Abstract
Tri-ponderal mass index (TMI) and fat mass index (FMI) have been proposed as alternative approaches for assessing body fat since BMI does not ensure an accurate screening for obesity and overweight status in children and adolescents. This study proposes thresholds of the TMI and FMI for the prediction of metabolic syndrome (MetS) in children and young people. For this purpose, a cross-sectional study was conducted on 4673 participants (57.1% females), who were 9–25 years of age. As part of the study, measurements of the subjects’ weight, waist circumference, serum lipid indices, blood pressure and fasting plasma glucose were taken. Body composition was measured by bioelectrical impedance analysis (BIA). The TMI and FMI were calculated as weight (kg)/height (m3) and fat mass (kg)/height (m3), respectively. Following the International Diabetes Federation (IDF) definition, MetS is defined as including three or more metabolic abnormalities. Cohort-specific thresholds were established to identify Colombian children and young people at high risk of MetS. The thresholds were applied to the following groups: (i) a cohort of children where the girls’ TMI ≥ 12.13 kg/m3 and the boys’ TMI ≥ 12.10 kg/m3; (ii) a cohort of adolescents where the girls’ TMI ≥ 12.48 kg/m3 and the boys’ TMI ≥ 11.19 kg/m3; (iii) a cohort of young adults where the women’s TMI ≥ 13.21 kg/m3 and the men’s TMI ≥ 12.19 kg/m3. The FMI reference cut-off values used for the different groups were as follows: (i) a cohort of children where the girls’ FMI ≥ 2.59 fat mass/m3 and the boys’ FMI ≥ 1.98 fat mass/m3; (ii) a cohort of adolescents where the girls’ FMI ≥ 3.12 fat mass/m3 and the boys’ FMI ≥ 1.46 fat mass/m3; (iii) a cohort of adults where the women’s FMI ≥ 3.27 kg/m3 and the men’s FMI ≥ 1.65 kg/m3. Our results showed that the FMI and TMI had a moderate discriminatory power to detect MetS in Colombian children, adolescents, and young adults.
Keywords: adiposity, fat mass, tri-ponderal mass index, fat mass index, metabolic syndrome, children
1. Introduction
Metabolic syndrome (MetS) is a set of metabolic abnormalities that are risk factors for cardiovascular disease (CVD), diabetes mellitus type 2 (DM-2), and atherosclerosis [1]. Central obesity and high blood pressure are frequent components of MetS, which are often associated with insulin resistance and metabolic disorders [2]. Thus, MetS is a strong, independent predictor of all-cause and CVD mortality [3]. In the cross-sectional Insulin Resistance Atherosclerosis Study (IRAS Study), Palaniappan et al. showed that every increase of 11 cm in waist circumference (WC) was associated with an adjusted 80% increased risk of developing MetS within five years [4]. Additionally, in a longitudinal adult population-based cohort study (PAMELA Study), the increased risk of cardiovascular and all-cause mortality of subjects was related to high blood pressure and glucose levels, which are components of metabolic disorder [5].
Obesity, especially visceral fat, is an important link between the components of the MetS, since central obesity is highly prevalent [6]. In Colombia, results from the 2016 Report Card on Physical Activity for Children and Youth [7] showed that 13.4% of children (5–11 years of age) were overweight and 4.1% of the adolescents (12–17 years of age) were obese. This obesity epidemic has been associated with obesogenic factors, such as increased intake of energy-dense diets, a sedentary lifestyle, and low levels of physical activity [8]. As obesity plays a central role in MetS and since the probability of childhood obesity persisting into adulthood is estimated to increase from ~20% at the age of four to 80% by adolescence, the epidemic of pediatric obesity can result in an increased prevalence of hypertension, DM-2, and CVD in adulthood [9]. In this context, the over-accumulation of body fat correlates epidemiologically with various pathophysiological sequelae, including a higher incidence of MetS, which is usually associated with CVD mortality [10]. Thus, epidemiological studies have reported an association of fat distribution and metabolic risk factors, including high blood pressure, hyperglycemia, and dyslipidemia, with a risk of MetS in Colombian children and young people [11,12].
Body mass index (BMI) is based on the finding that adult body weight should be proportional to height squared [13]. However, during childhood and adolescent development, weight is not proportional to height squared, thus undercutting the validity of BMI in adolescents [14]. To rectify the problem that these scaling powers (approximately 2.5–3.5) are inconsistent with BMI (approximately 2) prior to the age of 18 years, BMI z-scores are instead used for children and adolescents [15]. However, BMI percentiles for each age (BMI z-scores) do not consider that both body proportions and body fat levels change during growth in a way that is inconsistent with BMI. Therefore, this approach does not ensure an adequate classification of children and adolescents as normal weight vs. overweight or obese. To replace BMI, Peterson et al. [14] recently tested other body fat indices with the form of mass divided by heightn, including the tri-ponderal mass index TMI (as kilograms divided by meters cubed), which is based on the ponderal index and the Rohrer Index. Another simple and inexpensive approach for assessing body fat is the fat mass index (FMI, fat mass/(height)2, which is a surrogate marker of cardiovascular risk in young adults [16]. The FMI was initially conceived as a component of the BMI and is indeed useful in analyzing BMIs in terms of fat and non-fat (especially for men, though the optimal exponent can be close to one for women and three for children) [17]. However, as highlighted by Burton [18], a theoretically sound index, independent of population statistics, uses the formula of (fat mass in kg)/(D × height in m3), where D is the fat density. Burton [18] asserts that, because D is virtually constant, the index may be simplified as (fat mass)/height3. Unlike the FMI, this nameless index has the same value for individuals of different sizes that are identical in body composition and proportions. VanItallie et al. [19] proposed an FMI (fat mass/(height)2) that considers an individual’s height, while Johnson et al. [20] used a regression-based height exponent of 5.8 to adapt the FMI to nine-year-old children. To date, only one previous study has evaluated the applicability of FMI (fat mass/(height)2) to predict MetS, with results confirming its close relationship to the components of MetS [21]. However, there is no consensus as to the cut-off value that can be used to define excess adiposity based on TMI (kg)/height3) and FMI (fat mass)/height3).
As MetS is acknowledged to be a global public health problem and given the increasing prevalence of METs in the Colombian population, there is a clear need to establish gender-specific TMI and FMI cut-off values for estimating the fat mass and body adiposity dysfunction associated with MetS. Accordingly, the objective of this study was to explore thresholds of TMI and FMI (fat mass)/height3) for the prediction of MetS in a large population of Colombian children and youth.
2. Methods
2.1. Study Design and Sample Population
This research is based on a two-part relatively large-scale study (FUPRECOL) [22] of over 1000 children/adolescents and young adults [23]. We used the data from these two projects, which totaled 4673 participants that were 9–25 years old (n = 1047 children 9–12 years; n = 1830 adolescents 13–17 years; and n = 1796 young adults 18–25 years). All participants and their parents/guardians provided their written, informed consent. Each study was approved by the authorized institutional review boards (UMB N° 01-1802-2013 and UR N° CEI-ABN026-000262) and complied with the Declaration of Helsinki (as revised in Hong Kong in 1989 and in Edinburgh, Scotland, in 2000). The studies also complied with Colombian laws regulating clinical research on human subjects (Resolution 008430/1993 of the Ministry of Health). Exclusion factors included a clinical diagnosis of CVD, DM-1 and 2, pregnancy, the use of alcohol or drugs and in general, the presence of any disease not directly associated with nutrition. Volunteers received no compensation for their participation.
2.2. Data Collection
Body weight (kg) was measured using an electric scale (Model Tanita® BC-418® or BF-689®, Tokyo, Japan) and height (cm) with a portable stadiometer (Seca® 216, Hamburg, Germany). BMI was calculated as weight (kg)/height (m2) [24]. WC (cm) was measured as the narrowest point between the lower costal border and the iliac crest using a metal tape measure (Lufkin W606PM®, Parsippany, NJ, USA) in accordance with the guidelines of the International Society for the Advancement of Kinanthropometry [25]. BMI and TMI were calculated as weight (kg)/height (m2) and weight (kg)/height (m3), respectively, while the BMI z-score was estimated using the World Health Organization Growth Reference from 2007 [24]. For all anthropometric variables, a low technical error measurement was reported (less than 2%).
We determined body fat percentage (BF%) and FMI using bioelectrical impedance analyses (BIA) by whole-body impedance (Tanita Model BC-418®, Tokyo, Japan). Detailed information about the BIA technique has been provided in previous studies [26,27]. The FMI was then calculated by dividing each subject’s fat mass (kg) by the cubed value of his/her height (m), as described in Burton [18].
2.3. Metabolic Syndrome Diagnosis
Between 6:00 and 9:00 a.m. after 10–12 h of an overnight fast, blood samples were extracted from capillary sampling. Participants were asked not to engage in prolonged exercise 24 h prior to testing. Using enzymatic colorimetric methods, we measured (i) high density lipoprotein cholesterol (HDL-c); (ii) triglycerides; (iii) total cholesterol; and (iv) fasting blood glucose. Over a period of 15 days, the inter-assay reproducibility, estimated by the coefficient of variation, was determined from 16 replicate analyses of eight plasma pools. For all blood samples, we obtained errors of less than 4%.
We measured blood pressure levels on the left arm at the heart level using an automatic device Omron M6 Comfort (Omron® Healthcare Europe B.V., Hoofddorp, The Netherlands). Individuals were seated in a semi-reclined position with arms relaxed and supported, while the midpoint of the arm was positioned at the level of the heart.
In children and adolescents, MetS score was defined in accordance with the updated harmonized criteria of de Ferranti et al. [28] and Magge et al. [29] based on age/gender/height criteria. In young adults, MetS was defined in accordance with the updated harmonized criteria of the IDF [30]. According to the criteria of the IDF, participants were considered to have MetS if they showed three or more of the following: (1) abdominal obesity (WC ≥80 cm in women and ≥90 cm in men); (2) hypertriglyceridemia (≥150 g/dL); (3) low HDL-C (<50 mg/dL in women and <40 mg/dL in men); (4) high blood pressure (systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg); (5) high fasting glucose (≥100 mg/dL).
We calculated a MetS score that reflected a continuous score of the five MetS risk factors. The MetS score was calculated from the data collected for each participant, based on the IDF [30], Ferranti et al. [28], and Magge et al. [29], with standard deviations using data from the total cohort at baseline. The mean of this continuously distributed MetS was thus zero by definition.
2.4. Statistical Analysis
The characteristics of the participants were given as mean values and standard deviation (SD). Independent two-tailed t-tests for continuous variables and chi-square (χ2) tests for categorical variables were used to examine sex differences and age groupings. The relationships between BMI, FMI, TMI, and MetS were tested by means of partial correlation coefficients. This analysis was adjusted by age and sex. Locally weighted scatterplot smoothing curves were used to illustrate the shape of the relationship between TMI, FMI, and MetS. The results are given for the lineal form. A higher fraction of the explained variance (i.e., R2 values) in MetS indicates greater accuracy of the anthropometric indexes.
The area under the curve (AUC), ranging from 0 (worthless test) to 1 (perfect test), was used to predict MetS with the TMI and FMI. AUC has been reported to be a global indicator of diagnostic performance since it represents the ability of the test to correctly classify participants with a high risk of MetS (p-values < 0.01 and an AUC > 0.80) [31]. In addition, the positive likelihood ratio LR (+) and the negative likelihood ratio LR (−) were also determined. Cutoff points were chosen based on the highest J-Youden index, which uses the point on the receiver operating characteristic (ROC) curve that is farthest from the line of equality [32]. The analysis of the data was performed with the SPSS statistical software package, version 24.0 (IBM, Chicago, IL, USA) for Windows.
3. Results
3.1. Study Participants
Table 1 shows the results obtained for the anthropometric variables, body composition, blood pressure, and metabolic biomarker variables. The final sample comprised a total of 4673 children and young people. It was composed of 2001 males (42.9%) and 2672 females (57.1%) with a mean age of 17 ± 4.0 (a minimum age of 9 years and a maximum age of 25 years). In the cohort of children, girls were found to have a significantly lower WC, HDL, and glucose level as well as a greater quantity of body fat (%), FMI, and triglycerides compared to boys (p < 0.05).
Table 1.
Characteristics among a sample from Colombia (mean (standard deviation (SD)) or frequency (%)).
| Characteristic | Children 9–12 Years (n = 1047) | Adolescents 13–17 Years (n = 1830) | Young Adults 18–25 Years (n = 1796) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Girls (n = 582) | Boys (n = 465) | p-Value | Girls (n = 986) | Boys (n = 844) | p-Value | Women (n = 1104) | Men (n = 692) | p-Value | |
| Anthropometric variable | |||||||||
| Age (years) | 10.8 (1.1) | 10.7 (1.1) | 0.104 | 14.6 (1.3) | 14.7 (1.3) | 0.077 | 21.9 (1.9) | 22.6 (1.2) | 0.624 |
| Weight (kg) | 38.2 (8.8) | 37.6 (9.6) | 0.353 | 50.9 (8.6) | 53.0 (10.4) | <0.001 | 58.7 (10.3) | 68.9 (12.1) | <0.001 |
| Height (m) | 1.43 (0.09) | 1.42 (0.10) | 0.526 | 1.55 (0.06) | 1.63 (0.10) | <0.001 | 1.59 (0.05) | 1.72 (0.06) | <0.001 |
| WC (cm) | 60.1 (7.1) | 62.2 (7.7) | <0.001 | 65.9 (6.8) | 67.5 (6.8) | <0.001 | 71.5 (8.0) | 78.2 (8.0) | <0.001 |
| BMI (kg/m2) | 18.5 (2.8) | 18.4 (3.0) | 0.312 | 21.0 (3.0) | 19.9 (2.8) | <0.001 | 23.2 (3.7) | 23.1 (3.6) | 0.810 |
| BMI z | 0.91 (0.4) | 1.12 (0.7) | <0.001 | 0.51 (0.5) | 0.39 (0.3) | <0.001 | - | - | - |
| Overweight by BMI/z-BMI n (%) * | 141 (24.4) | 78 (16.9) | 0.001 | 221 (22.5) | 82 (9.8) | 0.001 | 236 (21.4) | 144 (20.8) | 0.724 |
| Obesity by BMI/z-BMI n (%) * | 51 (8.8) | 47 (10.2) | 0.001 | 43 (4.4) | 23 (2.7) | 0,001 | 61 (5.5) | 33 (4.8) | 0.722 |
| TMI (kg/m3) | 13.0 (1.9) | 12.9 (1.9) | 0.447 | 13.6 (1.9) | 12.2 (1.7) | <0.001 | 14.6 (2.4) | 13.4 (2.1) | <0.001 |
| Body fat (%) | 23.6 (5.8) | 19.3 (6.5) | <0.001 | 25.7 (6.0) | 15.1 (5.9) | <0.001 | 27.0 (7.2) | 15.6 (6.5) | <0.001 |
| FMI (fat mass)/height3) | 3.2 (1.2) | 2.6 (1.3) | <0.001 | 3.6 (1.3) | 1.9 (1.1) | <0.001 | 4.0 (1.7) | 2.2 (1.3) | <0.001 |
| Blood pressure | |||||||||
| Systolic blood pressure (mmHg) | 109.6 (13.8) | 111.0 (13.7) | 0.113 | 110.6 (11.5) | 114.4 (14.0) | <0.001 | 111.2 (11.1) | 120.2 (12.9) | <0.001 |
| Diastolic blood pressure (mmHg) | 67.1 (8.6) | 66.6 (8.9) | 0389 | 69.4 (8.6) | 68.9 (9.4) | 0.288 | 71.7 (9.3) | 74.1 (11.4) | <0.001 |
| Mean arterial pressure (mmHg) | 81.2 (8.7) | 81.4 (8.9) | 0.797 | 83.1 (8.2) | 84.0 (9.4) | 0.020 | 91.5 (8.9) | 97.2 (10.9) | <0.001 |
| Metabolic biomarkers | |||||||||
| Total cholesterol (mg/dL) | 151.3 (29.3) | 152.1 (30.3) | 0.656 | 148.3 (31.3) | 132.9 (30.3) | <0.001 | 146.3 (33.3) | 132.7 (30.2) | <0.001 |
| Triglycerides (mg/dL) | 96.0 (60.4) | 86.8 (44.7) | 0.006 | 96.7 (50.2) | 84.4 (35.8) | <0.001 | 88.5 (45.3) | 93.7 (48.5) | 0.020 |
| LDL-C (mg/dL) | 86.0 (26.6) | 86.6 (30.0) | 0.756 | 84.6 (29.4) | 78.6 (35.9) | <0.001 | 87.9 (26.1) | 81.0 (26.0) | <0.001 |
| HDL-C (mg/dL) | 48.4 (13.0) | 51.5 (13.1) | <0.001 | 46.9 (11.7) | 44.4 (11.2) | <0.001 | 43.9 (12.8) | 39.5 (10.6) | <0.001 |
| Glucose (mg/dL) | 83.3 (15.0) | 85.3 (16.2) | 0.038 | 80.5 (16.1) | 82.3 (15.5) | 0.015 | 86.0 (11.5) | 85.5 (11.7) | <0.001 |
| MetS score | -0.12 (0.13) | -0.14 (0.12) | 0.008 | -0.13 (0.11) | -0.14 (0.09) | 0.077 | −3.94 (2.66) | −3.90 (2.78) | 0.501 |
| Metabolic Syndrome n (%) * | |||||||||
| Yes | 85 (14.6) | 60 (12.9) | 0.428 | 80 (8.1) | 56 (6.5) | 0.229 | 82 (7.4) | 166 (9.2) | 0.001 |
Continuous variables are reported as mean values (standard deviations (SDs)) and categorical variables are reported as numbers and percentages in brackets. Significant between-sex differences (t-tests or * chi-square test χ2). WC: waist circumference; BMI: body mass index; TMI: tri-ponderal mass index, FMI: fat mass index; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol.
In the cohort of adolescents, the weight, height, WC, SBP, mean arterial pressure, and glucose level of the girls were significantly lower in comparison to the boys (p < 0.05). In contrast, their BMI, TMI, body fat, FMI, total cholesterol, triglycerides, LDL, and HDL were significantly higher (p < 0.001).
In the cohort of young adults, women were found to have a lower weight, height, WC, SBP, DBP, MAP, and triglycerides than men (p < 0.05). However, they had a higher TMI, body fat, FMI, total cholesterol, LDL, HDL, and glucose level in comparison to the men (p < 0.001). It is worth highlighting that, among young adults, the prevalence of MetS was greater in women than in men (p = 0.001).
3.2. Relationship between BMI, FMI, TMI, and MetS Score
Table 2 shows the partial correlation between BMI, FMI, TMI, and MetS score. Overall, we showed an acceptable to moderate positive correlation with MetS score (all p < 0.01).
Table 2.
Results of the partial correlation analysis between body mass index (BMI), fat mass index (FMI), Tri-ponderal Mass (TMI) and a continuous score of the five MetS.
| Group and Variable | MetS Score | TMI (kg/m3) | FMI (Fat Mass)/Height3) | BMI |
|---|---|---|---|---|
| Children 9–12 years (n = 1047) | ||||
| BMI | 0.534 * | 0.938 * | 0.942 * | 1 |
| FMI (fat mass)/height3) | 0.522 * | 0.911 * | 1 | |
| TMI (kg/m3) | 0.462 * | 1 | ||
| cMets | 1 | |||
| Adolescents 13–17 years (n = 1830) | ||||
| BMI | 0.455 * | 0.942 * | 0.882 * | 1 |
| FMI (fat mass)/height3) | 0.427 * | 0.846 * | 1 | |
| TMI (kg/m3) | 0.386 * | 1 | ||
| cMets | 1 | |||
| Young adults 18–25 years (n = 1796) | ||||
| BMI | 0.600 * | 0.971 * | 0.943 * | 1 |
| FMI (fat mass)/height3) | 0.602 * | 0.912 * | 1 | |
| TMI (kg/m3) | 0.554 * | 1 | ||
| cMets | 1 |
Analysis adjusted by co-variables: age and sex, * p < 0.01. MetS: metabolic syndrome; BMI: body mass index; TMI: tri-ponderal mass index; FMI: fat mass index.
3.3. Association between FMI, TMI, and MetS Score
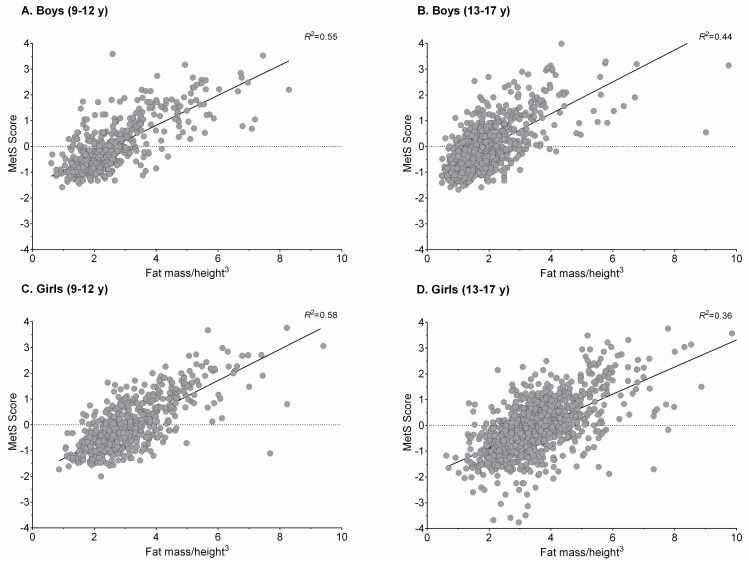
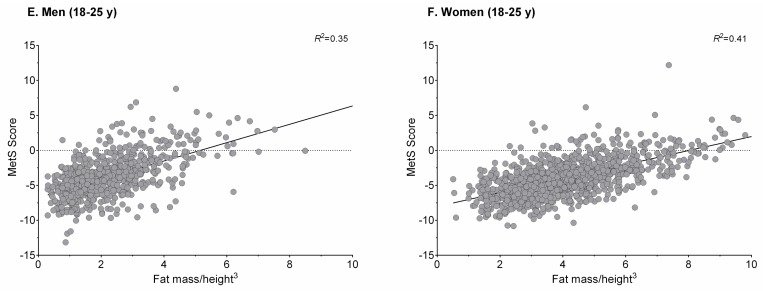
Figure 1 and Figure 2 show plots of the association between FMI, TMI, and MetS score for sex and age groups. As observed, the locally weighted scatterplot smoothing curves provide evidence of a well-defined threshold effect of high/low value indexes on MetS score, which is provided as a continuous outcome.
Figure 1.
Association between fat mass index and MetS score for sex and age groups. MetS: metabolic syndrome.
Figure 2.
Association between TMI and MetS score for sex and age groups. MetS: metabolic syndrome; TMI: tri-ponderal mass index.
3.4. Optimal Cut-Off Value in the Screening of MetS
The ROC curve analyses for the diagnostic performance of TMI and FMI in identifying a high risk of MetS are shown in Table 3 and Table 4, respectively. The ROC analyses showed that TMI and FMI parameters could be used to detect MetS in Colombian children, adolescents, and young adults.
Table 3.
Parameters of the receiver operating characteristic (ROC) curves analysis for the diagnostic performance of Tri-ponderal Mass (TMI) vs. fat mass index (FMI) in identifying high risk of metabolic syndrome (MetS) according to the Ferranti and Maggie criteria in children and adolescents.
| High Risk of MetS | |||
|---|---|---|---|
| Parameter | TMI (kg/m3) | FMI (Fat Mass)/Height3) | |
| Girls (9–12 years) | AUC | 0.674 | 0.698 |
| 95% CI | 0.608–0.740 | 0.634–0.763 | |
| p-value | <0.0001 | <0.0001 | |
| J-Youden | 0.19 | 0.18 | |
| Cut-off | 12.13 | 2.59 | |
| Sensitivity (%) | 80 | 85 | |
| Specificity (%) | 61 | 59 | |
| LR (+) | 2.04 | 2.05 | |
| LR (−) | 0.33 | 0.26 | |
| Boys (9–12 years) | AUC | 0.755 | 0.752 |
| 95% CI | 0.677–0.833 | 0.676–0.828 | |
| p value | <0.0001 | <0.0001 | |
| J-Youden | 0.17 | 0.19 | |
| Cut-off | 12.10 | 1.98 | |
| Sensitivity (%) | 85 | 82 | |
| Specificity (%) | 59 | 60 | |
| LR (+) | 2.05 | 2.04 | |
| LR (−) | 0.26 | 0.31 | |
| Girls (13–17 years) | AUC | 0.684 | 0.699 |
| 95% CI | 0.619–0.748 | 0.635–0.762 | |
| p-value | <0.0001 | <0.0001 | |
| J-Youden | 0.11 | 0.13 | |
| Cut-off | 12.48 | 3.12 | |
| Sensitivity (%) | 86 | 87 | |
| Specificity (%) | 70 | 66 | |
| LR (+) | 2.87 | 2.55 | |
| LR (−) | 0.20 | 0.19 | |
| Boys (13–17 years) | AUC | 0.729 | 0.745 |
| 95% CI | 0.654–0.797 | 0.675–0.816 | |
| p-value | <0.0001 | <0.0001 | |
| J-Youden | 0.19 | 0.18 | |
| Cut-off | 11.19 | 1.46 | |
| Sensitivity (%) | 93 | 84 | |
| Specificity (%) | 70 | 60 | |
| LR (+) | 3.09 | 2.10 | |
| LR (−) | 0.10 | 0.27 | |
AUC: area under the curve; CI: confidence interval; LR (+): positive likelihood ratio; LR (−): negative likelihood ratio. MetS: metabolic syndrome; TMI: tri-ponderal mass index; FMI: fat mass index.
Table 4.
Parameters of the receiver operating characteristic (ROC) curves analysis for the diagnostic performance of Tri-ponderal Mass (TMI) vs. fat mass index (FMI) in identifying high risk of metabolic syndrome (MetS) according to the International Diabetes Federation (IDF) criteria in men and women.
| High Risk of MetS | |||
|---|---|---|---|
| Parameter | TMI (kg/m3) | FMI (Fat Mass)/Height3) | |
| Women (18–25 years) | AUC | 0.854 | 0.882 |
| 95% CI | 0.805–0.903 | 0.840–0.924 | |
| p-value | <0.0001 | <0.0001 | |
| J-Youden | 0.14 | 0.12 | |
| Cut-off | 13.21 | 3.27 | |
| Sensitivity (%) | 94 | 95 | |
| Specificity (%) | 67 | 62 | |
| LR (+) | 2.81 | 2.52 | |
| LR (−) | 0.09 | 0.08 | |
| Men (18–25 years) | AUC | 0.814 | 0.848 |
| 95% CI | 0.759–0.869 | 0.800–0.896 | |
| p-value | <0.0001 | <0.0001 | |
| J-Youden | 0.10 | 0.15 | |
| Cut-off | 12.19 | 1.65 | |
| Sensitivity (%) | 94 | 93 | |
| Specificity (%) | 70 | 57 | |
| LR (+) | 3.11 | 2.14 | |
| LR (−) | 0.09 | 0.13 | |
AUC: area under the curve; CI: confidence interval; LR (+): positive likelihood ratio; LR (−): negative likelihood ratio. MetS: metabolic syndrome; TMI: tri-ponderal mass index; FMI: fat mass index
For the girls in the cohort of children, the TMI cut-off value of 12.13 kg/m3 provided a sensitivity of 80%, an LR (+) value of 2.04, a specificity of 61%, and an LR (−) value of 0.33. Regarding the FMI, the cut-off value of 2.59 fat mass/m3 provided a sensitivity of 85%, an LR (+) value of 2.05, a specificity of 59%, and an LR (−) value of 0.26. For the boys in the same cohort, the TMI cut-off value of 12.10 kg/m3 provided a sensitivity of 85%, an LR (+) value of 2.05, a specificity of 59%, and an LR (−) value of 0.26. The ROC curve for the FMI was also obtained using a cut-off value of 1.98 fat mass/m3, which achieved a sensitivity of 82%, an LR (+) of 2.04, a specificity of 60%, and an LR (−) of 0.31.
For the girls in the cohort of adolescents, the TMI cut-off value of 12.48 kg/m3 provided a sensitivity of 86%, an LR (+) value of 2.87, a specificity of 70%, and an LR (−) value of 0.20. For the FMI, the cut-off value of 3.12 fat mass/m3 provided a sensitivity of 87%, an LR (+) value of 2.55, a specificity of 66%, and an LR (−) value of 0.19. For the adolescent boys in this cohort, the TMI cut-off value of 11.19 kg/m3 provided a sensitivity of 93%, an LR (+) value of 3.09, a specificity of 70%, and an LR (−) value of 0.10. The ROC curve for FMI was also obtained using a cut-off value of 1.46 fat mass/m3, which achieved a sensitivity of 84%, an LR (+) of 2.10, a specificity of 60%, and an LR (−) of 0.27.
For the women in the young adult cohort, the TMI cut-off value of 13.21 kg/m3 provided a sensitivity of 94%, an LR (+) value of 2.81, a specificity of 67%, and an LR (−) value of 0.09. For FMI, the cut-off value of 3.27 fat mass/m3 provided a sensitivity of 97%, an LR (+) value of 2.52, a specificity of 62%, and an LR (−) value of 0.08. Regarding the TMI of the men in this cohort, the cut-off value of 12.19 kg/m3 provided a sensitivity of 94%, an LR (+) value of 3.11, a specificity of 70%, and an LR (−) value of 0.09. The ROC curve for FMI was also obtained using a cut-off value of 1.65 fat mass/m3, which achieved a sensitivity of 93%, an LR (+) of 2.14, a specificity of 57%, and an LR (−) of 0.13.
4. Discussion
MetS is considered a worldwide public health problem that increases the risk of cardiovascular morbidity and mortality [10]. In Colombia, the prevalence of MetS was found to be 7.8% and 11.0%, according to the definitions proposed by the Ford et al. and de Ferranti et al., respectively in a population-based sample of schoolchildren [11]. Our study found that the MetS prevalence was higher in girls in the cohorts of children and adolescents (14.6% and 8.1%), while the prevalence was higher in males for young adults. These findings differ from the results of Ruano-Nieto et al. [33], who found that the estimated prevalence of MetS was 8.4% for women and 6.1% for men in a population of Ecuadorian university students. These differences could be explained by the MetS cluster used, the design method, and the target population. Thus, the identification of useful screening tools to detect MetS early in life is of particular interest since this will facilitate more timely and effective interventions in those subjects who are at greater risk.
This study provides gender-specific TMI and FMI reference thresholds as a way of estimating the fat mass and body adiposity dysfunction associated with MetS during childhood and early adulthood. To the best of our knowledge, this is the first evaluation of the predictive capacity of TMI and FMI for MetS. As reflected in the data obtained in our study, both TMI and FMI have a moderate discriminating power for the detection of MetS in Colombian children and youth. This finding supports the diagnostic capabilities of both indices to identify children and young people at a high risk of MetS. This is clinically relevant, since it is known that MetS increases the risk of CVD.
The FMI (fat mass/m2) has been established as a valid predictor of fatness, which is based on fat mass and height in children and adolescents [34,35,36,37]. However, since previous research has indicated that the FMI does not predict adiposity, this research study focused on the prediction capacity of the FMI (fat mass/m3) as proposed by Burton [18]. The results of the MetS ROC analysis demonstrated that FMI showed the greatest AUC compared with the TMI for predicting MetS risk in all groups.
The thresholds established to detect a high risk of MetS in Colombian children and young people were as follows: (i) children cohort: FMI ≥ 2.59 fat mass/m3 (girls); FMI ≥ 1.98 fat mass/m3 (boys); (ii) adolescent cohort: FMI ≥ 3.12 fat mass/m3 (girls); FMI ≥ 1.46 fat mass/m3 (boys); (iii) young adults: FMI ≥ 3.27 kg/m3 (women); FMI ≥ 1.65 kg/m3 (men).
These results are consistent with those of a previous study that compared the BMI and BF% of a population of 1698 Chinese adults and their accuracy in predicting MetS [21]. Their conclusion was that FMI (fat mass/(height)2) was the best screening tool for the prediction of MetS [21]. Nevertheless, differences in ethnicity, genetic susceptibility, lifestyle, and age range of the two study populations signify that the data are not directly comparable [38]. Thus, there is a growing interest in proposing cut-off points for body fat levels for the early detection of CVD risk. Future longitudinal studies should be conducted that will lead to a deeper understanding of the role of different levels of adiposity in regard to CVD risk stratification in children, adolescents, and young adults.
Furthermore, it has been found that in adolescents, TMI estimates body fat levels more accurately than BMI [14]. As shown in our study, in addition to being an effective obesity screening tool, TMI can be used to detect MetS among Colombian children and young adults. The thresholds established to detect a high risk of MetS in Colombian children and young people were as follows: (i) children cohort: TMI ≥ 12.13 kg/m3 (girls); TMI ≥ 12.10 kg/m3 (boys); (ii) adolescent cohort: TMI ≥ 12.48 kg/m3 (girls); TMI ≥ 11.19 kg/m3 (boys); (iii) young adult cohort: TMI ≥ 13.21 kg/m3 (women); TMI ≥ 12.19 kg/m3 (men). These results indicate that TMI is a useful indicator for predicting MetS at early ages. However, the differences observed between boys and adolescent boys justify the need to consider sex- and age-specific thresholds as such thresholds are considered in BMI assessments for children and adolescents [39].
Interestingly, among the study cohorts (children, adolescents, and young adults), the greatest AUCs were observed for TMIs of 0.854 for females and 0.814 for males and for FMIs of 0.882 for females and 0.848 for males in the cohort of young adults. This highlights the fact that TMI and FMI are valuable tools that can be used to combat MetS risk in early adulthood. In this same line, previous studies have reported that age- and sex-associated variations of body fat stem from development during growth [40]. According to Rolland-Cachera [41], a developing maturational process and, thus, the absence of a well-defined fat pattern among the children and adolescents in this study could explain the differences in predictive capacity observed between cohorts.
The present study has certain limitations. Firstly, our analysis was of a cross-sectional sample and did not have a longitudinal design. Because the children and adolescents were still growing, the percentiles in height or BF% could vary markedly. Secondly, our study was limited to a well characterized sample of Colombian children and young people. Given that children and adolescents of different races or ethnic groups show important differences in body composition, which mainly occurs in body fat, our reported cut-off values may not be extrapolated to other populations. Nevertheless, this fact does not limit the interesting results obtained. Future population-based studies conducted in other ethnic or racial groups are required to provide TMI and FMI cut-off values for the detection of MetS during childhood and early adulthood. Finally, due to its cross-sectional design, it is not possible to examine whether TMI and FMI are predictors for future MetS. Therefore, further studies investigating their power longitudinally are required.
It should be highlighted that the main strength of our research is that it is the first study to investigate the predictive power of FMI and TMI in relation to MetS in a large population of 4673 subjects and to provide cut-off values for the identification of MetS by FMI and TMI in a population of children and young people. Accordingly, this research has relevant clinical implications in that it permits the early detection of increased cardiometabolic risk at young ages.
5. Conclusions
In summary, FMI and TMI were found to have a moderate discriminatory power to detect MetS in a Colombian population of children and young adults. For the first time, reference cut-off values were provided for the detection of MetS by FMI and TMI in children and young people. The reported thresholds could be used in clinical practice to identify Colombian children and youth at high risk of MetS. Furthermore, these results justify the need to incorporate FMI and TMI in daily clinical practice as indicators for predicting MetS and avoiding its associated complications. However, since most clinicians and researchers globally utilize BMI, there would have to be an overall proven increase in usefulness of TMI and FMI in order to convince the healthcare community to adopt these alternate measures. Future studies of different races and ethnic groups are needed to obtain reference values applicable to populations in countries throughout the world.
Acknowledgments
This study was part of the project entitled “Body Adiposity Index and Biomarkers of Endothelial and Cardiovascular Health in Adults”, which was funded by the Centre for Studies on Measurement of Physical Activity, School of Medicine and Health Sciences, Universidad del Rosario (Code N° FIUR DN-BG001), and the “FUPRECOL Study” was possible given the financial support provided by the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología “Francisco José de Caldas” COLCIENCIAS (Contract Number 671-2014 Code 122265743978). The funder had no role in the study design, data collection, data analysis and interpretation, the preparation of the manuscript, or the decision to publish.
Abbreviations
The following abbreviations are used in this manuscript:
| BF | body fat |
| BIA | bioelectrical impedance analysis |
| BMI | body mass index |
| CI | confidence interval |
| CVD | cardiovascular disease |
| FUPRECOL | in Spanish: Association between Muscular Strength and Metabolic Risk Factors in Colombia |
| FMI | fat mass index |
| HDL-C | high-density lipoprotein cholesterol |
| IDF | International Diabetes Federation |
| LDL-C | low-density lipoprotein cholesterol |
| MetS | metabolic syndrome |
| SD | standard deviation |
| TMI | tri-ponderal mass index |
| WC | waist circumference |
Author Contributions
Robinson Ramírez-Vélez, Jorge Enrique Correa-Bautista, Hugo Alejandro Carrillo, and Antonio García-Hermoso conceived and designed the study; María Correa-Rodríguez, Emilio González-Jiménez, and Jacqueline Schmidt-RioValle contributed to the data analysis; Robinson Ramírez-Vélez and Katherine González-Ruíz analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bonora E., Targher G., Formentini G., Calcaterra F., Lombardi S., Marini F., Zenari L., Saggiani F., Poli M., Perbellini S., et al. The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet. Med. 2004;21:52–58. doi: 10.1046/j.1464-5491.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 2.Despres J.-P., Lemieux I., Bergeron J., Pibarot P., Mathieu P., Larose E., Rodés-Cabau J., Bertrand O.F., Poirier P. Abdominal Obesity and the Metabolic Syndrome: Contribution to Global Cardiometabolic Risk. Arterioscler. Thromb. Vasc. Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 3.Zambon S., Zanoni S., Romanato G., Corti M.C., Noale M., Sartori L., Musacchio E., Baggio G., Crepaldi G., Manza E. Metabolic syndrome and all-cause and cardiovascular mortality in an Italian elderly population: The Progetto Veneto Anziani (Pro.V.A.) Study. Diabetes Care. 2009;32:153–159. doi: 10.2337/dc08-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palaniappan L., Carnethon M.R., Wang Y., Hanley A.J., Fortmann S.P., Haffner S.M., Wagenknecht L. Insulin Resistance Atherosclerosis Study. Predictors of the incident metabolic syndrome in adults: The Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:788–793. doi: 10.2337/diacare.27.3.788. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G., Bombelli M., Corrao G., Facchetti R., Madotto F., Giannattasio C., Trevano F.Q., Grassi G., Zanchetti A., Sega R. Metabolic Syndrome in the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) Study: Daily Life Blood Pressure, Cardiac Damage, and Prognosis. Hypertension. 2007;49:40–47. doi: 10.1161/01.HYP.0000251933.22091.24. [DOI] [PubMed] [Google Scholar]
- 6.Moreira G.C., Cipullo J.P., Ciorlia L.A., Cesarino C.B., Vilela-Martin J.F. Prevalence of Metabolic Syndrome: Association with Risk Factors and Cardiovascular Complications in an Urban Population. PLoS ONE. 2014;9:e105056. doi: 10.1371/journal.pone.0105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González S.A., Castiblanco M.A., Arias-Gómez L.F., Martinez-Ospina A., Cohen D.D., Holguin G.A., Almanza A., Lemos D.M., Correa-Bautista J.E., Escobar I.D., et al. Results From Colombia’s 2016 Report Card on Physical Activity for Children and Youth. J. Phys. Act. Health. 2016;13:S129–S136. doi: 10.1123/jpah.2016-0369. [DOI] [PubMed] [Google Scholar]
- 8.Ramírez-Vélez R., Correa-Bautista J.E., González-Ruíz K., Vivas A., Triana-Reina H.R., Martínez-Torres J., Prieto-Benavides D.H., Carrillo H.A., Ramos-Sepúlveda J.A., Villa-González E., et al. Body Adiposity Index Performance in Estimating Body Fat Percentage in Colombian College Students: Findings from the FUPRECOL-Adults Study. Nutrients. 2017;9:40. doi: 10.3390/nu9010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallare J.T., Karabell A.H., Velasquez-Mieyer P., Stender S.R.S., Christensen M.L. Current and Future Treatment of Metabolic Syndrome and Type 2 Diabetes in Children and Adolescents. Diabetes Spectr. 2005;18:220–228. doi: 10.2337/diaspect.18.4.220. [DOI] [Google Scholar]
- 10.Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Ramírez-Vélez R., Anzola A., Martinez-Torres J., Vivas A., Tordecilla-Sanders A., Prieto-Benavides D., Izquierdo M., Correa-Bautista J.E., Garcia-Hermoso A. Metabolic Syndrome and Associated Factors in a Population-Based Sample of Schoolchildren in Colombia: The FUPRECOL Study. Metab. Syndr. Relat. Disord. 2016;14:455–462. doi: 10.1089/met.2016.0058. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Torres J., Correa-Bautista J., González-Ruíz K., Vivas A., Triana-Reina H.R., Prieto-Benavidez D.H., Carrillo H.A., Ramos-Sepúlveda J.A., Villa-González E., García-Hermoso A., et al. A Cross-Sectional Study of the Prevalence of Metabolic Syndrome and Associated Factors in Colombian Collegiate Students: The FUPRECOL-Adults Study. Int. J. Environ. Res. Public Health. 2017;14:233. doi: 10.3390/ijerph14030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole T. Anthr Assess Nutrition Status. Wiley-Liss; New York, NY, USA: 1991. Weight-stature indices to measure underweight, overweight and obesity; pp. 83–111. [Google Scholar]
- 14.Peterson C.M., Su H., Thomas D.M., Golnabi A.H., Pietrobelli A., Heymsfield S.B. Tri-Ponderal Mass Index vs Body Mass Index in Estimating Body Fat During Adolescence. JAMA Pediatr. 2017;171:629–636. doi: 10.1001/jamapediatrics.2017.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole T.J. Weight/heightp compared to weight/height2 for assessing adiposity in childhood: Influence of age and bone age on p during puberty. Ann. Hum. Biol. 1986;13:433–451. doi: 10.1080/03014468600008621. [DOI] [PubMed] [Google Scholar]
- 16.Ramírez-Vélez R., Correa-Bautista J., Sanders-Tordecilla A., Ojeda-Pardo M.L., Cobo-Mejía E.A., Castellanos-Vega R.D.P., García-Hermoso A., González-Jiménez E., Schmidt-RioValle J., González-Ruíz K., et al. Percentage of Body Fat and Fat Mass Index as a Screening Tool for Metabolic Syndrome Prediction in Colombian University Students. Nutrients. 2017;9:1009. doi: 10.3390/nu9091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton R.F. Why is the body mass index calculated as mass/height2, not as mass/height3? Ann. Hum. Biol. 2007;34:656–663. doi: 10.1080/03014460701732962. [DOI] [PubMed] [Google Scholar]
- 18.Burton R.F. Measures of adiposity: The inappropriate use of the fat mass index. Int. J. Obes. 2010;34:213. doi: 10.1038/ijo.2009.202. [DOI] [PubMed] [Google Scholar]
- 19.VanItallie T.B., Yang M.U., Heymsfield S.B., Funk R.C., Boileau R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 20.Johnson L., Mander A.P., Jones L.R., Emmett P.M., Jebb S.A. A prospective analysis of dietary energy density at age 5 and 7 years and fatness at 9 years among UK children. Int. J. Obes. 2008;32:586–593. doi: 10.1038/sj.ijo.0803746. [DOI] [PubMed] [Google Scholar]
- 21.Liu P., Ma F., Lou H., Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. 2013;13:629. doi: 10.1186/1471-2458-13-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramírez-Vélez R., García-Hermoso A., Agostinis-Sobrinho C., Mota J., Santos R., Correa-Bautista J.E., Peña-Guzmán C.A., Domínguez-Sánchez M.A., Schmidt-RioValle J., González-Jiménez E. Pubertal Stage, Body Mass Index, and Cardiometabolic Risk in Children and Adolescents in Bogotá, Colombia: The Cross-Sectional Fuprecol Study. Nutrients. 2017;9:644. doi: 10.3390/nu9070644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramírez-Vélez R., Correa-Bautista J.E., Lobelo F., Izquierdo M., Alonso-Martínez A., Rodríguez-Rodríguez F., Cristi-Montero C. High muscular fitness has a powerful protective cardiometabolic effect in adults: Influence of weight status. BMC Public Health. 2016;16:1012. doi: 10.1186/s12889-016-3678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Onis M., Onyango A.W., Borghi E., Siyam A., Nishida C., Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marfell-Jones M., Olds T., Stewart A. International standards for anthropometric assessment. Int. Soc. Adv. Kinanthropometry. 2011 doi: 10.1152/japplphysiol.00187.2013. [DOI] [Google Scholar]
- 26.Rodríguez-Rodríguez F., Cristi-Montero C., González-Ruíz K., Correa-Bautista J.E., Ramírez-Vélez R. Bioelectrical Impedance Vector Analysis and Muscular Fitness in Healthy Men. Nutrients. 2016;8:407. doi: 10.3390/nu8070407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramírez-Vélez R., Correa-Bautista J., Martínez-Torres J., González-Ruíz K., González-Jiménez E., Schmidt-RioValle J., Garcia-Hermoso A. Performance of Two Bioelectrical Impedance Analyses in the Diagnosis of Overweight and Obesity in Children and Adolescents: The FUPRECOL Study. Nutrients. 2016;8:575. doi: 10.3390/nu8100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Ferranti S.D., Gauvreau K., Ludwig D.S., Neufeld E.J., Newburger J.W., Rifai N. Prevalence of the Metabolic Syndrome in American Adolescents: Findings From the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 29.Magge S.N., Goodman E., Armstrong S.C., Committee on Nutrition. Section On Endocrinology. Section on Obesity The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics. 2017:e20171603. doi: 10.1542/peds.2017-1603. [DOI] [PubMed] [Google Scholar]
- 30.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 31.Bewick V., Cheek L., Ball J. Statistics review 13: Receiver operating characteristic curves. Crit. Care. 2004;8:508–512. doi: 10.1186/cc3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fluss R., Faraggi D., Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 33.Ruano Nieto C.I., Melo Pérez J.D., Mogrovejo Freire L., De Paula Morales K.R., Espinoza Romero C.V. Prevalence of metabolic syndrome and associated risk factors in ecuadorian university students. Nutr. Hosp. 2015;31:1574–1581. doi: 10.3305/nh.2015.31.4.8371. [DOI] [PubMed] [Google Scholar]
- 34.Eissa M.A., Dai S., Mihalopoulos N.L., Day R.S., Harrist R.B., Labarthe D.R. Trajectories of fat mass index, fat free-mass index, and waist circumference in children: Project HeartBeat! Am. J. Prev. Med. 2009;37:S34–S39. doi: 10.1016/j.amepre.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maynard L.M., Wisemandle W., Roche A.F., Chumlea W.C., Guo S.S., Siervogel R.M. Childhood body composition in relation to body mass index. Pediatrics. 2001;107:344–350. doi: 10.1542/peds.107.2.344. [DOI] [PubMed] [Google Scholar]
- 36.Freedman D.S., Wang J., Maynard L.M., Thornton J.C., Mei Z., Pierson R.N., Dietz W.H., Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int. J. Obes. 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 37.Nakao T., Komiya S. Reference norms for a fat-free mass index and fat mass index in the Japanese child population. J. Physiol. Anthropol. Appl. Hum. Sci. 2003;22:293–298. doi: 10.2114/jpa.22.293. [DOI] [PubMed] [Google Scholar]
- 38.Gishti O., Kruithof C.J., Felix J.F., Raat H., Hofman A., Duijts L., Gaillard R., Jaddoe V.W. Ethnic disparities in general and abdominal adiposity at school age: A multiethnic population-based cohort study in the Netherlands. Ann. Nutr. Metab. 2014;64:208–217. doi: 10.1159/000365022. [DOI] [PubMed] [Google Scholar]
- 39.Lee J., Kubik M.Y. Child’s Weight Status and Parent’s Response to a School-Based Body Mass Index Screening and Parent Notification Program. J. Sch. Nurs. 2015;31:300–305. doi: 10.1177/1059840514556181. [DOI] [PubMed] [Google Scholar]
- 40.Laurson K.R., Eisenmann J.C., Welk G.J. Body Fat Percentile Curves for U.S. Children and Adolescents. Am. J. Prev. Med. 2011;41:S87–S92. doi: 10.1016/j.amepre.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 41.Rolland-Cachera M.F. Body composition during adolescence: Methods, limitations and determinants. Horm. Res. 1993;39:25–40. doi: 10.1159/000182782. [DOI] [PubMed] [Google Scholar]