10.1038/ejcn.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Rezaei R., Li P., Wu G. Composition of amino acids in feed ingredients for animal diets. Amino Acids. 2011;40:1159–1168. doi: 10.1007/s00726-010-0740-y. [DOI] [PubMed] [Google Scholar]
- Manso H.E., Manso Filho H.C., de Carvalho L.E., Kutschenko M., Nogueira E.T., Watford M. Glutamine and glutamate supplementation raise milk glutamine concentrations in lactating gilts. J Anim Sci Biotechnol. 2012;3:2. doi: 10.1186/2049-1891-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso Filho H.C., McKeever K.H., Gordon M.E., Costa H.E., Lagakos W.S., Watford M. Changes in glutamine metabolism indicate a mild catabolic state in the transition mare. J Anim Sci. 2008;86:3424–3431. doi: 10.2527/jas.2008-1054. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Sunde M.L., Harper A.E. Is L-glutamic acid nutritionally a dispensable amino acid for the young chick? Poult Sci. 1976;55:45–60. doi: 10.3382/ps.0550045. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Harper A.E., Sunde M.L. Effects of D-, DL-and L-glutamic acid on chicks. J Nutr. 1975;105:1012–1019. doi: 10.1093/jn/105.8.1012. [DOI] [PubMed] [Google Scholar]
- Meijer G.A., Van der Meulen J., Bakker J.G., Van der Koelen C.J., Van Vuuren A.M. Free amino acids in plasma and muscle of high yielding dairy cows in early lactation. J Dairy Sci. 1995;78:1131–1141. doi: 10.3168/jds.S0022-0302(95)76730-3. [DOI] [PubMed] [Google Scholar]
- Meijer G.A., van der Meulen J., van Vuuren A.M. Glutamine is a potentially limiting amino acid for milk production in dairy cows: a hypothesis. Metabolism. 1993;42:358–364. doi: 10.1016/0026-0495(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Pine A.P., Jessop N.S., Allan G.F., Oldham J.D. Maternal protein reserves and their influence on lactational performance in rats. 4. Tissue protein synthesis and turnover associated with mobilization of maternal protein. Br J Nutr. 1994;72:831–844. doi: 10.1079/bjn19940088. [DOI] [PubMed] [Google Scholar]
- Rose W.C., Oesterling M.J., Womack M. Comparative growth on diets containing ten and 19 amino acids, with further observations upon the role of glutamic and aspartic acids. J Biol Chem. 1948;176:753–762. [PubMed] [Google Scholar]
- Rose W.C., Smith L.C., Womack M., Shane M.J. The utilization of the nitrogen of ammonium salts, urea, and certain other compounds in the synthesis of non-essential amino acids in vivo. J Biol Chem. 1949;181:307–316. [PubMed] [Google Scholar]
- Santos de Aquino R., Dutra W.M., Manso H.E.C.C., Manso Filho H.C., Kutschenko M., Nogueira E.T. Glutamine and glutamate (AminoGut) supplementation influences sow colostrum and mature milk composition. LiveStock Sci. 2014;169:112–117. http://dx.doi.org/10.1016/j.livsci.2014.07.0091871-1413 [Google Scholar]
- Wang J., Chen L., Li P., Li X., Zhou H., Wang F. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. 2008;138:1025–1032. doi: 10.1093/jn/138.6.1025. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang Z.M., Nolan M.T., Jiang H., Han H.R., Yu K. The impact of glutamine dipeptide-supplemented parenteral nutrition on outcomes of surgical patients: a meta-analysis of randomized clinical trials. J Parenter Enter Nutr. 2010;34:521–529. doi: 10.1177/0148607110362587. [DOI] [PubMed] [Google Scholar]
- Watford M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr. 2008;138:2003S–2007S. doi: 10.1093/jn/138.10.2003S. [DOI] [PubMed] [Google Scholar]
- Wernerman J. Clinical use of glutamine supplementation. J Nutr. 2008;138:2040S–2044S. doi: 10.1093/jn/138.10.2040S. [DOI] [PubMed] [Google Scholar]
- Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. 2010;1:31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Knabe D.A. Free and protein-bound amino acids in sow׳s colostrum and milk. J Nutr. 1994;124:415–424. doi: 10.1093/jn/124.3.415. [DOI] [PubMed] [Google Scholar]
- Wu G., Meier S.A., Knabe D.A. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr. 1996;126:2578–2584. doi: 10.1093/jn/126.10.2578. [DOI] [PubMed] [Google Scholar]
- Yi G.F., Carroll J.A., Allee G.L., Gaines A.M., Kendall D.C., Usry J.L. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J Anim Sci. 2005;83:634–643. doi: 10.2527/2005.833634x. [DOI] [PubMed] [Google Scholar]
- Yoo S.S., Field C.J., McBurney M.I. Glutamine supplementation maintains intramuscular glutamine concentrations and normalizes lymphocyte function in infected early weaned pigs. J Nutr. 1997;127:2253–2259. doi: 10.1093/jn/127.11.2253. [DOI] [PubMed] [Google Scholar]