Abstract
Vacuolar ion channels in guard cells play important roles during stomatal movement and are regulated by many factors including Ca2+, calmodulin, protein kinases, and phosphatases. We report that physiological cytosolic and luminal Mg2+ levels strongly regulate vacuolar ion channels in fava bean (Vicia faba) guard cells. Luminal Mg2+ inhibited fast vacuolar (FV) currents with a Ki of approximately 0.23 mm in a voltage-dependent manner at positive potentials on the cytoplasmic side. Cytosolic Mg2+ at 1 mm also inhibited FV currents. Furthermore, in the absence of cytosolic Mg2+, cytosolic Ca2+ at less than 10 μm did not activate slow vacuolar (SV) currents. However, when cytosolic Mg2+ was present, submicromolar concentrations of cytosolic Ca2+ activated SV currents with a Kd of approximately 227 nm, suggesting a synergistic Mg2+-Ca2+ effect. The activation potential of SV currents was shifted toward physiological potentials in the presence of cytosolic Mg2+ concentrations. The direction of SV currents could also be changed from outward to both outward and inward currents. Our data predict a model for SV channel regulation, including a cytosolic binding site for Ca2+ with an affinity in the submicromolar range and a cytosolic low-affinity Mg2+-Ca2+ binding site. SV channels are predicted to contain a third binding site on the vacuolar luminal side, which binds Ca2+ and is inhibitory. In conclusion, cytosolic Mg2+ sensitizes SV channels to physiological cytosolic Ca2+ elevations. Furthermore, we propose that cytosolic and vacuolar Mg2+ concentrations ensure that FV channels do not function as a continuous vacuolar K+ leak, which would prohibit stomatal opening.
Mg2+ is an abundant cytoplasmic cation in higher plants (Epstein, 1965), with concentrations of 2 to 10 mm in leaf cells (Leigh and Wyn Jones, 1986). Mg2+ ions exist as free cations and are also sequestered in internal organelles, bound by cytosolic proteins, or complexed with small organic molecules. Many enzymes require or are strongly activated by Mg2+, for example, plasma membrane ATPases, protein kinases, type-2C phosphatases, glutathione synthase, and RuBP carboxylase (Marschner, 1995; Leube et al., 1998). The important role of Mg2+ as a regulator of various ion channels is well established in animal cells (Agus and Morad, 1991; Flatman, 1991; Murphy et al., 1991; Hille, 1992; Chuang et al., 1997; Kerschbaum and Cahalan, 1999). Matsuda et al. (1987) and Vandenberg (1987) demonstrated the direct blockage of inward rectifier K+ channels in animal cells by Mg2+; however, little is known about how Mg2+ affects ion channel activities in plant cells.
Two types of ion channels have been characterized in most plant vacuolar membranes studied to date. These are the Ca2+-permeable, cation-selective slow vacuolar (SV) channels and the cation-selective fast vacuolar (FV) channels (Hedrich and Neher, 1987; Weiser et al., 1991; Bethke and Jones, 1994; Ward and Schroeder, 1994; Allen and Sanders, 1996). SV channels are activated by cytosolic Ca2+, whereas FV channels are inhibited by elevations in cytosolic Ca2+ (Allen and Sanders, 1996).
FV channels show instantaneous currents in response to voltage pulses (Hedrich and Neher, 1987; Allen and Sanders, 1996; Tikhonova et al., 1997). FV channels are cation-selective (Allen and Sanders, 1996; Tikhonova et al., 1997). The functions of FV channels remain unknown (Allen and Sanders, 1997), although proposals of functions have been made on the basis of their properties, including mediating K+ release from guard cell vacuoles during stomatal closing (Allen and Sanders, 1996). However, at physiological resting cytosolic Ca2+ concentrations of 0.1 to 0.2 μm, FV current activities can be very high (Hedrich and Neher, 1987; Allen and Sanders, 1996; Tikhonova et al., 1997). This raised the possibility that FV channels need to be further down-regulated by factors other than Ca2+ in order to maintain vacuolar membrane ion gradients. Recently, physiological polyamine levels have been shown to partially down-regulate FV channels (Brüggemann et al., 1998; Dobrovinskaya et al., 1999).
Voltage- and time-dependent SV channels, as well as vacuolar K+ selective (VK) channels, are activated by cytosolic Ca2+ (Hedrich and Neher, 1987; Bethke and Jones, 1994; Ward and Schroeder, 1994; Allen and Sanders, 1996; Pottosin et al., 1997). In addition, SV channels are regulated by ATP, calmodulin, protein kinases, and phosphatases (Weiser et al., 1991; Bethke and Jones, 1994, 1997; Allen and Sanders, 1995).
Although a significant anion permeability of SV channels had been proposed (Coyaud et al., 1987; Hedrich and Kurkdjian, 1988; Schulz-Lessdorf and Hedrich, 1995), detailed studies unequivocally demonstrated the cation selectivity of SV channels with negligible anion permeability (Colombo et al., 1988; Ward and Schroeder, 1994; Ward et al., 1995; Allen and Sanders, 1996; Pottosin et al., 1997). Studies showed substantial Ca2+ and Mg2+ permeabilities of SV currents (Ward and Schroeder, 1994; Allen and Sanders, 1996). Therefore, SV channels are cation selective with poor selectivity among monovalent cations (K+, Na+, and Cs+) and divalent cations (Ca2+, Mg2+, and Ba2+). The finding that Ca2+-activated SV channels are Ca2+ permeable has led to the suggestion that these channels may provide an important mechanism not only for K+ transport but also for Ca2+-induced Ca2+ release (Ward and Schroeder, 1994). A recent study showed that conditions favoring Ca2+ release from vacuoles decrease the SV channel open probability, leading to a counter-hypothesis in which SV channels cannot mediate Ca2+-induced Ca2+ release from vacuoles (Pottosin et al., 1997).
At physiological cytosolic Ca2+ concentrations, SV channel activities are generally negligible in many plants (Hedrich and Neher, 1987; Ward and Schroeder, 1994; Barkla and Pantoja, 1996; Allen and Sanders, 1997; Allen et al., 1998). Moreover, the activation potentials of SV channels lie positive of physiological vacuolar membrane potentials of 0 to −40 mV (Hedrich and Neher, 1987; Sze et al., 1992; Bethke and Jones, 1994; Ward and Schroeder, 1994; Allen and Sanders, 1996; Pottosin et al., 1997; Allen et al., 1998). A study on fava bean (Vicia faba) guard cell vacuoles led to the suggestion that cytosolic Mg2+ activates SV channels in the absence of cytosolic Ca2+ (Allen and Sanders, 1996). However, a more recent study on barley mesophyll vacuoles suggested that Mg2+ does not activate SV channels (Pottosin et al., 1997).
The findings that physiological cytosolic Ca2+ concentrations do not activate SV channels and over-stimulate FV channels have led to difficulties in predicting their functions in vivo. In the present study, we demonstrate that at physiological concentrations, Mg2+ down-regulates vacuolar membrane FV channels in fava bean guard cells, which may provide an efficient down-regulation mechanism of FV channels in vivo. Interestingly, cytosolic Mg2+ sensitized SV channels to physiological concentrations of cytosolic Ca2+, and data presented here clarify the controversy of Mg2+ activation of SV channels raised previously.
MATERIALS AND METHODS
Isolation of Fava Bean Guard Cell Vacuoles
Fava bean (Vicia faba) plants were grown in a controlled environment growth chamber (model E15, Conviron, Asheville, NC) with 16-h light/8-h dark cycle. Guard cell protoplasts were isolated from 3- to 4-week-old plants by enzymatic digestion of leaf epidermal strips, as previously described (Kruse et al., 1989; Ward and Schroeder, 1994). Vacuoles were released from guard cell protoplasts by osmotic shock and purified using a Ficoll density gradient (Ward and Schroeder, 1994).
Patch Clamp and Data Acquisition
Patch-clamp pipettes were prepared from soft glass capillaries (Kimax 51, Kimble, Toledo, OH), and pulled on a multi-stage programmable puller. Giga-Ω seals between electrode and the vacuolar membrane (>15 GΩ) were obtained by gentle suction. The patch-clamp technique was applied to isolated guard cell vacuoles as previously described (Pei et al., 1996). The whole-vacuole configuration, analogous to the whole-cell configuration (Hamill et al., 1981), was attained by applying high-voltage pulses (usually ±500 mV, 25 ms for each) and slight suction to the interior of the pipette (Pei et al., 1996).
Vacuoles were voltage clamped using an amplifier (Axopatch 200, Axon Instruments, Foster City, CA). All membrane potentials are specified as the potential on the cytosolic side relative to the vacuolar side (Bertl et al., 1992). Data were analyzed using AXOGRAPH software (3.5, Axon Instruments). Statistical analyses were performed using EXCEL (5.0, Microsoft, Redmond, WA). Data are the means ± se. In Figure 1D, the average percentage of inhibition of SV currents at +100 mV by vacuolar Mg2+ is fitted to a Hill equation:
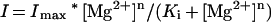 |
where I is the degree of current inhibition, Imax is the maximum current inhibition, [Mg2+] is the Mg2+ concentration on the vacuolar side, n is the Hill coefficient, and Ki is the inhibition constant.
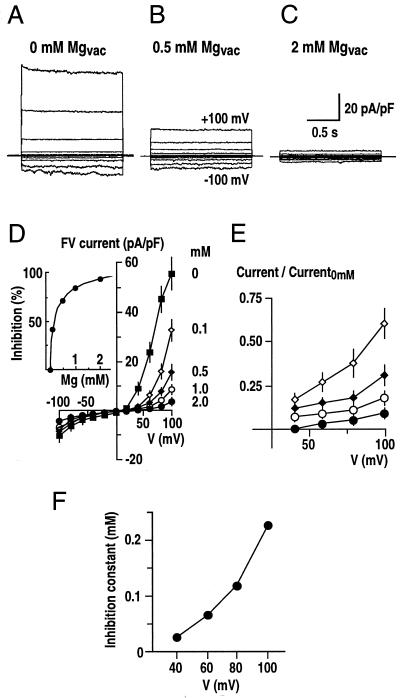
Figure 1.
Fast-activating vacuolar currents inhibited by vacuolar Mg2+ in fava bean guard cells. A through C, Three representative whole-vacuole recordings are shown at Mg2+ concentrations of 0 mm (A), 0.5 mm (B), and 2 mm (C) in the pipette (luminal) solution. Membrane potential was stepped from −100 to +100 mV in 20-mV increments from a holding potential of 0 mV. In all traces, the vacuolar ion currents have been normalized to the whole-vacuolar capacitance (pA/pF). The solutions for FV current measurement contained 100 mm KCl, 4 mm EGTA, 10 mm HEPES-Tris, pH 7.5, in the bathing medium (cytosolic side), and 100 mm KCl, 5 mm CaCl2, 5 mm MES-Tris, pH 5.5, with varied MgCl2 of 0 to 2 mm in the pipette (vacuolar side). D, Average current-voltage relationships from experiments performed as in A through C at Mg2+ concentrations of 0, 0.1, 0.5, 1.0, and 2.0 mm in the pipette solution. FV currents were measured as the instantaneous component of whole-vacuole currents (n = 3–5 vacuoles per Mg2+ concentration; whole-vacuole capacitance = 9.7 ± 3.4 pF). Inset, Average percentage of inhibition of SV currents at +100 mV is plotted as a function of the concentrations of vacuolar Mg2+ and fitted to a Hill equation. E, Voltage dependence of vacuolar Mg2+ block. Average whole-vacuole currents in the presence of vacuolar Mg2+ as in D were normalized to currents in the absence of Mg2+ (Current0 mm). Symbols are as in D. F, Mg2+ inhibition constant (Ki) plotted as a function of the applied membrane potentials. Inhibition constants at +40 to +100 mV were obtained using the Hill equation (see “Materials and Methods”).
Experimental Solutions
The standard solutions used in patch-clamp experiments were composed of 200 mm KCl and 20 mm HEPES-Tris, pH 8.0, in the bathing medium (cytosolic side), and 20 mm KCl, 2 mm EGTA, and 5 mm HEPES-Tris, pH 7.0, in the pipette (vacuolar side) unless otherwise noted. Free cytosolic Ca2+ concentrations ranging from 10 nm to 1 μm were buffered with EGTA. Total CaCl2 concentrations in bath solutions (Fig. 5) were changed to give the indicated cytosolic free Ca2+ of 10 nm (0.8 mm total CaCl2 concentration), 50 nm (2 mm), 150 nm (3 mm), and 1 μm (3.8 mm), pH 7.8, with 4 mm EGTA in all solutions. Free Ca2+ concentrations were calculated after accounting for 10 mm MgCl2, ionic strength, and temperature (24°C) with CALCV22 software (Foehr et al., 1993). For 10 and 50 μm Ca2+ in Figure 5, 10 and 50 μm CaCl2 were added to the bath solution without the addition of the Ca2+ buffer EGTA, as these concentrations lie outside the range of effective EGTA-buffering capacity. The bath solution was exchanged either by bath perfusion using a peristaltic pump (Rainin, Woburn, MA) or by a local perfusion pipette. Osmolalities of all solutions were adjusted to 600 mmol kg−1 by the addition of d-sorbitol.
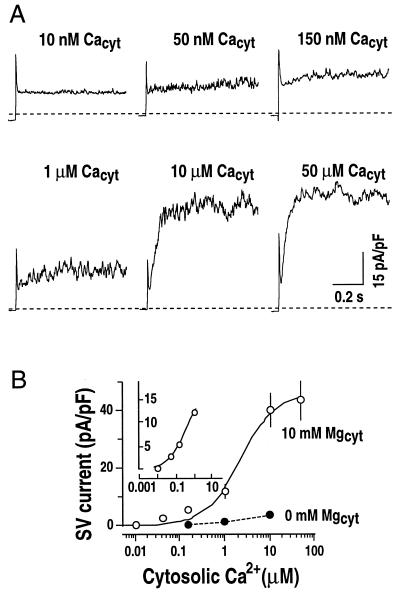
Figure 5.
Cytosolic Mg2+ sensitizes SV channels to cytosolic Ca2+. A, Representative whole-vacuole currents recorded at different cytosolic Ca2+ concentrations in two separate vacuoles. In one vacuole, cytosolic Ca2+ concentrations were changed from 10 nm to 1 μm by either local or bath perfusion (vacuolar capacitance = 3.5 pF). In another vacuole, 10 μm Ca2+ in the bath solution was replaced by 50 μm Ca2+ (vacuolar capacitance = 4.2 pF). Only current traces at +100 mV are shown. Dashed lines show zero current levels. Pipette solution contained 20 mm KCl, 2 mm EGTA, and 5 mm HEPES-Tris, pH 7. Bath solution contained 200 mm KCl, 10 mm MgCl2, and 20 mm HEPES-Tris, pH 8.0, with varying free Ca2+ concentrations of 0, 10 nm, 50 nm, 150 nm, 1 μm, 10 μm, and 50 μm (see “Materials and Methods” for details). B, Effect of cytosolic Mg2+ on cytosolic Ca2+ activation of SV currents at +100 mV as performed in A. In control experiments, SV currents were recorded at 0 mm Mg2+ in bath solutions (●). Values are from three to eight vacuoles (capacitance = 4.7 ± 1.2 pF). A Hill curve is fitted to the data for the SV currents activated by Ca2+ at 10 mm cytosolic Mg2+. Data obtained at 10 nm to 1 μm cytosolic Ca2+ are shown in the inset (Kd approximately 227 nm).
RESULTS
Inhibition of Vacuolar FV Channels by Luminal Mg2+
At zero cytosolic Ca2+, vacuolar currents were almost entirely instantaneous and were larger at positive potentials (on the cytoplasmic side of the membrane) compared with negative potentials (Fig. 1A). The activation of previously described Ca2+-activated VK channels (Ward and Schroeder, 1994) was avoided by buffering cytosolic Ca2+ to nominally zero. The steady-state current-voltage characteristics were similar to FV channel-mediated currents previously described in beet root vacuoles (Hedrich and Neher, 1987), barley mesophyll vacuoles (Tikhonova et al., 1997), and fava bean guard cell vacuoles (Allen and Sanders, 1996; Allen et al., 1998). The instantaneous FV currents were carried by monovalent cations including K+ and Cs+ (data not shown) as shown for FV currents (Allen and Sanders, 1996; Tikhonova et al., 1997).
Whole-vacuolar currents were analyzed at 0 to 2 mm vacuolar Mg2+ concentrations (Fig. 1). FV current amplitudes were reduced by increasing the vacuolar Mg2+ concentration from 0 (Fig. 1A) to 0.5 mm (Fig. 1B) or 2 mm (Fig. 1C). FV currents measured at five different vacuolar Mg2+ concentrations confirmed the strong down-regulation of FV currents by vacuolar Mg2+ (Fig. 1D). The average effect of vacuolar Mg2+ shows a 14.3- ± 2.1-fold decrease of FV currents at +100 mV by increasing vacuolar Mg2+ from 0 to 2 mm (Fig. 1D). FV currents at negative potentials were also reduced (2.4- ± 0.4-fold). A Hill curve could be fitted to the currents at +100 mV showing a Ki of approximately 0.23 mm and a Hill coefficient of 0.67 (Fig. 1D, inset), indicating that FV current amplitudes are inhibited by vacuolar Mg2+ within the physiological range (Yazaki et al., 1988). The Hill coefficient of 0.67 is consistent with one Mg2+ binding site per FV channel.
Whole-vacuole currents measured at different vacuolar Mg2+ concentrations were normalized to the control currents measured in the absence of Mg2+, and plotted as a function of applied voltage (Fig. 1E). Voltage-dependent block was observed at positive membrane potentials, with a continuous decrease in current by decreasing the voltage from +100 to +40 mV. Furthermore, the apparent Ki at different membrane potentials also shows the voltage dependence of Mg2+ block (Fig. 1F).
Inhibition of Vacuolar FV Channels by Cytosolic Mg2+
Experiments were designed to analyze whether, in addition to vacuolar Mg2+ (Fig. 1), cytosolic Mg2+ affects FV currents. A local perfusion system was used that allowed multiple changes of cytosolic solutions bathing single vacuoles. In the whole-vacuole configuration with zero Mg2+ on the cytosolic side, large instantaneous currents were recorded (Fig. 2A). When Mg2+ (1 mm) was applied to the cytosolic side, FV currents were decreased dramatically at both positive and negative vacuolar potentials (Fig. 2B). Activation of time-dependent SV currents in Figure 2B will be described later. Quantitative analysis showed a 3-fold inhibition of instantaneous currents by varying cytosolic Mg2+ from 0 to 1 mm at +100 mV (Fig. 2C). These results indicate that both luminal (Fig. 1) and cytosolic Mg2+ (Fig. 2) down-regulate FV currents at both positive and negative vacuolar potentials. As predicted, elimination of Mg2+ and Ca2+ from both the luminal and cytosolic sides gave rise to large FV currents (Fig. 2D), further illustrating the inhibitory effects of Mg2+ and Ca2+.
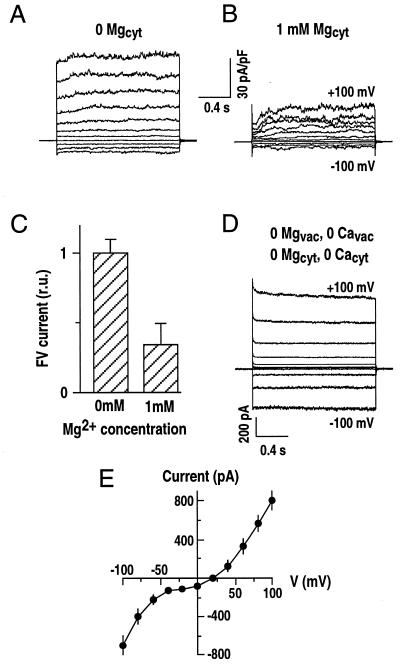
Figure 2.
Cytosolic Mg2+ inhibits FV current. A and B, Whole-vacuole currents recorded in the absence (0 Mgcyt; A) or presence of Mg2+ (1 mm Mgcyt; B) in bath (cytosolic) solutions of the same vacuole. The holding potential was −40 mV. The pipette solution contained 20 mm KCl, 2 mm EGTA, and 5 mm HEPES-Tris, and the bath solution contained 100 mm KCl, 20 mm HEPES-Tris, pH 8.0, with the addition of 10 μm CaCl2, in the absence or presence of 1 mm MgCl2. Whole-vacuole capacitance = 8.4 pF. C, Average FV currents at +100 mV as recorded in A and B. Currents recorded in the absence of cytosolic Mg2+ were normalized as 1 (64.2 ± 4.5 pA/pF; n = 11 vacuoles for each condition; whole-vacuole capacitance = 8.3 ± 1.2 pF). r.u., Relative unit. D, Representative whole-vacuole FV currents recorded in the absence of Mg2+ and Ca2+ on both cytosolic and vacuolar membrane sides (n > 15 vacuoles). The solution contained 200 mm KCl, 5 mm HEPES-Tris, pH 7.0, in the pipette and 50 mm KCl, 2 mm EGTA, and 20 mm HEPES-Tris, pH 8.0, in the bath. No Mg2+ or Ca2+ was added to solutions. E, Current-voltage relationships as recorded in D. Currents from five representative recordings are averaged and plotted as a function of the applied membrane potentials.
Does Cytosolic Mg2+ Activate SV Currents?
In fava bean guard cells, at high cytosolic Ca2+ concentrations SV currents are the major vacuolar conductance. However, the cytosolic Ca2+ concentration required for SV current activation is larger than known resting cytosolic Ca2+ levels and the upper limit of free cytosolic Ca2+ concentrations measured during Ca2+-dependent signal transduction (Ward and Schroeder, 1994; Bush, 1995; Allen and Sanders, 1996; McAinsh et al., 1997). This has contributed to difficulties in predicting the physiological roles of SV channels. We therefore designed experiments to determine whether the Ca2+ sensitivity of SV activation could be modified. At 10 μm cytosolic Ca2+, instantaneous currents were recorded in the absence of cytosolic Mg2+ (Fig. 3A).
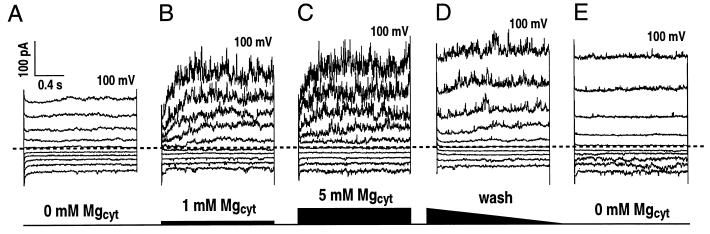
Figure 3.
Possible up-regulation of slowly activating vacuolar currents by cytosolic Mg2+ in fava bean guard cells. Whole-vacuole currents measured from one vacuole at different Mg2+ concentrations in the bath solution. The holding potential was −40 mV with an interval time between pulses of 1 s. Standard bath and pipette solutions (see “Materials and Methods”) were used with varying Mg2+ concentrations (0, 1, and 5 mm) in the bath. Note that 10 μm CaCl was added to the bath solution. A, Current recordings started in a bath solution containing no added Mg2+. B and C, Bath solutions containing 1 mm (B) and 5 mm Mg2+ (C) were subsequently added by local perfusion (see “Materials and Methods”). D and E, The vacuole was then perfused with a bath solution containing no added Mg2+. Similar experiments were repeated on eight vacuoles.
Cytosolic Mg2+ of 1 mm was applied by local perfusion in the continued presence of 10 μm Ca2+. Interestingly, time-dependent SV currents were increased dramatically at positive vacuolar potentials (Fig. 3B). The Mg2+ concentration was then further increased to 5 mm. SV currents were similar in magnitude to those at 1 mm cytosolic Mg2+ (Fig. 3C). Finally cytosolic Mg2+ was removed by slow bath perfusion, during which the time-dependent SV currents vanished (Fig. 3, D and E), while instantaneous currents increased (Fig. 3, D and E), also confirming the inhibitory effect of cytosolic Mg2+ on FV currents described in Figure 2. These data suggest that Mg2+ might up-regulate SV current as previously proposed (Allen and Sanders, 1996). However, in a recent study, no Mg2+ activation of SV currents was found in barley mesophyll vacuoles, and Mg2+ activation of SV channels described previously (Allen and Sanders, 1996) were concluded to be an artifact (Pottosin et al., 1997). To further examine these differences among previous reports, we investigated whether Mg2+ activation of SV currents depends on the presence of physiological levels of cytosolic Ca2+.
Mg2+ Sensitizes SV Currents to Cytosolic Ca2+
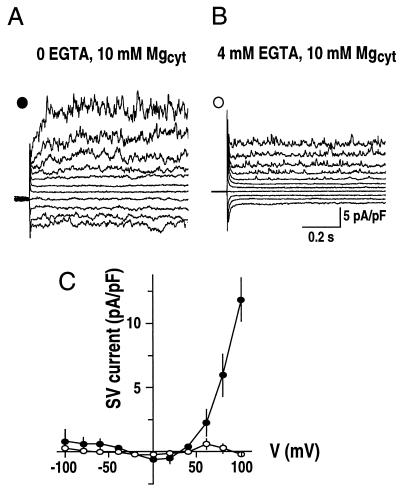
To test whether cytosolic Ca2+ is necessary for activation of SV currents by Mg2+ in fava bean guard cells, whole-vacuolar currents were measured at 10 mm cytosolic Mg2+ in the absence or presence of the Ca2+ buffer EGTA (4 mm) in the bath solution (Fig. 4). Small time-dependent SV currents were observed in the absence of EGTA (Fig. 4A). However, in the presence of EGTA, SV currents were reduced (Fig. 4B). Figure 4C (○) shows the dramatic reduction in time-dependent SV currents at positive potentials, when EGTA was added to the cytosolic side. These data (Figs. 3 and 4) indicate the possibility that cytosolic Mg2+ might modify the sensitivity of SV channels to cytosolic Ca2+.
Figure 4.
Cytosolic EGTA inhibits Mg2+ activation of SV currents. A and B, Whole-vacuole currents recorded in the absence (A) or presence (B) of 4 mm EGTA in the bath solutions in one vacuole. Standard pipette and bath solutions were used without or with the addition of 4 mm EGTA. C, Current-voltage relationships from experiments performed in the absence or presence of 4 mm EGTA as in A and B. SV currents were measured as time-dependent components of whole-vacuole currents. Symbols are as given in A and B (n = 8; whole-vacuole capacitance = 7.1 ± 2.6 pF).
To analyze quantitatively whether Mg2+ could shift the sensitivity of SV activation to physiological cytosolic Ca2+ concentrations and to determine cytosolic Ca2+ concentrations required for Mg2+ activation of SV currents, SV currents were measured over a range of cytosolic Ca2+ concentrations from 10 nm to 50 μm with a constant cytosolic Mg2+ concentration of 10 mm (Fig. 5). At 10 nm Ca2+, SV currents were not activated (Fig. 5A). Strikingly, when the Ca2+ concentration was subsequently increased to 50 nm, 150 nm, and up to 1 μm, SV currents measured in the same vacuole were gradually activated (Fig. 5). At 10 and 50 μm Ca2+, SV currents were close to saturation (Fig. 5). In contrast, in the absence of Mg2+ in the bath solution, physiological concentrations of Ca2+ could not activate SV currents (Fig. 5B, ●). A Hill curve could be fitted to the data for cytosolic Ca2+ concentrations from 10 nm to 1 μm, showing a Kd of approximately 227 nm for a Hill coefficient of 0.95 (Fig. 5B, inset). The Hill coefficient of approximately 1 indicates binding of one Ca2+ ion per SV channel. These data demonstrate that physiological concentrations of Ca2+ can activate SV currents, if Mg2+ is also present on the cytosolic side, showing a sensitization of the SV channel to Ca2+ by cytosolic Mg2+.
Differential Activation Time Course of SV Currents by Saturating Cytosolic Ca2+ or Mg2+
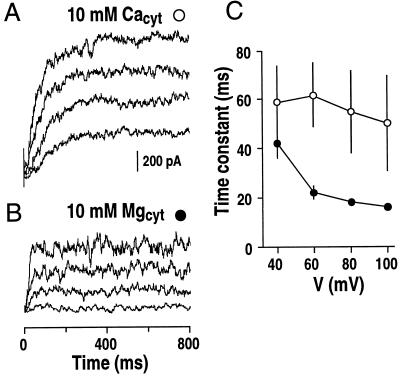
The data presented above suggested two ways to activate SV currents: first by high concentrations of cytosolic Ca2+ alone and second by combining cytosolic Mg2+ with low physiological concentrations of Ca2+. To test whether these two putative mechanisms of SV channel activation were kinetically distinguishable, experiments were designed using saturating Ca2+ (10 mm) in the absence of Mg2+; or using saturating Mg2+ (10 mm) in the presence of 10 μm cytosolic Ca2+. Under these two conditions, activation time courses for SV currents were different (Fig. 6, A and B). The time constants for SV current activation by Mg2+ in the presence of 10 μm Ca2+ were approximately three times more rapid than by Ca2+ alone (Fig. 6C), further supporting the hypothesis that there are two distinct mechanisms for the activation of SV channels (see “Discussion”).
Figure 6.
Effect of cytosolic Mg2+ on the activation time course of SV currents. A and B, SV currents recorded at saturated cytosolic 10 mm Ca2+ (A) are compared with SV currents at saturated cytosolic 10 mm Mg2+ (B). For Mg2+ activation of SV currents, 10 μm CaCl2 was added to the bath solution to saturate the proposed high-affinity Ca2+ binding site (see “Discussion”). Standard pipette and bath solutions (see “Materials and Methods”) were used with the addition of 10 mm CaCl2 in A and with the addition of 10 mm MgCl2 in B. C, Fitted time constants of the activation of SV currents plotted against the applied vacuolar membrane potentials (n = 3 vacuoles for each condition). Symbols are as in A and B.
Varying the luminal Mg2+ concentration had no effect on SV currents in fava bean guard cells (n = 6; data not shown), which was also demonstrated in barley mesophyll vacuoles (Pottosin et al., 1997), suggesting that SV channels are not regulated by luminal Mg2+.
Shifting SV Activation to Physiological Potentials and Modification of Outward-Rectifying Properties
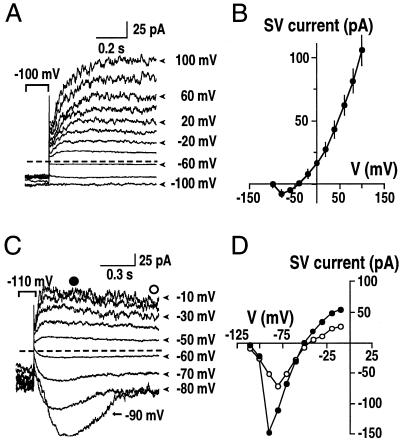
In previous studies, steady-state SV currents have only been activated at positive vacuolar potentials (Ward and Schroeder, 1994; Allen and Sanders, 1996; Barkla and Pantoja, 1996; Bethke and Jones, 1997; Pottosin et al., 1997), whereas at physiological vacuolar potentials (from 0 to −40 mV; Sze et al., 1992) SV currents are vanishingly small. Experiments were designed to test whether the activation potential of SV currents could be significantly shifted to negative vacuolar potentials within the physiological range. To maximize SV channel activation, we designed a pipette solution containing 20 mm KCl and 4 mm EGTA (to eliminate the inhibitory effect of Ca2+ from the luminal side on SV channels (Allen and Sanders, 1996; Pottosin et al., 1997), and a bath solution containing 200 mm KCl, 10 mm CaCl2, and 2 mm MgCl2. Under these conditions the activation potential was shifted to potentials of about −60 to −40 mV (Fig. 7, A and B). In some vacuoles (two out of nine), the activation potential was shifted dramatically to −90 mV. Both inward and outward currents were recorded, and the time-dependent activation of SV channels was not altered (Fig. 7, C and D). A similar modification of the rectification property of the SV current has also been found in barley mesophyll cells (Pottosin et al., 1997). These results suggest that SV currents can activate at physiological vacuolar potentials under specific ionic conditions, and that SV channels may carry both inward and outward currents in vivo depending on conditions. We used extreme experimental conditions to show that the activation potential of SV channels could be strongly shifted (Fig. 7). The variation in activation potential (Fig. 7) suggests that additional unknown factors exist that can greatly shift the activation potential of SV channels.
Figure 7.
Shifting SV activation to physiological vacuolar potentials and changing rectification property. A and B, Whole-vacuolar SV currents (A) and current-voltage relationship (B). The pipette solution contained 20 mm KCl, 2 mm EGTA, and 5 mm HEPES-Tris, pH 7.0. The bath contained 200 mm KCl, 10 mm CaCl2, 2 mm MgCl2, and 20 HEPES-Tris, pH 8.0. Similar currents were recorded in seven of nine vacuoles. C, Under the same conditions as in A, time-dependent inward SV currents were recorded occasionally (n = 2 of 9 vacuoles) at negative membrane potentials (C). D, Current-voltage relationship of vacuoles showing bi-directional SV currents. This behavior was observed in two of nine vacuoles recorded under these conditions. As symbolized in C, ○ and ● show the time-dependent peak and steady-state amplitudes of SV currents, respectively.
DISCUSSION
In animal cells, Mg2+ blocks many cation channels, which includes Ca2+ channels, various inward-rectifier K+ channels, N-methyl-d-Asp receptor channels, ryanodine receptor-Ca2+ release channels, and Ca2+ release-activated Ca2+ channels (Nowak et al., 1984; Matsuda et al., 1987; Vandenberg, 1987; Agus and Morad, 1991; Hille, 1992; Laver et al., 1997; Kerschbaum and Cahalan, 1999). However, in plant cells, regulation of ion channels by Mg2+ has not yet been studied in detail. In guard cells, inward-rectifying K+ currents are not blocked by cytosolic Mg2+ (Schroeder, 1995), and the only ionic current shown to be activated by Mg2+ is a cation current in beet vacuoles, which has been proposed to be a shunt conductance for the vacuolar H+-ATPase (Davies and Sanders, 1995). In this study, we show that in fava bean guard cells, Mg2+ strongly regulates two major vacuolar currents: down-regulating the vacuolar FV currents from both the cytosolic and luminal sides (Figs. 1 and 2) and up-regulating vacuolar SV currents from the cytosolic side (Figs. 4 and 6). The regulation of vacuolar ion channels by Mg2+ may play an important role in guard cells, as ion transport processes across the vacuolar membrane are essential for stomatal movements (MacRobbie, 1981, 1998; Assmann, 1993; Ward et al., 1995; Allen and Sanders, 1997).
Inhibition of Guard Cell Vacuolar FV Currents by Both Cytosolic and Luminal Mg2+
Systematic studies of the effect of Ca2+ on FV channels in fava bean guard cell and barley mesophyll vacuoles have shown that cytosolic and vacuolar Ca2+ inhibits FV channels (Allen and Sanders, 1996; Tikhonova et al., 1997). Higher concentrations of Mg2+ are required to inhibit FV currents (Figs. 1 and 2). For half inhibition from the luminal side, Mg2+ concentrations of approximately 230 μm were required (Fig. 1). In a previous study of VK currents, 2 mm Mg2+ was used to exclude FV currents (Ward and Schroeder, 1994), while Mg2+-free conditions result in large FV currents (Allen and Sanders, 1996). These results suggest a divalent ion-binding/block site on the luminal side of FV channels.
In animal cells, both Mg2+ and spermine block inward-rectifier K+ channels and cause voltage-dependent inward rectification (Matsuda et al., 1987; Vandenberg, 1987; Hille, 1992; Fakler et al., 1995). In the case of NMDA receptors in neurons, Mg2+ and spermine share a regulatory site (Paoletti et al., 1995). Similarly, FV channels are inhibited by both Mg2+, as shown here, and spermine in barley vacuoles (Figs. 1 and 2; Brüggemann et al., 1998; Dobrovinskaya, et al., 1999). The inhibition of FV channels by vacuolar Mg2+ is voltage dependent (Fig. 1), whereas spermine inhibition is voltage independent (Brüggemann et al., 1998; Dobrovinskaya, et al., 1999), suggesting that Mg2+ and spermine may not share the same binding site or that the inhibitory mechanisms are different. Whether the inhibitory effects of Mg2+ and spermine are additive in FV channel regulation or if Mg2+ and spermine share an inhibitory site will require further investigation.
Mg2+ Sensitizes SV Channels to Physiological Cytosolic Ca2+ Levels and a Model for SV Activation with Two Binding Sites
SV currents in many plant cell types are activated at cytosolic Ca2+ concentrations (for example ≥100 μm), which are >100-fold higher than known resting levels (Ward and Schroeder, 1994; Barkla and Pantoja, 1996; Allen and Sanders, 1996, 1997). The high cytosolic Ca2+ levels required for SV channel activation have contributed to the difficulty in assigning a physiological function to the channels. Information on mechanisms that modify the Ca2+ sensitivity of SV channel activation could further our understanding of SV function in guard cells. Mg2+ activation of SV channels has been proposed in fava bean guard cells (Allen and Sanders, 1996). However, Pottosin et al. (1997) reported that the SV channel activation in barley mesophyll vacuoles is due to Ca2+ contamination of the cytosolic bath solution, and that Mg2+ does not activate SV channels.
To clarify these controversial conclusions, our results show that in the presence of EGTA, Mg2+ does not activate SV currents in fava bean guard cells (Fig. 3, B and C), indicating that Mg2+ activation in the previous study can be explained by residual free Ca2+, because no Ca2+ chelators were added to the cytosolic membrane side (Allen and Sanders, 1996). The conclusion that cytosolic Mg2+ does not modulate SV channels was based on experiments with 1 to 2 mm EGTA and no Ca2+ added to the cytosolic solutions (Pottosin et al., 1997). Interestingly, however, in our experiments within the range of cytosolic Ca2+ concentrations at which SV currents were not normally activated, the addition of Mg2+ led to SV current activation (Fig. 4), indicating a synergistic effect between Mg2+ and Ca2+. Our data show that Mg2+ sensitizes SV channels to physiological levels of cytosolic Ca2+. Ba2+ did not activate SV channels in fava bean guard cell vacuoles (Schulz-Lessdorf and Hedrich, 1995), but did activate SV channels in beet vacuoles (Pantoja et al., 1992).
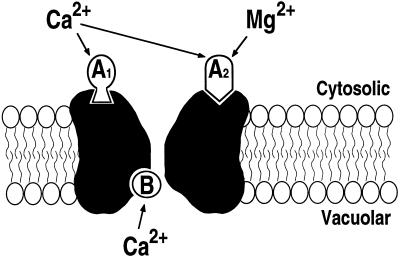
Based on our results, a simplified model for SV channel regulation in fava bean guard cells is proposed (Fig. 8), which includes two activating cytosolic sites and one inhibitory luminal site. First, low concentrations of cytosolic Ca2+ cannot activate SV channels in the absence of cytosolic Mg2+, whereas in the presence of cytosolic Mg2+, these low concentrations of Ca2+ (A1; Fig. 8) are necessary and sufficient to activate SV channels, implying a synergistic Mg2+-binding site (A2; Fig. 8). Second, Mg2+ alone cannot activate SV channels, indicating that a high-affinity (Kd of approximately 227 nm) Ca2+-binding site (A1) is required and is different from the Mg2+-binding site (A2). Mg2+ cannot compete with Ca2+ for A1 binding. Both A1 and A2 need to be occupied for SV channel activation. Third, a high concentration of cytosolic Ca2+ alone can activate SV channels (Ward and Schroeder, 1994; Allen and Sanders, 1996), suggesting (for a simple model) that cytosolic Ca2+ can bind to both sites A1 and A2. In addition, our results showed that the time course of SV current activation differed when using saturating Ca2+ (10 mm) to bind both sites (A1 and A2) in the absence of Mg2+ as opposed to using saturating Mg2+ (10 mm) binding to the low-affinity site (A2) in the presence of 10 μm Ca2+ to bind to the high-affinity site (A1) (Fig. 6), indicating additional ion-specific effects.
Figure 8.
Simplified model for the regulation of SV channels by cytosolic and luminal Ca2+ and Mg2+ in fava bean guard cells. A1, High-affinity Ca2+-binding site on the cytosolic side, which is not activated by Mg2+. A2, Low-affinity binding site on the cytosolic side, which can be occupied by either Mg2+ or Ca2+. B, Vacuolar Ca2+-binding site, which is not affected by vacuolar Mg2+. For the activation of SV channels, both activation sites A1 and A2 need to be occupied (see “Discussion”). The cytosolic and vacuolar membrane sides are labeled.
Finally, luminal Ca2+ inhibits SV currents by shifting the activation potential (Figs. 7B versus 4C; also see Allen and Sanders, 1996; Pottosin et al., 1997), indicating that Ca2+ ion binding on the luminal side (B; Fig. 8) is inhibitory and thus limits large Ca2+ release currents that could be toxic. Vacuolar Mg2+ did not affect SV (data not shown), indicating that Mg2+ does not compete for this inhibitory Ca2+-binding site (B).
Proposed Physiological Roles of Mg2+ Regulation of Vacuolar Currents
The vacuole constitutes ≥90% of the guard cell volume and functions as a storage organelle for solutes that are important for osmoregulation during stomatal movements (Boller and Wiemken, 1986; Assmann, 1993). More than 90% of the K+ and anions released from guard cells during stomatal closing must first be released from vacuoles into the cytosol (MacRobbie, 1981). Studies show that FV channels can mediate both inward and outward currents with large amplitudes (Fig. 2D), which would lead to vacuolar K+ release when V-type ATPases are active. Cytosolic Ca2+ and polyamines down-regulate FV channels (Hedrich and Neher, 1987; Allen and Sanders, 1996; Tikhonova et al., 1997). The half-inhibitory concentration of cytosolic Ca2+ for FV currents is about 6 μm (Tikhonova et al., 1997), which is higher than known physiological levels of free Ca2+ (Bush, 1995; McAinsh and Hetherington, 1998). In contrast to Ca2+, the half-inhibitory concentration of cytosolic Mg2+ was about 230 μm (Fig. 1), which lies within the physiological range of free Mg2+ concentrations (400 μm; Yazaki et al., 1988), suggesting that Mg2+ might play a major role in the down-regulation of FV channels in vivo during stomatal opening or cell expansion.
A recent study concluded that due to vacuolar Ca2+ block (Fig. 8B), SV channels cannot mediate Ca2+-induced Ca2+ release (Pottosin et al., 1997). However, this model represents a negative hypothesis based on a lack of observation, which, given the complexity of biological systems, may be oversimplified (for review, see Alberts, 1998). This hypothesis (Pottosin et al., 1997) did not consider shifts in the Ca2+ sensitivity of SV channels to cytosolic Mg2+ (Fig. 7), the effects of the K+ gradient across the vacuolar membrane (Fig. 7), nor effects of malate gradients proposed to shift SV activation (Hedrich et al., 1986). Cellular regulation mechanisms of the SV channel, such as calmodulin (Bethke and Jones, 1994), redox agents (Carpaneto et al., 1999), and protein phosphorylation (Allen and Sanders, 1995; Bethke and Jones, 1997) might also shift the voltage dependence of SV channels, and were not considered (Pottosin et al., 1997).
In addition, deactivating time-dependent (tail) SV currents have been shown to unequivocally mediate Ca2+ efflux from vacuoles (Ward and Schroeder, 1994; Ward et al., 1995). Therefore, the conclusion that SV channels cannot mediate Ca2+ release (Pottosin et al., 1997) is not consistent with these direct recordings. Transient stimulation of second-messenger (cADPR and InP3)-activated Ca2+ selective channels in the vacuolar membrane (Allen et al., 1995; Leckie et al., 1998; Cancela et al., 1999) will polarize the vacuolar potential to positive voltages, which in turn activates SV channels. Subsequently, deactivation of second-messenger-activated Ca2+-selective channels (Allen et al., 1995; Leckie et al., 1998) could produce Ca2+-induced Ca2+ release via tail currents. Rapid vacuolar membrane repolarization could also be mediated by the combination of activated VK channels and proton pumps and/or anion efflux from vacuoles (Ward et al., 1995). In addition, data in Figure 7 show yet to be identified conditions that shift the voltage dependence of SV channels. Further research on mechanisms that could shift the voltage dependence of SV channels, such as cytosolic Mg2+ (Fig. 7), calmodulin, redox agents (Bethke and Jones, 1994; Carpaneto et al., 1999), and other regulators, may lead to the identification of additional mechanisms that allow Ca2+-induced Ca2+ release. Taken together, our data show that Mg2+ can play an important role in the regulation of vacuolar ion channels. These findings raise an additional question of whether cytosolic Mg2+ activities change during stomatal movements.
CONCLUSION
The effects of both cytosolic and vacuolar Mg2+ on SV and FV channels have been systematically investigated. The present study shows that even if cytosolic Mg2+ concentrations do not change, physiological levels of Mg2+ ions provide a major mechanism for sensitizing SV channels to stimulus-induced elevations in cytosolic Ca2+ during signal transduction. Furthermore, both vacuolar and cytosolic Mg2+ ensure that FV channels do not function as a continuous leak for K+ ions, which would prevent stomatal opening.
ACKNOWLEDGMENTS
We thank Gethyn J. Allen for comments, Sébastien Thomine and David A. Lee for reading the manuscript, and Walter Gassmann, Martin Schwarz, and Walter B. Kelly for technical support during experiments and helpful discussions.
Footnotes
This work was supported by the National Science Foundation (grant no. MCB–9506191 to J.I.S.).
LITERATURE CITED
- Agus ZS, Morad M. Modulation of cardiac ion channels by magnesium. Annu Rev Physiol. 1991;53:299–307. doi: 10.1146/annurev.ph.53.030191.001503. [DOI] [PubMed] [Google Scholar]
- Alberts B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell. 1998;92:291–294. doi: 10.1016/s0092-8674(00)80922-8. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Amtmann A, Sanders D. Calcium-dependent and calcium-independent K+ mobilization channels in Vicia faba guard cell vacuoles. J Exp Bot. 1998;49:305–318. [Google Scholar]
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. Calcineurin, a type 2B protein phosphatase, modulates the Ca2+-permeable slow vacuolar ion channel of stomatal guard cells. Plant Cell. 1995;7:1473–1483. doi: 10.1105/tpc.7.9.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996;10:1055–1069. doi: 10.1046/j.1365-313x.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. Vacuolar ion channels of higher plants. Adv Bot Res. 1997;25:217–252. [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Barkla BJ, Pantoja O. Physiology of ion transport across the tonoplast of higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:159–184. doi: 10.1146/annurev.arplant.47.1.159. [DOI] [PubMed] [Google Scholar]
- Bertl A, Blumwald E, Coronado R, Eisenberg R, Findlay G, Gradman D, Hille B, Kohler K, Kolb HA, MacRobbie E. Electrical measurements on endomembranes. Science. 1992;258:873–874. doi: 10.1126/science.1439795. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Jones RL. Ca2+-calmodulin modulates ion channel activity in storage protein vacuoles of barley aleurone cells. Plant Cell. 1994;6:277–285. doi: 10.1105/tpc.6.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Jones RL. Reversible protein phosphorylation regulates the activity of the slow-vacuolar ion channel. Plant J. 1997;11:1227–1235. [Google Scholar]
- Boller T, Wiemken A. Dynamics of vacuolar compartmentation. Annu Rev Plant Physiol. 1986;37:137–164. [Google Scholar]
- Brüggemann LI, Pottosin II, Schönknecht G. Cytoplasmic polyamines block the fast-activating vacuolar cation channel. Plant J. 1998;16:101–106. [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Carpaneto A, Cantù AM, Gambale F. Redox agents regulate ion channel activity in vacuoles from higher plant cells. FEBS Lett. 1999;442:129–132. doi: 10.1016/s0014-5793(98)01642-1. [DOI] [PubMed] [Google Scholar]
- Chuang H-h, Jan YN, Jan LY. Regulation of IRK3 inward rectifier K+ channel by m1 acetylcholine receptor and intracellular magnesium. Cell. 1997;89:1121–1132. doi: 10.1016/s0092-8674(00)80299-8. [DOI] [PubMed] [Google Scholar]
- Colombo R, Cerana R, Lado P, Peres A. Voltage-dependent channels permeable to K+ and Na+ in the membrane of Acer pseudoplatanus vacuoles. J Membr Biol. 1988;103:227–236. [Google Scholar]
- Coyaud L, Kurkdjian A, Kado R, Hedrich R. Ion channels and ATP-driven pumps involved in ion transport across the tonoplast of sugar beet vacuoles. Biochim Biophys Acta. 1987;902:263–268. [Google Scholar]
- Davies JM, Sanders D. ATP, pH and Mg2+ modulate a cation current in Beta vulgaris vacuoles: a possible shunt conductance for the vacuolar H+-ATPase. J Membr Biol. 1995;145:75–86. doi: 10.1007/BF00233308. [DOI] [PubMed] [Google Scholar]
- Dobrovinskaya OR, Muniz J, Pottosin II. Inhibition of vacuolar ion channels by polyamines. J Membr Biol. 1999;167:127–140. doi: 10.1007/s002329900477. [DOI] [PubMed] [Google Scholar]
- Epstein E. Mineral metabolism. In: Bonner J, Varner JE, editors. Plant Biochemistry. London: Academic Press; 1965. pp. 438–466. [Google Scholar]
- Fakler B, Brändle U, Glowatzki E, Weidemann S, Zenner H-P, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Flatman PW. Mechanisms of magnesium transport. Annu Rev Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- Foehr KJ, Warchol W, Gratzl M. Calculation and control of free divalent cations in solutions used for membrane fusion studies. Methods Enzymol. 1993;221:149–157. doi: 10.1016/0076-6879(93)21014-y. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording form cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Flügge UI, Fernandez JM. Patch-clamp studies of ion transport in isolated plant vacuoles. FEBS Lett. 1986;204:228–232. [Google Scholar]
- Hedrich R, Kurkdjian A. Characterization of an anion-permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J. 1988;7:3661–3666. doi: 10.1002/j.1460-2075.1988.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Neher E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature. 1987;329:833–836. [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Ed 2. Sunderland, MA: Sinauer Associates; 1992. [Google Scholar]
- Kerschbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 1999;283:836–839. doi: 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- Kruse T, Tallman G, Zeiger E. Isolation of guard cell protoplasts from mechanically prepared epidermis of Vicia faba leaves. Plant Physiol. 1989;90:1382–1386. doi: 10.1104/pp.90.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Owen VJ, Junankar PR, Taske NL, Dulhunty AF, Lamb GD. Reduced inhibitory effect of Mg2+ on ryanodine receptor Ca2+ release channels in malignant hyperthermia. Biophys J. 1997;73:1913–1924. doi: 10.1016/S0006-3495(97)78222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RA, Wyn Jones RG. Cellular compartmentation in plant nutrition: the selective cytoplasm and the promiscuous vacuole. In: Tinker B, Lauchli A, editors. Advances in Plant Nutrition 2. New York: Praeger Scientific; 1986. pp. 249–279. [Google Scholar]
- Leube MP, Grill E, Amrhein N. ABI1 of Arabidopsis is a protein serine/threonine phosphatase highly regulated by the proton and magnesium ion concentration. FEBS Lett. 1998;424:100–104. doi: 10.1016/s0014-5793(98)00149-5. [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. Effects of ABA on “isolated” guard cells of Commelina communis L. J Exp Bot. 1981;32:563–572. [Google Scholar]
- MacRobbie EAC. Signal transduction and ion channels in guard cells. Phil Trans R Soc Lond B. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. Ed 2. London: Academic Press; 1995. [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inward rectifying K+ channel and blocking by Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Calcium ions as second messengers in guard cell signal transduction. Physiol Plant. 1997;100:16–29. [Google Scholar]
- McAinsh MR, Hetherington AM. Encoding specificity in Ca2+ signaling systems. Trends Plant Sci. 1998;3:32–36. [Google Scholar]
- Murphy E, Freudenrich CC, Lieberman M. Cellular magnesium and Na+/Mg2+ exchange in heart cells. Annu Rev Physiol. 1991;53:273–287. doi: 10.1146/annurev.ph.53.030191.001421. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pantoja O, Gelli A, Blumwald E. Voltage-dependent calcium channels in plant vacuoles. Science. 1992;255:1567–1570. doi: 10.1126/science.255.5051.1567. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+ Neuron. 1995;15:1109–1120. doi: 10.1016/0896-6273(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Ward JM, Harper JF, Schroeder JI. A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 1996;15:6564–6574. [PMC free article] [PubMed] [Google Scholar]
- Pottosin II, Tikhonova LI, Hedrich R, Schönknecht G. Slowly activating vacuolar channels can not mediate Ca2+-induced Ca2+ release. Plant J. 1997;12:1387–1398. [Google Scholar]
- Schroeder JI. Magnesium-independent activation of inward-rectifying K+ channels in Vicia faba guard cells. FEBS Lett. 1995;363:157–160. doi: 10.1016/0014-5793(95)00306-t. [DOI] [PubMed] [Google Scholar]
- Schulz-Lessdorf B, Hedrich R. Protons and calcium modulate SV-type channels in the vacuolar-lysosomal compartment: channel interaction with calmodulin inhibitors. Planta. 1995;197:655–671. [Google Scholar]
- Sze H, Ward JM, Lai S. Vacuolar H+-translocating ATPases from plants: structure, function, and isoforms. J Bioenerg Bio-membr. 1992;24:371–381. doi: 10.1007/BF00762530. [DOI] [PubMed] [Google Scholar]
- Tikhonova LI, Pottosin II, Dietz K-J, Schönknecht G. Fast-activating cation channel in barley mesophyll vacuoles: inhibition by calcium. Plant J. 1997;11:1059–1070. [Google Scholar]
- Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci USA. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Pei Z-M, Schroeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+ channel and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser T, Blum W, Bentrup FW. Calmodulin regulates the Ca2+-dependent slow-vacuolar ion channel in the tonoplast of Chenopodium rubrum suspension cells. Planta. 1991;185:440–442. doi: 10.1007/BF00201069. [DOI] [PubMed] [Google Scholar]
- Yazaki Y, Asukagawa N, Ishikawa Y, Ohta E, Sakata M. Estimation of cytoplasmic free Mg2+ levels and phosphorylation potentials in mung bean root tips by in vivo 31P NMR spectroscopy. Plant Cell Physiol. 1988;29:919–924. [Google Scholar]