Abstract
In recent years, the in ovo feeding in fertilized broiler (Gallus gallus) eggs approach was further developed and currently is widely applied in the evaluation process of the effects of functional foods (primarily plant origin compounds) on the functionality of the intestinal brush border membrane, as well as potential prebiotic properties and interactions with the intestinal microbial populations. This review collates the information of potential nutrients and their effects on the mineral absorption, gut development, brush border membrane functionality, and immune system. In addition, the advantages and limitations of the in ovo feeding method in the assessment of potential prebiotic effects of plant origin compounds is discussed.
Keywords: intra-amniotic administration, in ovo feeding, prebiotics, mineral absorption, gut development, immune system
1. Introduction
Functional foods, supplemented with bioactive substances (such as bioactive peptides, prebiotics, and polyphenols), provide health benefits and decrease the risk of chronic diseases [1,2,3,4]. Extensive research related to functional foods suggested numerous health benefits, including the decrease of cancer risk, improvement of heart health, enhancement of the immune system, improvement of gut health, diminution of blood pleasure, and decline of osteoporosis [5,6,7,8]. Among these functional foods are prebiotics, which are non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth or activity of gut bacterial, and thereby exerting a health-promoting effect [9,10,11]. To date, prebiotics have been confirmed to have functional benefits such as the: (1) inhibition of acute gastroenteritis via the alteration of gut health and the immune system [1,12,13]; (2) reduction of cancer risk via the decrease of genotoxic enzyme production [14,15,16]; (3) promotion of uptake of minerals and release of bone-modulating factors [17,18,19]; and (4) the regulation of lipids [14,20].
Inulin, oligofructose, and galactooligosaccharides are the most intensively investigated prebiotics with regard to prebiotic effects [17,18,21,22]. Most of the studies on the effect of prebiotics have been performed in rats; the results showed that lactic acid bacteria increased in the intestine in the oligo-fructose treatment group after two weeks. However, in the long-term, any effect was lost in the rat animal model [23]. One study conducted in rats demonstrated that only inulin, alone, significantly (p < 0.05) increased bone mineral content (BMC) and density (BMD), and decreased the urinary excretion of collagen cross-links, which is a marker of bone resorption. However, oligo-fructose alone or oligo-fructose combined with inulin did not have an effect on BMC and BMD [24]. Silvi et al. (1999) used a human flora-associated rats model to look at the effects of resistant starch administration, and found that β-glucosidase increased, while β-glucuronidase and ammonia levels decreased [25]. In addition, a study on diabetic rats found that when xylooligosaccharides (XOS) replaced simple carbohydrates in the diet, the increase of serum cholesterol and triglyceride in diabetes were reduced, and liver triacylglycerols increased to a comparable level to that seen in healthy rats [26]. Moreover, human studies concluded no significant results [27,28], while others found that prebiotics stimulated calcium or magnesium absorption [29,30]. Tahiri et al. (2003) applied the metabolic balance and the stable isotope techniques in parallel, and found no significant effects (p > 0.05) of short-chain oligofructose on calcium absorption in postmenopausal women [27]. Ito et al. (1990) reported that feeding galactooligosaccharides to humans resulted in a decrease of nitroreductase, which is a metabolic activator carcinogenic substance. Meanwhile, the levels of indole and isovaleric acid as markers of putrefaction decreased in the galactooligosaccharide treatment groups [31]. Current research utilizes both animal and human models to evaluate the prebiotic potential effects of various nutrients; however, there are still inconsistencies in the results. In ovo exogenous nutrients administration was first applied in the 1980s for vaccination against Marek’s disease [32]. Over the years, further research on in ovo nutrients administration was conducted in order to potentially improve poultry production [33]. For example, numerous nutrients that have been applied for in ovo feeding, including amino acids [34], carbohydrates [35], and vitamins [36], are used to improve the quality of broiler chickens, specifically in the context of hatch weights, feed utilization, growth, and marketing size, all of which were observed to improve and increase post in ovo feeding [33]. Ohta and Kidd (2001) demonstrated that in ovo feeding site and time affect hatchability [34]. Figure 1 shows the various compartments that surround the poultry embryo (i.e., air chamber, albumen, yolk, allantoic fluid, and amniotic fluid).
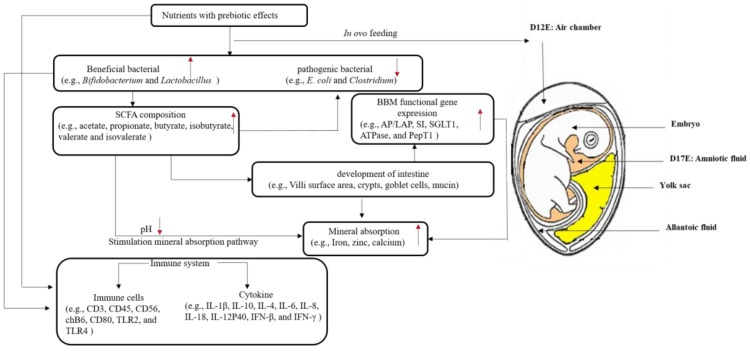
Figure 1.
Schematic diagram depicting proposed mechanisms by which the in ovo feeding approach of nutrients with prebiotic properties may affect the Gallus gallus developing embryo. Processes described as follows: post in ovo administration, the gut bacterial populations are affected, mostly as the beneficial bacterial population’s increase (1). The increase of beneficial bacterial (such as Bifidobacterium and Lactobacillus) promote the production of short-chain fatty acids (SCFA) (2). The increased production of SCFA due to bacterial activity leads to a luminal pH reduction (3); moreover, intestinal morphology (such as villi height, crypts, goblet cells, and mucin) is affected (4), and the mineral absorption (iron, zinc, and calcium) is increased due to their pH reduction and their increased solubility (5). The morphological affects (increased villi surface area and goblet cell numbers) can potentially stimulate the intestinal functional genes expressions, primarily proteins that are required for intestinal mineral absorption. In addition, the in ovo prebiotic administration seemed to affect the immune system (6). Black arrow: the relationship between two factors; red arrow: increased or decreased levels. Injection target: the injection site is air chamber at Day 12 (D12;) the injection target is amniotic fluid at D17. BBM: Brush Border Membrane; AP: Aminopeptidase; LAP: leucine aminopeptidase; SI: Sucrase-isomaltase; SGLT1: Sodium glucose transporter 1; PepT1 peptide transporter 1; TLR: toll-like receptor; IL: interleukin; IFN: interferon; ATP: adenosine triphosphate.
Previously, two time points during embryonic development were suggested and for the in ovo procedure. Both of these are on Day 12 (D12) of embryonic development, when the chorioallantoic membrane is fully developed and vascularized, and the embryo is surrounded by the amniotic fluid that remains in contact with the embryonic gastrointestinal tract, which allows the transport of substances from the air chamber into the intestine [37]. Villaluenga C.M. et al. (2004) demonstrated that the optimal time for the injection of a prebiotic is the 12th day of embryonic development. In comparison with injections on D1, 8, and 17, D12 injection resulted in a significantly (p < 0.05) increased relative abundance of intestinal bifidobacteria populations [38]. However, Uni and Ferket (2003) illustrated that in ovo feeding must be applied while the embryo consumes the amniotic fluid at 17–18 days of the embryonic development, just prior to the embryo’s oral consumption of the amniotic fluid, which occurs by Day 19 [39]. Salahi et al. (2011) provided evidence that the best in ovo injection time might be at 453 h of incubation [40]. It should be noted that the embryos are transferred from the setter to the hatching basket at D17–18, which should be an appropriate time to administer nutrients practically. Thus, the injection targeted egg compartment is amniotic fluid, on Day 17 of embryonic development.
On either D12 or D17 (days of embryonic development), eggs were weighed and divided into relevant treatment groups. All of the treatment groups were assigned eggs of a similar weight frequency distribution. Next, each group was injected with a specified solution (1 mL per egg) with a 21-gauge needle into the air chamber or the amniotic fluid (days 12 or 17, respectively). The solution should maintain an osmolality value of ≤320 osmolality (OSM) in order to ensure that the embryo is not dehydrated. After all of the eggs were injected, the injection holes were sterilized and sealed with cellophane tape, and the eggs were placed in hatching baskets [35,39].
Currently, the in ovo feeding model is widely used as an in vivo method to assess the potential prebiotic effects, as shown in Table 1. Thus, the goal of this review is to focus on how nutrients present potential prebiotic effects by using the in ovo feeding method, particularly with reference to mineral absorption, gut microflora population, intestinal development, and short-chain fatty acids (SCFA) content. Hence, the potential of the in ovo feeding approach, as a technique for the evaluation of prebiotic effects is discussed.
Table 1.
Studies of in ovo nutrients administration. BBM: brush border membrane.
| Injected Substances | Aims | Injected Target | Infection Time | References |
|---|---|---|---|---|
| Extract of Laminaria species of seaweed | development of duodenum | air chamber | Day 12 | [37] |
| Raffinose and stachyose | iron bioavailability, BBM functionality, gut microflora population | amniotic fluid | Day 17 | [41] |
| Extract of chickpea and lentil, duck egg white peptides | calcium bioavailability, BBM functionality, gut microflora population | amniotic fluid | Day 17 | [42] |
| Extract of beta-glucans, Transgalactooligosaccharides | hatchability, gut microflora population | air chamber | Day 12 | [43] |
| Extract containing laminarin and fucoidan; Transgalactooligosaccharides from milk lactose | muscle, lipid oxidation of meat | air chamber | Day 12 | [44] |
| Inulin, Galactooligosaccharides (GOS), Lactococcus lactis | transcriptomic prolife of spleen, cecal tonsils, and large intestine | air chamber | Day 12 | [45] |
| Raffinose | gut health and immune system | air chamber | Day 12 | [46] |
| Silybum marianum extract | immune system | amniotic fluid | Day 17 | [47] |
| Inulin, Enterococcus faecium | BBM functionality, gut microflora population, short-chain fatty acid content | amniotic fluid | Day 17 | [48] |
| Inulin, transgalactooligosaccharides, Lactococcus lactis | gut health and short-chain fatty acid content | air chamber | Day 12 | [49] |
| Inulin, Lactococcus lactis | immune-related gene expression | air chamber | Day 12 | [50] |
| Inulin, transgalactooligosaccharides, Lactococcus lactis | digestive potency of pancreas | air chamber | Day 12 | [51] |
| Wheat prebiotics | iron bioavailability, gut microflora population | amniotic fluid | Day 17 | [52] |
| Daidzein | BBM functionality, gut microflora population | amniotic fluid | Day 17 | [53] |
| Inulin | iron bioavailability, gut functionality | amniotic fluid | Day 17 | [54] |
| Raffinose, Lactococcus lactis | muscle fiber | air chamber | Day 12 | [55] |
| Mannan oligosaccharides | small intestine development | amniotic fluid | Day 17 | [56] |
| Dextrin, maltose, sucrose | mucin gene expression | amniotic fluid | Day 17 | [57] |
| Zinc-methionine | zinc status, small intestine development | amniotic fluid | Day 17 | [58] |
| β-hydroxy-β-methyl butyrate, Dextrin, maltose, sucrose | small intestine development | amniotic fluid | Day 17 | [35] |
2. In Ovo Administration and Mineral Absorption
2.1. Iron Status
Iron is a vital trace element for most life forms, and plays an important role in human health. Iron contributes to numerous biologic processes such as oxygen transport, DNA biosynthesis, and energy metabolism [59,60,61]. However, iron deficiency is the most common nutrient deficiency; it affects about two billion people worldwide [62]. The major causes of iron deficiency are the low iron content plant-based diets and low iron bioavailability [63]. Currently, a wealth of research aimed at exploring the effects of some substances (bioactive peptides and prebiotics) in the promotion of iron bioavailability and uptake is available [41,64]. For example, peptides from barley proteins were shown to increase iron uptake and ferritin levels in Caco-2 cells. Its SVNVPLY (Ser-Val-Asn-Val-Pro-Leu-Tyr) hexapeptide formed a chelate with Fe2+, which could increase Fe2+ uptake four-fold compared to FeSO4 [65]. β-lactoglobulin hydrolysate-iron complex could normalize hematocrit and hemoglobin, and improve serum iron levels in anemic rats [66]. Inulin and oligofructose have been shown to have benefits on the regeneration of hemoglobin mass, and increase intestinal iron absorption in anemic rats [67].
The intra-amniotic administration approach was used to evaluate the effect of natural occurring prebiotics in staple food crops on Fe bioavailability and absorption [54,68]. Previous research demonstrated that intra-amniotic administration and dietary inulin could increase 58Fe uptake, divalent metal transporter 1 (DMT1) gene expression, and liver ferritin amounts. In addition, the intestinal beneficial bacterial populations were also improved by inulin [54]. These results suggested that inulin improved iron status via changes in the bacterial population and the overall health of the intestine.
However, the study of intra-amniotic administration of wheat prebiotics demonstrated that there was no significant differences (p > 0.05) in the hatching Fe status and the intestinal expressions of DMT1, ferroportin, and duodenal cytochrome B (DyctB) between the treatment groups [52]. These results suggested that the iron status was not affected by the short-term exposure. Nevertheless, the study found an increase in the relative amounts of bifidobacteria and lactobacilli in the wheat prebiotics extract treatment group. This indicated that the iron bioavailability might be affected by wheat prebiotics in long-term studies via the increased production of short-chain fatty acids, due to bacterial activity, which lowers intestinal lumen pH, and hence increases iron solubility.
Further, the intra-amniotic administration of raffinose and stachyose suggested that the prebiotic treatments up-regulated the relative expression of brush border membrane (BBM) functionality proteins, down-regulated the iron metabolism proteins, increased the relative abundance of beneficial probiotics and villi surface area, and decreased the pathogenic bacteria (Clostridium and E. coli) [41]. These results suggested that the intra-amniotic administration of raffinose and stachyose, compounds that are found in staple food crops such as chickpea and lentil [69,70], may improve iron status via bacterial activity.
2.2. Zinc Status
Zinc is a required cofactor for the function of over 300 different enzymes, and participates in a wide variety of biochemical processes [71]. Zinc plays an important role in the regulation of genes involved in nucleic metabolism, cell signaling, and apoptosis [72,73]. Zinc deficiency is a major cause of stunting among children, who then run a risk of compromised cognitive development and physical capability [74].
Zinc cannot cross biological membranes by simple diffusion since it is a highly charged, hydrophilic ion [58]. Therefore, the uptake system in the intestine, such as the transport proteins, is paramount to zinc absorption [75,76]. Tako et al. (2005) used intra-amniotic zinc-methionine administration to evaluate the changes of the intestinal zinc exporter mRNA expression and small intestinal functionality. Authors found an approximately 200% mRNA increase of zinc transporter 1 (ZnT1) from 48 h post-ZnMet (zinc-methionine) injection compared to the control. Moreover, the gene expressions of the brush border enzymes and transporters showed increases of sucrase-isomaltase, leucine aminopeptidase, sodium–glucose cotransporter, and Na+/K+ATPase (Na+ and K+-stimulated adenosine triphosphatase) transporter (Na+/K+ATPase) from 48 h post-ZnMet injection. Meanwhile, the jejunal villus surface area increased significantly (p < 0.05) from the day of hatch (96 h post ZnMet injection). This study was the first introduction of the intra-amniotic administration approach in the evaluation of zinc digestion and BBM functionality [58].
Recently, the Gallus gallus was used to evaluate a proposed emerging physiological zinc status biomarker (the linoleic acid: dihomo-γ-linolenic acid ratio); this biomarker was assessed in the context of dietary zinc bioavailability in zinc biofortified staple food crops [77,78]. The broiler chicken model was used to explore the relationship between the dietary zinc deficiency and the red blood cell linoleic acid: dihomo-γ-linolenic acid ratio [78]. It was found that the linoleic acid: dihomo-γ-linolenic acid ratio significantly increased in the zinc dietary deficient group compared to that in the zinc adequate group (p < 0.001). Thus, the linoleic acid: dihomo-γ-linolenic acid ratio may be used as a biomarker of Zn status, specifically for the detection of marginal zinc deficiency status [79].
2.3. Calcium Status
Calcium (Ca2+), is an essential nutrient in the human body; as such, it participates in various biological pathways such as: intracellular metabolism, nerve conduction, blood muscle concentration, bone growth, and skeletal structural support [80,81]. Insufficient calcium uptake will cause bone resorption and the decrease of bone mass, which may lead to metabolic bone diseases, such as rickets in children and osteoporosis in the elderly [82]. Currently, some animal models, such as the calcium-deficient rat, are used for the in vivo assessment of calcium dietary bioavailability [8,17,19].
Several studies have shown positive effects of dietary prebiotics on calcium metabolism and bone composition [83,84,85]. The mechanisms by which prebiotics stimulate calcium absorption have been described and reviewed, and were suggested to be as follows [86,87,88,89]: (1) increased mineral solubility in the intestine due to the bacterial production of short-chain fatty acids; (2) enlargement of the absorption surface area by the promoting enterocytes proliferation; (3) stabilization of the intestinal flora and stimulation of gut beneficial prebiotics levels; (4) probiotic degradation of mineral-complexing phytic acid; and (5) increased expression of calcium-binding proteins.
Additional research suggested that prebiotics improve bone heath by: (1) the release of bone-modulating factors; (2) the impact of modulating growth factors; and (3) the suppression of the bone resorption rate relative to the bone formation rate [90].
Recently, the intra-amniotic administration model was used to evaluate the effects of prebiotics and duck egg white peptides on the promotion of calcium uptake [42]. It was found that the prebiotics and peptides increased the relative abundance of beneficial probiotics, the intestinal villus surface area, and goblet cell diameters, as well as regulated the calcium-related gene expressions. This suggested that the chickpea prebiotic, lentil prebiotic, and duck egg white peptides are promising in improving Ca2+ status, and as was demonstrated by the in ovo feeding approach. Prebiotics from chickpea and lentil improve calcium bioavailability by promotion of gut beneficial prebiotics levels, the enlargement of gut villus surface area, and the improvement of BBM functionality. Duck egg white peptides promote calcium uptake through the reaction with calcium to act as calcium carriers and maintain gut health [42].
As shown in Figure 1, the administration of nutrients with potential prebiotics may increase the intestinal bacterial populations (such as Bifidobacterium and Lactobacillus); the fermentation activity of these populations leads to increased SCFA synthesis. The increased production of SCFA lowers the intestinal pH, and hence may increase mineral solubility [91].
3. In Ovo Administration and Small Intestinal Morphology
3.1. Intestinal Morphometric Parameters
The small intestine is highly specialized in the hydrolysis and absorption of nutrients, and constitutes the barrier between the host’s external and internal environment [37]. The intestinal villi play an essential role in the digestion and absorption processes of nutrients [37], as the villi increase the internal surface area, as well as the digestive and absorptive capacities of the brush border membrane (BBM) [92]. The intestinal epithelium that covers the villi is invaginated into the lamina propria, forming tubular glands called intestinal crypts [37]. The crypts are comprised of populations of continuously proliferating stem cells, which are responsible for the formation of various types of intestinal epithelial cells [93]. Amongst these cells are the enterocytes, which have a key role due to their nutrients’ absorptive ability from the intestinal lumen into blood vessels [93]. Deeper crypts lead to an increase in the secretion of digestive enzymes [94]. Thus, the surface area of the villi, the crypts’ depth, and the ratio between villi height and crypts’ depth are common indictors of intestinal developmental and functional status [94,95,96]. Hence, an increase of any of these morphometric parameters is expected to improve the digestive and absorptive capabilities of the BBM.
In this context, the in ovo feeding of DiNovo (extract of Laminaria species of seaweed) significantly increased the width of duodenal villi and the depth of the crypts [37]. The villi surface area was also observed to increase post intra-amniotic administration of raffinose and stachyose [41,46], chickpea and lentil prebiotics [42], egg white peptides [42], symbiotic (inulin, Enterococcus faecium) [48], mannan oligosaccharides [56], carbohydrates (dextrin, maltose, sucrose) [57], and zinc-methionine [58]. However, in ovo injected probiotics (inulin, transgalactooligosaccharides, Lactococcus lactis) did not affect the villi heights, but rather changed the crypts’ depth [49]. The crypts’ depth increased after the injection of inulin combined with Enterococcus faecium [48] and mannan oligosaccharides [56], while the crypts’ depth was not affected by the injection of raffinose [46].
In the late embryonic and immediate post-hatch period, the small intestinal mucus-producing and secreting cells (goblet cell) begin to develop [39]. Since the intra-amniotic nutrients administration enhanced intestinal enterocytes proliferation, it may also affect the proliferation of goblet cells populations; this may further reflect on the intestinal digestive and absorptive capabilities. Pacifici et al. (2017) reported that the goblet cells’ diameters significantly increased post raffinose and stachyose administration [41]. A similar result was also observed post administration of mannan oligosaccharides [56]. In contrast, Calik et al. (2016) found that intra-amniotic symbiotic (0.5% inulin + 1 × 106 Enterococcus faecium) administration had no effect on the goblet cell numbers, while dietary symbiotic treatments increased goblet cell numbers significantly [48]. In the case of mucin content, Smirnov et al. (2006) reported that carbohydrates injection led to an increased proportion of goblet cells containing acidic mucin compared with controls. On Day 19 of incubation (36 h after injection), the number of goblet cells containing acidic mucins was 50% greater than that in the controls [57]. More importantly, the mucin-secretion system was the first one to respond to the administration of the mannan oligosaccharides and the MUC2 (Mucin 2) gene expression increased three-fold compared to the control [56].
3.2. Microbial Populations
The intestinal microbial populations play an essential role in human and animal health [97,98,99,100]. It has been reported that the gut microbiome contains an estimated 3–8 million unique genes, which expands the genetic capacity of humans by >100-fold [101]. In recent years, it was demonstrated that the gut microbiome community participates in abundant bioactivities, such as the: (1) maturation and regulation of the immune system [102]; (2) digestion and release of essential nutrients [103]; (3) improvement of intestinal barrier function [90]; and the (4) potential inhibition of pathogenic bacteria [90].
Gallus gallus harbors a complex and dynamic gut microbiota [104], which is heavily influenced by host genetics, the environment, and diet [105]. There is considerable similarity at the phylum level between the gut microbiota of broilers (Gallus gallus) and humans, with Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria representing the four dominant bacterial phyla in both [77,106]. Due to its rapid maturation and well-characterized phenotype, Gallus gallus has been used extensively as a model of human nutrition, especially as it pertains to assessing gut health and mineral absorption [58,77].
Recent studies suggested that cecal microbial populations are a useful indicator of gut health; this hypothesis was also confirmed in recent in ovo prebiotics administration studies [41,42,48,52,53,54]. For example, an early study showed that Bifidobacterium and Lactobacillus genera proportions were higher (p < 0.05) in intestinal contents of Gallus gallus after the intra-amniotic administration and dietary inulin treatment [54]. The increase of Bifidobacterium and Lactobacillus genera proportions were also observed in the intra-amniotic administration of wheat prebiotics [52], raffinose, stachyose [41], and chickpea and lentil extracts [42].
However, in the intra-amniotic administration of soy bean daidzein, there were no significant increases in Bifidobacterium, Lactobacillus, and Clostridium genera relative abundance in the 2.5 mg/mL daidzein treatment group. However, the relative E. coli abundance was significantly elevated [53]. The authors thought that E. coli might represent a candidate bacterial species involved in the biotransformation of daidzein to the bioactive metabolites, equol and O-desmethylangolensin [53]. Two prebiotics (DN, an extract of beta-glucans; and BI, transgalactooligosaccharides) both numerically increased the relative abundance of Bifidobacterium and Lactobacillus in chicken feces [43]. Similarly, the number of Bifidobacterium and Lactobacillus was consistently higher for both intra-amniotic administration and dietary symbiotic (inulin, Enterococcus faecium) treatments [48]. Moreover, the relative abundance of Clostridium significantly (p < 0.05) decreased in the presence of both concentrations of stachyose and raffinose compared to the controls, while the relative abundance of E. coli was not affected [41]. Interestingly, the relative abundance of E. coli and Clostridium significantly increased (p < 0.05) in the 18 MΩ H2O and Ca groups, and significantly decreased (p < 0.05) in peptide treatment groups compared to the non-injected group. The possible reason might be that prebiotics from chickpea and lentil and peptides from egg white could limit the presence of potentially pathogenic bacterial populations [42].
3.3. Short-Chain Fatty Acid Composition
The composition of SCFA in the intestine is significant to the mineral absorption of calcium, iron, zinc, and other micronutrients [90,92,107,108]. Previous research demonstrated that many prebiotics (i.e., soluble corn fiber, inulin, and agave fructans) increased the cecal content of SCFA, such as acetate, propionate, butyrate, isobutyrate, valerate, and isovalerate [4,90,109]. As the production of SCFA increases the intestinal lumen acidity, it also thus lowers the intestinal lumen pH and enhances minerals solubility (as Ca), which may lead to increased absorption [110,111].
The in ovo feeding model has been used to evaluate prebiotics and synbiotics (inulin with Lactococcus lactis subsp. lactis IBB SL1) on the cecal fermentation. The results showed that the propionate molar proportion was the highest in the groups treated with synbiotics, especially in the inulin with Lactococcus lactis subsp. lactis IBB SL1 group (Syn-1) (p < 0.01). In addition, the molar proportion of acetate was the lowest in the Syn-1 group (p < 0.05). However, the total cecal SCFA concentrations were similar in all of the groups, and the inulin group exhibited the lowest SCFA level. Other SCFAs, such as isobutyrate, isovalerate, valerate, isocaproate, and caproate, were low and not affected by the in ovo injections. Interestingly, the SCFA proportions varied over time in that study. The acetate molar proportion decreased, while the propionate and butyrate proportion increased [49]. Calik et al. (2016) used an approach that combined intra-amniotic with dietary administration to evaluate the effect of inulin with Enterococcus faecium on the SCFA composition [48]. Authors found that the butyrate concentration in the synbiotic group increased by 14.6% in comparison to the control group; however, this increase was not significant. In the dietary study, symbiotic (1.0% inulin + 2 × 109 Enterococcus faecium cfu/kg feed) supplementation significantly increased the butyrate concentration at the end of the experiment [48]. Butyric acid has been shown to be the preferred energy source for enterocytes, and takes part in cellular differentiation and proliferation with the intestinal mucosa [112]. Additional research is needed in order to further investigate the efficacy and efficiency of the combined administration of prebiotics and synbiotics to increased SCFA synthesis, its potential effect on the intestinal probiotic populations, and in the context of intestinal functionality and development.
3.4. Brush Border Membrane (BBM) Gene Expressions
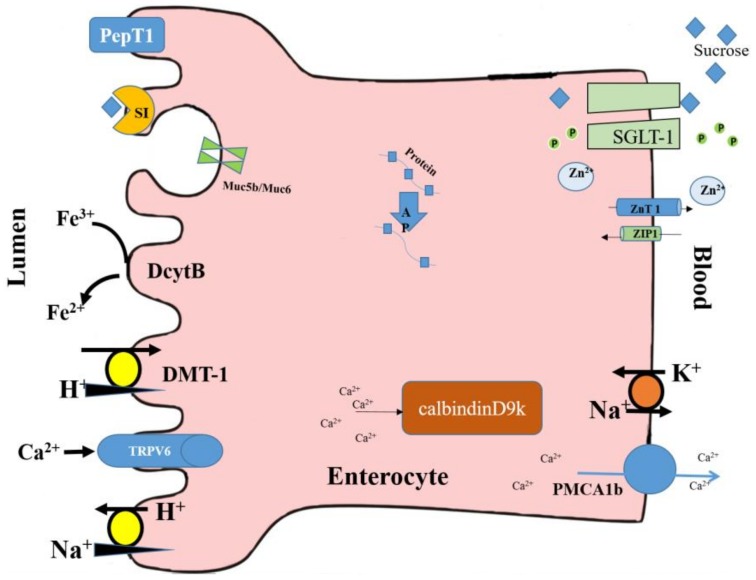
BBM functional genes expressions are used as biomarkers of BBM digestive and absorptive capabilities and overall tissue functionality [77,113]. Aminopeptidase (AP) and leucine aminopeptidase (LAP) are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides. Sucrase-isomaltase (SI) is a glucosidase enzyme that is located on the brush border of the small intestine. Sodium glucose transporter 1 (SGLT1) is a glucose transporter that is found in the intestinal mucosa (enterocytes) of the small intestine. ATPase is an enzyme that catalyzes the decomposition of ATP into ADP and a free phosphate ion. PepT1 (peptide transporter 1) is a solute carrier for oligopeptides; it functions in renal oligopeptide reabsorption, and in the intestines in a proton-dependent way. These functional proteins are all located on the enterocyte’s brush border and basal membranes, as shown in Figure 2.
Figure 2.
Schematic diagram of the discussed functional proteins located on the small intestinal enterocyte’s brush border and basolateral membranes. PepT1: peptide transporter 1; Dcyt B: duodenal cytochrome B; DMT-1: divalent metal transporter 1; AP: aminopeptidase; LAP: leucine aminopeptidase; SI: sucrase-isomaltase; SGLT1: sodium glucose transporter 1; TRPV6: transient receptor potential cation channel, subfamily V, member 6; PMCA1b: plasma membrane calcium ATPase 1b; calbindinD9k: calcium-binding protein.
One or more gene expressions from AP, SI, ATPase, and SGLT1 were significantly up-regulated by the intra-amniotic administration of chickpea and lentil prebiotics [42], stachyose and raffinose [41], daidzein [53], and zinc-methionine [58]. Cheled-Shoval et al. (2011) found that there was a five-fold increase in AP mRNA expression, and a two-fold increase in SI mRNA expression post mannan oligosaccharides in ovo treatment [56]. Chicken embryos have a limited ability to digest and absorb nutrients prior to hatching due to the low functional mRNA expression, such as AP, SI, ATPase, and SGLT1 in the small intestinal mucosa [39]. In the Gallus gallus model, immediate feeding post hatch is critical for the intestinal development [114]; therefore, nutrients supply via in ovo feeding enhances the intestinal development during embryonic development [33]. As shown in Figure 1, the up-regulation of the BBM functional genes expressions reflects the intestinal development and digestive capabilities. Thus, it also affects the potential increased absorption of nutrients as Fe, thus improving the poor Fe status of the late term embryo and Fe status post hatch.
4. In Ovo Administration and the Immune System
Previous research indicated that the in ovo feeding approach improved early immune response [33]. Bhanja et al. (2010) reported that a higher expression of genes associated with humoral immunity, IL-6, and TNF-α, was observed after the treatment with lysine, threonine, or methionine and cystine [115]. Additionally, in ovo treatment of 10% glucose improved humoral immune response [116].
Schley et al. (2002) reported that the immunity system was modulated by prebiotics directly through the interaction with immune cell receptors, stimulation of endocytosis, phagocytosis, respiratory burst, and the production of numerous cytokines and chemokines [117]. Probiotics cross the intestinal barrier through intestinal epithelial cells, are processed and presented to the immune system, and modulate both the innate and adaptive responses [118]. As shown in Table 2 (section B), the expression of CD3, CD45, CD56, chB6, CD80, (toll-like receptor) TLR2, and TLR4 is frequently used as an indicator of immune response post prebiotic in ovo administration. In addition, some cytokines, such as IL-1β, IL-10, IL-4, IL-6, IL-8, IL-18, IL-12P40, IFN-β, and IFN-γ, are also used as indicators of immune status (Table 2, section C). CD3 is membrane protein that is expressed in T cells and used as a biomarker of T-cell activity [119]. CD56 is expressed on the surface of neurons, glia, skeletal muscle, and natural killer cells (NK), and is used as markers of NK cells with TLR2 and TLR4 [46]. chB6, which is also used as marker, is expressed in mature B cells [120]. CD80 is a costimulatory molecular marker that is expressed in T cells or B cells [50]. IL-1β and IL-10 are known as a pro-inflammatory cytokine and an anti-inflammatory cytokine, respectively.
Table 2.
Functional gene expression and immune system response in the in ovo prebiotic administration model.
| Gene | References |
|---|---|
| Section A: Functional Gene Expression | |
| Aminopeptidase (AP)/leucine aminopeptidase (LAP) | [41,42,53,56,58,122] |
| Sucrose isomaltase (SI) | [35,41,42,53,56,58,122] |
| Sodium glucose transporter 1 (SGLT1) | [41,53,56,58] |
| ATPase | [53,58] |
| Peptide transporter 1 (PepT1) | [56] |
| Section B: Immune system | |
| CD3, CD45, CD56, chB6 | [46] |
| CD80 | [50] |
| TLR2, TLR4 | [46,56] |
| Section C: Cytokine | |
| IL-1β, IL-10 | [46] |
| IL-4, IL-6, IL-8, IL-18, IL-12P40 | [50] |
| IFN-β, IFN-γ | [50] |
T helper-1 genes (IFN-β, IFN-γ, and IL-18), T helper-4 gene (IL-4), pro-inflammatory cytokine (IL-6 and IL-12P40), and a chemokine (IL-8) are all markers of the immune system. Berrocoso et al. (2016) reported that the expression levels of CD3 and chB6 in the small intestine of broilers was significantly (p < 0.05) up-regulated by raffinose administration [46]. Additionally, no significant difference was observed in the expression levels of CD56, TLR4, IL-1β, and IL-10 post raffinose injected broilers [46]. Madej and Bednarczyk (2016) studied the effect of in ovo feeding of prebiotics and synbiotics (inulin, transgalactooligosaccharides, Lactococcus lactis subsp. lactis IBB SL1 or Lactococcus lactis subsp. cremoris IBB SC1) on the composition of T cells and B cells in gut-associated lymphoid tissue. They found that the number of CD3-expressed cells was increased by some symbiotic; however, there was no significant difference on the population of CD3 or chB6-expressing cells in only prebiotics-treated birds [121]. TLR2 and TLR4 mRNA expression were significantly (p < 0.05) higher after the treatment with mannan oligosaccharides [56]. However, in ovo administration of inulin or inulin supplemented with L lactis subsp lactis 2955 on Day 12 of embryonic development resulted in a general down-regulation of immune-related genes in the spleen and cecal tonsils of broilers during the 35 days after hatching. The magnitude of that down-regulation increased with age, and was most likely caused by the stabilization of the gastrointestinal microbiota [50].
5. Conclusions and Future work
The evidence provided in this review demonstrate that in ovo feeding (primarily the intra-amniotic fluid administration) approach is a useful and time–cost effective in vivo method to evaluate the probiotic effects of nutrients. To date, research has shown that utilizing the in ovo feeding model of various plant origin prebiotics, peptides, isoflavones, carbohydrates, and synbiotics resulted in an in vivo indication of these compounds’ prebiotic effects (such as: mineral absorption, gut microflora population, intestinal development, short-chain fatty acid content, and immune system response).
Future research via the utilizing the in ovo feeding model will be focused on the further identification of plant origin nutrients and bioactive compounds, which may improve intestinal overall health, and specifically the functionality of the digestive and absorptive surface, and beneficial bacterial populations. Current evidence indicates that the in ovo approach allows the investigation of a single nutrient or in combination of other ingredients, as previously described. This suggests that the in ovo feeding approach is an emerging in vivo method that can assess bioactive compounds with potential nutritional benefits.
Author Contributions
T.H. and E.T. conducted the literature search, conceptualized and synthetized information, and wrote the review paper. All authors have proofread the manuscript and approved the final version of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Al-Sheraji S.H., Ismail A., Manap M.Y., Mustafa S., Yusof R.M., Hassan F.A. Prebiotics as functional foods: A review. J. Funct. Foods. 2013;5:1542–1553. doi: 10.1016/j.jff.2013.08.009. [DOI] [Google Scholar]
- 2.Orsolic N., Goluza E., Dikic D., Lisicic D., Sasilo K., Rodak E., Jelec Z., Lazarus M.V., Orct T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014;53:1217–1227. doi: 10.1007/s00394-013-0622-7. [DOI] [PubMed] [Google Scholar]
- 3.Whisner C.M., Martin B.R., Nakatsu C.H., McCabe G.P., McCabe L.D., Peacock M., Weaver C.M. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: A randomised controlled trial using dual stable isotopic tracers. Br. J. Nutr. 2014;112:446–456. doi: 10.1017/S0007114514000981. [DOI] [PubMed] [Google Scholar]
- 4.García-Vieyra M.I., del Real A., López M.G. Agave fructans: Their effect on mineral absorption and bone mineral content. J. Med. Food. 2014;17:1247–1255. doi: 10.1089/jmf.2013.0137. [DOI] [PubMed] [Google Scholar]
- 5.Shahidi F. Functional foods: Their role in health promotion and disease prevention. J. Food Sci. 2004 doi: 10.1111/j.1365-2621.2004.tb10727.x. [DOI] [Google Scholar]
- 6.Chalamaiah M., Yu W., Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins D.J.A., Srichaikul K., Wong J.M.W., Kendall C.W.C., Bashyam B., Vidgen E., Lamarche B., Rao A.V., Jones P.J.H., Josse R.G., et al. Supplemental barley protein and casein similarly affect serum lipids in hypercholesterolemic women and men. J. Nutr. 2010;140:1633–1637. doi: 10.3945/jn.110.123224. [DOI] [PubMed] [Google Scholar]
- 8.Choi I., Jung C., Choi H., Kim C., Ha H. Effectiveness of phosvitin peptides on enhancing bioavailability of calcium and its accumulation in bones. Food Chem. 2005;93:577–583. doi: 10.1016/j.foodchem.2004.10.028. [DOI] [Google Scholar]
- 9.Roberfroid M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Chin. Nutr. 2000;71:1682S–1687S. doi: 10.1093/ajcn/71.6.1682S. [DOI] [PubMed] [Google Scholar]
- 10.Catry E., Bindels L.B., Tailleux A., Lestavel S., Neyrinck A.M., Goossens J.-F., Lobysheva I., Plovier H., Essaghir A., Demoulin J.-B., et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67:271–283. doi: 10.1136/gutjnl-2016-313316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chunchai T., Thunapong W., Yasom S., Wanchai K., Eaimworawuthikul S., Metzler G., Lungkaphin A., Pongchaidecha A., Sirilun S., Chaiyasut C., et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018 doi: 10.1186/s12974-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park S.F., Kroll R.G. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol. Microbiol. 1993;8:653–661. doi: 10.1111/j.1365-2958.1993.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 13.Roller M., Rechkemmer G., Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J. Nutr. 2004;134:153–156. doi: 10.1093/jn/134.1.153. [DOI] [PubMed] [Google Scholar]
- 14.Bornet F., Brouns F., Tashiro Y., Duvillier V. Nutritional aspects of short-chain fructooligosaccharides: Natural occurrence, chemistry, physiology and health implications. Dig. Liver Dis. 2002;34:S111–S120. doi: 10.1016/S1590-8658(02)80177-3. [DOI] [PubMed] [Google Scholar]
- 15.Burns A., Rowland I. Anti-carcinogenicity of probiotics and prebiotics. Curr. Issues Intest. Microbiol. 2000;1:13–24. [PubMed] [Google Scholar]
- 16.McBain A., Macfarlane G. Modulation of genotoxic enzyme activities by non-digestible oligosaccharide metabolism in in-vitro human gut bacterial ecosystems. J. Med. Microbiol. 2001;50:833–842. doi: 10.1099/0022-1317-50-9-833. [DOI] [PubMed] [Google Scholar]
- 17.Bryk G., Coronel M.Z., Pellegrini G., Mandalunis P., Rio M.E., de Portela M.L.P.M., Zeni S.N. Effect of a combination GOS/FOS® prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur. J. Nutr. 2015;54:913–923. doi: 10.1007/s00394-014-0768-y. [DOI] [PubMed] [Google Scholar]
- 18.Roberfroid M.B., Cumps J., Devogelaer J. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. J. Nutr. 2002;132:3599–3602. doi: 10.1093/jn/132.12.3599. [DOI] [PubMed] [Google Scholar]
- 19.Tenorio M.D., Espinosa-Martos I., Préstamo G., Rupérez P. Soybean whey enhance mineral balance and caecal fermentation in rats. Eur. J. Nutr. 2010;49:155–163. doi: 10.1007/s00394-009-0060-8. [DOI] [PubMed] [Google Scholar]
- 20.Roberfroid M. Functional food concept and its application to prebiotics. Dig. Liver Dis. 2002;34:S105–S110. doi: 10.1016/S1590-8658(02)80176-1. [DOI] [PubMed] [Google Scholar]
- 21.Holloway L., Moynihan S., Abrams S.A., Kent K., Hsu A.R., Friedlander A.L. Effects of oligofructose-enriched inulin on intestinal absorption of calcium and magnesium and bone turnover markers in postmenopausal women. Br. J. Nutr. 2007;97:365–372. doi: 10.1017/S000711450733674X. [DOI] [PubMed] [Google Scholar]
- 22.Weaver C.M., Martin B.R., Nakatsu C.H., Armstrong A.P., Clavijo A., McCabe L.D., McCabe G.P., Duignan S., Schoterman M.H., van den Heuvel E.G. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011;59:6501–6510. doi: 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- 23.Le Blay G., Michel C., Blottière H.M., Cherbut C. Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 1999;129:2231–2235. doi: 10.1093/jn/129.12.2231. [DOI] [PubMed] [Google Scholar]
- 24.Kruger M.C., Brown K.E., Collett G., Layton L., Schollum L.M. The effect of fructooligosaccharides with various degrees of polymerization on calcium bioavailability in the growing rat. Exp. Biol. Med. 2003;228:683–688. doi: 10.1177/153537020322800606. [DOI] [PubMed] [Google Scholar]
- 25.Silvi S., Rumney C., Cresci A., Rowland I. Resistant starch modifies gut microflora and microbial metabolism in human flora-associated rats inoculated with faeces from Italian and UK donors. J. Appl. Microbiol. 1999;86:521–530. doi: 10.1046/j.1365-2672.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- 26.Imaizumi K., Nakatsu Y., Sato M., Sedarnawati Y., Sugano M. Effects of xylooligosaccharides on blood glucose, serum and liver lipids and cecum short-chain fatty acids in diabetic rats. Agric. Biol. Chem. 1991;55:199–205. [Google Scholar]
- 27.Tahiri M., Tressol J.C., Arnaud J., Bornet F.R., Bouteloup-Demange C., Feillet-Coudray C., Brandolini M., Ducros V., Pépin D., Brouns F., et al. Effect of short-chain fructooligosaccharides on intestinal calcium absorption and calcium status in postmenopausal women: A stable-isotope study. Am. J. Chin. Nutr. 2003;77:449–457. doi: 10.1093/ajcn/77.2.449. [DOI] [PubMed] [Google Scholar]
- 28.López-Huertas E., Teucher B., Boza J.J., Martínez-Férez A., Majsak-Newman G., Baró L., Carrero J.J., González-Santiago M., Fonollá J., Fairweather-Tait S. Absorption of calcium from milks enriched with fructo-oligosaccharides, caseinophosphopeptides, tricalcium phosphate, and milk solids. Am. J. Chin. Nutr. 2006;83:310–316. doi: 10.1093/ajcn/83.2.310. [DOI] [PubMed] [Google Scholar]
- 29.Griffin I., Davila P., Abrams S. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes. Br. J. Nutr. 2002;87:S187–S191. doi: 10.1079/BJN/2002536. [DOI] [PubMed] [Google Scholar]
- 30.Abrams S.A., Griffin I.J., Hawthorne K.M., Liang L., Gunn S.K., Darlington G., Ellis K.J. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Chin. Nutr. 2005;82:471–476. doi: 10.1093/ajcn/82.2.471. [DOI] [PubMed] [Google Scholar]
- 31.Ito M., Deguchi Y., Miyamori A., Matsumoto K., Kikuchi H., Matsumoto K., Kobayashi Y., Yajima T., Kan T. Effects of administration of galactooligosaccharides on the human faecal microflora, stool weight and abdominal sensation. Microb. Ecol. Health Dis. 1990;3:285–292. doi: 10.3109/08910609009140251. [DOI] [Google Scholar]
- 32.Sharma J., Burmester B. Resistance of marek’s disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian. Dis. 1982;26:134–149. doi: 10.2307/1590032. [DOI] [PubMed] [Google Scholar]
- 33.Kadam M.M., Barekatain M.R., K Bhanja S., Iji P.A. Prospects of in ovo feeding and nutrient supplementation for poultry: The science and commercial applications—A review. J. Sci. Food Agric. 2013;93:3654–3661. doi: 10.1002/jsfa.6301. [DOI] [PubMed] [Google Scholar]
- 34.Ohta Y., Kidd M. Optimum site for in ovo amino acid injection in broiler breeder eggs. Poult. Sci. 2001;80:1425–1429. doi: 10.1093/ps/80.10.1425. [DOI] [PubMed] [Google Scholar]
- 35.Tako E., Ferket P., Uni Z. Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult. Sci. 2004;83:2023–2028. doi: 10.1093/ps/83.12.2023. [DOI] [PubMed] [Google Scholar]
- 36.Effect of in Ovo Injection of Vitamins on the Chick Weight and Post-Hatch Growth Performance in Broiler Chickens. [(accessed on 24 February 2018)]; Available online: https://www.cabi.org/animalscience/worlds-poultry-science-association-wpsa/wpsa-france-2007/
- 37.Sobolewska A., Elminowska-Wenda G., Bogucka J., Dankowiakowska A., Kułakowska A., Szczerba A., Stadnicka K., Szpinda M., Bednarczyk M. The influence of in ovo injection with the prebiotic DiNovo® on the development of histomorphological parameters of the duodenum, body mass and productivity in large-scale poultry production conditions. J. Anim. Sci. Biotechnol. 2017;8 doi: 10.1186/s40104-017-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villaluenga C.M., Wardeńska M., Pilarski R., Bednarczyk M., Gulewicz K. Utilization of the chicken embryo model for assessment of biological activity of different oligosaccharides. Folia Boil. 2004;52:135–142. doi: 10.3409/1734916044527502. [DOI] [PubMed] [Google Scholar]
- 39.Uni Z., Ferket P.R. Enhancement of Development of Oviparous Species by in Ovo Feeding. 6,592,878. U.S. Patent. 2003 Jul 15;
- 40.Salahi A., Mozhdeh M., Seyed N. Optimum time of in ovo injection in eggs of young broiler breeder flock; Proceedings of the 18th Eur. Symp. on Poultry Nutrition; Izmir, Turkey. 31 October–4 November 2011; Izmir, Turkey: World’s Poultry Science Association; 2011. pp. 557–559. [Google Scholar]
- 41.Pacifici S., Song J., Zhang C., Wang Q., Glahn R.P., Kolba N., Tako E. Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut microflora populations. Nutrients. 2017;9 doi: 10.3390/nu9030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou T., Kolba N., Glahn R.P., Tako E. Intra-amniotic administration (Gallus Gallus) of cicer arietinum and lens culinaris prebiotics extracts and duck egg white peptides affects calcium status and intestinal functionality. Nutrients. 2017;9 doi: 10.3390/nu9070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bednarczyk M., Stadnicka K., Kozłowska I., Abiuso C., Tavaniello S., Dankowiakowska A., Sławińska A., Maiorano G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal. 2016;10:1271–1279. doi: 10.1017/S1751731116000173. [DOI] [PubMed] [Google Scholar]
- 44.Maiorano G., Stadnicka K., Tavaniello S., Abiuso C., Bogucka J., Bednarczyk M. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult. Sci. 2017;96:511–518. doi: 10.3382/ps/pew311. [DOI] [PubMed] [Google Scholar]
- 45.Slawinska A., Plowiec A., Siwek M., Jaroszewski M., Bednarczyk M. Long-term transcriptomic effects of prebiotics and synbiotics delivered in ovo in broiler chickens. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0168899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berrocoso J., Kida R., Singh A., Kim Y., Jha R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2016;96:1573–1580. doi: 10.3382/ps/pew430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarei A., Morovat M., Chamani M., Sadeghi A., Dadvar P. Effect of in ovo feeding and dietary feeding of silybum marianum extract on performance, immunity and blood cation-anion balance of broiler chickens exposed to high temperatures. Iran. J. Appl. Anim. Sci. 2016;6:697–705. [Google Scholar]
- 48.Calik A., Ceylan A., Ekim B., Adabi S.G., Dilber F., Bayraktaroglu A.G., Tekinay T., Özen D., Sacakli P. The effect of intra-amniotic and posthatch dietary synbiotic administration on the performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult. Sci. 2016;96:169–183. doi: 10.3382/ps/pew218. [DOI] [PubMed] [Google Scholar]
- 49.Miśta D., Króliczewska B., Pecka-Kiełb E., Kapuśniak V., Zawadzki W., Graczyk S., Kowalczyk A., Łukaszewicz E., Bednarczyk M. Effect of in ovo injected prebiotics and synbiotics on the caecal fermentation and intestinal morphology of broiler chickens. Anim. Prod. Sci. 2016 doi: 10.1071/AN16257. [DOI] [Google Scholar]
- 50.Płowiec A., Sławińska A., Siwek M.Z., Bednarczyk M.F. Effect of in ovo administration of inulin and Lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015;76:975–982. doi: 10.2460/ajvr.76.11.975. [DOI] [PubMed] [Google Scholar]
- 51.Pruszynska-Oszmalek E., Kolodziejski P., Stadnicka K., Sassek M., Chalupka D., Kuston B., Nogowski L., Mackowiak P., Maiorano G., Jankowski J., et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015;94:1909–1916. doi: 10.3382/ps/pev162. [DOI] [PubMed] [Google Scholar]
- 52.Tako E., Glahn R.P., Knez M., Stangoulis J.C.R. The effect of wheat prebiotics on the gut bacterial population and iron status of iron deficient broiler chickens. Nutr. J. 2014;13 doi: 10.1186/1475-2891-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartono K., Reed S., Ankrah N.A., Glahn R.P., Tako E. Alterations in gut microflora populations and brush border functionality following intra-amniotic daidzein administration. RSC Adv. 2015;5:6407–6412. doi: 10.1039/C4RA10962G. [DOI] [Google Scholar]
- 54.Tako E., Glahn R. Intra-amniotic administration and dietary inulin affect the iron status and intestinal functionality of iron-deficient broiler chickens. Poult. Sci. 2012;91:1361–1370. doi: 10.3382/ps.2011-01864. [DOI] [PubMed] [Google Scholar]
- 55.Maiorano G., Sobolewska A., Cianciullo D., Walasik K., Elminowska-Wenda G., Sławińska A., Tavaniello S., Żylińska J., Bardowski J., Bednarczyk M. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 2012;91:2963–2969. doi: 10.3382/ps.2012-02208. [DOI] [PubMed] [Google Scholar]
- 56.Cheled-Shoval S., Amit-Romach E., Barbakov M., Uni Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre-and posthatch periods in chickens. Poult. Sci. 2011;90:2301–2310. doi: 10.3382/ps.2011-01488. [DOI] [PubMed] [Google Scholar]
- 57.Smirnov A., Tako E., Ferket P., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- 58.Tako E., Ferket P.R., Uni Z. Changes in chicken intestinal zinc exporter mRNA expression and small intestinal functionality following intra-amniotic zinc-methionine administration. J. Nutr. Biochem. 2005;16:339–346. doi: 10.1016/j.jnutbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira F., Rocha S., Fernandes R. Iron metabolism: From health to disease. J. Chin. Lab. Anal. 2014;28:210–218. doi: 10.1002/jcla.21668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Da Cunha M.D.S.B., Campos Hankins N.A., Arruda S.F. Effect of vitamin A supplementation on iron status in humans: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018 doi: 10.1080/10408398.2018.1427552. [DOI] [PubMed] [Google Scholar]
- 61.Young I., Parker H.M., Rangan A., Prvan T., Cook R.L., Donges C.E., Steinbeck K.S., O’Dwyer N.J., Cheng H.L., Franklin J.L., et al. Association between haem and non-haem iron intake and serum ferritin in healthy young women. Nutrients. 2018;10 doi: 10.3390/nu10010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou X., Wang D., Sun P., Bucheli P., Li L., Hou Y., Wang J. Effects of soluble tea polysaccharides on hyperglycemia in alloxan-diabetic mice. J. Agric. Food Chem. 2007;55:5523–5528. doi: 10.1021/jf070699t. [DOI] [PubMed] [Google Scholar]
- 63.Dwivedi S., Sahrawat K., Puppala N., Ortiz R. Plant prebiotics and human health: Biotechnology to breed prebiotic-rich nutritious food crops. Electr. J. Biotechnol. 2014;17:238–245. doi: 10.1016/j.ejbt.2014.07.004. [DOI] [Google Scholar]
- 64.Cian R.E., Garzon A.G., Betancur Ancona D., Chel Guerrero L., Drago S.R. Chelating properties of peptides from red seaweed pyropia columbina and its effect on iron bio-accessibility. Plant. Foods Hum. Nutr. 2016;71:96–101. doi: 10.1007/s11130-016-0533-x. [DOI] [PubMed] [Google Scholar]
- 65.Eckert E., Lu L., Unsworth L.D., Chen L., Xie J., Xu R. Biophysical and in vitro absorption studies of iron chelating peptide from barley proteins. J. Funct. Foods. 2016;25:291–301. doi: 10.1016/j.jff.2016.06.011. [DOI] [Google Scholar]
- 66.Zhou J., Mao X.-Y., Wang X., Ai T., Ma J.-J., Li Y.-H. Anti-anaemia efficacy of β-lactoglobulin hydrolysate-iron complex on iron-deficient anaemic rats. Eur. J. Nutr. 2014;53:877–884. doi: 10.1007/s00394-013-0591-x. [DOI] [PubMed] [Google Scholar]
- 67.De Cássia Freitas K., Amancio O.M.S., de Morais M.B. High-performance inulin and oligofructose prebiotics increase the intestinal absorption of iron in rats with iron deficiency anaemia during the growth phase. Br. J. Nutr. 2012;108:1008–1016. doi: 10.1017/S0007114511006301. [DOI] [PubMed] [Google Scholar]
- 68.Tako E., Rutzke M., Glahn R. Using the domestic chicken (Gallus Gallus) as an in vivo model for iron bioavailability. Poult. Sci. 2010;89:514–521. doi: 10.3382/ps.2009-00326. [DOI] [PubMed] [Google Scholar]
- 69.Gangola M.P., Jaiswal S., Kannan U., Gaur P.M., Baga M., Chibbar R.N. Galactinol synthase enzyme activity influences raffinose family oligosaccharides (RFO) accumulation in developing chickpea (Cicer arietinum L.) seeds. Phytochemistry. 2016;125:88–98. doi: 10.1016/j.phytochem.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Tahir M., Lindeboom N., Baga M., Vandenberg A., Chibbar R.N. Composition and correlation between major seed constituents in selected lentil (Lens culinaris. Medik) genotypes. Can. J. Plant. Sci. 2011;91:825–835. doi: 10.4141/cjps2011-010. [DOI] [Google Scholar]
- 71.Gaither L.A., Eide D.J. Zinc Biochemistry, Physiology, and Homeostasis. Springer; Dordrecht, The Netherlands: 2001. Eukaryotic zinc transporters and their regulation; pp. 65–84. [DOI] [PubMed] [Google Scholar]
- 72.Salgueiro M.A.J., Zubillaga M.B., Lysionek A.E., Caro R.A., Weill R., Boccio J.R. The role of zinc in the growth and development of children. Nutrition. 2002;18:510–519. doi: 10.1016/S0899-9007(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 73.Tian K., Wang Y.-X., Li L.-X., Liu Y.-Q. Neuronal death/apoptosis induced by intracellular zinc deficiency associated with changes in amino-acid neurotransmitters and glutamate receptor subtypes. J. Inorg. Biochem. 2018;179:54–59. doi: 10.1016/j.jinorgbio.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Trijatmiko K.R., Dueñas C., Tsakirpaloglou N., Torrizo L., Arines F.M., Adeva C., Balindong J., Oliva N., Sapasap M.V., Borrero J., et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016;6 doi: 10.1038/srep19792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaither L.A., Eide D.J. Functional expression of the human hzip2 zinc transporter. J. Biol. Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 76.Palmiter R.D., Findley S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reed S., Neuman H., Moscovich S., Glahn R.P., Koren O., Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7:9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reed S., Qin X., Ran-Ressler R., Brenna J.T., Glahn R.P., Tako E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-γ-linolenic acid (la: Dgla) ratio; a sensitive physiological marker of zinc status in vivo (Gallus Gallus) Nutrients. 2014;6:1164–1180. doi: 10.3390/nu6031164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knez M., Tako E., Glahn R.P., Kolba N., de Courcy-Ireland E., Stangoulis J.C.R. Linoleic acid: Dihomo-γ-linolenic acid ratio predicts the efficacy of Zn-biofortified wheat in chicken (Gallus Gallus) J. Agric. Food Chem. 2018;66:1394–1400. doi: 10.1021/acs.jafc.7b04905. [DOI] [PubMed] [Google Scholar]
- 80.Purali N. Fast calcium transients translate the distribution and conduction of neural activity in different regions of a single sensory neuron. Invert. Neurosci. 2017;13 doi: 10.1007/s10158-017-0201-3. [DOI] [PubMed] [Google Scholar]
- 81.Wang S., Noda K., Yang Y., Shen Z., Chen Z., Ogata Y. Calcium hydroxide regulates transcription of the bone sialoprotein gene via a calcium-sensing receptor in osteoblast-like ROS 17/2.8 cells. Eur. J. Oral Sci. 2018;126:13–23. doi: 10.1111/eos.12392. [DOI] [PubMed] [Google Scholar]
- 82.Hou T., Liu W., Shi W., Ma Z., He H. Desalted duck egg white peptides promote calcium uptake by counteracting the adverse effects of phytic acid. Food Chem. 2017;219:428–435. doi: 10.1016/j.foodchem.2016.09.166. [DOI] [PubMed] [Google Scholar]
- 83.Rodrigues F.C., Barbosa Castro A.S., Rodrigues V.C., Fernandes S.A., Filomeno Fontes E.A., de Oliveira T.T., Duarte Martino H.S., de Luces Fortes Ferreira C.L. Yacon flour and bifidobacterium longum modulate bone health in rats. J. Med. Food. 2012;15:664–670. doi: 10.1089/jmf.2011.0296. [DOI] [PubMed] [Google Scholar]
- 84.Scholz-Ahrens K.E., Ade P., Marten B., Weber P., Timm W., Aςil Y., Glüer C.-C., Schrezenmeir J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 2007;137:838S–846S. doi: 10.1093/jn/137.3.838S. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y., Zeng T., Wang S.-E., Wang W., Wang Q., Yu H.-X. Fructo-oligosaccharides enhance the mineral absorption and counteract the adverse effects of phytic acid in mice. Nutrition. 2010;26:305–311. doi: 10.1016/j.nut.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 86.Scholz-Ahrens K.E., Schaafsma G., van den Heuvel E.G., Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am. J. Chin. Nutr. 2001;73:459s–464s. doi: 10.1093/ajcn/73.2.459s. [DOI] [PubMed] [Google Scholar]
- 87.Scholz-Ahrens K.E., Schrezenmeir J. Inulin, oligofructose and mineral metabolism-experimental data and mechanism. Br. J. Nutr. 2002;87:179S–S186. doi: 10.1079/BJN/2002535. [DOI] [PubMed] [Google Scholar]
- 88.Afsana K., Shiga K., Ishizuka S., Hara H. Ingestion of an indigestible saccharide, difructose anhydride III, partially prevents the tannic acid-induced suppression of iron absorption in rats. J. Nutr. 2003;133:3553–3560. doi: 10.1093/jn/133.11.3553. [DOI] [PubMed] [Google Scholar]
- 89.Whisner C.M., Castillo L.F. Prebiotics, bone and mineral metabolism. Calcif. Tissue Int. 2017 doi: 10.1007/s00223-017-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCabe L., Britton R.A., Parameswaran N. Prebiotic and probiotic regulation of bone health: Role of the intestine and its microbiome. Curr. Osteoporos. Rep. 2015;13:363–371. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 92.Yamauchi K.-E. Review on chicken intestinal villus histological alterations related with intestinal function. J. Poult. Sci. 2002;39:229–242. doi: 10.2141/jpsa.39.229. [DOI] [Google Scholar]
- 93.Potten C.S., Loeffler M. A comprehensive model of the crypts of the small intestine of the mouse provides insight into the mechanisms of cell migration and the proliferation hierarchy. J. Theor. Biol. 1987;127:381–391. doi: 10.1016/S0022-5193(87)80136-4. [DOI] [PubMed] [Google Scholar]
- 94.Xu Z., Hu C., Xia M., Zhan X., Wang M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- 95.Fan Y., Croom J., Christensen V., Black B., Bird A., Daniel L., McBride B., Eisen E. Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poult. Sci. 1997;76:1738–1745. doi: 10.1093/ps/76.12.1738. [DOI] [PubMed] [Google Scholar]
- 96.Samanya M., Yamauchi K.-E. Histological alterations of intestinal villi in chickens fed dried bacillus subtilis var. Natto. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;133:95–104. doi: 10.1016/S1095-6433(02)00121-6. [DOI] [PubMed] [Google Scholar]
- 97.Bäckhed F., Fraser C.M., Ringel Y., Sanders M.E., Sartor R.B., Sherman P.M., Versalovic J., Young V., Finlay B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell. Host Microb. 2012;12:611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Hsu C.-K., Liao J.-W., Chung Y.-C., Hsieh C.-P., Chan Y.-C. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J. Nutr. 2004;134:1523–1528. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- 99.Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., van den Brandt P.A., Stobberingh E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 100.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Consortium H.M.P. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486 doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Partida-Rodriguez O., Serrano-Vazquez A., Nieves-Ramirez M.E., Moran P., Rojas L., Portillo T., Gonzalez E., Hernandez E., Finlay B.B., Ximenez C. Human intestinal microbiota: Interaction between parasites and the host immune response. Arch. Med. Res. 2017 doi: 10.1016/j.arcmed.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Z., Li D., Refaey M.M., Xu W. High spatial and temporal variations of microbial community along the southern catfish gastrointestinal tract: Insights into dynamic food digestion. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu X.Y., Zhong T., Pandya Y., Joerger R.D. 16s rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 2002;68:124–137. doi: 10.1128/AEM.68.1.124-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong J.M., de Souza R., Kendall C.W., Emam A., Jenkins D.J. Colonic health: Fermentation and short chain fatty acids. J. Chin. Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 108.Lutz T., Scharrer E. Effect of short-chain fatty acids on calcium absorption by the rat colon. Exp. Physiol. 1991;76:615–618. doi: 10.1113/expphysiol.1991.sp003530. [DOI] [PubMed] [Google Scholar]
- 109.Weaver C.M., Martin B.R., Story J.A., Hutchinson I., Sanders L. Novel fibers increase bone calcium content and strength beyond efficiency of large intestine fermentation. J. Agric. Food Chem. 2010;58:8952–8957. doi: 10.1021/jf904086d. [DOI] [PubMed] [Google Scholar]
- 110.Trinidad T.P., Wolever T., Thompson L.U. Effect of acetate and propionate on calcium absorption from the rectum and distal colon of humans. Am. J. Chin. Nutr. 1996;63:574–578. doi: 10.1093/ajcn/63.4.574. [DOI] [PubMed] [Google Scholar]
- 111.Yang L.-C., Wu J.-B., Lu T.-J., Lin W.-C. The prebiotic effect of anoectochilus formosanus and its consequences on bone health. Br. J. Nutr. 2013;109:1779–1788. doi: 10.1017/S0007114512003777. [DOI] [PubMed] [Google Scholar]
- 112.Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—Implications for broiler chicken health and performance1. J. Appl. Poult. Res. 2013;22:647–658. doi: 10.3382/japr.2013-00742. [DOI] [Google Scholar]
- 113.Uni Z., Tako E., Gal-Garber O., Sklan D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult. Sci. 2003;82:1747–1754. doi: 10.1093/ps/82.11.1747. [DOI] [PubMed] [Google Scholar]
- 114.Noy Y., Sklan D. Yolk utilisation in the newly hatched poult. Br. Poult. Sci. 1998;39:446–451. doi: 10.1080/00071669889042. [DOI] [PubMed] [Google Scholar]
- 115.Modulation of Immunity Genes through in Ovo Supplemented Amino Acids in Broiler Chickens. [(accessed on 26 March 2018)]; Available online: https://www.researchgate.net/publication/258255600_Modulation_of_Immunity_Genes_through_in_ovo_Supplemented_Amino_Acids_in_Broiler_Chickens.
- 116.Bhattacharya D., Boppana V., Roy R., Roy J. Method for Automated Design of Integrated Circuits with Targeted Quality Objectives Using Dynamically Generated Building Blocks. 7,225,423. U.S. Patent. 2007 May 29;
- 117.Schley P., Field C. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 2002;87:S221–S230. doi: 10.1079/BJN/2002541. [DOI] [PubMed] [Google Scholar]
- 118.Galdeano C.M., Perdigon G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 2004;97:673–681. doi: 10.1111/j.1365-2672.2004.02353.x. [DOI] [PubMed] [Google Scholar]
- 119.Bernot A., Auffray C. Primary structure and ontogeny of an avian CD3 transcript. Proc. Natl. Acad. Sci. USA. 1991;88:2550–2554. doi: 10.1073/pnas.88.6.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Igyártó B., Nagy N., Magyar A., Oláh I. Identification of the avian B-cell-specific Bu-1 alloantigen by a novel monoclonal antibody. Poult. Sci. 2008;87:351–355. doi: 10.3382/ps.2007-00365. [DOI] [PubMed] [Google Scholar]
- 121.Madej J., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2016;95:19–29. doi: 10.3382/ps/pev291. [DOI] [PubMed] [Google Scholar]
- 122.Foye O., Ferket P., Uni Z. The effects of in ovo feeding arginine, β-hydroxy-β-methyl-butyrate, and protein on jejunal digestive and absorptive activity in embryonic and neonatal turkey poults. Poult. Sci. 2007;86:2343–2349. doi: 10.3382/ps.2007-00110. [DOI] [PubMed] [Google Scholar]