Abstract
Many studies have reported harmful effects of red meat or processed meat on chronic diseases including cancer and diabetes, but epidemiological evidence for metabolic syndrome is limited and remains controversial. Therefore, we performed a meta-analysis of observational studies to assess the association between various meat consumption and risk of metabolic syndrome. The PubMed and ISI Web of Science databases were searched through June 2017, and further included unpublished results from Korea National Health and Nutrition Examination Survey 2012–2015, including 8387 Korean adults. Sixteen studies were suitable for meta-analysis, which included 19,579 cases among 76,111 participants. We used a random-effects model to calculate the pooled relative risks (RR) and 95% confidence intervals (CI). The pooled RR for metabolic syndrome of the highest versus lowest category of meat intake was 1.14 (95% CI: 1.05, 1.23) for total meat, 1.33 (95% CI: 1.01, 1.74) for red meat, 1.35 (95% CI: 1.18, 1.54) for processed meat, and 0.86 (95% CI: 0.76, 0.97) for white meat. All of these associations did not differ significantly by study design and adjustment factors. Our findings indicated that total, red, and processed meat intake is positively associated with metabolic syndrome, and white meat intake is inversely associated with metabolic syndrome.
Keywords: metabolic syndrome, processed meat, white meat, meta-analysis, red meat
1. Introduction
A significant increase in the prevalence of metabolic syndrome has been observed worldwide [1]. Metabolic syndrome consists of an aggregation of metabolic abnormalities including central obesity, hypertriglyceridemia, hyperglycemia, low HDL cholesterol levels, and high blood pressure. These metabolic disorders are also risk factors of type 2 diabetes and cardiovascular disease (CVD), and a large body of evidence suggested that metabolic syndrome is positively related to high risks of CVD [2], type 2 diabetes [3], specific cancers [4], and total mortality [5]. Due to its high prevalence, the development of a preventive strategy against metabolic syndrome is needed to improve public health. The identification of modifiable factors affecting metabolic syndrome may help decrease the burden of death from CVD, type 2 diabetes, and several cancers.
Accumulating evidence from previous studies indicated that a high intake of red meat and processed meat could raise the risk of chronic diseases, including type 2 diabetes [6], CVD [7,8], and several types of cancer [9,10,11,12]. However, the associations between the consumption of red meat, processed meat and white meat and the risk of metabolic syndrome remain largely inconclusive. One cross-sectional study first provided a non-significant positive association between total meat intake and metabolic syndrome in data from an Epidemiology study on the Insulin Resistance Syndrome (DESIR) [13]. Several subsequent observational studies have investigated the relationship between red, processed, and white meat consumption and metabolic syndrome, but the results are sparse and inconsistent [14,15,16,17,18,19,20,21,22,23,24].
Therefore, we conducted a systematic review and comprehensive meta-analysis to quantitatively evaluate the relationship between meat intake and metabolic syndrome. In addition, we analyzed the association using the data of Korea National Health and Nutrition Examination Survey (KNHANES) and included these new results in our meta-analysis.
2. Materials and Methods
2.1. Literature Search
We searched the electronic databases (PubMed and ISI Web of Science) through June 2017 to identify eligible studies published in English as full-length articles. The search terms were the following: “(meat OR beef OR pork OR veal OR lamb OR steak OR hamburger OR ham OR bacon OR sausage OR poultry OR chicken OR turkey) combined with (metabolic syndrome OR insulin resistant syndrome OR syndrome X).” The reference lists of retrieved articles or published reviews were also manually screened to search further relevant studies. ‘Red meat’ included red meat and processed red meat. ‘White meat’ included poultry. In the study conducted by Cocate et al. [14], fish was included in the white meat consumption group and thus we performed a sensitivity analysis by removing this study. ‘Processed meat’ included offal, ham, sausages, pate, hamburgers, bacon and sundae. ‘Total meat’ included the total of these three categories. If the study did not report information for the meat category, we included the results in the meta-analysis of total meat consumption.
2.2. Study Selection
Studies were included in the current meta-analysis when they met the following criteria: (1) they were epidemiological studies; (2) the exposure of interest was meat consumption; (3) the outcome of interest was defined as prevalence or incidence of metabolic syndrome; and (4) they provided relative risks (RR) and 95% confidence intervals (CI). Studies targeting people with disease were excluded.
2.3. Data Extraction
Data were extracted according to the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [25] by two investigators (Y.K. and Y.J.). The following information from all studies was extracted: first author name; year of publication; design of study; country; study period or follow-up period; number of events/participants or person-years; sex; covariates used for adjustment; RRs and 95% CIs for the relationship between meat intake and metabolic syndrome across all categories of exposure. If the studies reported various RRs on this association, the RR from the most fully adjustment was used.
To include all available data in the meta-analysis, we investigated the association between meat consumption and metabolic syndrome in KNHANES 2012–2015 and conducted a meta-analysis including these results. The KNHANES is a cross-sectional, nationally representative sample of the South Korean population that uses a multistage probability sampling design to represent the non-institutionalized civilian South Korean population. The KNHANES assessed the health and nutritional status of Koreans via 3 surveys including a health examination, a health interview, and a nutrition survey. The procedures of human subjects for KNHANES were approved by the Institutional Review Board of Korea Centers for Disease Control and Prevention, and informed consent was received from all subjects. Dietary consumption was assessed using the 112-item dish-based semi-quantitative food frequency questionnaires (FFQ) of KNHANES. Details of the study populations and methods are provided in the online-only Data Supplement.
2.4. Statistical Analysis
In the present meta-analysis, odds ratios (ORs) and hazard ratios (HRs) were considered equivalent to RRs [26]. The natural logarithm values of the RRs from the original study were combined using the DerSimonian and Laird random-effects models, which take into account within- and between-study variations, to obtain the pooled estimate of metabolic syndrome for the highest vs. the lowest levels of meat intake [27]. We recalculated the RR and its 95% CI when a study did not report the lowest level as a reference [23]. We presented the summary estimates as forest plots where the extent of data markers (squares) is consistent with the inverse of the variance of the natural logarithm of RR from an individual study, and the diamond displays a pooled RR. Statistical heterogeneity between studies was assessed through the Q [28] and I2 statistics [29]. The subgroup analysis was performed by design of study, geographical region, and differences in adjustment factors. To test whether the results were not simply attributable to the inclusion of a single study, we conducted sensitivity analyses by removing one study at a time. We evaluated publication bias through Egger’s test [30] and Begg’s test [31]. All statistical analyses were performed using Stata software (version 14.2; StataCorp (College Station, TX, USA)). A two-tailed p-value less than 0.05 was deemed statistically significant.
3. Results
3.1. KNHANES Analysis
The characteristics of study populations by meat consumption are present in Supplementary Table S1. Subjects in the highest quintile of meat intake were younger, drank more alcohol, and had a high education level than those in the lowest quintile. The association between white meat intake and metabolic syndrome is shown in Supplementary Table S2. In the overall population, subjects in the highest quintile of white meat consumption had a decreased prevalence of hypertriglyceridemia (OR = 0.77, 95% CI: 0.61, 0.99, P-trend = 0.010) and elevated blood pressure (OR = 0.67, 95% CI: 0.50, 0.89, P-trend= 0.005) compared to those in the lowest quintile after controlling for potential confounders. The association between red meat consumption and metabolic syndrome is shown in Supplementary Table S3. In a comparison of highest vs. lowest consumption, we found no significant association between red meat intake and the prevalence of metabolic syndrome and its components. The association between processed meat consumption and metabolic syndrome is shown in Supplementary Table S4. Compared to those in the first quintile, subjects in the fifth quintile of processed meat consumption had a higher prevalence of hyperglycemia (OR = 1.27, 95% CI: 1.00, 1.60).
3.2. Systematic Review and Meta-Analysis
3.2.1. Study Characteristics
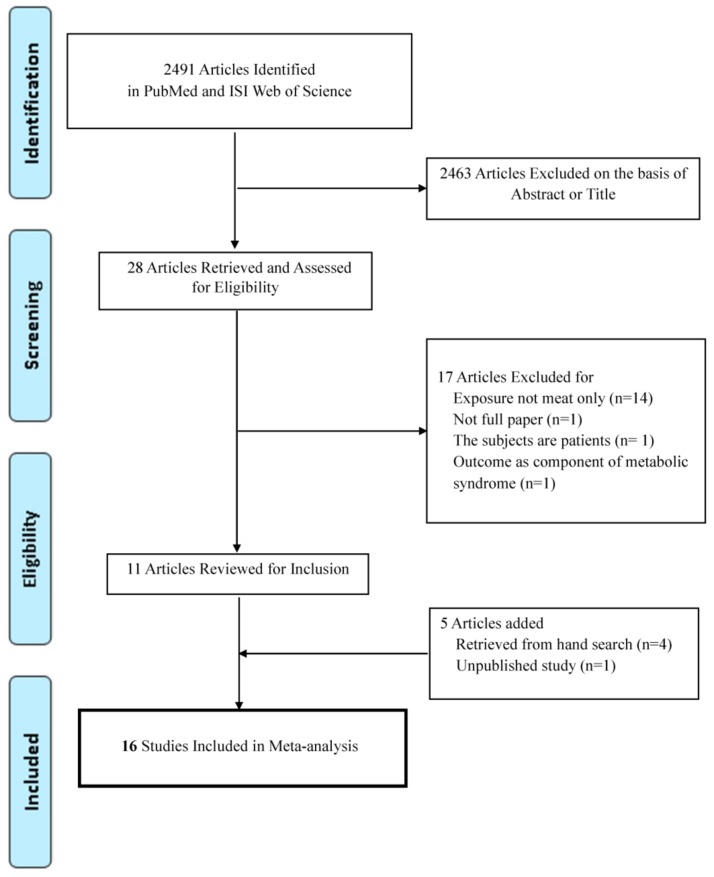
Sixteen studies including 19,579 cases among 76,111 participants were suitable for meta-analysis (Figure 1) [13,14,15,16,17,18,19,21,22,23,24,32,33,34,35]. The characteristics of studies which were included in meta-analysis are presented in Table 1. All articles were published from 2000 to 2017. By geographic region, 7 studies were performed in Asia [15,23,32,33,34,35], 5 in Europe [13,16,17,19,24], 3 in North or South America [14,21,22], and 1 in the Middle East [18]. Most of the studies investigated food intake using FFQ, and some studies used a food record [17,24] or questionnaire [23,32,34]. The criteria used to define metabolic syndrome were mostly NCEP ATP III criteria or Harmonized criteria from the International Diabetes Federation and the American Heart Association/National Heart, Lung, and Blood Institute [3]. Most studies reported risk estimates adjusted for age [13,14,15,16,17,18,19,21,22,24,32,33,34,35], smoking [14,15,16,17,19,21,22,24,35], alcohol [13,14,15,16,17,18,19,22,24,35], and physical activity [13,14,15,16,18,19,21,22,24,35].
Figure 1.
Flow chart of the selection of studies included in the meta-analysis.
Table 1.
Characteristics of epidemiological studies included in the meta-analysis of meat consumption and metabolic syndrome.
| First Author (year) | Country | Study Design | Age (years) | Subjects | Criteria for Metabolic Syndrome | MEAT Consumption | Relative Risk (95% CI) | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|---|
| Damiao (2006) [22] | Brazil | Cohort | 30–64 | 57/151 | NCEP ATP III | Red meat (g/day) 144.2 vs. 19.5 |
3.18 (0.87, 11.5) | Age, sex, physical activity, smoking, education level, alcohol, total energy intake, total fat intake, fried foods |
| White meat (g/day) 28.7 vs. 4.6 |
1.36 (0.38, 4.78) | |||||||
| Lutsey (2008) [21] | USA | Cohort | 45–64 | 3782/9514 | American Heart Association guidelines | Total meat (servings/day) 1.94 vs. 0.25 |
1.26 (1.11, 1.43) | Age, sex, race, education, center, total calories, smoking status, pack-years, physical activity, intakes of meat, dairy, fruits and vegetables, whole grains, and refined grains |
| Baik (2013) [35] | Korea | Cohort | 40–69 | 1325/5251 | The definition given by Alberti et al. [3] | Red meat (servings/day) 1.0 vs. 0 |
1.01 (0.79, 1.29) | Age, sex, income, occupation, education, smoking status, alcohol intake, quartiles of MET-hours/day, study sites, FTO genotypes, quartiles of energy intake, quintiles of healthy dietary pattern, unhealthy dietary pattern, refined grains and starches, mixed grain rice and cereal, fish and other seafood, eggs, legumes, nuts, vegetables and seaweed, fruits, dairy products, sweetened carbonated beverage, green tea and coffee. Types of meats were mutually adjusted for each other. |
| White meat (servings/day) 0.4 vs. 0 |
0.88 (0.71, 1.09) | |||||||
| Becerra-Tomas (2016) [19] | Spain | Cohort | 55–80 (male) 60–80 (female) | 930/1868 | The definition given by Alberti et al. [3] | Tertiles (g/day) Total meat 158.9 vs. 87.0 |
1.23 (1.03, 1.45) | Age, sex, intervention group, leisure time physical activity, BMI, current smoker, former smoker, average consumption quintiles of vegetables, fruit, legumes, cereals, fish, dairy products, alcohol, biscuits, olive oil and nuts, the prevalence of metabolic syndrome components at baseline |
| Red meat 96.4 vs. 38.4 |
1.46 (1.22, 1.74) | |||||||
| Processed meat 35.3 vs. 12.3 |
1.37 (1.15, 1.62) | |||||||
| White meat 79.4 vs. 28.9 | 0.83 (0.70, 0.99) | |||||||
| Mennen (2000) [13] | France | Cross-sectional | 30–64 | 1601/4976 | The presence of at least two of the following factors in the upper (or lower in the case of HDL cholesterol) sex-specific quartile: fasting glucose, serum triglycerides, HDL cholesterol and DBP. | Total meat (portion/day) >2 vs. <1 |
Male 1.39 (0.92, 2.28) | Age, waist- hip ratio, energy intake |
| Female 1.05 (0.67, 1.65) | ||||||||
| Yen (2006) [32] | China | Cross-sectional | 30–79 | 3957/19,839 | NCEP ATP III | Total meat (times/day) ≥3 vs. never or seldom | 1.13 (1.08, 1.18) | Age, betel-quid chewing habit, education level, physical activity, occupation, smoking habit, alcohol habit, dietary intake, family history of diabetes, hypertension, cerebrovascular and CVD in second degree relatives |
| Ruidavets (2007) [24] | France | Cross-sectional | 45–64 (male) | 214/912 | NCEP ATP III | Quintiles (g/day) Total meat 29.7 vs. 20.0 |
2.29 (1.30, 4.02) | Age, physical activity, centre, level of education, alcohol intake, smoking habits, drugs for dyslipidaemia and hypertension, energy intake (without alcohol), diet quality index, dieting |
| Azadbakht (2009) [18] | Iran | Cross-sectional | 18–74 (female) | 145/482 | NCEP ATP III | Red meat (g/day) ≥63.7 vs. <27.3 |
1.99 (1.09, 3.89) | Age, physical activity, total energy intake, current estrogen use, menopausal status, family history of diabetes or stroke, intakes of dietary fiber and cholesterol, percent of energy from fat, fruit, and vegetables, white meats and fish, dairy, partially hydrogenated and nonhydrogenated vegetable oils, and whole- and refined-grains, BMI |
| Kouki (2011) [17] | Finland | Cross-sectional | 57–78 | 351/1334 | NCEP ATP III | Processed meat (g/day) Male >42.5 vs. 0 |
1.38 (0.85, 2.22) | Age, smoking, alcohol consumption, education and VO2 max |
| Female >23.0 vs. 0 |
1.72 (1.08, 2.74) | |||||||
| Babio (2012) [16] | Spain | Cross-sectional | 55–80 (male), 60–80 (female) | 447/717 | NCEP ATP III | Quartiles (g/day) Red meat 150.6 vs. 35.9 |
2.3 (1.4, 3.9) | Age, sex, smoking, BMI, physical activity, total energy intake, dietary baseline variables (alcohol, dietary fibre, magnesium and potassium) |
| Strand (2015) [23] | China | Cross-sectional | 44–52 | 368/793 | NCEP ATP III | Total meat Often vs. rarely |
0.9 (0.6, 1.4) | Unadjusted |
| Aekplakorn (2015) [15] | Thailand | Cross-sectional | 30–59 | 1268/5872 | The definition given by Alberti et al. [3] | Total meat Q4 vs. Q1 |
Male 1.01 (0.82, 1.23) |
Age, alcohol drinking, family history of diabetes and smoking, leisure time physical activity, BMI |
| Female 0.94 (0.72, 1.21) | ||||||||
| Cocate (2015) [14] | Brazil | Cross-sectional | 50.5 (male) | 94/296 | The definition given by Alberti et al. [3] | Tertiles (g/day) Red meat ≥81.5 vs. <56.0 |
1.90 (1.06, 3.44) | Age, habitual physical activity, smoking habit, excessive alcohol intake, daily caloric intake |
| White meat ≥39.4 vs. <24.0 | 1.12 (0.64, 1.97) | |||||||
| Kim (2017) [33] | Korea | Cross-sectional | 30–64 | 3143/11,029 | NCEP ATP III | Red meat T3 vs. T1 |
0.89 (0.79, 1.00) | Age, sex, total energy intake, diet modification(receipt of dietary advice), education level |
| KNHANES | Korea | Cross-sectional | 19–64 | 1325/8387 | NCEP ATP III | Quintiles (servings/week) Total meat 16.2 vs. 1.4 |
0.85 (0.59, 1.24) | Age, sex, household income, education, smoking, alcohol, total energy intake, survey year, physical activity, BMI, intakes of coffee, green tea, soda, vegetables, legumes, whole grains, fish, nuts, dairy. |
| Red meat 9.6 vs. 0.8 |
0.84 (0.59, 1.21) | |||||||
| Processed meat 3.1 vs. 0.0 |
1.18 (0.9, 1.56) | |||||||
| White meat 3.8 vs. 0.0 |
0.80 (0.58, 1.09) | |||||||
| Chiu (2007) [34] | Taiwan | Case-control | ≥20 | 572 cases/4690 controls | NCEP ATPIII | Total meat Usually vs. infrequent |
1.08 (0.89, 1.30) | Age, gender, place of residence of case and control proband |
Abbreviations: NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; KNHANES, Korea National Health and Nutrition Examination Survey; DBP, diastolic blood pressure.
3.2.2. Total Meat Consumption and Metabolic Syndrome
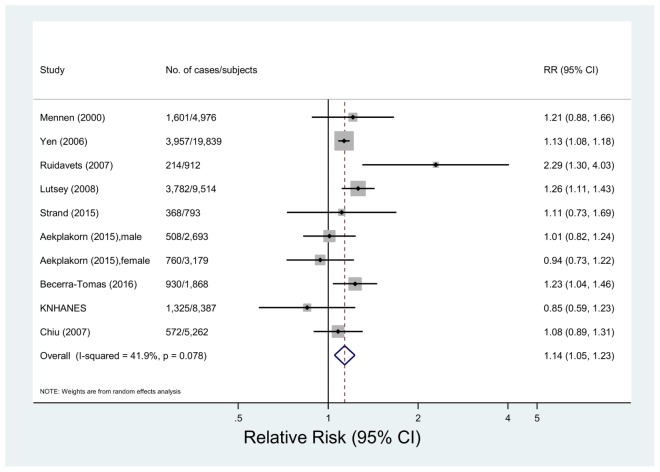
Nine studies including 52,733 subjects and 18,135 events of metabolic syndrome were eligible for the meta-analysis of total meat consumption and metabolic syndrome [13,15,19,21,23,24,32,34]. The multivariable-adjusted pooled RR was 1.14 (95% CI 1.05, 1.23) with no significant heterogeneity among studies (p = 0.08, I2 = 41.9%) (Figure 2). In a sensitivity analysis by removing one study at a time, the pooled RRs were between 1.11 (95% CI: 1.02, 1.21) and 1.15 (95% CI: 1.06, 1.25) (data not shown). The variances by study design, region, adjustment factors or meat intake assessment were insignificant (p > 0.10 for all comparisons) (Table 2).
Figure 2.
Forest plot of observational studies of metabolic syndrome for the highest vs. lowest levels of total meat consumption, using a random-effects model.
Table 2.
Summary of pooled relative risks for meat consumption and risk of metabolic syndrome for highest vs. lowest meat consumption.
| Factor | No. of Studies | Relative Risk | 95% CIs | p for Difference |
|---|---|---|---|---|
| Total meat | ||||
| All studies | 9 | 1.14 | 1.05, 1.23 | |
| Stratified by study design | ||||
| Cohort study | 2 | 1.25 | 1.13, 1.38 | |
| Cross-sectional study | 6 | 1.09 | 0.96, 1.24 | 0.17 a |
| Case-control study | 1 | 1.08 | 0.89, 1.31 | 0.33 a |
| Stratified by geographical region | ||||
| Asia | 5 | 1.11 | 1.06, 1.16 | |
| Europe | 3 | 1.36 | 1.04, 1.78 | 0.10 b |
| North America | 1 | 1.26 | 1.11, 1.43 | 0.14 b |
| Adjusted for BMI | ||||
| Yes | 2 | 1.07 | 0.91, 1.26 | 0.52 |
| No | 7 | 1.16 | 1.05, 1.28 | |
| Adjusted for smoking, alcohol and physical activity | ||||
| Yes | 5 | 1.11 | 0.97, 1.26 | 0.41 |
| No | 4 | 1.20 | 1.09, 1.32 | |
| Meat intake assessment | ||||
| Grams c | 2 | 1.58 | 0.87, 2.87 | 0.19 |
| Servings d | 7 | 1.12 | 1.05, 1.19 | |
| Red meat | ||||
| All studies | 8 | 1.33 | 1.01, 1.74 | |
| Stratified by study design | ||||
| Cohort study | 3 | 1.31 | 0.91, 1.89 | 0.97 |
| Cross-sectional study | 5 | 1.36 | 0.90, 2.07 | |
| Stratified by geographical region | ||||
| Asia | 3 | 0.91 | 0.82, 1.00 | |
| Europe | 2 | 1.72 | 1.12, 2.63 | 0.01 e |
| South America | 2 | 2.08 | 1.22, 3.55 | 0.04 e |
| Middle East | 1 | 1.99 | 1.05, 3.76 | 0.08 e |
| Adjusted for BMI | ||||
| Yes | 4 | 1.47 | 0.99, 2.18 | 0.60 |
| No | 4 | 1.13 | 0.83, 1.55 | |
| Adjusted for smoking, alcohol and physical activity | ||||
| Yes | 5 | 1.54 | 1.06, 2.24 | 0.33 |
| No | 3 | 1.04 | 0.79, 1.38 | |
| Meat intake assessment | ||||
| Grams c | 5 | 1.71 | 1.37, 2.12 | 0.002 |
| Servings d | 3 | 0.91 | 0.82, 1.00 | |
| White meat | ||||
| All studies | 5 | 0.86 | 0.76, 0.97 | |
| Stratified by study design | ||||
| Cohort study | 3 | 0.85 | 0.75, 0.98 | 0.93 |
| Cross-sectional study | 2 | 0.87 | 0.65, 1.16 | |
| Stratified by geographical region | ||||
| South America | 2 | 1.16 | 0.69, 1.93 | |
| Europe | 1 | 0.83 | 0.70, 0.99 | 0.35 f |
| Asia | 2 | 0.85 | 0.72, 1.02 | 0.39 f |
| Adjusted for BMI | ||||
| Yes | 2 | 0.82 | 0.71, 0.96 | 0.46 |
| No | 3 | 0.92 | 0.75, 1.12 | |
| Adjusted for smoking, alcohol and physical activity | ||||
| Yes | 4 | 0.85 | 0.73, 0.98 | 0.78 |
| No | 1 | 0.88 | 0.71, 1.09 | |
| Meat intake assessmen | ||||
| Grams c | 3 | 0.86 | 0.73, 1.01 | 0.97 |
| Servings d | 2 | 0.85 | 0.72, 1.02 | |
a p value for difference in RRs of total meat consumption for cross-sectional study vs. cohort study (p = 0.17) and case-control study vs. cohort study (p = 0.33). b p value for difference in RRs of total meat consumption for Europe vs. Asia (p = 0.10) and North America vs. Asia (p = 0.14). c Studies assessed meat intakes by grams. d Studies assessed meat intake by servings or frequencies e p value for difference in RRs of red meat consumption for Europe vs. Asia (p = 0.01), South America vs. Asia (p = 0.04), and Middle East vs. Asia (p = 0.08). f p value for difference in RRs of white meat consumption for Europe vs. South America (p = 0.35) and Asia vs. South America (p = 0.39).
3.2.3. Red Meat Consumption and Metabolic Syndrome
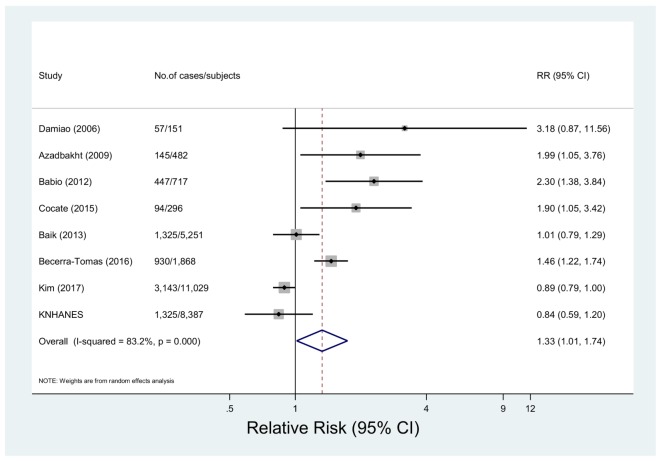
Eight studies with 7466 cases among 28,181 subjects investigated the relationship between red meat intake and metabolic syndrome [14,16,18,19,22,33,35]. The multivariable-adjusted pooled RR was 1.33 (95% CI 1.01, 1.74) showing a high degree of heterogeneity (p < 0.001, I2 = 83.2%) (Figure 3). In the sensitivity analysis, the summary RRs were between 1.22 (95% CI: 0.94, 1.60) and 1.44 (95% CI: 1.07, 1.95) (data not shown). By geographical region, a positive association was found for the studies carried out in Europe (RR = 1.72, 95% CI: 1.12, 2.63), South America (RR = 2.08, 95% CI: 1.22, 3.55), and the Middle East (RR = 1.99, 95% CI: 1.05, 3.76), while the inverse association was shown for studies conducted in Asia (RR = 0.91, 95% CI: 0.82, 1.00) (p for Europe, South America, or Middle East vs. Asia = 0.01, 0.04, and 0.08, respectively). By meat intake assessment, studies which assessed meat intake by grams showed a positive association (RR=1.71, 95% CI: 1.37, 2.12), while studies which assessed meat intake by servings or frequencies showed the inverse association (RR = 0.91, 95% CI: 0.82, 1.00) (p = 0.002). There was no significant difference by study design or adjustment factors (p > 0.33 for all comparisons) (Table 2).
Figure 3.
Forest plot of observational studies of metabolic syndrome for the highest vs. lowest levels of red meat consumption, using a random-effects model.
3.2.4. Processed Meat Consumption and Metabolic Syndrome
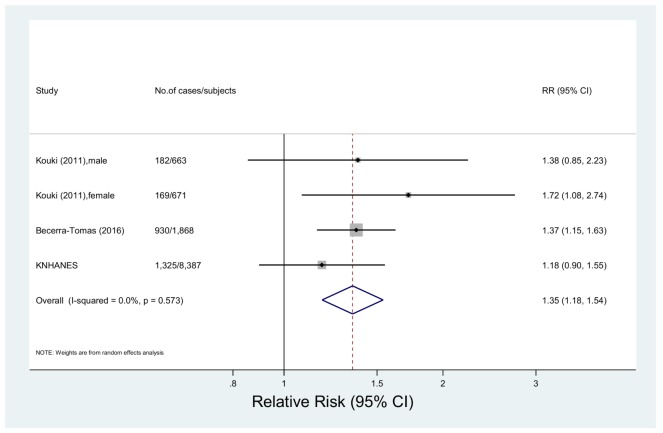
Three studies including 11,589 subjects and 2606 cases were included in the meta-analysis of processed meat intake and metabolic syndrome [17,19]. The multivariable-adjusted pooled RR was 1.35 (95% CI: 1.18, 1.54) with no significant heterogeneity (p = 0.57, I2 = 0.0%) (Figure 4). The summary RRs were between 1.32 (95% CI: 1.06, 1.63) and 1.40 (95% CI: 1.21, 1.64) in the sensitivity analysis (data not shown).
Figure 4.
Forest plot of observational studies of metabolic syndrome for the highest vs. lowest levels of processed meat consumption, using a random-effects model.
3.2.5. White Meat Consumption and Metabolic Syndrome
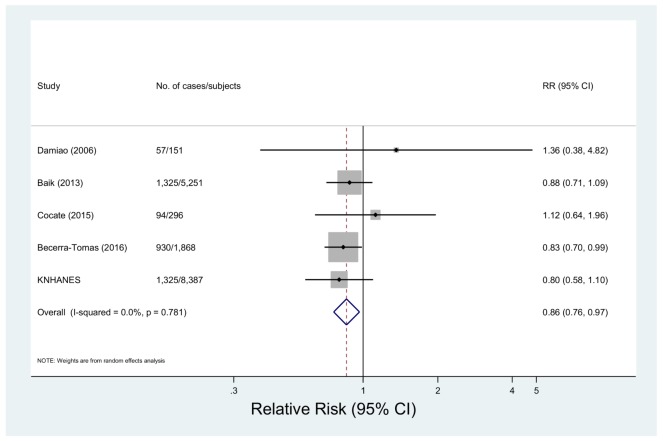
Five studies with 3731 cases among 15,953 subjects investigated the association between white meat intake and metabolic syndrome [14,19,22,35]. The multivariable-adjusted pooled RR was 0.86 (95% CI: 0.76, 0.97) with non-significant heterogeneity (p = 0.78, I2 = 0.0%) (Figure 5). In the sensitivity analysis, the summary RRs were between 0.85 (95% CI: 0.73, 0.98) and 0.88 (95% CI: 0.74, 1.04) (data not shown). The differences by study design, region, adjustment factors or meat intake assessment were insignificant (p > 0.3 for all comparisons) (Table 2).
Figure 5.
Forest plot of observational studies of metabolic syndrome for the highest vs. lowest levels of white meat consumption, using a random-effects model.
3.2.6. Publication Bias
We found no evidence of publication bias for the meta-analysis of total meat (Begg’s p = 0.72; Egger’s p = 0.90), red meat (Begg’s p = 0.90; Egger’s p = 0.09), processed meat (Begg’s p > 0.99; Egger’s p = 0.70), and white meat (Begg’s p = 0.22; Egger’s p = 0.13) intake.
4. Discussion
In this meta-analysis, a high intake of total meat, red meat and processed meat was associated with increased risk of metabolic syndrome. People in the highest category of total meat, red meat and processed meat intake had an increased risk of metabolic syndrome of 14%, 33%, and 35%, respectively, compared with those in the lowest intake category. On the other hand, white meat intake was inversely associated with metabolic syndrome risk. People who have a high consumption of white meat had a 14% reduced risk of metabolic syndrome compared to those who have a low consumption of white meat. All of these associations did not differ significantly by study design and adjustment factors.
The evidence of heterogeneity between studies was found in the meta-analysis of red meat intake and risk of metabolic syndrome. The possible reason for the observed heterogeneity was found in the subgroup analysis by geographic region. The results from the Asian population showed an inverse association between red meat intake and metabolic syndrome unlike the results from other populations. The observed heterogeneity in the analysis of red meat consumption disappeared after excluding studies targeting the Asian population (I2 = 17.0%, p = 0.31) [33,35]. Moreover, the relationship between red meat intake and the risk of metabolic syndrome became stronger when these studies were excluded (RR = 1.71, 95% CI: 1.37, 2.12). This result is consistent with the result from a recent meta-analysis of CVD mortality and red meat intake, which showed a stronger positive association after excluding Asian studies [8]. Relatively low red meat consumption in Asian population compared to Western population might explain the different results [36].
Several meta-analyses reported that a high intake of red and processed meat would raise the risk of diabetes [6,37], CVD [7,8], cancers [9,38,39], and mortality [8,40]. One portion per day increase in red and processed meat intake had a 14 and 32% increased risk, respectively, of type 2 diabetes [6]. In the case of mortality, high processed meat consumption was associated with a high risk of mortality from all-causes and CVD of 22% and 18%, respectively [8]. Also, people in the highest category of red meat intake had 29% and 16% increased risk of death from all-causes and CVD, respectively [8,40]. These findings are in agreement with our results in that a high intake of both red and processed meat might have a harmful effect on health. There were a relatively small number of studies that reported the relationship between white meat consumption and risk of disease. One meta-analysis including 4 studies suggested that a high intake of white meat was related to a reduction of total mortality of 5% in women, and they found a non-significant inverse association in relation to mortality from CVD and ischemic heart disease [8]. In addition, a pooled analysis of 8 Asian prospective cohort studies reported a lower risk of total mortality in people with high poultry intake [36]. More studies examining the association between white meat and the risk of diseases including metabolic syndrome are required to identify effects of white meat consumption on the incidence and development of disease.
In our nationally representative study including 8387 Korean adults aged 19–64 years, the intake of red and processed meat was not significantly associated with the prevalence of metabolic syndrome. These non-significant associations are similar to previous results from Asian studies. A cross-sectional study on the Thai population reported a non-significant association between meat intake and the prevalence of metabolic syndrome in men (OR = 1.01, 95% CI: 0.82, 1.23) and women (OR = 0.94, 95% CI: 0.72, 1.21) in the analysis of the highest vs. lowest quartile of meat intake [15]. A pooled analysis of 8 Asian prospective cohort studies also found a non-significant inverse association or significant inverse association between total or red meat consumption and death from CVD, cancer, or all-causes in male and female [36]. These results are in contrast with our meta-analysis results, which showed positive associations between total and red meat consumption and the risk of metabolic syndrome. This difference in the effect of meat consumption might be attributable to the lower intakes of red meat in Asian countries compared to Western countries [36]. There were a relatively small number of studies which examined the relationship between meat intake and metabolic syndrome in the Asian population compared to the Western population, and thus more well-designed studies targeting large populations are warranted to identify the effect of meat intake on metabolic syndrome in Asian countries. For processed meat consumption, we found that people in the highest quintile had a 32% higher prevalence of hyperglycemia compared to those in the lowest quintile. These results correspond to former studies, which reported an increased risk of diabetes by high processed meat intake [41,42]. We did not observe a significant association between white meat intake and the prevalence of metabolic syndrome, but inverse associations were observed in relation to the prevalence of hypertriglyceridemia and elevated blood pressure. Korean adults in the highest quintile of white meat intake had a lower prevalence of hypertriglyceridemia and elevated blood pressure, i.e., 24% and 32%, respectively, compared to those in the lowest quintile of white meat intake. There is a possibility that high white meat consumption was associated with low red meat intake, and this fact could have affected the results. However, we observed that people with high white meat intake also consumed more red meat, and the inverse association between white meat intake and hypertriglyceridemia and elevated blood pressure remained after further adjustment for dietary intakes including red meat.
Some potential mechanisms might explain a harmful effect of red and processed meat intake on metabolic syndrome. Red meat contains large amounts of total fat, saturated fat and haem-iron. Total and saturated fat might increase metabolic syndrome risk by obesity, hyperinsulinaemia and hyperglycemia [43,44]. Iron is a strong pro-oxidant, and thus can promote oxidative stress, which can damage tissues such as pancreatic beta cells. Furthermore, a high iron level may inhibit glucose metabolism and decrease synthesis and secretion of pancreatic insulin [37,45]. Nitrate used as a preservative in processed meat can be converted into nitrosamines. Nitrosamines have been observed to be poisonous to pancreatic cells and result in insulin resistance [8,46]. High levels of inflammatory mediators including C-reactive protein in people with high red and processed meat consumption also might be another reason for the increased risk of metabolic syndrome [18,47]. Unlike red meat, white meat contains a high proportion of polyunsaturated fatty acids and a low proportion of saturated fatty acids [10]. These differences in fat content might be the reason for the opposite effect of red and white meat on the risk of metabolic syndrome. In addition, the positive association between total meat consumption and metabolic syndrome, which was observed in the current meta-analysis, might be attributable to the high proportion of red meat in meat intake.
To the best of our knowledge, this is the first comprehensive meta-analysis to examine the relationship between total, red, processed and white meat consumption and the risk of metabolic syndrome. We conducted the analyses by types of meat. Most of the studies that were included in the meta-analysis were controlled for confounding factors including age, alcohol consumption, smoking, or physical activity. In addition, the relationships of total, white, red, and processed meat consumption and metabolic syndrome risk did not substantially differ by study design and adjustment factors, and we found no evidence of publication bias in all analyses.
Despite these strengths, there are several limitations which should be considered. First, most of the studies that were included in our meta-analysis evaluated meat intake using FFQ, and thus, our results are susceptible to some degree of misclassification. However, the misclassification in the evaluation of exposure would more likely be non-differential, and the results would have biased toward the null. Second, a meta-analysis of observational studies cannot address problems of confounders that could be inherent in the original studies. Although most of the studies included in the meta-analysis controlled for other known risk factors including age, alcohol consumption, smoking or physical activity, residual confounding cannot be totally ruled out. People consuming more meat tended to have a low fruit and vegetable consumption. Some of the studies included in the meta-analysis controlled other food intake, but other studies did not. More well-designed studies that include various food consumption as an adjustment variable are required to confirm the association between meat consumption and the risk of metabolic syndrome. Third, because our study in Korean adults had a cross-sectional design, which assessed information of exposure and outcome simultaneously, the direction of causality cannot be inferred. However, we tried to eliminate a possible reverse causation bias by excluding both participants with a self-reported diagnosis of hypertension, diabetes, dyslipidemia, stroke, myocardial infarction, or cancer and those who were taking medications to control diabetes, hypertension, or dyslipidemia. Lastly, the results of the Korean adults were significant only in relation to the components of the metabolic syndrome such as hypertriglyceridemia, elevated blood pressure, and hyperglycemia.
5. Conclusions
In conclusion, we observed that total, red, and processed meat consumption was associated with a high risk of metabolic syndrome, while white meat consumption was inversely associated with the risk of metabolic syndrome. White meat intake was inversely associated with the prevalence of hypertriglyceridemia and elevated blood pressure, and high processed meat intake was associated with the raised prevalence of hyperglycemia in Korean adults. Our findings suggest that the effect on health is different by the types of meat and also support current common dietary guidelines to decrease the intake of red and processed meat and increase the consumption of white meat. The results of this study need to be confirmed in well-designed prospective cohort studies and randomized controlled trials on a large population.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A1A1A05001362). The funders had no role in the study design, analysis, and data collection, preparation of the manuscript or decision to publish.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/10/4/390/s1, Table S1, Demographic and dietary intake profiles according to meat consumption in adults (KNHANES)a. Table S2, Multivariate adjusted odds ratio and 95% CI values for metabolic syndrome components according to white meat consumption. Table S3, Multivariate adjusted odds ratio and 95% CI values for metabolic syndrome components according to red meat consumption. Table S4, Multivariate adjusted odds ratio and 95% CI values for metabolic syndrome components according to processed meat consumption.
Author Contributions
Y.J. developed the study design and concept and contributed to the critical revision of the manuscript for important intellectual content; Y.K. wrote the draft of the manuscript and performed statistical analyses; Y.K. and Y.J. extracted the data, contributed to discussion and reviewed/edited the manuscript. All authors have read and approved the final version submitted for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kolovou G.D., Anagnostopoulou K.K., Salpea K.D., Mikhailidis D.P. The prevalence of metabolic syndrome in various populations. Am. J. Med. Sci. 2007;333:362–371. doi: 10.1097/MAJ.0b013e318065c3a1. [DOI] [PubMed] [Google Scholar]
- 2.Galassi A., Reynolds K., He J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Esposito K., Chiodini P., Colao A., Lenzi A., Giugliano D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S.H., Liu Z., Ho S.C. Metabolic syndrome and all-cause mortality: A meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2010;25:375–384. doi: 10.1007/s10654-010-9496-7. [DOI] [PubMed] [Google Scholar]
- 6.Pan A., Sun Q., Bernstein A.M., Schulze M.B., Manson J.E., Willett W.C., Hu F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011;94:1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micha R., Wallace S.K., Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abete I., Romaguera D., Vieira A.R., Lopez de Munain A., Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014;112:762–775. doi: 10.1017/S000711451400124X. [DOI] [PubMed] [Google Scholar]
- 9.Guo J., Wei W., Zhan L. Red and processed meat intake and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2015;151:191–198. doi: 10.1007/s10549-015-3380-9. [DOI] [PubMed] [Google Scholar]
- 10.Kolahdooz F., van der Pols J.C., Bain C.J., Marks G.C., Hughes M.C., Whiteman D.C., Webb P.M., Australian Cancer Study. The Australian Ovarian Cancer Study Group Meat, fish, and ovarian cancer risk: Results from 2 australian case-control studies, a systematic review, and meta-analysis. Am. J. Clin. Nutr. 2010;91:1752–1763. doi: 10.3945/ajcn.2009.28415. [DOI] [PubMed] [Google Scholar]
- 11.Xue X.J., Gao Q., Qiao J.H., Zhang J., Xu C.P., Liu J. Red and processed meat consumption and the risk of lung cancer: A dose-response meta-analysis of 33 published studies. Int. J. Clin. Exp. Med. 2014;7:1542–1553. [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson S.C., Wolk A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int. J. Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 13.Mennen L.I., Lafay L., Feskens E.J.M., Novak M., Lepinay P., Balkau B. Possible protective effect of bread and dairy products on the risk of the metabolic syndrome. Nutr. Res. 2000;20:335–347. doi: 10.1016/S0271-5317(00)00127-5. [DOI] [Google Scholar]
- 14.Cocate P.G., Natali A.J., de Oliveira A., Alfenas R.D.G., Peluzio M.D.G., Longo G.Z., dos Santos E.C., Buthers J.M., de Oliveira L.L., Hermsdorff H.H.M. Red but not white meat consumption is associated with metabolic syndrome, insulin resistance and lipid peroxidation in Brazilian middle-aged men. Eur. J. Prev. Cardiol. 2015;22:223–230. doi: 10.1177/2047487313507684. [DOI] [PubMed] [Google Scholar]
- 15.Aekplakorn W., Satheannoppakao W., Putwatana P., Taneepanichskul S., Kessomboon P., Chongsuvivatwong V., Chariyalertsak S. Dietary pattern and metabolic syndrome in Thai adults. J. Nutr. Metab. 2015;2015:468759. doi: 10.1155/2015/468759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babio N., Sorli M., Bullo M., Basora J., Ibarrola-Jurado N., Fernandez-Ballart J., Martinez-Gonzalez M.A., Serra-Majem L., Gonzalez-Perez R., Salas-Salvado J., et al. Association between red meat consumption and metabolic syndrome in a mediterranean population at high cardiovascular risk: Cross-sectional and 1-year follow-up assessment. Nutr. Metab. Cardiovasc. 2012;22:200–207. doi: 10.1016/j.numecd.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Kouki R., Schwab U., Hassinen M., Komulainen P., Heikkila H., Lakka T.A., Rauramaa R. Food consumption, nutrient intake and the risk of having metabolic syndrome: The DR’S extra study. Eur. J. Clin. Nutr. 2011;65:368–377. doi: 10.1038/ejcn.2010.262. [DOI] [PubMed] [Google Scholar]
- 18.Azadbakht L., Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J. Nutr. 2009;139:335–339. doi: 10.3945/jn.108.096297. [DOI] [PubMed] [Google Scholar]
- 19.Becerra-Tomas N., Babio N., Martinez-Gonzalez M.A., Corella D., Estruch R., Ros E., Fito M., Serra-Majem L., Salaverria I., Lamuela-Raventos R.M., et al. Replacing red meat and processed red meat for white meat, fish, legumes or eggs is associated with lower risk of incidence of metabolic syndrome. Clin. Nutr. 2016;35:1442–1449. doi: 10.1016/j.clnu.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Asghari G., Yuzbashian E., Mirmiran P., Mahmoodi B., Azizi F. Fast food intake increases the incidence of metabolic syndrome in children and adolescents: Tehran lipid and glucose study. PLoS ONE. 2015;10:e0139641. doi: 10.1371/journal.pone.0139641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutsey P.L., Steffen L.M., Stevens J. Dietary intake and the development of the metabolic syndrome—The atherosclerosis risk in communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 22.Damiao R., Castro T.G., Cardoso M.A., Gimeno S.G.A., Ferreira S.R.G. Dietary intakes associated with metabolic syndrome in a cohort of Japanese ancestry. Br. J. Nutr. 2006;96:532–538. [PubMed] [Google Scholar]
- 23.Strand M.A., Perry J., Wang P., Liu S., Lynn H. Risk factors for metabolic syndrome in a cohort study in a north China urban middle-aged population. Asia Pac. J. Public Health. 2015;27:NP255–NP265. doi: 10.1177/1010539512438609. [DOI] [PubMed] [Google Scholar]
- 24.Ruidavets J.B., Bongard V., Dallongeville J., Arveiler D., Ducimetiere P., Perret B., Simon C., Amouyel P., Ferrieres J. High consumptions of grain, fish, dairy products and combinations of these are associated with a low prevalence of metabolic syndrome. J. Epidemiol. Community Health. 2007;61:810–817. doi: 10.1136/jech.2006.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 29.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 32.Yen A.M., Chiu Y.H., Chen L.S., Wu H.M., Huang C.C., Boucher B.J., Chen T.H. A population-based study of the association between betel-quid chewing and the metabolic syndrome in men. Am. J. Clin. Nutr. 2006;83:1153–1160. doi: 10.1093/ajcn/83.5.1153. [DOI] [PubMed] [Google Scholar]
- 33.Kim O.Y., Kwak S.Y., Kim B., Kim Y.S., Kim H.Y., Shin M.J. Selected food consumption mediates the association between education level and metabolic syndrome in Korean adults. Ann. Nutr. Metab. 2017;70:122–131. doi: 10.1159/000470853. [DOI] [PubMed] [Google Scholar]
- 34.Chiu Y.H., Lin W.Y., Wang P.E., Chen Y.D., Wang T.T., Warwick J., Chen T.H. Population-based family case-control proband study on familial aggregation of metabolic syndrome: Finding from Taiwanese people involved in Keelung community-based integrated screening (KCIS No. 5) Diabetes Res. Clin. Pract. 2007;75:348–356. doi: 10.1016/j.diabres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Baik I., Lee M., Jun N.R., Lee J.Y., Shin C. A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr. Res. Pract. 2013;7:233–241. doi: 10.4162/nrp.2013.7.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J.E., McLerran D.F., Rolland B., Chen Y., Grant E.J., Vedanthan R., Inoue M., Tsugane S., Gao Y.T., Tsuji I., et al. Meat intake and cause-specific mortality: A pooled analysis of Asian prospective cohort studies. Am. J. Clin. Nutr. 2013;98:1032–1041. doi: 10.3945/ajcn.113.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aune D., Ursin G., Veierod M.B. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52:2277–2287. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- 38.Alexander D.D., Weed D.L., Cushing C.A., Lowe K.A. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur. J. Cancer. Prev. 2011;20:293–307. doi: 10.1097/CEJ.0b013e328345f985. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z., Yin Z., Pu Z., Zhao Q. Association between consumption of red and processed meat and pancreatic cancer risk: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2017;15:486–493. doi: 10.1016/j.cgh.2016.09.143. [DOI] [PubMed] [Google Scholar]
- 40.Larsson S.C., Orsini N. Red meat and processed meat consumption and all-cause mortality: A meta-analysis. Am. J. Epidemiol. 2014;179:282–289. doi: 10.1093/aje/kwt261. [DOI] [PubMed] [Google Scholar]
- 41.Lajous M., Tondeur L., Fagherazzi G., de Lauzon-Guillain B., Boutron-Ruaualt M.C., Clavel-Chapelon F. Processed and unprocessed red meat consumption and incident type 2 diabetes among French women. Diabetes Care. 2012;35:128–130. doi: 10.2337/dc11-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannisto S., Kontto J., Kataja-Tuomola M., Albanes D., Virtamo J. High processed meat consumption is a risk factor of type 2 diabetes in the alpha-tocopherol, beta-carotene cancer prevention study. Br. J. Nutr. 2010;103:1817–1822. doi: 10.1017/S0007114510000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips C.M., Kesse-Guyot E., McManus R., Hercberg S., Lairon D., Planells R., Roche H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J. Nutr. 2012;142:824–831. doi: 10.3945/jn.111.153460. [DOI] [PubMed] [Google Scholar]
- 44.Schulz M., Kroke A., Liese A.D., Hoffmann K., Bergmann M.M., Boeing H. Food groups as predictors for short-term weight changes in men and women of the EPIC-Potsdam cohort. J. Nutr. 2002;132:1335–1340. doi: 10.1093/jn/132.6.1335. [DOI] [PubMed] [Google Scholar]
- 45.Rajpathak S.N., Crandall J.P., Wylie-Rosett J., Kabat G.C., Rohan T.E., Hu F.B. The role of iron in type 2 diabetes in humans. Biochim. Biophys. Acta. 2009;1790:671–681. doi: 10.1016/j.bbagen.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Risch H.A. Pancreatic cancer: Helicobacter pylori colonization, N-nitrosamine exposures, and ABO blood group. Mol. Carcinog. 2012;51:109–118. doi: 10.1002/mc.20826. [DOI] [PubMed] [Google Scholar]
- 47.Ley S.H., Sun Q., Willett W.C., Eliassen A.H., Wu K., Pan A., Grodstein F., Hu F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014;99:352–360. doi: 10.3945/ajcn.113.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.