Summary
Adult neurogenesis declines with aging due to the depletion and functional impairment of neural stem/progenitor cells (NSPCs). An improved understanding of the underlying mechanisms that drive age‐associated neurogenic deficiency could lead to the development of strategies to alleviate cognitive impairment and facilitate neuroregeneration. An essential step towards this aim is to investigate the molecular changes that occur in NSPC aging on a genomewide scale. In this study, we compare the transcriptional, histone methylation and DNA methylation signatures of NSPCs derived from the subventricular zone (SVZ) of young adult (3 months old) and aged (18 months old) mice. Surprisingly, the transcriptional and epigenomic profiles of SVZ‐derived NSPCs are largely unchanged in aged cells. Despite the global similarities, we detect robust age‐dependent changes at several hundred genes and regulatory elements, thereby identifying putative regulators of neurogenic decline. Within this list, the homeobox gene Dbx2 is upregulated in vitro and in vivo, and its promoter region has altered histone and DNA methylation levels, in aged NSPCs. Using functional in vitro assays, we show that elevated Dbx2 expression in young adult NSPCs promotes age‐related phenotypes, including the reduced proliferation of NSPC cultures and the altered transcript levels of age‐associated regulators of NSPC proliferation and differentiation. Depleting Dbx2 in aged NSPCs caused the reverse gene expression changes. Taken together, these results provide new insights into the molecular programmes that are affected during mouse NSPC aging, and uncover a new functional role for Dbx2 in promoting age‐related neurogenic decline.
Keywords: DNA methylation, epigenetics, histone methylation, neural stem/progenitor cells, neurospheres, subventricular zone
1. INTRODUCTION
In the adult mouse forebrain, neurogenesis persists in two restricted niches located in the subventricular zone (SVZ) close to the lateral ventricles and in the subgranular zone (SGZ) within the dentate gyrus. In these regions, neural stem/progenitor cells (NSPCs) are maintained throughout adulthood and give rise to daughter cells that undergo differentiation into neurons and glia. Increasing evidence supports a role for adult neurogenesis in normal brain function and suggests that neurological disorders and neurodegeneration may be caused, at least in part, by reduced neuronal output of adult NSPCs (Goncalves, Schafer & Gage, 2016; Lledo & Valley, 2016).
Aging is a physiological process substantially affecting, in a time‐dependent manner, the function of somatic stem cells in multiple tissues (Signer & Morrison, 2013). Aging is associated with reduced neurogenesis in the mouse SVZ and SGZ (Encinas et al., 2011; Enwere et al., 2004; Lugert et al., 2010; Luo, Daniels, Lennington, Notti & Conover, 2006), which might lead to decreased olfactory function and cognitive hippocampus‐dependent impairment (Goncalves et al., 2016; Lledo & Valley, 2016). This age‐associated neurogenic decline appears to be caused both by a depletion in the NSPC pool of the aged niche (Ahlenius, Visan, Kokaia, Lindvall & Kokaia, 2009; Bouab, Paliouras, Aumont, Forest‐Berard & Fernandes, 2011; Corenblum et al., 2016; Enwere et al., 2004; Luo et al., 2006; Maslov, Barone, Plunkett & Pruitt, 2004; Molofsky et al., 2006; Stoll et al., 2011) and by the decreased capacity of the remaining NSPCs to sustain proliferation and neuronal differentiation, as revealed by in vitro studies (Ahlenius et al., 2009; Apostolopoulou et al., 2017; Corenblum et al., 2016; Daynac, Morizur, Chicheportiche, Mouthon & Boussin, 2016; Daynac et al., 2014; L'Episcopo et al., 2013; Shi et al., 2017; Zhu et al., 2014). NSPCs undergo cell autonomous age‐related changes that affect intracellular molecular pathways, including the altered expression of telomerase and cell cycle regulators, which have been linked to the decline in NSPC proliferation upon aging (Caporaso, Lim, Alvarez‐Buylla & Chao, 2003; Molofsky et al., 2006; Nishino, Kim, Chada & Morrison, 2008). Transcriptional analysis of the aged whole SVZ cell population (including NSPCs, differentiated cells and non‐neural cell types) identified several misregulated genes that are associated with NSPC proliferation and differentiation, suggesting that intrinsic gene expression changes in aged NSPCs can alter adult neurogenesis (Apostolopoulou et al., 2017; Shi et al., 2017). The transcriptional regulators that cause these effects and their roles in relaying extrinsic niche signals remain largely unclear.
Attenuating the age‐associated decline in somatic stem cell function can be achieved by modulating the extracellular environment (Adler et al., 2007; Conboy et al., 2005; Villeda et al., 2011), which implicates epigenetic regulation as a key component of stem cell aging (Beerman & Rossi, 2015; O'Sullivan & Karlseder, 2012; Rando & Chang, 2012). To date, the epigenomes of three stem cell populations have been examined upon mouse and human aging: blood stem cells, mesenchymal stem cells and muscle satellite cells (Beerman et al., 2013; Bocker et al., 2011; Bork et al., 2010; Fernandez et al., 2015; Liu et al., 2013; Sun et al., 2014). Together, these studies conclude that the stem cell epigenome is relatively stable during aging, with a small number of potentially important loci that are significantly altered (Beerman & Rossi, 2015). Genes encoding self‐renewal and differentiation factors are particularly vulnerable to age‐dependent alterations, and, although often do not have an immediate impact on transcriptional changes; they might alter the potential or future decisions of the stem cells (Beerman & Rossi, 2015). Notably, the global epigenetic profiles have not been examined in aging NSPCs or in any aged somatic stem cell from nonmesoderm derivatives. This knowledge gap is important to address to identify potential age‐associated drivers of neurogenic decline, and to understand the commonalities in intrinsic mechanisms that might underpin somatic stem cell aging.
To address these deficits, we have generated molecular profiles of NSPCs derived from the SVZ of young adult and aging mice. We identified age‐dependent changes at several hundred genes and regulatory elements, thereby identifying putative regulators of neurogenic decline. Among them, we focused our attention on the transcription factor‐encoding gene Dbx2, which was upregulated in aged NSPCs and has not been associated previously with aging. Functional assays revealed that increased Dbx2 transcript levels in young adult NSPCs promote age‐related phenotypes, including the reduced proliferation of NSPC cultures and the altered expression levels of age‐associated regulators of NSPC proliferation and differentiation. Partial reduction of Dbx2 levels in aged NSPCs caused an opposite transcriptional response. Taken together, these results provide new insights into the molecular programmes that are affected during mouse NSPC aging, and identify a Dbx2‐dependent transcriptional programme involved in the functional decline of NSPCs in the aged SVZ.
2. RESULTS
2.1. Comparative transcriptomic analysis of NSPCs derived from the young adult and aged SVZ
We derived three cohorts of NSPCs from the SVZ of young adult (3 months old, 3 months) and aged (18 months) mice and expanded the cells for two passages (2–3 weeks) in nonadherent culture conditions. We used neurospheres that formed during the second passage to generate expression profiles by RNA sequencing (RNA‐seq). Analysis of these data revealed that the majority of genes were expressed at similar levels between adult and aged samples, although 254 genes were differentially expressed (Figure 1a and Table S1; False Discovery Rate (FDR) < .05). As an initial confirmation of our dataset, we detected changes in Dlx2 and Let‐7b expression, which are misregulated in aged NSPCs (Nishino et al., 2008; Shi et al., 2017). Furthermore, many cell type‐specific genes were detected at comparable levels in adult and aged samples, indicating that the cellular composition of the neurospheres was unchanged with age (Figure S1A).
Figure 1.
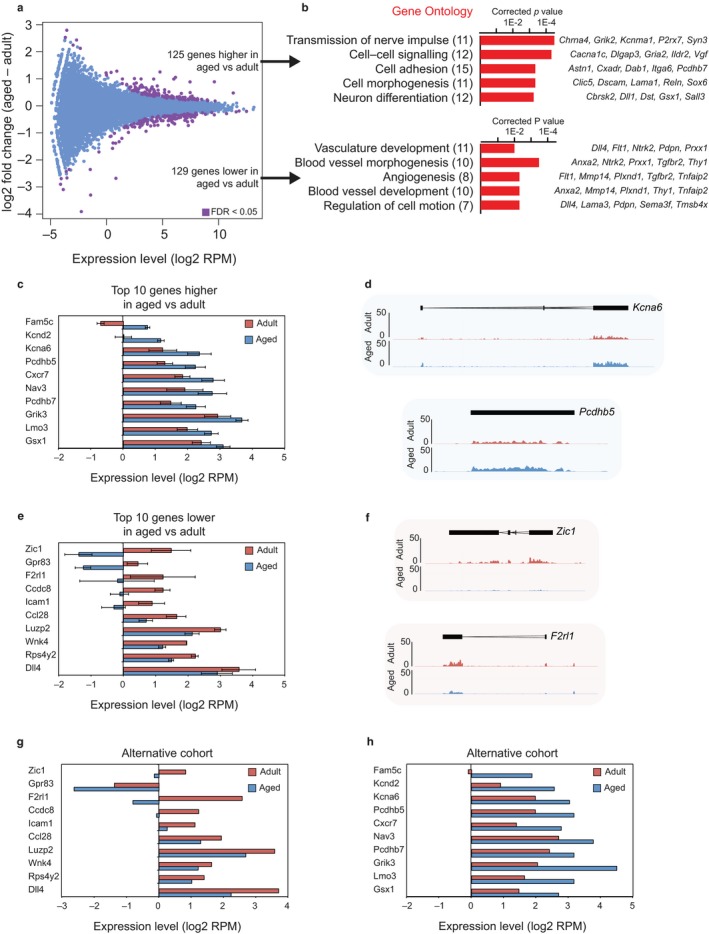
Identification of differentially expressed genes in NSPCs derived from the SVZ of young adult and aged mice. (a) MA plot of RNA‐seq data from NSPCs derived from the SVZ of aged and young adult mice. For each age, three independent derivations of SVZ NSPCs were used for this analysis. A Limma moderated t test with multiple testing corrections identified genes with a false discovery rate (FDR) of <.05, and these genes were classified as differentially expressed. (b) Top GO terms of differentially expressed gene sets that are upregulated (upper) and downregulated (lower) in aged compared to young adult NSPCs. Numbers of genes are shown; example genes within each GO category are listed (right). Corrected p‐values were calculated using a modified Fisher's exact test followed by Bonferroni's multiple comparison test. (c–f) Summary of the ten most strongly upregulated (c, d) or downregulated (e, f) genes in NSPCs from aged mice. Charts in (c, e) show log2 RPM expression levels in adult and aged NSPCs. Data show mean ± SD; n = 3 biological replicates. Genome browser representations in (d, f) show the density of RNA‐seq reads across transcribed regions of representative genes. (g–h) RNA‐seq analysis of an alternative set of NSPC derivations from mice that were housed in a separate facility and from a different strain (C57BL/10). The results show consistent gene expression changes for upregulated (g) and downregulated (h) genes, demonstrating that the observed age‐related transcriptional changes were independent of the genetic background and laboratory conditions of the mice
To gain insight into the biological function of the identified genes, we performed Gene Ontology (GO) analysis of the 125 genes that were upregulated in the aged NSPCs compared with the young adult NSPCs. This analysis revealed an enrichment for processes associated with neuron differentiation, cell signalling and cell morphogenesis (Figure 1b, top). GO terms associated with the 129 genes that were downregulated in the aged NSPCs included vasculature development, angiogenesis and regulation of cell motion (Figure 1b, bottom). Misregulated genes with the largest fold change are shown in Figure 1c–f. We validated our findings with a separate cohort of adult and aged NSPCs from mice that were housed in a different facility, thereby affirming that aging itself was the main driver of the observed gene expression changes (Figure 1g,h). These results provide a robust and comprehensive gene expression data set for young adult and aged NSPCs.
2.2. Epigenome profiling identifies differences between young adult and aged SVZ NSPCs
Epigenetic control of gene regulation through DNA and histone modifications has a pivotal role to ensure the appropriate transcriptional programmes during embryonic and adult neurogenesis (Cacci, Negri, Biagioni & Lupo, 2017) and can be misregulated in aged stem cell compartments (Beerman & Rossi, 2015). Altered epigenetic mechanisms could also be involved in age‐associated neurogenic decline, but little is known about the epigenetic changes that occur during the aging of adult NSPCs.
We first investigated DNA methylation levels by profiling 5‐methylcytosine (5mC) and 5‐hydroxymethylcytosine (5hmC) using whole‐genome bisulphite (BS‐seq) and oxidative bisulphite (oxBS‐seq) sequencing. Globally, the levels of 5mC and 5hmC were very similar between the young adult and aged NSPCs (Figure 2a,b). In keeping with other mammalian cell types, low 5mC levels were found in CpG islands (CGIs), and high methylation levels were found in gene bodies and repetitive elements such as L1 and LTR (Figure 2a). Furthermore, the distribution of 5mC across various genome compartments was indistinguishable between the adult and aged samples (Figure 2c). To look for localized differences in DNA methylation between adult and aged NSPCs, we applied a chi‐squared test with multiple testing corrections to define differentially methylated regions (DMRs; p adj < .05). Using this approach, we identified 330 DMRs that overlapped with CGIs (Figure 2d and Table S2). There were 119 DMRs with reduced 5mC levels (hypo‐DMRs) and 211 DMRs with increased 5mC levels (hyper‐DMRs) in the aged NSPCs compared with young adult NSPCs. The majority of hypo‐DMRs (67%) and hyper‐DMRs (80%) were within 2kb of a gene (Figure 2e) and were associated with 79 and 162 genes, respectively (Figure 2f).
Figure 2.
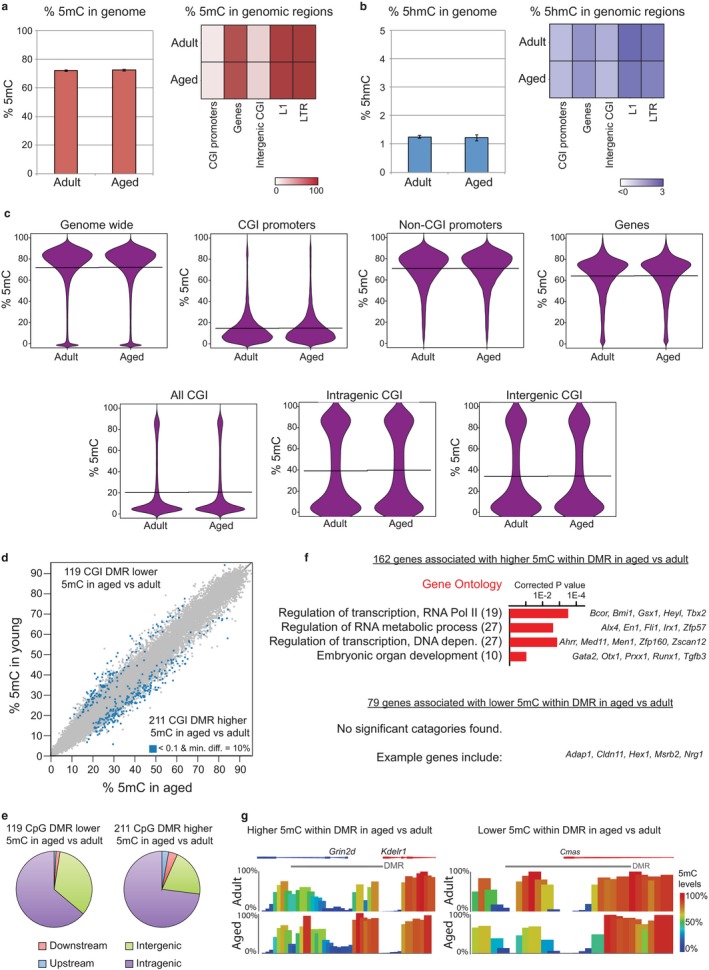
Genomewide profiling of DNA methylation in young adult and aged NSPCs identifies differentially methylated regions that are associated with gene promoters. (a–b) BS‐seq and oxBS‐seq analysis of 5mC (a) and 5hmC (b) in adult and aged NSPCs. Boxplots show the average levels of 5mC and 5hmC over the whole genome (mean ± SD; n = 3 biological replicates). Heatmaps show the percentage of 5mC and 5hmC in genes, CGIs located within promoters or intergenic regions, and transposon elements (L1, LTR). (c) Bean plots showing the distribution of 5mC levels for different genomic features in adult and aged NSPCs. (d) Scatter plot comparing 5mC levels at individual CGIs between adult and aged NSPCs. CGIs that correspond to differentially methylated regions (DMRs) are highlighted in blue (p < .05, chi‐squared test with multiple testing corrections, and a minimum difference in methylation of 10%). (e) Pie charts showing the percentages of DMRs that are located within less than 2 kb upstream or downstream of a gene, or that are found in intragenic or intergenic regions. (f) Top GO terms of genes that are associated with differentially methylated regions either hypomethylated (upper) or hypermethylated (lower) in aged compared to adult NSPCs. Numbers of genes are shown; example genes within each GO category are listed (right). Corrected p‐values were calculated using a modified Fisher's exact test followed by Bonferroni's multiple comparison test. (g) Genome browser tracks of 5mC levels in adult and aged NSPCs across representative differentially methylated CGIs (indicated by grey line) that overlap with gene promoters
GO analysis of the genes with hyper‐DMRs revealed an enrichment for biological processes associated with transcription factors including Bmi1, Prdm1, Tbx2 and with multicellular organism development (Figure 2f). Genes associated with hypo‐DMRs were not significantly enriched for any GO categories, but example genes include Arx, Dlg2 and Slmo1. Genome browser representations are shown for DMRs associated with Grin2d and Cmas (Figure 2g). Overall, there was no correlation between the changes in DNA methylation and the changes in the expression of the nearest gene (not shown), although there were several examples where the gain of DNA methylation was associated with a transcriptional downregulation upon aging (e.g. Chl1, Zfp536) and where the loss of DNA methylation was associated with a transcriptional upregulation (e.g. Fam179a, Tmcc3). These findings show that DNA methylation levels and distribution are very similar between adult and aged NSPCs, although we have identified several hundred regions that are differentially methylated and are near to genes.
We also profiled the genomewide localization of histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 lysine 27 trimethylation (H3K27me3) using chromatin immunoprecipitation combined with sequencing (ChIP‐seq). We identified 30,406 H3K4me3 peaks (MACS, p < 1E−9) that were associated with 13,030 transcriptional start sites (TSS) in adult NSPCs, and 28,854 peaks associated with 12,955 TSS in aged NSPCs. The majority of H3K4me3 signal was centred on the TSS of genes, and this distribution profile was identical for the adult and aged NSPCs (Figure 3a). We did not observe the broadening of H3K4me3 peaks that have been reported in aged blood stem cells (Sun et al., 2014). Focusing on gene promoters (−2 kb to +0.5 kb from TSS), we identified 27 regions with increased H3K4me3 levels in the aged NSPCs compared with the adult NSPCs, and 51 regions with the opposite trend (Figure 3b and Table S3). Gene promoters with the largest changes in H3K4me3 upon NSPC aging are shown in Figure 3c–f. Overall, there was a significant correlation between the changes in H3K4me3 promoter levels and the changes in the expression of the associated gene (Figure 3g; Pearson correlation test, p < .0001).
Figure 3.
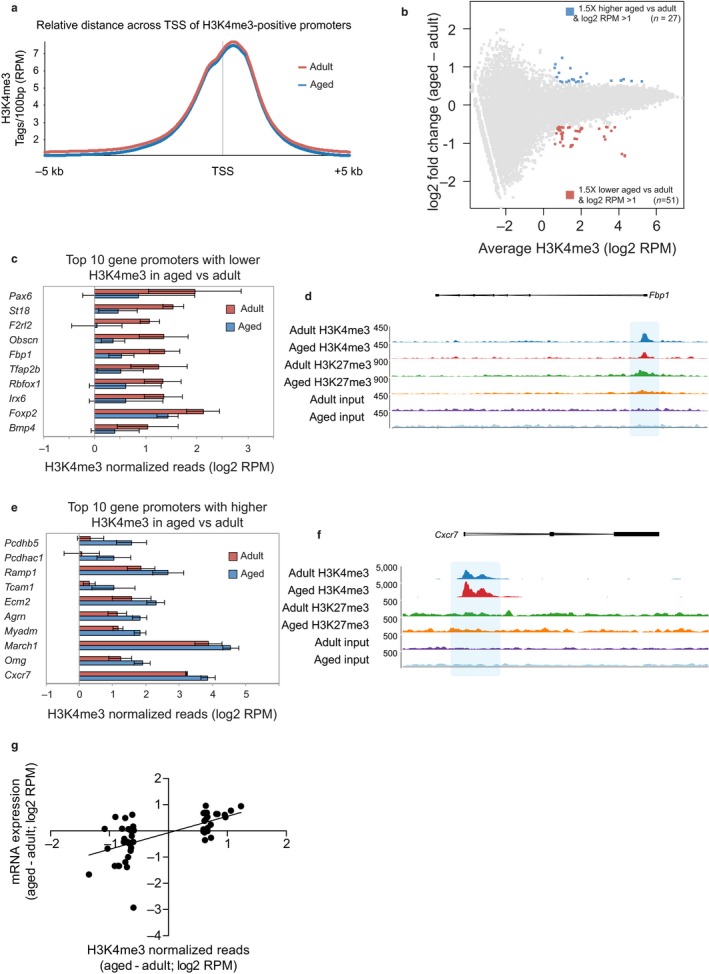
Genomewide analysis of H3K4me3 levels in young adult and aged NSPCs. (a) Quantitative trend plot of H3K4me3 normalized ChIP‐seq reads over TSS ± 5 kb. (b) MA plot of H3K4me3 ChIP‐seq data for adult and aged NSPCs. Data are shown as the average of three biological replicates. (c–f) Summary of the ten gene promoters showing the strongest decrease (c, d) or increase (e, f) in H3K4me3 signal in aged NSPCs compared to adult NSPCs. Charts in (c, e) show H3K4me3 normalized reads (log2 RPM) in adult and aged NSPCs. Data show mean ± SD; n = 3 biological replicates. Genome browser representations in (d, f) show the density of H3K4me3 ChIP‐seq reads across two example genes. (g) Comparison of RNA‐seq log2 fold change (y‐axis) and H3K4me3 ChIP‐seq log2 fold change (x‐axis) for the 78 transcripts that have differential H3K4me3 promoter levels between adult and aged NSPCs. There is a significant correlation between gene expression changes and differences in H3K4me3 levels according to Pearson correlation test (p < .0001)
Analysis of the H3K27me3 ChIP‐seq data identified 43,640 peaks (MACS, p < 1E−9) that were associated with 3,819 TSS in adult NSPCs, and 43,569 peaks associated with 3,836 TSS in aged NSPCs. The distribution of H3K27me3 across gene promoter regions was similar for the adult and aged NSPCs, with a broad peak centred on the TSS of genes (Figure 4a). Quantitation of these regions identified a set of 202 gene promoters with increased H3K27me3 levels in the aged NSPCs compared with the adult NSPCs, and 92 gene promoters with the opposite pattern (Figure 4b and Table S4). Gene promoters with the largest change in H3K27me3 upon NSPC aging are shown in Figure 4c–f. There was no correlation between the changes in H3K27me3 promoter levels and the changes in the expression of the associated gene (Figure 4g; Pearson correlation test, p = .18), although there were several examples where the gain of H3K27me3 was associated with a transcriptional downregulation (Igf2bp2, Prrg4, Trp73) and also for the converse situation (Tnfrsf13b, Laptm5, Rhcg).
Figure 4.
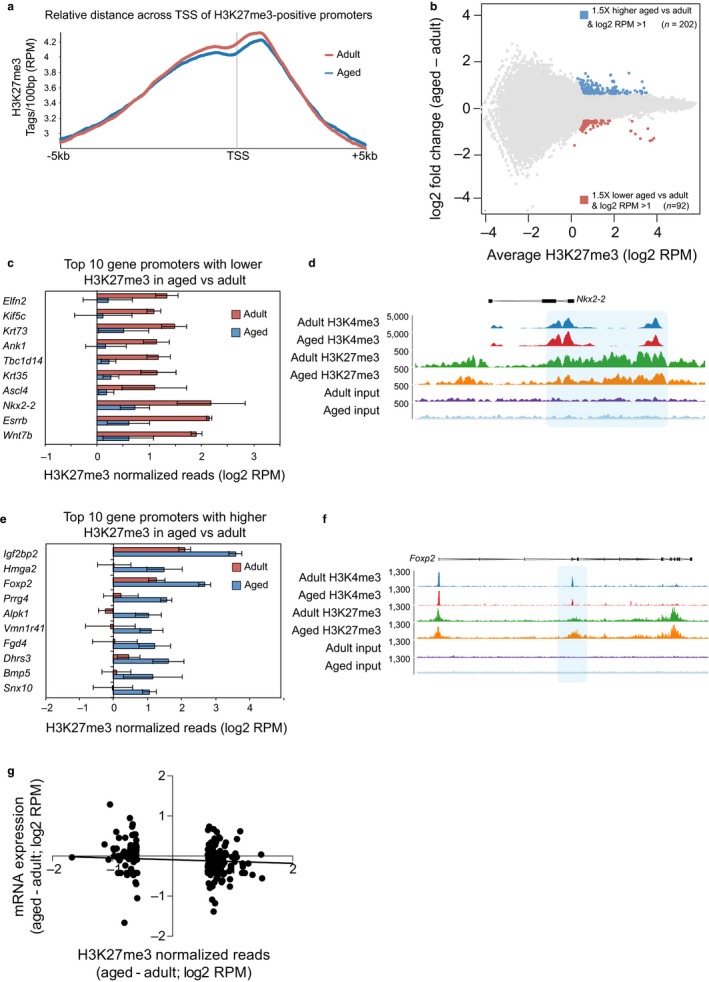
Genomewide analysis of H3K27me3 levels in young adult and aged NSPCs. (a) Quantitative trend plot of H3K27me3 normalized ChIP‐seq reads over TSS ± 5 kb. (b) MA plot of H3K27me3 ChIP‐seq data for adult and aged NSPCs. Data are shown as the average of three biological replicates. (c–f) Summary of the ten gene promoters showing the strongest decrease (c, d) or increase (e, f) in H3K27me3 signal in aged compared to adult NSPCs. Charts in (c, e) show H3K27me3 normalized reads (log2 RPM) in adult and aged NSPCs. Data show mean ± SD; n = 3 biological replicates. Genome browser representations in (d, f) show the density of H3K27me3 ChIP‐seq reads across two example genes. (g) Comparison of RNA‐seq log2 fold change (y‐axis) and H3K27me3 ChIP‐seq log2 fold change (x‐axis) for the 294 transcripts that have differential H3K27me3 promoter levels between adult and aged NSPCs. No significant correlation between gene expression changes and differences in H3K27me3 levels is present according to Pearson correlation test (p < .18)
Our results suggest that the transcriptional and epigenetic signatures of SVZ NSPCs are remarkably well preserved during aging. Nonetheless, we identified loci with significant gene expression and epigenetic differences between young adult and aged NSPCs, suggesting that the functional impairment of aged NSPCs might be caused by the altered regulation of a limited set of influential genes.
2.3. Identification of Dbx2 as a candidate gene underlying age‐dependent decline of SVZ NSPCs
As an initial step towards defining the molecular modifiers of neurogenic decline, we mined our data set for genes that might play an instructive role in the altered proliferation and/or differentiation of aged NSPCs. First, we identified the genes that showed consistent transcriptional and epigenetic changes between adult and aged samples (Table S5). Second, we used qRT‐PCR to screen for genes that maintained an expression difference between adult and aged NSPCs after prolonged expansion in vitro. Third, we focused on genes showing expression changes when NSPCs were switched from proliferating to differentiating conditions. Fourth, we verified the expression of candidate genes in the adult SVZ in vivo either according to previous literature or by experimental analysis.
This strategy led us to focus on Dbx2, which encodes for a homeodomain‐containing transcription factor (Shoji et al., 1996). Dbx2 is implicated in spinal cord development (Pierani, Brenner‐Morton, Chiang & Jessell, 1999), but has not been previously associated with adult neurogenesis or NSPC aging. Dbx2 expression was significantly increased in aged NSPCs compared with young adult samples (p adj = .04; limma moderated t test). Corresponding epigenetic changes were present near to the Dbx2 promoter, including increased H3K4me3 signal and reduced DNA methylation at an overlapping CGI (Figure 5a). Dbx2 levels remained higher in aged neurospheres than in young adult cultures after prolonged passaging in vitro (Figure 5b). Furthermore, Dbx2 was upregulated in adult NSPC cultures upon switching from proliferating to differentiating conditions (Figure 5c), suggesting that elevated Dbx2 expression is associated with reduced NSPC proliferation.
Figure 5.
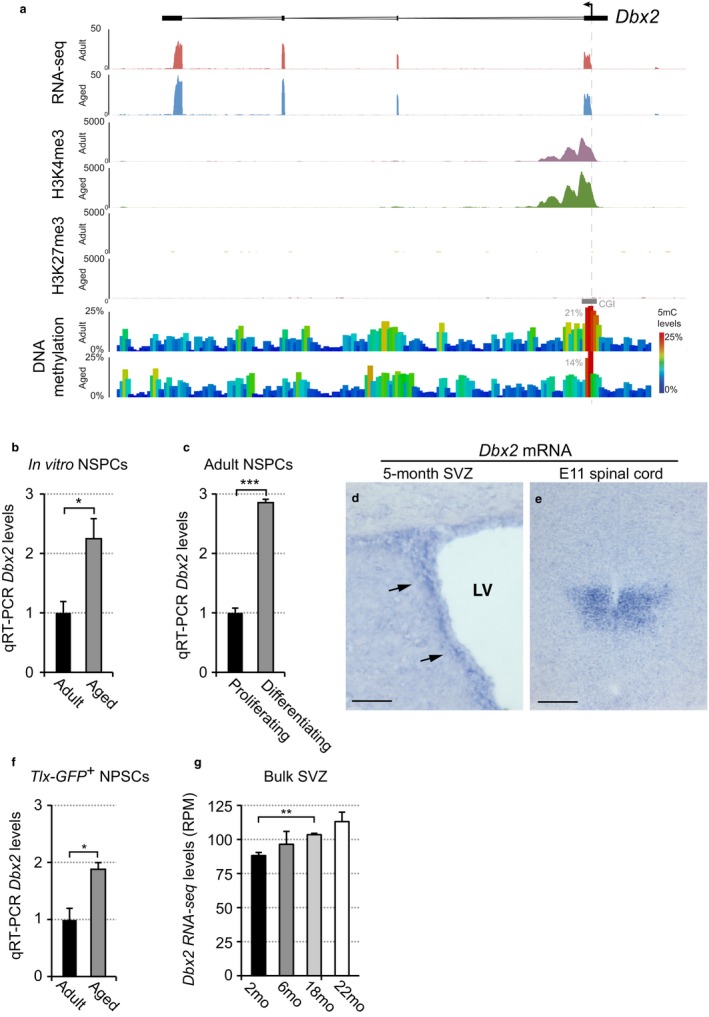
Identification of Dbx2 as a candidate gene implicated in SVZ NSPC aging. (a) Genomewide data track for the Dbx2 locus in young adult and aged NSPCs. The dashed line indicates the TSS; the solid grey line indicates the position of the DMR, and the percentage 5mC levels across the DMR are shown. Data represent the average of three biological replicates. Genomic coordinates for the region shown are as follows: chr15:95619911‐95661537 (GRCm38). (b) qRT‐PCR analysis of Dbx2 expression in nonadherent cultures of adult or aged NSPCs that were expanded for 5–6 passages in vitro from dissected SVZ tissues. Data show mean ± SEM following normalization to adult NSPC samples; n = 3 biological replicates. *p < .05, Student's t test. (c) qRT‐PCR analysis of Dbx2 expression in adherent cultures of adult SVZ NSPCs that were maintained in proliferating conditions or cultured for 24 hr in differentiation conditions devoid of EGF. Data show mean ± SEM following normalization to proliferating NSPC samples; n = 4 biological replicates. ***p < .001, Student's t test. (d, e) Representative images of in situ hybridization assays with a Dbx2 probe performed on (d) coronal sections of adult (5 months) mouse brain cut at the level of the lateral ventricle (LV) or (e) on transversal sections of E11 mouse embryonic spinal cord. Dbx2 staining is detectable in the SVZ region adjacent to the LV (arrows in d) of the adult brain and in the intermediate embryonic spinal cord region (e). Scale bars, 75 μm (d) and 160 μm (e). (f) qRT‐PCR analysis of Dbx2 expression in Tlx‐GFP‐positive NSPCs freshly cell sorted from adult (7 months) and aged (18 months) mouse brain. Data show mean ± SEM following normalization to adult NSPC samples; n = 3 biological replicates. *p < .05, Student's t test. (g) Analysis of published (Apostolopoulou et al., 2017) RNA‐seq data shows that Dbx2 levels increase with SVZ age. Data show mean ± SEM; n = 3 biological replicates. *p < .05, **p < .01, Student's t test. mo, months old
We next examined Dbx2 expression in SVZ NSPCs in vivo. In situ hybridization assays on sections of mouse telencephalon suggested that Dbx2 is transcribed in adult SVZ (Figure 5d), in addition to its previously described expression domain in the embryonic spinal cord (Figure 5e; Shoji et al., 1996). To quantitatively assess Dbx2 levels, we used qRT‐PCR to compare Tlx‐GFP‐positive SVZ NSPCs freshly isolated from adult (7 months) and aged (18 months) mice (Feng et al., 2013). The results showed that Dbx2 levels were higher in the aged sorted NSPCs compared with adult, with a similar fold change to that detected in aged SVZ‐derived NSPC cultures (Figure 5f). Finally, by re‐analysing a recently published RNA‐seq data set of SVZ tissues dissected at different ages, we detected a significant increase in Dbx2 levels between 2‐months and 18‐months SVZ samples (Figure 5g) (Apostolopoulou et al., 2017). These results show that Dbx2 is upregulated in aged SVZ NSPCs in vitro and in vivo, and raise the possibility that Dbx2 has a functional role in driving age‐associated changes in NSPC proliferation.
2.4. Dbx2 overexpression impairs neurosphere growth in young adult SVZ NSPCs
Aged SVZ NSPCs form smaller neurospheres and adherent colonies compared with young adult NSPCs, and this correlates with their decreased proliferation capacity in vitro (Ahlenius et al., 2009; Corenblum et al., 2016; Daynac et al., 2014, 2016; L'Episcopo et al., 2013; Zhu et al., 2014). We also observed a proliferation defect in NSPCs derived from the aged mice (Figures S1B,C).
To address whether elevated levels of Dbx2 could recapitulate this proliferation phenotype, we generated young adult NSPC lines that constitutively expressed either a Dbx2 transgene (Dbx2‐NSPCs) or a GFP control transgene (GFP‐NSPCs). Immunofluorescence microscopy using an anti‐Nestin antibody confirmed the homogeneous NSPC identity of the cultures when maintained in adherent or nonadherent proliferating culture conditions (Figure S2A–H). Furthermore, an anti‐Dbx2 antibody detected increased Dbx2 protein in Dbx2‐NSPCs compared to GFP‐NSPCs (Figure S2I–P). Quantification of nonviable (trypan blue‐positive) cells and proliferating (Ki67‐positive) cells showed a slight increase in cell death and a slight decrease in the proportion of cycling cells in Dbx2‐NSPC cultures (Figure S2Q,R), suggesting that elevated Dbx2 levels may reduce the self‐renewing capacity of NSPCs. To explore this further, we cultured GFP‐NSPCs and Dbx2‐NSPCs in nonadherent conditions and, after 4–7 days, quantitated the number and the size of the resulting neurospheres. Dbx2‐NSPCs produced smaller neurospheres compared with the GFP‐NSPCs (Figure 6a–e). The mean cell number of individual neurospheres in Dbx2‐NSPC cultures was approximately 30%–40% that of GFP‐NSPCs, with a similar decrease in the mean total cell count (Figure 6f,g). The average number of neurospheres formed did not differ between the GFP‐NSPC and Dbx2‐NSPC cultures (Figure 6h). These proliferation defects were confirmed with an independent pair of GFP‐NSPC and Dbx2‐NSPC lines (Figure S3A–E).
Figure 6.
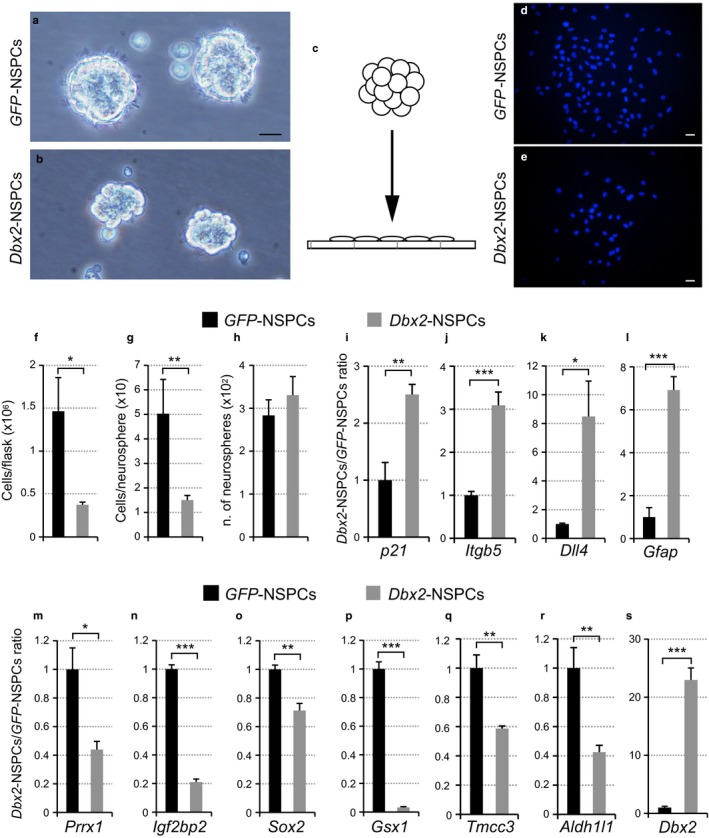
Elevated Dbx2 expression in young adult SVZ NSPCs impairs neurosphere growth and alters the expression of genes involved in NSPC self‐renewal and differentiation. (a–b) Neurospheres from Dbx2‐NSPCs are smaller in comparison with GFP‐expressing aggregates. Scale bar, 20 μm. (c) Schematic of the procedure used for quantification of neurosphere size. Neurospheres were allowed to attach on glass coverslips for approximately six hours, followed by nuclear staining and quantification of the number of nuclei contained in each neurosphere‐derived adherent colony. (d, e) Hoechst nuclear staining of representative colonies generated by nonadherent culture of transgenic NSPCs, followed by attachment of neurospheres to glass coverslips. Scale bar, 20 μm. (f–h) Quantification of the average number of cells per 25 cm2 flask (f), of the average number of cells per neurosphere (g) and of the average number of neurospheres (h) found in nonadherent cultures of transgenic NSPCs. Data show mean ± SEM; n = 4 (f) and n = 6 (g,h) biological replicates. *p < .05; **p < .01, Mann–Whitney test. (i–s) qRT‐PCR analysis of gene expression in nonadherent cultures of Dbx2‐NSPCs. (i–l), upregulated genes; (m–r), downregulated genes; (s) confirmation of increased Dbx2‐expression in Dbx2‐NSPC cultures. Data show mean ± SEM following normalization to GFP‐NSPC samples; n = 4 biological replicates. *p < .05; **p < .01; ***p < .001, Student's t test
2.5. Dbx2 modulates the expression of age‐associated regulators of NSPC proliferation and differentiation
We investigated the Dbx2‐mediated transcriptional programme in SVZ NSPCs by focusing on a cohort of genes that are transcriptionally altered upon aging in NSPCs (our data) and SVZ tissue (Apostolopoulou et al., 2017) and are associated with NSPC proliferation and differentiation. p21, a key cell cycle inhibitor and age‐associated negative regulator of NSPC proliferation (Akizu et al., 2016), was upregulated in Dbx2‐NSPCs (Figure 6i) as were Itgb5, Dll4 and Gfap (Figure 6j–l). Conversely, Prrx1, Igf2bp2 and Sox2 were downregulated in Dbx2‐NSPCs (Figure 6l–n), which is a change that is consistent with the positive influence of these genes on NSPC proliferation (Fujii, Kishi & Gotoh, 2013; Nishino, Kim, Zhu, Zhu & Morrison, 2013; Shimozaki, Clemenson & Gage, 2013). Additional genes that were expressed at lower levels in Dbx2‐NSPCs included Gsx1, Tmcc3 and Aldh1 l1 (Figure 6p–s).
Many of these transcriptional responses were mirrored by corresponding changes in aged NSPCs and SVZ tissue. For example, Prrx1 and Igfbp2 were downregulated in aged NSPCs and in aged SVZ, similar to Dbx2‐NSPCs (Figure 1b, Figure S4A–D, Table S1). Dll4 was less expressed, and Tmcc3 more expressed, in aged (18 months) NSPCs (Figure S4E,F), recapitulating the changes observed in 18‐months SVZ (Figure S4G,H). Of note, even older SVZ samples (22 months) showed a significant increase of Dll4 transcripts and a reduction in Tmcc3 expression (Figure S4G,H), and this pattern matches the changes detected in Dbx2‐NSPCs (Figure 6k,q). Finally, Gsx1 and Itgb5 showed transcriptional differences between young adult and aged SVZ (Figure S4K–L) that were similar to those observed in Dbx2‐NSPCs (Figure 6p,j), even though the expression changes did not match the aged NSPCs (Figure S4I,J).
We confirmed these transcriptional changes in four ways. First, the above described gene expression differences were reproducible in an independent pair of Dbx2‐NSPC and GFP‐NSPC lines (Figure S3F–P). Second, similar effects were detected in adherent proliferating or differentiating NSPC cultures (Figure S5), indicating that the changes were not due to differences in the size or composition of Dbx2 and GFP neurospheres. Third, NSPCs with doxycycline‐controlled expression of Dbx2 phenocopied the proliferation defects (Figure S6A–D) and the gene expression changes (Figure S6E–N) that were detected in NSPCs that constitutively overexpressed Dbx2. Finally, the opposite gene expression changes were observed for a subset of genes (Itgb5, Dll4, Gsx1, Aldh1l1) following a partial (35%) reduction in Dbx2 mRNA by means of a shRNA plasmid (Figure S7).
These results lead us to propose that altered levels of Dbx2 can affect adult NSPC self‐renewal and function by inducing a specific transcriptional programme that includes age‐associated regulators of NSPC proliferation and differentiation.
3. DISCUSSION
Mouse SVZ and SGZ are complex niches where neurogenesis persists throughout adult life due to a finely regulated balance among NSPC self‐renewal, expansion and differentiation. During aging, this regulation is progressively altered, and neurogenic output is curtailed. To investigate the intrinsic molecular changes upon NSPC aging, we report here a comprehensive set of transcriptional and epigenetic maps in young adult and aged SVZ NSPCs. We found that the NSPC epigenome was largely unchanged upon aging, which is broadly consistent with prior studies that profiled somatic stem cells from other aged tissues (Beerman et al., 2013; Bocker et al., 2011; Bork et al., 2010; Fernandez et al., 2015; Liu et al., 2013; Sun et al., 2014). In contrast to previous reports, we did not detect an age‐associated broadening of H3K4me3 (as in blood stem cells; Sun et al., 2014) and H3K27me3 (as in muscle satellite stem cells; Liu et al., 2013) domains in NSPCs, nor a global acquisition in DNA methylation levels (as in blood stem cells; Beerman et al., 2013; Sun et al., 2014). It is currently unclear what impact these epigenetic changes might have on the aged stem cells, and whether the differences are due to the varied stem cell properties from each tissue, such as cellular potential or the turnover rate, or perhaps influenced by external signals from their niches.
Despite the overall similarities, we did identify several hundred genes with significant differences in the levels of transcription and/or epigenetic modifications in NSPCs from the aged SVZ. These data will serve as an important resource for normal and aging studies and could be used to identify biomarkers and candidate regulators of NSPC aging. As an initial step, we identified and characterized the homeobox gene Dbx2 as a putative age‐associated regulator of NSPC functional decline. Increased Dbx2 expression in young adult NSPCs promoted age‐related phenotypes, including the reduced proliferation of NSPC cultures and the altered expression levels of age‐associated regulators of NSPC proliferation and differentiation. In particular, elevated Dbx2 expression decreased the size, but not the number, of neurospheres generated from adult SVZ NSPCs, supporting a causal link between Dbx2 upregulation and the defective proliferation of aged NSPCs. Dbx2 overexpression slightly increased the fraction of nonviable cells and decreased the fraction of cycling cells, suggesting that cell death and cell cycle exit contribute to the reduced expansion of the transgenic neurospheres. Nonetheless, as the majority of the cells remained positive for proliferation markers, alternative changes, including lengthening of the cell cycle, could be a main driver of the growth phenotype elicited by Dbx2. This hypothesis is consistent with the previously described alterations in the cell cycle of aged NSPCs (Apostolopoulou et al., 2017; Daynac et al., 2014, 2016; Stoll et al., 2011). Dbx2 is part of a cohort of transcription factor genes that are enriched in quiescent NSPCs of the SGZ and SVZ and are downregulated in NSPCs actively engaged in cell proliferation and neurogenesis (Codega et al., 2014; Shin et al., 2015), thus suggesting that Dbx2 may negatively regulate NSPC proliferation in both adult neurogenic niches. Elevated Dbx2 expression in adult NSPCs also phenocopied several of the molecular effects of aging, including the altered expression of key genes such as Sox2 and p21 that are associated with proliferation and differentiation. Thus, Dbx2 may act as a crucial regulator of age‐dependent transcriptional programmes, as also suggested by the reversed trend of some molecular markers of SVZ aging following moderate Dbx2 knockdown in aged NSPCs. As cell proliferation was not affected by this partial knockdown, an exciting future line of research will be to abrogate Dbx2 function in aged NSPCs by knockout approaches in vitro and in vivo. Furthermore, investigating the direct targets of Dbx2 might help to expand the gene regulatory networks involved in NSPC dysfunction.
Prrx1 and Igf2bp2 are additional candidate regulators of NSPC functional decline during SVZ aging. Both genes were downregulated in NSPCs from the aged SVZ, and for Igf2bp2 this correlated with increased levels of repressive epigenetic marks at the promoter. Prrx1 is expressed in NSPCs of the SVZ and the SGZ and promotes proliferation of SGZ NSPCs at the expense of neuronal differentiation (Shimozaki et al., 2013). Igf2bp2 is expressed in embryonic cortical progenitors where it promotes neurogenesis at the expense of astrogenesis (Fujii et al., 2013), but its role in the postnatal SVZ has not been investigated. Igf2bp1 supports proliferation and prevents premature differentiation of embryonic cortical progenitors, but its expression is extinguished postnatally (Nishino et al., 2008). It is tempting to speculate that the role of Igf2bp1 in maintaining embryonic NSPC proliferation is superseded by Igf2bp2 in the postnatal SVZ, and that the combined decay of Igf2bp1/2 levels underlies the progressive decline of NSPC proliferation during postnatal life. Increased Dbx2 expression in adult NSPCs caused the downregulation of Prrx1 and Igf2bp2, providing a potential explanation, along with the transcriptional effects on Sox2 and p21, for the antiproliferative activity of Dbx2.
In conclusion, by comparing the molecular profiles of young adult and aged SVZ, our work suggests that aging does not cause widespread gene dysregulation in NSPCs, but acts through the modulation of specific gene expression programmes. Among them, we have identified a transcriptional pathway involving the upregulation of the homeodomain protein Dbx2 in aged NSPCs. Dbx2, in turn, can influence a cohort of age‐associated regulators of NSPC function, and restrain NSPC proliferation. These results provide a platform to investigate how changes in extrinsic signals acting in the SVZ niche due to aging, injury or disease could impinge on intrinsic transcriptional mechanisms controlling adult neurogenesis.
4. EXPERIMENTAL PROCEDURES
4.1. Mouse NSPC isolation, culture and manipulation
Young adult and aged SVZ NSPCs were derived from 3 months or 18 months male C57BL/6 J/Babr mice, respectively. Methods for their derivation and subsequent in vitro culture were reported previously (Soldati et al., 2015) and are described in the Supplemental Experimental Procedures. We combined SVZ tissues from five mice to form one NSPC derivation, and we repeated this procedure three times on different days to obtain three independent replicates. The neurospheres used for the transcriptomic and epigenomic assays were harvested 5–6 days after seeding SVZ NSPCs at the second passage postdissection. Summing the first and second passages, SVZ NSPCs were cultured for a total of 13–20 days postdissection before being processed for molecular analyses. Experimental procedures for NSPC derivation were performed in accordance with EU Directive 2010/63/EU and were approved by the Ethical Committee for Animal Research of the Italian Ministry of Health according to national regulations (Art. 7, D.Lgs. n. 116/1992). Detailed methods for the transcriptional and epigenetic profiling are provided in the Supplemental Experimental Procedures.
ACCESSION NUMBERS
Sequencing data are available at GEO under accession number GSE101610.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
G.L. performed conceptualization, resources, funding acquisition, investigation, methodology, supervision, validation, visualization and writing. P.S.N. performed investigation. P.E. and M.A.K. were involved in investigation and visualization. Y.‐L.P. and C.L.N. performed investigation and methodology. B.S. carried out methodology and formal analysis. S.B. performed conceptualization and funding acquisition. H‐K.L. involved in investigation and supervision. P.B. was involved in visualization, resources and funding acquisition. E.C. performed conceptualization, resources, funding acquisition, investigation, methodology, visualization and writing. P.J.R.‐G. performed conceptualization, resources, formal analysis, funding acquisition, supervision, validation, visualization and writing.
Supporting information
ACKNOWLEDGMENTS
We thank Maria Jesus Martin‐Bermejo for help with in situ hybridization assays, Amanda Collier, Natasha Morgan and Viviana Orlando for assistance with qRT‐PCR and cell culture, Alessandro Rosa for providing the epB‐Puro‐TT plasmid, and Felix Krueger (Babraham Bioinformatics) for help with the DNA methylation analysis. This work was supported by grants to P.J.R.‐G. from the Wellcome Trust (WT093736) and the BBSRC (BB/P013406/1, BB/M022285/1), by funding from Sapienza University of Rome, calls 2013‐2016 (G.L, S.B., E.C) and by a grant from the Spanish Ministry of Economy to P.B. (BFU2016‐75412‐R, co‐financed by FEDER). The Babraham Institute Biological Services Unit is supported by Campus Capability Grant funding from the BBSRC.
Lupo G, Nisi PS, Esteve P, et al. Molecular profiling of aged neural progenitors identifies Dbx2 as a candidate regulator of age‐associated neurogenic decline. Aging Cell. 2018;17:e12745 10.1111/acel.12745
Contributor Information
Emanuele Cacci, Email: emanuele.cacci@uniroma1.it.
Peter J. Rugg‐Gunn, Email: peter.rugg-gunn@babraham.ac.uk
REFERENCES
- Adler, A. S. , Sinha, S. , Kawahara, T. L. , Zhang, J. Y. , Segal, E. , & Chang, H. Y. (2007). Motif module map reveals enforcement of aging by continual NF‐kappaB activity. Genes & Development, 21, 3244–3257. 10.1101/gad.1588507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenius, H. , Visan, V. , Kokaia, M. , Lindvall, O. , & Kokaia, Z. (2009). Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. The Journal of Neuroscience, 29, 4408–4419. 10.1523/JNEUROSCI.6003-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akizu, N. , Garcia, M. A. , Estaras, C. , Fueyo, R. , Badosa, C. , de la Cruz, X. , & Martinez‐Balbas, M. A. (2016). EZH2 regulates neuroepithelium structure and neuroblast proliferation by repressing p21. Open Biology, 6, 150227 10.1098/rsob.150227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulou, M. , Kiehl, T. R. , Winter, M. , Cardenas De La Hoz, E. , Boles, N. C. , Bjornsson, C. S. , … Temple, S. (2017). Non‐monotonic changes in progenitor cell behavior and gene expression during aging of the adult V‐SVZ neural stem cell niche. Stem Cell Reports, 9, 1931–1947. 10.1016/j.stemcr.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman, I. , Bock, C. , Garrison, B. S. , Smith, Z. D. , Gu, H. , Meissner, A. , & Rossi, D. J. (2013). Proliferation‐dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell, 12, 413–425. 10.1016/j.stem.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Beerman, I. , & Rossi, D. J. (2015). Epigenetic Control of Stem Cell Potential during Homeostasis, Aging, and Disease. Cell Stem Cell, 16, 613–625. 10.1016/j.stem.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocker, M. T. , Hellwig, I. , Breiling, A. , Eckstein, V. , Ho, A. D. , & Lyko, F. (2011). Genome‐wide promoter DNA methylation dynamics of human hematopoietic progenitor cells during differentiation and aging. Blood, 117, e182–e189. 10.1182/blood-2011-01-331926 [DOI] [PubMed] [Google Scholar]
- Bork, S. , Pfister, S. , Witt, H. , Horn, P. , Korn, B. , Ho, A. D. , & Wagner, W. (2010). DNA methylation pattern changes upon long‐term culture and aging of human mesenchymal stromal cells. Aging Cell, 9, 54–63. 10.1111/j.1474-9726.2009.00535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouab, M. , Paliouras, G. N. , Aumont, A. , Forest‐Berard, K. , & Fernandes, K. J. (2011). Aging of the subventricular zone neural stem cell niche: Evidence for quiescence‐associated changes between early and mid‐adulthood. Neuroscience, 173, 135–149. 10.1016/j.neuroscience.2010.11.032 [DOI] [PubMed] [Google Scholar]
- Cacci, E. , Negri, R. , Biagioni, S. , & Lupo, G. (2017). Histone methylation and microRNA‐dependent regulation of epigenetic activities in neural progenitor self‐renewal and differentiation. Current Topics in Medicinal Chemistry, 17, 794–807. 10.2174/1568026616666160414124456 [DOI] [PubMed] [Google Scholar]
- Caporaso, G. L. , Lim, D. A. , Alvarez‐Buylla, A. , & Chao, M. V. (2003). Telomerase activity in the subventricular zone of adult mice. Molecular and Cellular Neuroscience, 23, 693–702. 10.1016/S1044-7431(03)00103-9 [DOI] [PubMed] [Google Scholar]
- Codega, P. , Silva‐Vargas, V. , Paul, A. , Maldonado‐Soto, A. R. , Deleo, A. M. , Pastrana, E. , & Doetsch, F. (2014). Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron, 82, 545–559. 10.1016/j.neuron.2014.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy, I. M. , Conboy, M. J. , Wagers, A. J. , Girma, E. R. , Weissman, I. L. , & Rando, T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature, 433, 760–764. 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- Corenblum, M. J. , Ray, S. , Remley, Q. W. , Long, M. , Harder, B. , Zhang, D. D. , … Madhavan, L. (2016). Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle‐age period. Aging Cell, 15, 725–736. 10.1111/acel.12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynac, M. , Morizur, L. , Chicheportiche, A. , Mouthon, M. A. , & Boussin, F. D. (2016). Age‐related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Scientific Reports, 6, 21505 10.1038/srep21505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynac, M. , Pineda, J. R. , Chicheportiche, A. , Gauthier, L. R. , Morizur, L. , Boussin, F. D. , & Mouthon, M. A. (2014). TGFbeta lengthens the G1 phase of stem cells in aged mouse brain. Stem Cells, 32, 3257–3265. 10.1002/stem.1815 [DOI] [PubMed] [Google Scholar]
- Encinas, J. M. , Michurina, T. V. , Peunova, N. , Park, J. H. , Tordo, J. , Peterson, D. A. , … Enikolopov, G. (2011). Division‐coupled astrocytic differentiation and age‐related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell, 8, 566–579. 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere, E. , Shingo, T. , Gregg, C. , Fujikawa, H. , Ohta, S. , & Weiss, S. (2004). Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. The Journal of Neuroscience, 24, 8354–8365. 10.1523/JNEUROSCI.2751-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, W. , Khan, M. A. , Bellvis, P. , Zhu, Z. , Bernhardt, O. , Herold‐Mende, C. , & Liu, H. K. (2013). The chromatin remodeler CHD7 regulates adult neurogenesis via activation of SoxC transcription factors. Cell Stem Cell, 13, 62–72. 10.1016/j.stem.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Fernandez, A. F. , Bayón, G. F. , Urdinguio, R. G. , Toraño, E. G. , García, M. G. , Carella, A. , … García‐Castro, J. (2015). H3K4me1 marks DNA regions hypomethylated during aging in human stem and differentiated cells. Genome Research, 25, 27–40. 10.1101/gr.169011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii, Y. , Kishi, Y. , & Gotoh, Y. (2013). IMP2 regulates differentiation potentials of mouse neocortical neural precursor cells. Genes to Cells, 18, 79–89. 10.1111/gtc.12024 [DOI] [PubMed] [Google Scholar]
- Goncalves, J. T. , Schafer, S. T. , & Gage, F. H. (2016). Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell, 167, 897–914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- L'Episcopo, F. , Tirolo, C. , Testa, N. , Caniglia, S. , Morale, M. C. , Impagnatiello, F. , … Marchetti, B. (2013). Aging‐induced Nrf2‐ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K‐Wnt/beta‐catenin dysregulation. The Journal of Neuroscience, 33, 1462–1485. 10.1523/JNEUROSCI.3206-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Cheung, T. H. , Charville, G. W. , Hurgo, B. M. , Leavitt, T. , Shih, J. , … Rando, T. A. (2013). Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Reports, 4, 189–204. 10.1016/j.celrep.2013.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P. M., Valley M. (2016) Adult Olfactory Bulb Neurogenesis. Cold Spring Harbor Perspectives in Biology 8, pii: a018945 10.1101/cshperspect.a018945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert, S. , Basak, O. , Knuckles, P. , Haussler, U. , Fabel, K. , Gotz, M. , … Giachino, C. (2010). Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell, 6, 445–456. 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Luo, J. , Daniels, S. B. , Lennington, J. B. , Notti, R. Q. , & Conover, J. C. (2006). The aging neurogenic subventricular zone. Aging Cell, 5, 139–152. 10.1111/j.1474-9726.2006.00197.x [DOI] [PubMed] [Google Scholar]
- Maslov, A. Y. , Barone, T. A. , Plunkett, R. J. , & Pruitt, S. C. (2004). Neural stem cell detection, characterization, and age‐related changes in the subventricular zone of mice. The Journal of Neuroscience, 24, 1726–1733. 10.1523/JNEUROSCI.4608-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky, A. V. , Slutsky, S. G. , Joseph, N. M. , He, S. , Pardal, R. , Krishnamurthy, J. , … Morrison, S. J. (2006). Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during aging. Nature, 443, 448–452. 10.1038/nature05091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino, J. , Kim, I. , Chada, K. , & Morrison, S. J. (2008). Hmga2 promotes neural stem cell self‐renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell, 135, 227–239. 10.1016/j.cell.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J., Kim S., Zhu Y., Zhu H., Morrison S. J. (2013) A network of heterochronic genes including Imp1 regulates temporal changes in stem cell properties. eLife, 2, e00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan, R. J. , & Karlseder, J. (2012). The great unravelling: Chromatin as a modulator of the aging process. Trends in Biochemical Sciences, 37, 466–476. 10.1016/j.tibs.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani, A. , Brenner‐Morton, S. , Chiang, C. , & Jessell, T. M. (1999). A sonic hedgehog‐independent, retinoid‐activated pathway of neurogenesis in the ventral spinal cord. Cell, 97, 903–915. 10.1016/S0092-8674(00)80802-8 [DOI] [PubMed] [Google Scholar]
- Rando, T. A. , & Chang, H. Y. (2012). Aging, rejuvenation, and epigenetic reprogramming: Resetting the aging clock. Cell, 148, 46–57. 10.1016/j.cell.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z. , Geng, Y. , Liu, J. , Zhang, H. , Zhou, L. , Lin, Q. , … Sun, Y. E. (2017). Single‐cell transcriptomics reveals gene signatures and alterations associated with aging in distinct neural stem/progenitor cell subpopulations. Protein Cell. 10.1007/s13238-017-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozaki, K. , Clemenson, G. D. Jr , & Gage, F. H. (2013). Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. The Journal of Neuroscience, 33, 4066–4075. 10.1523/JNEUROSCI.4586-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J. , Berg, D. A. , Zhu, Y. , Shin, J. Y. , Song, J. , Bonaguidi, M. A. , … Song, H. (2015). Single‐Cell RNA‐Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell, 17, 360–372. 10.1016/j.stem.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji, H. , Ito, T. , Wakamatsu, Y. , Hayasaka, N. , Ohsaki, K. , Oyanagi, M. , … Takahashi, N. (1996). Regionalized expression of the Dbx family homeobox genes in the embryonic CNS of the mouse. Mechanisms of Development, 56, 25–39. 10.1016/0925-4773(96)00509-6 [DOI] [PubMed] [Google Scholar]
- Signer, R. A. , & Morrison, S. J. (2013). Mechanisms that regulate stem cell aging and life span. Cell Stem Cell, 12, 152–165. 10.1016/j.stem.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati, C. , Caramanica, P. , Burney, M. J. , Toselli, C. , Bithell, A. , Augusti‐Tocco, G. , … Cacci, E. (2015). RE1 silencing transcription factor/neuron‐restrictive silencing factor regulates expansion of adult mouse subventricular zone‐derived neural stem/progenitor cells in vitro. Journal of Neuroscience Research, 93, 1203–1214. 10.1002/jnr.23572 [DOI] [PubMed] [Google Scholar]
- Stoll, E. A. , Habibi, B. A. , Mikheev, A. M. , Lasiene, J. , Massey, S. C. , Swanson, K. R. , … Horner, P. J. (2011). Increased re‐entry into cell cycle mitigates age‐related neurogenic decline in the murine subventricular zone. Stem Cells, 29, 2005–2017. 10.1002/stem.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D. , Luo, M. , Jeong, M. , Rodriguez, B. , Xia, Z. , Hannah, R. , … Goodell, M. A. (2014). Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self‐renewal. Cell Stem Cell, 14, 673–688. 10.1016/j.stem.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda, S. A. , Luo, J. , Mosher, K. I. , Zou, B. , Britschgi, M. , Bieri, G. , … Lucin, K. M. (2011). The aging systemic milieu negatively regulates neurogenesis and cognitive function. Nature, 477, 90–94. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Demidov, O. N. , Goh, A. M. , Virshup, D. M. , Lane, D. P. , & Bulavin, D. V. (2014). Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. Journal of Clinical Investigation, 124, 3263–3273. 10.1172/JCI73015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


