Abstract
BACKGROUND
Optimal management of patients with stable chest pain relies on the prognostic information provided by noninvasive cardiovascular testing, but there are limited data from randomized trials comparing anatomic with functional testing.
METHODS
In the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain), patients with stable chest pain and intermediate pretest probability for obstructive coronary artery disease (CAD) were randomly assigned to functional testing (exercise electrocardiography, nuclear stress, or stress echocardiography) or coronary computed tomography angiography (CTA). Site-based diagnostic test reports were classified as normal or mildly, moderately, or severely abnormal. The primary end point was death, myocardial infarction, or unstable angina hospitalizations over a median follow-up of 26.1 months.
RESULTS
Both the prevalence of normal test results and incidence rate of events in these patients were significantly lower among 4500 patients randomly assigned to CTA in comparison with 4602 patients randomly assigned to functional testing (33.4% versus 78.0%, and 0.9% versus 2.1%, respectively; both P<0.001). In CTA, 54.0% of events (n=74/137) occurred in patients with nonobstructive CAD (1%–69% stenosis). Prevalence of obstructive CAD and myocardial ischemia was low (11.9% versus 12.7%, respectively), with both findings having similar prognostic value (hazard ratio, 3.74; 95% confidence interval [CI], 2.60–5.39; and 3.47; 95% CI, 2.42–4.99). When test findings were stratified as mildly, moderately, or severely abnormal, hazard ratios for events in comparison with normal tests increased proportionally for CTA (2.94; 7.67–10.13; all P<0.001) but not for corresponding functional testing categories (0.94 [P=0.87], 2.65 [P=0.001], 3.88 [P<0.001]). The discriminatory ability of CTA in predicting events was significantly better than functional testing (c-index, 0.72; 95% CI, 0.68–0.76 versus 0.64; 95% CI, 0.59–0.69; P=0.04). If 2714 patients with at least an intermediate Framingham Risk Score (>10%) who had a normal functional test were reclassified as being mildly abnormal, the discriminatory capacity improved to 0.69 (95% CI, 0.64–0.74).
CONCLUSIONS
Coronary CTA, by identifying patients at risk because of nonobstructive CAD, provides better prognostic information than functional testing in contemporary patients who have stable chest pain with a low burden of obstructive CAD, myocardial ischemia, and events.
CLINICAL TRIAL REGISTRATION
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01174550.
Keywords: coronary artery disease; diagnostic tests, routine; prognosis
Evaluation of chest pain is a fundamental element of cardiology patient care. On a daily basis, many physicians experience the clinical pressures to accurately rule out obstructive coronary artery disease (CAD) or myocardial ischemia as a cause of chest pain, while limiting the performance of unnecessary diagnostic testing. This difficulty is compounded by the fact that presenting symptoms are often unspecific, and traditional risk factors, while associated with CAD and myocardial ischemia, do not alone permit accurate diagnosis in the vast majority of patients. Hence, knowledge about the prognostic implications of imaging-based findings is imperative to properly assess, prognosticate, and treat these patients. In this climate, functional cardiac testing (exercise electrocardiography, stress nuclear single-photon emission computed tomography, stress echocardiography) has been the traditional way (>4 million patients each year in the United States) to assess stable outpatients with suspected but not previously diagnosed CAD.1,2 However, major changes in referral patterns, improvements in lifestyle, and preventive medical therapy over the past 40 years have contributed to decreasing rates of functional tests positive for myocardial ischemia3 and lower cardiovascular event rates.4,5 With fewer patients demonstrating classical findings of myocardial ischemia indicating the need for interventional therapy, the latest American Heart Association/American College of Cardiology guidelines recommend stress electrocardiography or stress imaging for patients with intermediate to high likelihood of CAD and emphasize the importance of prognostic assessment by cardiovascular imaging to predict future cardiovascular events and to guide medical therapy.6
Observational studies and registries provide ample evidence that traditional assessment with functional testing, especially the detection of myocardial ischemia using echocardiography and myocardial perfusion imaging, provides excellent prognostic value to predict future cardiovascular events. Historically, such findings were associated with a 5- to 10-fold increase in risk for cardiovascular events.5,7–18 Coronary computed tomography angiography (CTA) is a relatively new test that enables direct and noninvasive visualization of the presence and extent of coronary plaque and stenosis. Consistent with previous studies in invasive coronary angiography, a finding of obstructive CAD in coronary CTA is associated with a significant (6- to 12-fold) increase in the risk of future cardiovascular events, independent of traditional cardiovascular risk factors.19,20 In addition, the absence of CAD carries a nearly perfect negative predictive value (>99%).21–25 These data suggest that both anatomic (coronary CTA) and functional assessment provide excellent risk prediction for cardiovascular events. However, the number of diagnostic tests that are positive for myocardial ischemia or obstructive CAD is relatively low in contemporary practice (10%–15%).3 Instead, the detection of nonobstructive CAD defined as coronary atherosclerosis causing between 1% and 69% luminal narrowing has emerged as a significant and frequent finding that, although often not associated with myocardial ischemia, carries a substantial risk for major adverse cardiovascular events (MACE) in comparison with patients without any CAD.19,26
Moreover, a randomized comparison of the ability of anatomic and functional testing to correctly classify risk in symptomatic patients has not been performed. To accomplish this, we performed a prespecified secondary analysis of the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain), comparing the prognostic value of an anatomic versus a functional testing strategy in stable symptomatic patients with suspected CAD.
METHODS
Study Design and Population
PROMISE (URL: http://clinicaltrials.gov. Unique identifier: NCT01174550) is a pragmatic comparative effectiveness trial that enrolled 10 003 patients at 193 sites in North America with expertise in the fields of cardiology, primary care, radiology, and anesthesia and represented both community practices and academic medical centers. Details regarding the PROMISE study population, selection criteria, design, and primary results have been described elsewhere.27,28 In brief, the study participants were stable symptomatic outpatients without known CAD who were referred to noninvasive cardiovascular testing for further evaluation. Local or central institutional review boards approved the study at the coordinating centers and each of the 193 enrolling sites in North America.
For this analysis, we included patients who received the initial diagnostic test as randomly assigned. We excluded subjects who received other tests as their first test, did not undergo any diagnostic test, or received noncontrast CTA only. In addition, we excluded patients whose test results could not be assigned to prespecified test strata because of indeterminate test results, including patients who underwent functional testing with exercise but achieved <75% of maximum predicted heart rate. The flow of patients is described in Figure 1.
Figure 1. Patient flow and analytic population.
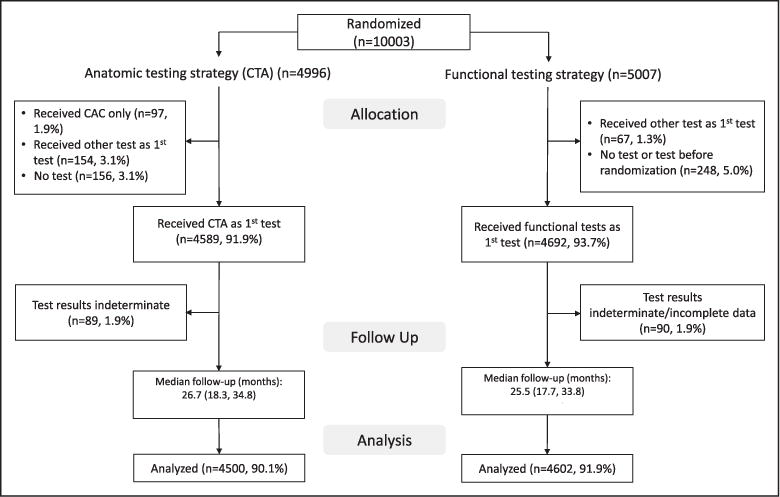
CAC indicates coronary artery calcium; and CTA, computed tomography angiography.
Study Procedures
After providing written informed consent, participants were randomly assigned to either the CTA group or the functional testing group, with stratification according to study site and according to the choice, as indicated before randomization by the site clinician, of the intended functional test if the patient were to be assigned to that study group.28 Enrollment began July 27, 2010, and was completed on September 19, 2013. Tests were performed and interpreted by local physicians who made all subsequent clinical decisions. Appropriate medical therapy was encouraged, and guideline-based educational materials were provided to patients and providers. Follow-up visits were performed at 60 days at the study sites and centrally by means of telephone or mail at 6-month intervals after randomization, for a minimum of 1 year until October 31, 2014. Diagnostic testing was performed in compliance with professional society guidelines. Functional testing included exercise electrocardiography, exercise or pharmacological nuclear myocardial perfusion imaging, and exercise or pharmacological stress echocardiography. Coronary CTA was performed with at least 64-slice multidetector computed tomographic technology.
Diagnostic Test Results
Site-reported test results were prospectively classified as normal or mildly, moderately, or severely abnormal. Broadly, for coronary CTA, we defined nonobstructive CAD (stenosis of 1%–69% for primary and 1%–49% for secondary analysis) as mildly abnormal, single-vessel obstructive CAD as moderately abnormal (stenosis of >70% for primary and >50% for secondary analysis), and multivessel or proximal left anterior descending (>70%), or left main obstructive CAD >50% as severely abnormal. For functional testing, late positive treadmill or abnormal electrocardiography in the absence of reversible ischemia was defined as mildly abnormal, inducible ischemia, or mixed defect with perfusion or wall motion in one coronary territory for myocardial perfusion imaging and stress echocardiography, respectively, or early positive treadmill was defined as moderately abnormal, and multivessel, large territory inducible ischemia or mixed defect was defined as severe. A more detailed description of the classification of test results can be found in Table 1.
Table 1.
Prospective Risk Stratification of Noninvasive Imaging Test Results in the Anatomic (Coronary Computed Tomographic Angiography) and Functional (Exercise Treadmill Test, Stress Myocardial Perfusion Imaging, and Stress Echocardiography) Testing Arms of the Study
| Test Strata | Anatomic Testing | Functional Testing | ||
|---|---|---|---|---|
| Coronary Computed Tomographic Angiography | Exercise Treadmill Test | Stress Myocardial Perfusion Imaging | Stress Echocardiography | |
| Severely abnormal | High-risk coronary artery disease: ≥2 vessel disease (≥70%) OR ≥50% left main stenosis OR ≥70% proximal left anterior descending stenosis | Ischemic electrocardiography: ST changes consistent with ischemia during stress + either severe ventricular arrhythmia OR hypotension | Large territory inducible ischemia or mixed defect: septal/anterior/apical territory or other single territory with transient ischemic dilatation or ≥2 coronary territories with ischemia | Large territory inducible ischemia or mixed defect: wall motion abnormality or mixed abnormality (infarct and ischemia) OR isolated septal/anterior/apical or other single territory +↓EF <35% during stress or ≥2 coronary territories |
| Moderately abnormal | Obstructive coronary artery disease: ≥70% stenosis in 1 major vessel/branch | Early positive TM: failure to reach stage 2 (<3:00 min) with ST changes OR symptoms reproduced OR any arrhythmia or hypotension | Inducible ischemia or mixed defect: perfusion abnormality in 1 coronary territory (lateral or inferior/posterior) OR normal imaging but early positive TM: failure to reach stage 2 (<3:00 min) with ST changes or symptoms reproduced or any arrhythmia or hypotension | Inducible ischemia or mixed defect: wall motion abnormality or mixed abnormality (infarct and ischemia) in 1 coronary territory (lateral or inferior/posterior) OR normal imaging but early positive TM: failure to reach stage 2 (<3:00 min) with ST changes or symptoms reproduced OR any arrhythmia or hypotension |
| Mildly abnormal | Nonobstructive coronary artery disease:* 1%–69% stenosis in any major vessels/branch OR <50% left main stenosis | Late positive TM: more than stage 2 (>3:00 min) but failure to finish protocol or target heart rate achieved as a result of ST changes OR symptoms reproduced OR any arrhythmia or hypotension | Positive electrocardiography: normal perfusion or fixed perfusion defect (scar) OR normal imaging but late positive TM: more than stage 2 (>3:00 min) but failure to finish protocol or target heart rate achieved because of ST changes OR symptoms reproduced OR any arrhythmia or hypotension | Positive electrocardiography: normal wall motion or resting wall motion abnormality without inducible ischemia OR normal imaging but late positive TM: morethan stage 2 (>3:00 min) but failure to finish protocol or target heart rate achieved as a result of ST changes OR symptoms reproduced OR any arrhythmia or hypotension |
| Normal | Absence of coronary atherosclerosis | Normal electrocardiography, absence of symptoms during exercise, and normal exercise duration† | Normal electrocardiography, absence of symptoms during exercise, normal exercise duration, and normal imaging (absence of any findings suggesting myocardial abnormalities including fixed perfusion defects)† | Normal electrocardiography, absence of symptoms during exercise, normal exercise duration, and normal imaging (absence of any findings suggesting myocardial abnormalities including fixed wall motion abnormalities)† |
To standardize test reporting, site-reported test results were abstracted by a cardiology faculty or senior fellow physician using a prospectively designed protocol to deal with ambiguous test results, thereby standardizing interpretation of ambiguous test reports and harmonizing data across imaging modalities. TM indicates treadmill test.
For secondary risk stratification, nonobstructive coronary artery disease was defined as 1% to 49% luminal narrowing.
For secondary risk stratification, normal functional testing was defined as normal imaging plus a Framingham Risk Score >10%
Cardiovascular Risk Factors
Patient demographics and traditional cardiovascular risk factors were assessed and documented in a standard fashion at the time of enrollment into the PROMISE trial.27
Study End Points
The primary end point was a composite of time to MACE including death from any cause, myocardial infarction, or hospitalization for unstable angina. The secondary end point was defined as a composite of cardiovascular death, myocardial infarction, or hospitalization for unstable angina, and the tertiary end point was a composite of cardiovascular death or myocardial infarction. An independent clinical events committee adjudicated all primary and secondary end point events in a blinded fashion on the basis of standard, prospectively determined definitions.27,28
Statistical Analysis
Descriptive statistics are presented as mean and standard deviation for continuous variables and frequencies and percentages of patients for categorical variables. The Cox proportional hazards regression model was used to assess the relationship of test results to the time to the first clinical event (or censoring) for each composite end point.29 To appropriately account for heterogeneity among the subjects, analyses were adjusted for a prespecified set of baseline covariates, including age, sex, CAD risk equivalent (history of either diabetes mellitus, peripheral artery disease, or cerebrovascular disease), and the prespecification of the intended functional test (if randomly assigned to the functional testing arm). For each testing strategy, adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were computed using Cox models to characterize the relative risks of patients with normal versus mildly, moderately, and severely abnormal test results.29 For secondary analyses, we reclassified patients with at least an intermediate Framingham Risk Score (>10%) who had a normal functional test as mildly abnormal. In addition, we compared the predictive value of the absence (normal and mildly abnormal) and presence (moderately and severely abnormal) of obstructive CAD and myocardial ischemia. Cumulative event rates based on test results were computed for each testing strategy (CTA and functional testing) using the method of Kaplan and Meier.30 On the basis of the test results, the ability of each testing strategy to discriminate between patients who subsequently experienced an event versus those who did not was assessed by using the C statistic.31 The C statistic was calculated based on the predicted probability of 26-month risk from the Cox regression model. Data from each testing strategy were analyzed separately using the Cox model. A C-statistic comparison between the 2 testing strategies (anatomic versus functional) was based on z statistics. Analyses were performed for the primary end point and for the secondary and tertiary end points. All P values are 2-sided, and were considered significant if <0.05. All analyses were performed using SAS software, version 9.4 (SAS Institute).
RESULTS
Study Population
Overall, 91% of all patients enrolled in the PROMISE trial were eligible for this analysis (n=9102/10 003). Major reasons for exclusion were receiving no test or a test other than the randomized test (Figure 1). The demographics, cardiovascular risk factors, and cardiovascular event rates were similar between patients included in this analysis and those excluded (online-only Data Supplement Table I). Of the patients included, 4500 were randomly assigned to and received coronary CTA, and 4602 were randomly assigned to functional testing. There were no clinically meaningful differences in baseline patient demographics, cardiovascular risk, medication, or clinical presentation between coronary CTA and functional testing (Table 2). Overall, patients were on average 61 years of age, 53% were women, 78% were white, >90% had an intermediate or high Framingham Risk Score, about half were on at least 1 preventive medication, and the majority had atypical chest pain at presentation.
Table 2.
Characteristics of the Trial Participants at Baseline, by Study Group
| Variable | Anatomic Testing (N=4500) |
Functional Testing (N=4602) |
|---|---|---|
| Demographics | ||
| Age, y | 60.4±8.2 | 61.0±8.3 |
| Female sex | 2332 (51.8) | 2458 (53.4) |
| Racial or ethnic minority* | 1018 (22.8) | 983 (21.5) |
| Cardiac risk factors | ||
| Body mass index, kg/m2† | 30.4±5.9 | 30.5±6.1 |
| Hypertension | 2893 (64.3) | 2999 (65.2) |
| Diabetes mellitus | 936 (20.8) | 999 (21.7) |
| Dyslipidemia | 3029 (67.3) | 3127 (67.9) |
| Family history of premature CAD‡ | 1460 (32.6) | 1426 (31.1) |
| Peripheral or cerebrovascular disease | 228 (5.1) | 264 (5.7) |
| CAD equivalent§ | 1097 (24.4) | 1189 (25.8) |
| History of heart failure | 163 (3.6) | 176 (3.8) |
| Metabolic syndrome‖ | 1673 (37.2) | 1763 (38.3) |
| Current or past tobacco use | 2292 (50.9) | 2367 (51.4) |
| Sedentary lifestyle# | 2179 (48.5) | 2229 (48.5) |
| History of depression | 885 (19.7) | 992 (21.6) |
| Risk factor burden and risk score** | ||
| No risk factors | 116 (2.6) | 130 (2.8) |
| Risk factor burden | 2.4±1.1 | 2.4±1.1 |
| Combined Diamond-Forrester and coronary artery surgery risk score†† | 53.2±21.3 | 53.3±21.2 |
| Framingham Risk Score | ||
| Low risk (<10%) | 1028 (22.9) | 1036 (22.5) |
| Intermediate risk (10%–20%) | 1632 (36.3) | 1591 (34.6) |
| High risk (>20%) | 1832 (40.8) | 1971 (42.9) |
| Atherosclerotic cardiovascular disease pooled cohort risk prediction (2013) | ||
| Low risk (<7.5%) | 1471 (33.0) | 1444 (31.7) |
| Elevated risk (≥7.5%) | 2980 (67.0) | 3118 (68.3) |
| Relevant medications | ||
| β-Blocker | 1065 (24.8) | 1095 (24.9) |
| Angiotensin-converting enzyme or angiotensin-receptor blocker | 1860 (43.2) | 1952 (44.3) |
| Statin | 1973 (45.9) | 2008 (45.6) |
| Aspirin | 1945 (45.2) | 1941 (44.1) |
| Clopidogrel | 56 (1.3) | 69 (1.6) |
| Prasugrel | 1 (<0.1) | 1 (<0.1) |
| Warfarin | 68 (1.6) | 82 (1.9) |
| Primary presenting symptom and anginal type | ||
| Chest pain | 3322 (73.9) | 3299 (71.7) |
| Dyspnea on exertion | 633 (14.1) | 734 (16.0) |
| Anginal type, site-reported | ||
| Typical | 521 (11.6) | 521 (11.3) |
| Atypical | 3501 (77.8) | 3595 (78.1) |
| Nonanginal | 478 (10.6) | 486 (10.6) |
There were no significant between-group differences at baseline, except with respect to racial or ethnic minority group and history of depression. Values are mean±SD or n (%). CAD indicates coronary artery disease.
Racial or ethnic minority group was self-reported, with the status of minority being defined by the patient.
Body mass index is the weight in kilograms divided by the square of the height in meters.
A family history of premature CAD was defined as diagnosis of the disease in a male first-degree relative before 55 years of age or in a female first-degree relative before 65 years of age.
CAD risk equivalent was defined as diabetes mellitus, peripheral vascular disease, or cerebrovascular disease.
The metabolic syndrome was defined according to consensus criteria of the American Heart Association and the National Heart, Lung, and Blood Institute.
Sedentary lifestyle was defined by the patient as not participating in regular physical activities at least 1 time per week over the previous month.
Risk factors included hypertension, diabetes mellitus, dyslipidemia, family history of premature CAD, and tobacco use.
Combined Diamond and Forrester and Coronary Artery Surgery Study risk scores range from 0 to 100, with higher scores indicating a greater likelihood of obstructive CAD.
Outcomes
During the median follow-up of 26.1 months (interquartile range: 18.0–34.4), event rates were similar in the anatomic and functional arms: overall, 137 (3.1%) versus 132 (3.0%); death, 62 (1.4%) versus 66 (1.4%); myocardial infarction, 26 (0.6%) versus 31 (0.7%); and unstable angina, 52 (1.2%) versus 41 (0.9%).
Test Results
The distribution of test results was significantly different between coronary CTA and functional testing. There were twice as many patients who had completely normal functional testing in comparison with a normal coronary CTA (78.0% versus 33.4%; P<0.001).
HRs for events increased proportionally for mildly, moderately, and severely abnormal CTA results in comparison with normal CTA tests (HRs, 2.94; 7.67–10.13; all P<0.001) (Table 3). In contrast, the increase in risk for functional testing is only significant in moderately and severely abnormal categories (HRs, 2.65 [P=0.001], 3.88 [P<0.001]), with no difference in risk between normal and mildly abnormal test results (HR, 0.94 [P=0.87]). The discriminatory ability of CTA in predicting events was significantly better than functional testing (c-index, 0.72; 95% CI, 0.68–0.76 versus 0.64; 95% CI, 0.59–0.69; P=0.04) (Figure 2). The results were similar for secondary analyses in which nonobstructive CAD was defined as 1% to 49% (online-only Data Supplement Table II; c-index, 0.73; 95% CI, 0.69–0.78; P=0.01). The better discrimination of events by coronary CTA was the result of the ability to define a very low risk group using a normal CTA which nearly excluded events (14 of 137 events; 10.2% of all events), corresponding to an incidence rate of 0.93% over 2 years. In contrast, the majority of events in the functional arm (75 of 132 events; 56.8% of all events), corresponding to an incidence rate of 2.09%, occurred in those with completely normal functional tests (including no reversible or irreversible ischemia, normal electrocardiography, normal duration of exercise without symptoms). A second reason was that detection of nonobstructive CAD by coronary CTA identified an at-risk group of patients (62.1% of CTAs; n=2461/3966), in which the majority of events in the CTA arm occurred (54%, n=74/137; 3.0% event rate). If nonobstructive CAD was defined as 1% to 49% luminal stenosis, a still significant 33.6% of the events (n=46/137) occurred in this group (online-only Data Supplement Table II). In contrast, very few patients had mildly abnormal tests in the functional arm (9.4%, n=432/4602). Similar results were seen for the secondary end points of cardiovascular death/myocardial infarction/unstable angina and tertiary end points of cardiovascular death and myocardial infarction (Table 2 and online-only Data Supplement Table II).
Table 3.
Frequency of Test Findings and Association With Clinical Events for Anatomic and Functional Testing*
| Initial Test Results | Anatomic Testing (N=4500) | Functional Testing (N=4602) | ||||||
|---|---|---|---|---|---|---|---|---|
| Frequency n/N (%) |
Event Rate n/N (%) |
HR (95% CI) | P Value | Frequency n/N (%) |
Event Rate n/N (%) |
HR (95% CI) | P Value | |
| All-cause death/myocardial infarction/unstable angina | ||||||||
| Severely abnormal | 266/4500 (5.91) | 28/266 (10.53) | 10.13 (5.15–19.92) | <0.0001 | 365/4602 (7.93) | 35/365 (9.59) | 3.88 (2.58–5.85) | <0.0001 |
| Moderately abnormal | 268/4500 (5.96) | 21/268 (7.84) | 7.67 (3.83–15.37) | <0.0001 | 217/4602 (4.72) | 13/217 (5.99) | 2.65 (1.46–4.83) | 0.0014 |
| Mildly abnormal | 2461/4500 (54.69) | 74/2461 (3.01) | 2.94 (1.64–5.26) | 0.0003 | 432/4602 (9.39) | 9/432 (2.08) | 0.94 (0.47–1.89) | 0.8666 |
| Normal | 1505/4500 (33.44) | 14/1505 (0.93) | 3588/4602 (77.97) | 75/3588 (2.09) | ||||
| Cardiovascular death/myocardial infarction/unstable angina | ||||||||
| Severely abnormal | 266/4500 (5.91) | 26/266 (9.77) | 17.26 (7.55–39.46) | <0.0001 | 365/4602 (7.93) | 31/365 (8.49) | 4.59 (2.93–7.19) | <0.0001 |
| Moderately abnormal | 268/4500 (5.96) | 18/268 (6.72) | 12.03 (5.14–28.19) | <0.0001 | 217/4602 (4.72) | 13/217 (5.99) | 3.50 (1.89–6.48) | <0.0001 |
| Mildly abnormal | 2461/4500 (54.69) | 57/2461 (2.32) | 4.08 (1.93–8.66) | 0.0002 | 432/4602 (9.39) | 8/432 (1.85) | 1.11 (0.53–2.34) | 0.7834 |
| Normal | 1505/4500 (33.44) | 8/1505 (0.53) | 3588/4602 (77.97) | 56/3588 (1.56) | ||||
| Cardiovascular death/myocardial infarction | ||||||||
| Severely abnormal | 266/4500 (5.91) | 9/266 (3.38) | 4.87 (1.72–13.75) | 0.0028 | 365/4602 (7.93) | 14/365 (3.84) | 2.13 (1.16–3.91) | 0.0141 |
| Moderately abnormal | 268/4500 (5.96) | 5/268 (1.87) | 3.09 (0.96–9.97) | 0.0594 | 217/4602 (4.72) | 5/217 (2.30) | 1.53 (0.60–3.90) | 0.3681 |
| Mildly abnormal | 2461/4500 (54.69) | 39/2461 (1.58) | 2.73 (1.20–6.25) | 0.0170 | 432/4602 (9.39) | 5/432 (1.16) | 0.81 (0.32–2.04) | 0.6542 |
| Normal | 1505/4500 (33.44) | 7/1505 (0.47) | 3588/4602 (77.97) | 48/3588 (1.34) | ||||
CI indicates confidence interval; and HR, hazard ratio. Nonobstructive coronary artery disease is defined as 1% to 69% of stenosis.
Secondary test result stratification sets computed tomographic angiography threshold for moderate abnormality to 70%.
Figure 2. Kaplan-Meier curves demonstrating cumulative event rates for the primary end point based on test results (normal or mildly, moderately, or severely abnormal) for anatomic testing (using 1%–69% criterion for nonobstructive CAD on CTA) (A), functional testing (B), and functional testing including the Framingham Risk Score (C).
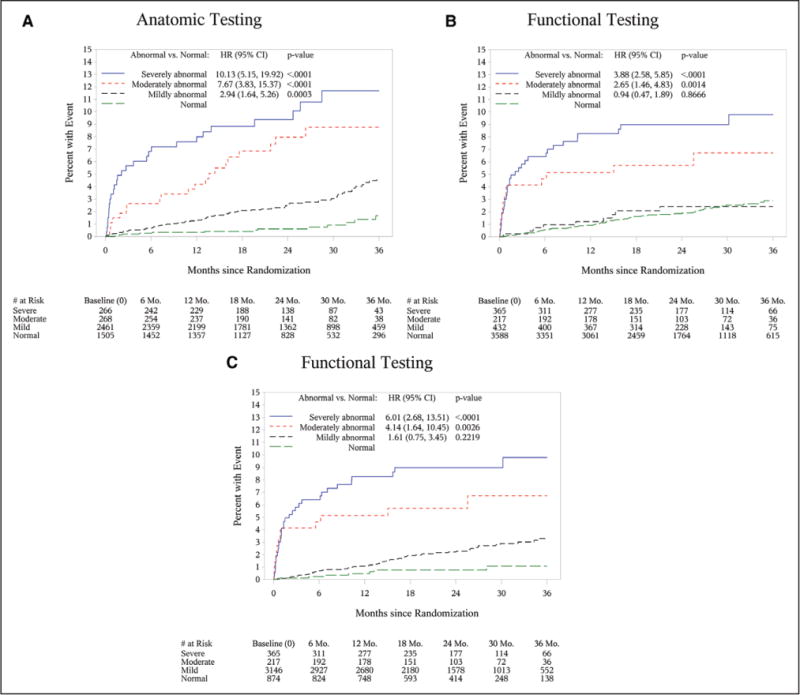
CAD indicates coronary artery disease; CI, confidence interval; CTA, computed tomography angiography; HR, hazard ratio; and Mo., months.
Important from a clinical management standpoint, when we compared anatomic versus functional binary test results (absence or presence of ≥50% LM or ≥70% stenosis elsewhere versus presence or absence of reversible myocardial ischemia in any segment), we found that both the prevalence (obstructive CAD: 11.9% [n=534/4500] versus myocardial ischemia: 12.6% [n=582/4602]; P=0.257) and the event rates (9.2% versus 8.2%, respectively; P=0.58) were similar. Thus, obstructive CAD and reversible myocardial ischemia were each associated with a similarly significantly increased relative risk for cardiovascular events for the primary end point (HR, 3.74; 95% CI, 2.60–5.39 versus HR, 3.47; 95% CI, 2.42–4.99; P<0.0001 for both) (Table 4), and with a similar discriminatory ability in predicting the primary end point, as well (c-index, 0.65; 95% CI, 0.60–0.69 versus 0.65; 95% CI, 0.60–0.69, respectively; P=0.946; online-only Data Supplement Figure I).
Table 4.
Frequency of Obstructive Coronary Artery Disease and Myocardial Ischemia and Association With Clinical Events
| Initial Test Results | Anatomic Testing (N=4500) | Functional Testing (N=4602) | ||||||
|---|---|---|---|---|---|---|---|---|
| Frequency n/N (%) |
Event Rate n/N (%) |
HR (95% CI) | P Value | Frequency n/N (%) |
Event Rate n/N (%) |
HR (95% CI) | P Value | |
| All-cause death/myocardial infarction/unstable angina | ||||||||
| Abnormal | 534/4500 (11.87) | 49/534 (9.18) | 3.74 (2.60–5.39) | <0.0001 | 582/4602 (12.65) | 48/582 (8.25) | 3.47 (2.42–4.99) | <0.0001 |
| Normal | 3966/4500 (88.13) | 88/3966 (2.22) | 4020/4602 (87.35) | 84/4020 (2.09) | ||||
| Cardiovascular death/myocardial infarction/unstable angina | ||||||||
| Abnormal | 534/4500 (11.87) | 44/534 (8.24) | 4.63 (3.10–6.92) | <0.0001 | 582/4602 (12.65) | 44/582 (7.56) | 4.15 (2.80–6.14) | <0.0001 |
| Normal | 3966/4500 (88.13) | 65/3966 (1.64) | 4020/4602 (87.35) | 64/4020 (1.59) | ||||
| Cardiovascular death/myocardial infarction | ||||||||
| Abnormal | 534/4500 (11.87) | 14/534 (2.62) | 1.76 (0.95–3.25) | 0.0730 | 582/4602 (12.65) | 19/582 (3.26) | 1.98 (1.16–3.37) | 0.0120 |
| Normal | 3966/4500 (88.13) | 46/3966 (1.16) | 4020/4602 (87.35) | 53/4020 (1.32) | ||||
CI indicates confidence interval; and HR, hazard ratio. Obstructive coronary artery disease is defined as >50% stenosis in the left main coronary artery and >70% stenosis elsewhere.
Functional Testing Stratified by Modality
Online-only Data Supplement Table III lists the distribution of test results and events among the 4602 patients randomly assigned in the functional arm by modality. The data demonstrate that only 10.1% of patients underwent exercise treadmill testing, whereas the vast majority (67.8%) underwent nuclear perfusion stress testing. The observed event rate was much lower in the patients undergoing exercise treadmill testing (n=6/467; 1.3%) in comparison with those undergoing either stress echocardiography (n=20/1019; 2%) or stress nuclear testing (n=106/3116; 3.4%), most likely reflecting the fact that physicians very appropriately referred patients at lowest risk to exercise treadmill testing and those at highest to stress nuclear testing. Last, only a minority of events occurred in those with normal or mildly abnormal exercise treadmill testing (n=4, 3, and 2 for the primary, secondary, and tertiary end points, respectively).
Stress Nuclear Perfusion Testing Versus Coronary CTA
Although we used an aggregate of the functional tests as the primary approach to compare the prognostic value of an anatomic versus a functional strategy, online-only Data Supplement Table IV provides a comparison of nuclear stress testing with coronary CTA based on the prerandomization intended functional test. In that analysis, only patients whose prerandomization intended functional test was nuclear were included in the analysis in both arms. In brief, the data demonstrate that the HRs for coronary CTA are higher than for functional testing across test result categories and end points, with differences being higher in comparison with the overall cohort.
Functional Testing Plus Risk Factors
Given the large number of normal functional tests and the importance of pretest probability in interpreting test results, we performed a secondary analysis in which we reclassified patients with at least an intermediate Framingham Risk Score (>10%) who had a normal functional test as being mildly abnormal. As a result, 2714 patients were reclassified from normal to a mildly abnormal functional test, whereas only 874 patients remained in the normal category. This reclassification resulted in stronger association of functional test strata with clinical outcomes in comparison with test results only, although there was still no significant difference in event rates between normal and mildly abnormal test results with the new classification for functional imaging (HR, 1.61; 95% CI, 0.75–3.45; P=0.22) (online-only Data Supplement Table V and Table 5). The discriminatory value of functional testing strata improved from a c-index of 0.64 (95% CI, 0.59–0.69) using test data only to a c-index of 0.69 (95% CI, 0.64–0.74) using the Framingham Risk Score for reclassification. The inclusion of Framingham Risk Score to stratify functional testing results rendered no significant difference in discriminatory capacity between anatomic and functional testing (c-index for CTA, 0.72; 95% CI, 0.68–0.76; versus c-index for functional testing including the Framing-ham Risk Score, 0.69; 95% CI, 0.64–0.74; P=0.29) (Figure 2C).
Table 5.
Frequency of Test Findings and Association With Clinical Events for Anatomic Test Strata and for Functional Test Strata Including the Framingham Risk Score
| Initial Test Results | Anatomic Testing (N=4500) | Functional Testing* (N=4602) | ||||||
|---|---|---|---|---|---|---|---|---|
| Frequency n/N (%) |
Event Rate n/N (%) |
HR (95% CI) | P Value | Frequency n/N (%) |
Event Rate n/N (%) |
HR (95% CI) | P Value | |
| All-cause death/myocardial infarction/unstable angina | ||||||||
| Severely abnormal | 266/4500 (5.91) | 28/266 (10.53) | 10.13 (5.15–19.92) | <0.0001 | 365/4602 (7.93) | 35/365 (9.59) | 6.01 (2.68–13.51) | <0.0001 |
| Moderately abnormal | 268/4500 (5.96) | 21/268 (7.84) | 7.67 (3.83–15.37) | <0.0001 | 217/4602 (4.72) | 13/217 (5.99) | 4.14 (1.64–10.45) | 0.0026 |
| Mildly abnormal | 2461/4500 (54.69) | 74/2461 (3.01) | 2.94 (1.64–5.26) | 0.0003 | 3146/4602 (68.36) | 76/3146 (2.42) | 1.61 (0.75–3.45) | 0.2219 |
| Normal | 1505/4500 (33.44) | 14/1505 (0.93) | 874/4602 (18.99) | 8/874 (0.92) | ||||
| Cardiovascular death/myocardial infarction/unstable angina | ||||||||
| Severely abnormal | 266/4500 (5.91) | 26/266 (9.77) | 17.26 (7.55–39.46) | <0.0001 | 365/4602 (7.93) | 31/365 (8.49) | 6.05 (2.54–14.41) | <0.0001 |
| Moderately abnormal | 268/4500 (5.96) | 18/268 (6.72) | 12.03 (5.14–28.19) | <0.0001 | 217/4602 (4.72) | 13/217 (5.99) | 4.63 (1.76–12.24) | 0.0020 |
| Mildly abnormal | 2461/4500 (54.69) | 57/2461 (2.32) | 4.08 (1.93–8.66) | 0.0002 | 3146/4602 (68.36) | 57/3146 (1.81) | 1.38 (0.61–3.15) | 0.4433 |
| Normal | 1505/4500 (33.44) | 8/1505 (0.53) | 874/4602 (18.99) | 7/874 (0.80) | ||||
| Cardiovascular death/myocardial infarction | ||||||||
| Severely abnormal | 266/4500 (5.91) | 9/266 (3.38) | 4.87 (1.72–13.75) | 0.0028 | 365/4602 (7.93) | 14/365 (3.84) | 2.22 (0.83–5.88) | 0.1103 |
| Moderately abnormal | 268/4500 (5.96) | 5/268 (1.87) | 3.09 (0.96–9.97) | 0.0594 | 217/4602 (4.72) | 5/217 (2.30) | 1.60 (0.47–5.38) | 0.4490 |
| Mildly abnormal | 2461/4500 (54.69) | 39/2461 (1.58) | 2.73 (1.20–6.25) | 0.0170 | 3146/4602 (68.36) | 46/3146 (1.46) | 1.02 (0.43–2.39) | 0.9678 |
| Normal | 1505/4500 (33.44) | 7/1505 (0.47) | 874/4602 (18.99) | 7/874 (0.80) | ||||
CI, confidence interval; HR, hazard ratio. Nonobstructive coronary artery disease is defined as 1% to 69% of stenosis.
Normal functional testing is defined as completely normal functional testing and a Framingham Risk Score of <10%.
DISCUSSION
Overall, our study provides comparative evidence on the prognostic value of findings of the most commonly performed diagnostic tests, including presence and extent of myocardial ischemia and CAD and the associated absolute and relative risks for future cardiovascular events in a contemporary patient stable chest pain population at intermediate risk for CAD using the PROMISE randomized trial data from 193 North American sites. Our results may contribute to a better understanding of how to use information from these tests to guide management of this large group of patients. We found that anatomic assessment with coronary CTA provided significantly better prognostic information than functional testing (c-index: 0.72 versus 0.64; P=0.04), which was a result of the detection of an at-risk group of patients with nonobstructive CAD by coronary CTA and the indiscriminatory nature of a normal functional test. Adding the Framingham Risk Score to functional test results significantly improved the prognostic value of functional testing.
The strengths of this study include the following: (1) this is the first large (N>9000) prospective randomized comparison of the prognostic value of anatomic imaging by coronary CTA with functional exercise- or stress-based testing results in patients with stable chest pain; (2) this uniquely allows for a direct comparison of test findings of anatomic and functional testing, and the understanding of which particular diagnostic findings are differential between the strategies in their ability to identify patients at risk for MACE; and (3) the multicenter nature of this study recruiting patients at 193 North American sites provides generalizable data from a contemporary stable chest pain population. One of the insights from the data is the low prevalence of obstructive CAD (11.9%) and myocardial ischemia (12.6%), which only 2 decades ago was between 30% and 40% in patients undergoing nuclear stress perfusion imaging.3 A related important observation is that the majority of clinical events over a 2-year follow-up occurred in patients without obstructive CAD or myocardial ischemia, indicating a significant risk burden undetected by conventional measures of test positivity. For coronary CTA, our data demonstrate that a finding of nonobstructive CAD identifies a large additional group of at-risk patients, in which the majority of events occurred (n=74/137, 54.0%) with similar observations made in smaller studies from Japan.16,19,32–35 In contrast, parameters from the exercise portion of functional tests (symptoms and duration of exercise, and electrocardiography changes, as well) and imaging findings, such as fixed defects without ischemia, did not identify patients at risk for events. Mechanistically, these data corroborate many years of research in interventional cardiology, suggesting that at least one-third, in the context of aggressive medical therapy, or up to two-thirds of future cardiovascular events occur at locations in the coronary artery tree where previously no obstructive CAD was present.34–36 Thus, in an era of imaging patients with a relatively low burden of demonstrable myocardial ischemia or obstructive CAD, the relative importance of detecting subclinical atherosclerotic disease becomes substantially greater and is an important consideration for test choice.37
Our results further emphasize the importance of cardiovascular risk profile in contemporary populations with stable chest pain, especially in those patients with completely normal functional testing. We demonstrate that addition of the Framingham Risk Score, as an accepted global risk estimation tool, improved the discriminatory capacity of functional assessment (c-index, 0.64–0.69), which rendered the comparison with anatomic testing nonsignificant (P=0.29).
Another important implication of our study is that a normal CTA, in contrast to a completely normal functional test, is highly unlikely to be associated with MACE for at least 2 years. Because similar findings have been reported in the acute chest pain setting,38 this determination of a warranty period is an important additional benefit of coronary CTA for patients and providers.
Our results confirm evidence from observational studies that both findings of obstructive CAD and reversible myocardial ischemia are associated with significantly increased relative risk for future MACE,14,16–18,32,33 but we extend these studies by demonstrating that the prognostic power of both findings is essentially equivalent in identifying patients with a substrate that can explain symptoms (HR, 3.74; 95% CI, 2.60–5.39 versus HR, 3.47; 95% CI, 2.42–4.99). The primary results of the PROMISE trial demonstrated that the aggregate of actions taken by physicians and patients based on a strategy of either initial anatomic or functional testing did not yield a difference in clinical outcomes.28 In contrast, this prespecified secondary analysis is the first to report on the ability of diagnostic test results to accurately distinguish patients who subsequently experience a clinical event from patients who do not. Although this is presum ably related to events, it represents a different, relevant clinical question.
Our study has limitations. Although the PROMISE trial was designed to compare 2 fundamentally different approaches to the management of patients with stable chest pain, anatomic versus functional testing, we acknowledge that the sensitivities, specificities, predictive values, and prognostic values can vary between different functional testing modalities and by age, sex, and other patient characteristics (eg, body mass index). We further acknowledge that the choice of functional test was dictated by physician preferences and patient presentation, and thus will vary by individual clinician choices. However, because physicians in the PROMISE trial had to prespecify before randomization their preference for which functional test the patient should undergo if he or she were randomly assigned to the functional arm, we were able to perform a matched comparison of CTA with nuclear testing, which demonstrated results similar to results seen for the entire population. Unfortunately, the much smaller numbers of patients receiving treadmill exercise or stress echocardiography precluded a valid subanalysis for these 2 modalities.
It is further important to note that treatments based on imaging results were not accounted for in our analysis, but may have affected the cardiovascular outcomes assessed. However, one could argue that, based on the intention of the trial as a strategy comparison, it may be desirable to include the effects of medical treatments or interventions and their effect on prognosis as a result of the study. Indeed, in keeping with the results of this analysis, it has been shown that the prognostic importance of coronary anatomic information is maintained and that of functional testing is lost or markedly attenuated when aggressive medical therapy and either elective or as-needed revascularization is pursued.39
Our study had a relatively small number of events and a short median follow-up of 26 months. In addition, we stratified test results for functional testing and coronary CTA based on site reads collected on case report forms to identify abnormal and normal tests. Further, the study excluded patients with abnormal left ventricular function or a history of myocardial infarction, and hence the prognostic value of diagnostic hallmarks of functional testing such as left ventricular function or fixed perfusion defects could not be assessed.
CONCLUSIONS
Contemporary stable chest pain populations present with a low prevalence of myocardial ischemia and obstructive CAD. In this population, the detection of non-obstructive CAD identifies additional at-risk patients, whereas consideration of the Framingham Risk Score is important for proper risk stratification of patients with normal stress testing. These results may contribute to a better understanding of how to use this information to guide management of these patients.
Supplementary Material
Clinical Perspective.
What Is New?
This was a large (N>9000) randomized comparison of the prognostic value of anatomic imaging by coronary computed tomography angiography with functional stress testing in patients with stable chest pain.
Contemporary chest pain populations referred for testing have a low burden of obstructive coronary artery disease and myocardial ischemia, and both findings have similar prognostic value.
Coronary computed tomography angiography, by visualizing nonobstructive coronary artery disease, identifies additional at-risk patients and imparts better prognostic and discriminatory information than functional testing.
Consideration of the Framingham Risk Score as an accepted, global risk estimation significantly improves the prognostic value of functional assessment.
What Are the Clinical Implications?
This study provides generalizable comparative evidence on the relative prognostic value of the diagnostic tests most commonly used to evaluate patients with stable chest pain.
This may improve the use of this information to guide management of these patients.
Given the low prevalence of myocardial ischemia and obstructive coronary artery disease in contemporary chest pain populations, the detection of nonobstructive coronary artery disease identifies additional at-risk patients.
A normal functional test result, including information on exercise and symptoms, has moderate prognostic value, and consideration of the Framingham Risk Score improves risk stratification.
Acknowledgments
The authors thank all the patients who participated in the PROMISE trial, and Sarah Hayden, Peter Hoffmann, Beth Martinez, Stephanie Wu, and Qinghong Yang for their important contributions.
SOURCES OF FUNDING
This project was supported by grants R01HL098237, R01HL098236, R01HL98305, and R01HL098235 from the National Heart, Lung, and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the article, and its final contents. This article does not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
Dr Hoffmann reports receiving grants from American College of Radiology Imaging Network and HeartFlow Inc. during the conduct of the study, and from Siemens Healthcare outside the submitted work. Dr Ferencik reports receiving grant support from the American Heart Association. Dr Patel reports receiving grants from HeartFlow Technologies, Janssen, John-son & Johnson, AstraZeneca, and the Agency for Healthcare Research and Quality, and personal fees from AstraZeneca, Bayer, Genzyme, and Janssen outside the submitted work. Dr Mark reports receiving grants from the National Institutes of Health during the conduct of the study, and personal fees, as well, from Medtronic, Inc., grants from Eli Lilly and Company, Bristol-Myers Squibb, Gilead Sciences, Inc., AGA Medical Corporation, Merck & Company, Oxygen Therapeutics, and Astra-Zeneca, and personal fees from CardioDx and St. Jude Medical outside the submitted work. Dr Budoff reports receiving grant support from the National Institutes of Health and General Electric. Dr Nahhas reports ownership of stocks in Johnson & John-son with value >$10 000. Dr Chow reports receiving research support from GE Healthcare and educational support from TeraRecon. Dr Lee reports receiving grants from the National Institutes of Health. Dr Douglas reports receiving grant support from HeartFlow and service on a data and safety monitoring board for GE HealthCare outside the submitted work.
Footnotes
This paper was handled by a guest editor.
DISCLOSURES
The other authors report no potential conflicts of interest.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.116.024360/-/DC1.
References
- 1.Ladapo JA, Blecker S, Douglas PS. Physician decision making and trends in the use of cardiac stress testing in the United States: an analysis of repeated cross-sectional data. Ann Intern Med. 2014;161:482–490. doi: 10.7326/M14-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–1065. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 4.SCOT-HEART Investigators. CT Coronary Angiography in Patients With Suspected Angina Due to Coronary Heart Disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 5.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, Marwick TH, Pandey DK, Wicklund RH, Thisted RA. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Rohatgi A, Ayers CR, Willis BL, Haskell WL, Khera A, Drazner MH, de Lemos JA, Berry JD. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–1383. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauer MS. How will exercise capacity gain enough respect? Circulation. 2011;123:1364–1366. doi: 10.1161/CIRCULATIONAHA.111.023218. [DOI] [PubMed] [Google Scholar]
- 10.Lauer MS, Pothier CE, Magid DJ, Smith SS, Kattan MW. An externally validated model for predicting long-term survival after exercise treadmill testing in patients with suspected coronary artery disease and a normal electrocardiogram. Ann Intern Med. 2007;147:821–828. doi: 10.7326/0003-4819-147-12-200712180-00001. [DOI] [PubMed] [Google Scholar]
- 11.Mark DB, Hlatky MA, Harrell FE, Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106:793–800. doi: 10.7326/0003-4819-106-6-793. [DOI] [PubMed] [Google Scholar]
- 12.Mark DB, Shaw L, Harrell FE, Jr, Hlatky MA, Lee KL, Bengtson JR, McCants CB, Califf RM, Pryor DB. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–853. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 13.Hachamovitch R, Berman DS, Kiat H, Cohen I, Lewin H, Amanullah A, Kang X, Friedman J, Diamond GA. Incremental prognostic value of adenosine stress myocardial perfusion single-photon emission computed tomography and impact on subsequent management in patients with or suspected of having myocardial ischemia. Am J Cardiol. 1997;80:426–433. doi: 10.1016/s0002-9149(97)00390-1. [DOI] [PubMed] [Google Scholar]
- 14.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 15.Marwick TH, Case C, Sawada S, Rimmerman C, Brenneman P, Kovacs R, Short L, Lauer M. Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol. 2001;37:754–760. doi: 10.1016/s0735-1097(00)01191-8. [DOI] [PubMed] [Google Scholar]
- 16.Marwick TH, Case C, Vasey C, Allen S, Short L, Thomas JD. Prediction of mortality by exercise echocardiography: a strategy for combination with the duke treadmill score. Circulation. 2001;103:2566–2571. doi: 10.1161/01.cir.103.21.2566. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11:171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Yao SS, Qureshi E, Sherrid MV, Chaudhry FA. Practical applications in stress echocardiography: risk stratification and prognosis in patients with known or suspected ischemic heart disease. J Am Coll Cardiol. 2003;42:1084–1090. doi: 10.1016/s0735-1097(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 19.Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Callister TQ, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Maffei E, Cademartiri F, Kaufmann P, Shaw LJ, Raff GL, Chinnaiyan KM, Villines TC, Cheng V, Nasir K, Gomez M, Min JK, CONFIRM Investigators Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) Circulation. 2012;126:304–313. doi: 10.1161/CIRCULATIONAHA.111.081380. [DOI] [PubMed] [Google Scholar]
- 20.Hadamitzky M, Achenbach S, Al-Mallah M, Berman D, Budoff M, Cademartiri F, Callister T, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Cury R, Delago A, Dunning A, Feuchtner G, Gomez M, Kaufmann P, Kim YJ, Leipsic J, Lin FY, Maffei E, Min JK, Raff G, Shaw LJ, Villines TC, Hausleiter J, CONFIRM Investigators Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry) J Am Coll Cardiol. 2013;62:468–476. doi: 10.1016/j.jacc.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 21.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 22.Min JK, Shaw LJ, Berman DS, Gilmore A, Kang N. Costs and clinical outcomes in individuals without known coronary artery disease undergoing coronary computed tomographic angiography from an analysis of Medicare category III transaction codes. Am J Cardiol. 2008;102:672–678. doi: 10.1016/j.amjcard.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 23.Ostrom MP, Gopal A, Ahmadi N, Nasir K, Yang E, Kakadiaris I, Flores F, Mao SS, Budoff MJ. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335–1343. doi: 10.1016/j.jacc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Min JK, Kang N, Shaw LJ, Devereux RB, Robinson M, Lin F, Legorreta AP, Gilmore A. Costs and clinical outcomes after coronary multidetector CT angiography in patients without known coronary artery disease: comparison to myocardial perfusion SPECT. Radiology. 2008;249:62–70. doi: 10.1148/radiol.2483071453. [DOI] [PubMed] [Google Scholar]
- 25.Koenig W, Bamberg F, Lee H, Truong QA, Nichols JH, Trischler G, Morrow DA, Nagurney TJ, Hoffmann U. High-sensitivity troponin reliably excludes acute coronary syndrome in patients with acute chest pain: Results from the rule out myocardial infarction by computed tomography (ROMICAT) Study. Circulation. 2008;118(18 suppl 2):S637. [Google Scholar]
- 26.Hadamitzky M, Täubert S, Deseive S, Byrne RA, Martinoff S, Schömig A, Hausleiter J. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J. 2013;34:3277–3285. doi: 10.1093/eurheartj/eht293. [DOI] [PubMed] [Google Scholar]
- 27.Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K, Dolor RJ, Kosinski A, Krucoff MW, Mudrick DW, Patel MR, Picard MH, Udelson JE, Velazquez EJ, Cooper L, PROMISE investigators PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J. 2014;167:796–803.e1. doi: 10.1016/j.ahj.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL, PROMISE Investigators Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. doi: 10.1056/NEJ-Moa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 32.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, COURAGE Investigators Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Out-comes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 33.Bamberg F, Sommer WH, Hoffmann V, Achenbach S, Nikolaou K, Conen D, Reiser MF, Hoffmann U, Becker CR. Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J Am Coll Cardiol. 2011;57:2426–2436. doi: 10.1016/j.jacc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 34.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 35.Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation. 2001;103:3142–3149. doi: 10.1161/01.cir.103.25.3142. [DOI] [PubMed] [Google Scholar]
- 36.Mancini GB, Hartigan PM, Bates ER, Sedlis SP, Maron DJ, Spertus JA, Berman DS, Kostuk WJ, Shaw LJ, Weintraub WS, Teo KK, Dada M, Chaitman BR, O’Rourke RA, Boden WE, COURAGE Investigators and Coordinators Angiographic disease progression and residual risk of cardiovascular events while on optimal medical therapy: observations from the COURAGE Trial. Circ Cardiovasc Interv. 2011;4:545–552. doi: 10.1161/CIRCINTERVENTIONS.110.960062. [DOI] [PubMed] [Google Scholar]
- 37.Maurovich-Horvat P, Schlett CL, Alkadhi H, Nakano M, Otsuka F, Stolzmann P, Scheffel H, Ferencik M, Kriegel MF, Seifarth H, Virmani R, Hoffmann U. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging. 2012;5:1243–1252. doi: 10.1016/j.jcmg.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Schlett CL, Banerji D, Siegel E, Bamberg F, Lehman SJ, Ferencik M, Brady TJ, Nagurney JT, Hoffmann U, Truong QA. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. JACC Cardiovasc Imaging. 2011;4:481–491. doi: 10.1016/j.jcmg.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancini GB, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, Maron DJ, Teo K, Sedlis SP, Chaitman BR, Weintraub WS, Spertus JA, Kostuk WJ, Dada M, Booth DC, Boden WE. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


