Abstract
Variegated coleus (Coleus blumei Benth.) plants were exposed to a restricted water supply for 21 d. The relative water content in leaf tissues was reduced from 80% (control) to 60% (drought-stressed). Under drought conditions, the stomatal conductance and leaf photosynthetic rate were reduced. In green leaf tissues, drought stress also greatly decreased the diurnal light-period levels of the raffinose family oligosaccharides (RFOs) stachyose and raffinose, as well as those of other non-structural carbohydrates (galactinol, sucrose, hexoses, and starch). However, drought had little effect on soluble carbohydrate content of white, non-photosynthetic leaf tissues. In green tissues, galactinol synthase activity was depressed by drought stress. An accumulation of O-methyl-inositol was also observed, which is consistent with the induction of myoinositol-6-O-methyltransferase activity seen in the stressed green tissues. In source tissues, RFO metabolism is apparently reduced by drought stress through a combined effect of decreased photosynthesis and reduced galactinol synthase activity. Moreover, a further reduction in RFO biosynthesis may have been due to a switch in carbon partitioning to O-methyl-inositol biosynthesis, creating competition for myoinositol, a metabolite shared by both biochemical pathways.
Water deficit stress, which can arise from many environmental conditions, including drought, salinity, or extremes in temperature, induces numerous biochemical and physiological responses in plants (for review, see Hanson and Hitz, 1982). Under water deficit conditions, plant growth is substantially reduced, partly because lower turgor pressure in the cells results in a lower cell expansion rate. Plant growth under water deficit can also be affected by changes in gene expression, leading to the synthesis and activation of novel proteins under water deficit conditions (Shinozaki and Yamaguchi-Shinozaki, 1997). A change in the plant growth rate, regardless of the cause, will also be reflected in a change in carbon partitioning between source and sink organs of the plant. However, our understanding of the effects of stress on carbon partitioning processes is still very poor.
The production and partitioning of metabolically important non-structural carbohydrates (sugars, starch, and sugar alcohols) have been found to be altered by drought in a number of different ways (Vyas et al., 1985; Jacomini et al., 1988; Keller and Ludlow, 1993; Volaire and Thomas, 1995). Some of the observed effects of water deficit may be the result of a stress-induced impairment of the biosynthetic machinery required for photosynthetic assimilation of carbon and/or its conversion to metabolically usable forms. In other cases, stress-induced changes may reflect adaptations for stress tolerance. For example, cyclic carbohydrates (cyclitols) have been reported to accumulate during drought (Keller and Ludlow, 1993; Wanek and Richter, 1997), a linear polyhydric alcohol, mannitol, has been found to increase in response to salt stress (Pharr et al., 1995), and transgenic tobacco overproducing mannitol exhibited greater salt tolerance than non-transformed plants (Tarczynski et al., 1993). These compounds have been generally proposed to act as compatible solutes or osmoprotectants to allow osmotic adjustment of plant cells exposed to water deficit. Other proposed roles include free radical scavenging, protection from photoinhibition, and metabolic detoxification (Orthen et al., 1994; Pharr et al., 1995), all of which may help a plant to survive various environmental stresses. Additionally, non-protective roles, such as functioning as storage reserves during stress (Hanson and Hitz, 1982), have also been proposed for these compounds.
Most research on water deficit stress has been done using Suc-translocating species, and it has generally been shown that carbohydrate levels (Suc, hexose, and starch) are altered by drought (Keller and Ludlow, 1993). The effects of water deficit on species that translocate raffinose family oligosaccharides (RFOs), however, have not been fully investigated. Since these oligosaccharides are thought to be involved in dessication tolerance in seeds (Koster and Leopold, 1988) and in low-temperature tolerance in cold-acclimated leaves (Bachmann et al., 1995), the study of water deficit stress effects on RFO metabolism in RFO-translocating plants is warranted. The present study was therefore conducted to examine the effects of drought stress on diurnal RFO metabolism in source and sink leaf tissues of a variegated variety of coleus (Coleus blumei Benth).
Coleus, a heat- and drought-tolerant ornamental, is an ideal experimental system because the variegated leaf (white center with green borders) provides a very simple but elegant source-sink system. Previous work from our laboratory has shown that RFO metabolism in coleus source and sink leaf tissues is altered during prolonged salinity stress (Gilbert et al., 1997). Moreover, the synthesis of a novel O-methyl-inositol (OMI) was induced by salinity stress (Gilbert et al., 1997), which may represent yet another carbohydrate-linked stress tolerance mechanism (Vernon et al., 1993). Both the OMI and the RFO metabolic pathways share myoinositol as a common biochemical intermediate, but the means by which myoinositol is partitioned between these two competing pathways during a stress period is not clear. The aims of the present study were therefore to examine the effects of water deficit on diurnal patterns of carbohydrate metabolism in coleus source and sink leaf tissues and to determine the interactions between RFO and OMI metabolism during drought stress in this species.
MATERIALS AND METHODS
Plant Material
Mature greenhouse-grown coleus (Coleus blumei Benth. cv Fairway White) plants were used for all experiments. Ten rooted clonal cuttings were potted in a soil:sand:peat mix (1:1:1, v/v) and kept in a greenhouse under natural lighting conditions and approximately 30°C/20°C day/night temperatures. Plants were watered daily with one-half-strength Hoagland solution and grown under these conditions for 2 months, by which time they had reached a height and canopy diameter of about 2 feet, were well branched, and had abundant mature source leaves (>60 per plant). For the drought stress treatment, five plants were stressed by lightly watering once at the start of the week and then depriving them of water for the rest of the week. This process was repeated weekly for a 3-week period, and the described experiments were run at the end of week 3. The five control plants were watered daily with tap water throughout 21-d period.
Photosynthesis Measurements
Photosynthetic rate and stomatal conductance were measured hourly from 6 am (early morning) to 6 pm (beginning of the dark period) using a portable photosynthesis system (model 6200, LI-COR, Lincoln, NE), operated essentially as described previously (Gilbert et al., 1997). The leaf was allowed to equilibrate in the leaf chamber for at least 1 min before a measurement was taken. Five leaves were selected at random from plants in each treatment and the final leaf area was corrected by subtracting the leaf area corresponding to white (non-photosynthetic) tissues.
Determination of Relative Water Content
Using a conventional hand-held paper punch, 15 leaf discs (7-mm diameter; average fresh weight: control, 86.9 ± 0.1 mg and drought, 78.6 ± 0.1 mg) were collected from green areas of mature leaves from well-watered and water-restricted plants. Samples were taken from randomly selected mature leaves every 2 h from 6 am to 6 pm for determination of leaf tissue water status, which was evaluated by calculating the relative water content (Turner, 1981). After fresh weights were measured, the leaf discs were floated on degassed distilled water for 12 h to allow rehydration, blotted, then reweighed to give the rehydrated fresh weight. Samples were then oven-dried at 85°C for 12 h and the dry weight was determined. The relative water content of leaf tissues was calculated by the formula:
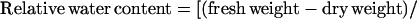 |
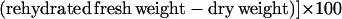 |
Diurnal Carbohydrate Analysis
Ten leaf discs were excised as described above from green and white areas of randomly selected leaves from control and stressed plants. Samples were collected every 2 h from 6 am until 6 pm and were immediately placed in foil packets on dry ice for transport back to the laboratory. Samples were stored frozen at −20°C until analysis. Soluble sugars were extracted with 80% (v/v) ethanol, deionized, and analyzed by HPLC (Sugar-Pak I column, Waters, Milford, MA), essentially as described previously (Madore et al., 1988). Starch remaining in the extracted discs was digested with amyloglucosidase and released Glc was quantified spectrophotometrically using a commercially available Glc detection kit (HK 20, Sigma, St. Louis) as described previously (Madore, 1990).
Enzyme Extraction and Assay
Leaf tissue (1 g) from green areas of mature leaves was excised with a razor blade from control and stressed plants. The leaf material was ground on ice using a mortar and pestle in 2 mL of grinding buffer (for galactinol synthase [GS]: 50 mm HEPES, 50 mm ascorbic acid, 1 mm dithiothreitol [DTT], 1 mm MnCl2, and 10% [v/v] ethylene glycol [pH 7.5]; for myo-inositol 6-O-methyl transferase [IMT]: 150 mm Tris-HCl, 100 mm NaCl, 20 mm EDTA, 10 mm 2-mercaptoethanol, and 5 mm DTT [pH 8.0]). The extracts were filtered through cheesecloth, then transferred to microfuge tubes and centrifuged for 2 min at 10,000g. Portions (1 mL) of the supernatant were desalted on Sephadex G25 columns equilibrated with grinding buffer. Protein content was determined by the Bradford method (Bradford, 1976).
GS activity was determined as previously described (Smith et al., 1991) with a few modifications. The reaction was carried out in a microfuge tube in a final volume of 100 μL, with the following concentrations of substrates: 20 mm myoinositol, 3 mm DTT, 10 mm MnCl2, 2 mm UDP-Gal containing 30,000 dpm UDP-[14C]Gal (specific activity = 59 mCi mmol−1; ICN, Costa Mesa, CA). Blank reactions were run by adding water in place of the myoinositol. The reaction was incubated at 30°C for 1 h and stopped by boiling for 1 min. Unreacted UDP-[14C]Gal was removed by adding 0.1 g of Dowex-1 (formate) resin. This mixture was placed on an orbital shaker for 20 min. One milliliter of water was added and the tube was vortexed vigorously and centrifuged for 1 min to pellet the resin containing unreacted UDP-[14C]Gal. The radioactivity in 0.5 mL of the supernatant was counted with a scintillation counter. The amount of galactinol formed was determined from the specific activity of the UDP-[14C]Gal in the reaction mixture.
IMT activity was assayed using the modified radioisotope assay as previously described (Vernon et al., 1993; Gilbert et al., 1997). Sixty microliters of enzyme was assayed in a microfuge tube containing 100 μL of a reaction mixture consisting of 50 mm Tris-HCl, pH 8.0, 10 mm MgCl2, 10 mm myoinositol, and 0.5 mm S-adenosyl-Met (SAM) containing 20,000 dpm methyl-[14C]SAM (specific activity = 56 mCi mmol−1; ICN). Blank reactions were run by adding water in place of the myoinositol. The reactions were run at 30°C for 3 h and stopped by boiling for 1 min. Unreacted SAM was then removed by adding 0.1 g of Dowex-1 (H+) ion-exchange resin and shaking the reaction tubes on a orbital shaker for 20 min. One milliliter of water was added and the tubes were vortexed vigorously and centrifuged for 1 min in a microfuge to pellet the resin containing unreacted methyl-[14C]SAM. The radioactivity in 0.5 mL of the supernatant was then determined by scintillation counting, and the amount of methyl-inositol formed was determined from the specific activity of the methyl-[14C]SAM in the reaction mix.
RESULTS
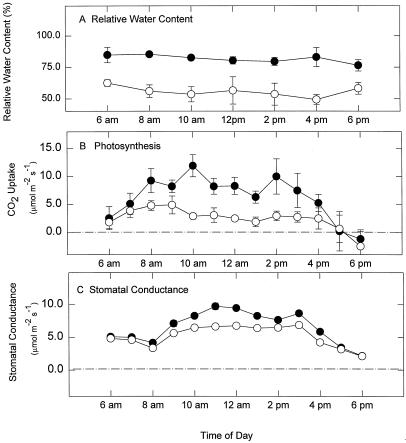
A 21-d water restriction on coleus plants decreased the leaf relative water content from a control value of 80% to 60% in the stressed plants (Fig. 1A). Although the stressed plants showed wilting at the time of sampling, the relative water content of the stressed plants had not yet reached the lethal level, because the plants quickly recovered to full turgor after re-watering (data not shown). Relative water contents of both control and stressed plants were unchanged throughout the whole daytime period from 6 am to 6 pm (Fig. 1A). Water restriction also resulted in an approximately 3-fold decrease in the maximum photosynthetic rate, from 6.25 μmol m−2 s−1 (control) to about 2.5 μmol m−2 s−1 (droughted) (Fig. 1B). The general pattern of photosynthetic activity throughout the day period was similar in both treatments (Fig. 1B), but droughted plants had a consistently lower photosynthetic rate than control plants at all experimental time points (Fig. 1B). Stomatal conductance of the stressed plants was also somewhat reduced compared with control plants, but followed the same general daytime photosynthetic pattern (Fig. 1C).
Figure 1.
Effects of water deficit stress on relative water content (A), photosynthetic rate (B), and stomatal conductance (C) in mature variegated coleus source leaves. Data represent the means ± se of five measurements per data point. ●, Control; ○, droughted.
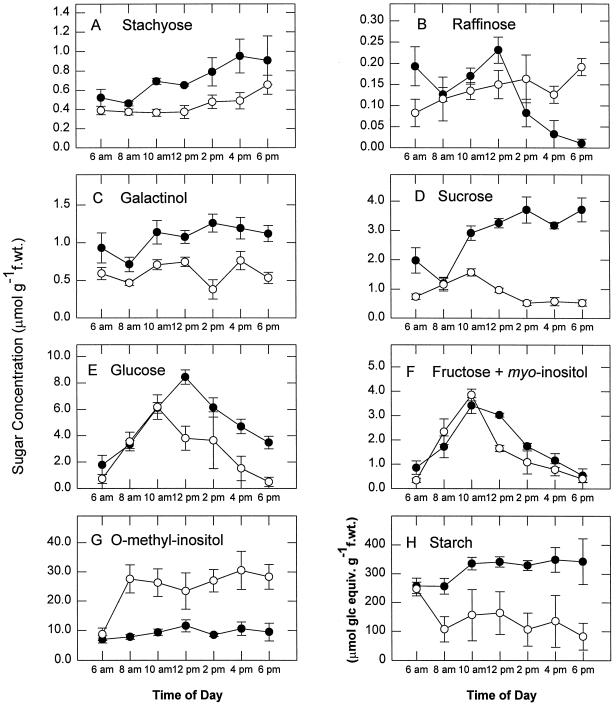
Drought stress altered the daytime patterns of non-structural carbohydrates in photosynthetic tissues (Fig. 2). In particular, levels of stachyose (Fig. 2A), galactinol (Fig. 2C), Suc (Fig. 2D), Glc (Fig. 2E), and starch (Fig. 2H), and to some extent Fru/myoinositol (Fig. 2F), were reduced by drought stress. Raffinose levels, which peaked at midday and declined to near zero levels at the end of the daytime period in control plants (Fig. 2B), remained constant throughout the daytime period in stressed plants (Fig. 2B). The most significant differences in carbohydrate levels were seen for O-methyl-inositol (Fig. 2G) which showed a 3-fold increase in the stressed plant tissues.
Figure 2.
Effects of water deficit stress on carbohydrate content of green photosynthetic tissues of mature variegated coleus source leaves. Data represent the means ± se of five measurements per data point. ●, Control; ○, droughted. f.wt., Fresh weight.
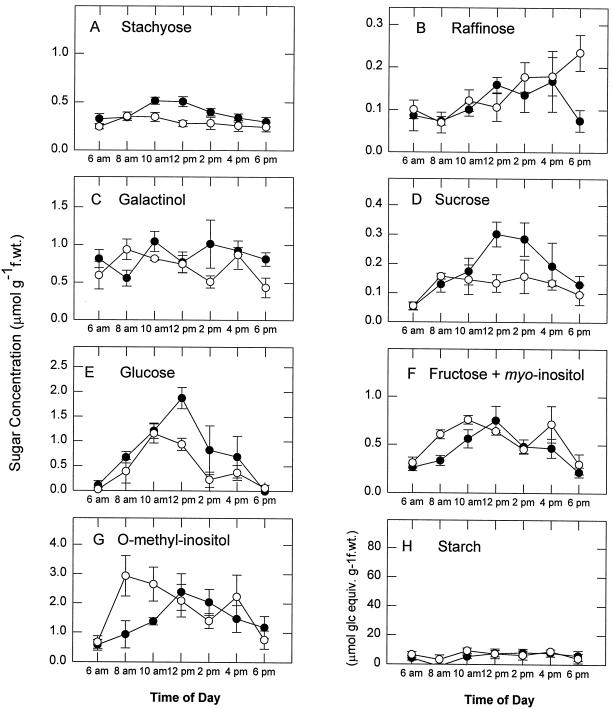
In contrast to what was observed in green source tissues, drought stress had a far less pronounced effect on overall carbohydrate levels in non-photosynthetic white leaf tissues (Fig. 3). Sink tissues contained similar levels of stachyose (Fig. 3A), raffinose (Fig. 3B), and galactinol (Fig. 3C) as were found in green source tissues, while other carbohydrates, including Suc (Fig. 3D), Glc (Fig. 3E), Fru/myoinositol (Fig. 3F), O-methyl-inositol (Fig. 3G), and starch (Fig. 3H), were present at only very low levels compared with the green tissue (Fig. 3). Drought stress reduced the midday levels of stachyose (Fig. 3A), Suc (Fig. 3D), Glc (Fig. 3E), and Fru/myoinositol (Fig. 3F) in the sink tissues, and increased levels of O-methyl-inositol in the early part of the light period (Fig. 3G).
Figure 3.
Effects of water deficit stress on carbohydrate content of white nonphotosynthetic tissues of mature variegated coleus source leaves. Data represent the means ± se of five measurements per data point. ●, Control; ○, droughted. f.wt., Fresh weight.
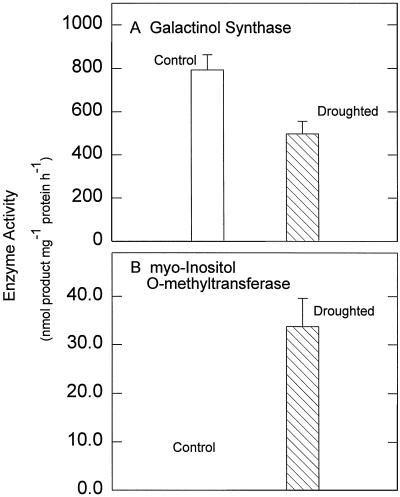
In the source tissues, the reduction in galactinol levels (Fig. 2C) levels was also reflected in a decrease in the extractable GS activity (Fig. 4A) in these tissues. Similarly, the large increase in OMI content (Fig. 2G) was reflected in a pronounced increase in extractable IMT activity in the stressed tissues (Fig. 4B).
Figure 4.
Effects of water deficit stress on activities of galactinol synthase (A) or myoinositol 6-O-methyltransferase (B) in green photosynthetic tissues of mature variegated coleus source leaves. Data represent the means ± se of five measurements per data point.
DISCUSSION
In Suc-transporting species, Suc and hexose levels increase, while the starch level is decreased by water stress (Vyas et al., 1985; Pelleschi et al., 1997; Vu et al., 1998). The increase in Suc and hexose amounts was suggested to be due to the increase in starch hydrolysis and the synthesis of Suc. Suc and hexose accumulation was proposed to play a role in osmotic adjustment in these species (Westgate and Boyer, 1985). Although starch levels were reduced in coleus in response to drought stress, no similar rise in Suc or hexoses was observed. Instead, the photosynthetic tissues accumulated OMI.
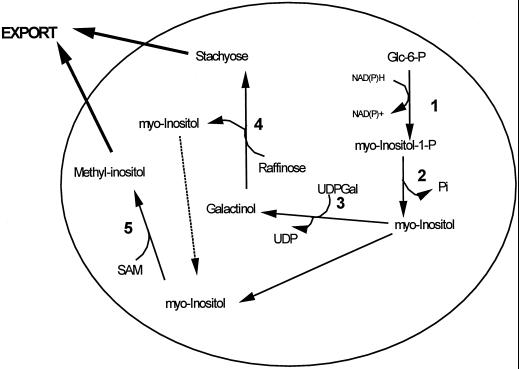
In coleus leaf tissues RFO metabolism was significantly altered by the imposition of drought stress. The observed reduction in RFO levels, and carbohydrate levels in general, may be at least partially explained by the reduction of overall photosynthesis rates in the stressed plants. However, drought stress also had direct effects on the activity of GS, and the lowered activity of this enzyme observed under stress conditions would also be expected to lower overall RFO levels. Additionally, the data presented here indicate that the induction of OMI synthesis, which diverts myoinositol away from the RFO biosynthetic pathway, may also be part of the process leading to the observed reduction in RFO levels under stress.
As indicated in Figure 5, conversion of myoinositol to its methyl derivative effectively removes it from the pathway leading to galactinol biosynthesis and therefore leads to a reduction in galactinol synthesis. Thus, activation of the IMT enzyme, coupled with the observed drought-induced depression of GS activity, would be expected to substantially impact the ability of these leaves to synthesize RFOs by limiting the formation of galactinol. It should also be noted that, since crude enzyme preparations were utilized in this study and no attempt was made to optimize the enzyme assay conditions for either enzyme, the values reported here are only estimates of the actual enzyme activities present in the leaves. Determination of the true magnitude of the differences in enzyme activities between control and water-restricted tissues will await further studies with purified enzymes from these sources.
Figure 5.
Hypothetical model depicting the effects of water deficit stress on carbon flow between raffinose family oligosaccharides and O-methyl-inositol in carbohydrate metabolism in green photosynthetic tissues of mature variegated coleus source leaves. 1, Myoinositol-1-P synthase; 2, myoinositol-1-P phosphatase; 3, galactinol synthase; 4, stachyose synthase; 5, myoinositol 6-O-methyltransferase.
On a molar basis, OMI accounted for over 60% of the total soluble sugar recovered from the stressed green tissues at midday. The level of OMI observed in this study, approximately 30 μmol g−1 fresh weight (or approximately 300 μmol g−1 dry weight), was more than 30-fold higher than levels of either the RFOs or galactinol in the same tissues. In other research, pinitol in soybean leaves (Guo and Ossterhuis, 1997) has been reported to accumulate to a level of approximately 800 μmol g−1 dry weight, while in Vigna umbellata leaves (Wanek and Richter, 1997) d-ononitol was reported at levels of 88 μmol g−1 dry weight following a 9-d drought stress treatment. These authors suggested that cyclitols act as osmolytes, allowing osmotic adjustment to occur during dessication stress. The levels of these cyclitols reported in these plants were similar to those reported here for drought-stressed coleus. Assuming an even cellular distribution of the OMI sugar reported here, this would correspond to an overall concentration of about 30 mm in coleus leaf tissues. The actual concentration would of course be much higher, perhaps on the order of 300 mm, if this sugar was restricted to the cytosol. However, we also know from previous studies (Gilbert et al., 1997) that OMI is translocated in the phloem. The sieve tubes of the leaf minor vein phloem are therefore likely to contain a substantial proportion of the total leaf OMI. Thus, not knowing the exact cellular distribution of OMI in the leaf, it is difficult to distinguish the true importance of the leaf cyclitols in osmotic adjustment during water deficit conditions. It is equally likely that OMI serves some other role in addition to its putative role as an osmolyte.
Many biotic and abiotic stresses are now thought to exert their effects by causing an enhanced generation of oxygen free radicals, such as the highly reactive hydroxyl radical. Water deficit stress is in fact a series of complex processes involving not only dehydration stress, but also other stresses imparted by depriving leaf tissues of water, including photooxidative damage, changes in ion concentration, and increased heat load, all of which may give rise to processes that generate oxygen free radicals. Cyclitols such as 1-d-1-O-methyl-muco-inositol, pinitol (3-O-methyl-d-chiro-inositol) and ononitol (1d-4-O-methyl-myoinositol) have been reported to be capable of scavenging hydroxyl radicals (Orthen et al., 1994). Myoinositol, a ubiquitous plant cyclitol and one of the RFO precursors, has also been found to be an effective hydroxyl radical scavenger (Smirnoff and Cumbes, 1989). Since these compounds accumulate in response to stress conditions, it is highly likely that cyclitols and the related linear polyhydroxy alcohols perform important biological functions involving oxygen radical scavenging and detoxification in plants (Pharr et al., 1995).
In many biological systems, free radical generation is actually capitalized upon as a means of destroying cellular pathogens. Many pathogenic fungi can in turn circumvent this defense system by synthesizing polyhydroxy compounds such as the polyol mannitol to counteract these free radicals (Chaturvedi et al., 1996). Recently, it has been shown that plants invaded by fungal pathogens may be induced to produce mannitol-degrading enzymes that in turn counteract this fungal defense system (Jennings et al., 1998). Cyclitol production may therefore represent a ubiquitous first line of defense aimed at preventing free radical oxidative damage in biological organisms. However, if the primary role of cyclitols is to scavenge free radicals, it is curious that coleus and also other species such as Mesembryanthemum crystallinum (Vernon and Bohnert, 1992) produce a methylated inositol derivative if unmethylated compounds are equally effective free radical scavengers.
An explanation for this may lie in the previous observation that OMI is phloem mobile and readily catabolized by sink tissues (Gilbert et al., 1997). Production of OMI requires SAM, a major substrate in the ethylene biosynthetic pathway, as a methyl donor (Bohnert and Jensen, 1996). OMI synthesis and phloem transport may therefore decelerate leaf senescence under stress conditions by removing methyl groups from potentially deleterious pathways, such as those leading to synthesis of stress ethylene, from the source leaf tissues (Gilbert et al., 1997). Thus, OMI induced by drought stress may act at several levels in plant stress metabolism, serving not only as an osmoprotectant and free radical scavenger, as was previously thought, but also as a phloem-mobile anti-senescence metabolite.
The effects of water deficit on carbohydrate metabolism in coleus appeared to differ in key ways from the effects of salinity stress previously observed (Gilbert et al., 1997). Most notable was the absence of any induction of high-molecular-mass RFOs by water stress, which was readily observed in response to salinity stress (Gilbert et al., 1997) and has been reported in response to low temperatures (Bachmann et al., 1994). The high-molecular-mass sugars are localized within the vacuole (Bachmann et al., 1995) and are part of an overwintering strategy in several members of the family Lamiaceae, to which coleus also belongs. Additionally, both the activity and mRNA message for GS have been reported to increase in response to low temperature (Liu et al., 1998), indicating an up-regulation of RFO metabolism due to cold.
RFOs have been proposed to be osmoprotectants when plant tissues are exposed to cold (Bachmann et al., 1995) and desiccation (Koster and Leopold, 1988). In the present study, however, both RFO levels and GS activity were depressed by drought stress, which suggests that for drought stress alone RFOs may not play a direct role in inducing tolerance in vegetative tissues, although they may be important in allowing plant tissues to achieve the level of dessication required in a dry seed (Koster and Leopold, 1988). Instead, production of these sugars in response to salinity stress or low temperature in vegetative tissues may simply represent a mechanism for carbon storage in the vacuole. In the case of salinity stress in particular, conversion of sugars to high-molecular-mass forms would be a suitable strategy for carbon storage in a vacuole already osmotically burdened by a large accumulation of salts and ions (Gilbert et al., 1997).
In summary, water deficit stress causes pronounced effects on carbohydrate metabolism in coleus source leaf tissues. Photosynthetic declines result in significantly less carbon being fixed overall, with the result that there is less carbon available to form substantial starch storage reserves. Secondly, far less carbon is channeled to form the RFO transport sugars, not only because less carbon is being fixed photosynthetically, but also because enzymes such as GS, which catalyze RFO synthesis, are also apparently down-regulated by water stress. The consequence of water deficit is therefore a loss of storage reserves and translocatable RFOs to support sink growth and development. However, the benefit of these alterations in carbon metabolism appears to be the switch toward another myoinositol-utilizing pathway, which leads to the production of OMI, a valuable stress-tolerance molecule.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Eugene A. Nothnagel for help with the OMI analysis and Rick Miranda for helping with the photosynthesis measurements.
Footnotes
This work was supported in part by a U.S. Department of Agriculture Competitive Research Grant (no. 9601050 to M.A.M.) and by a graduate student fellowship from the government of Thailand (to W.P.).
LITERATURE CITED
- Bachmann M, Inan C, Keller F. Raffinose oligosaccharide storage. In: Madore MA, Lucas WJ, editors. Carbon Partitioning and Source-Sink Interactions in Plants. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 215–225. [Google Scholar]
- Bachmann M, Matile P, Keller F. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Cold acclimation, translocation and sink to source transition: discovery of the chain elongation enzyme. Plant Physiol. 1994;105:1335–1345. doi: 10.1104/pp.105.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Jensen RG. Strategies for engineering water stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaturvedi VP, Wong B, Newman SL. Oxidative killing of Cryptococcus neoformans by human neutrophils: evidence that fungal mannitol protects by scavenging oxidants. J Immunol. 1996;156:3836–3840. [PubMed] [Google Scholar]
- Gilbert GA, Wilson C, Madore MA. Root-zone salinity alters raffinose oligosaccharide metabolism and transport in coleus. Plant Physiol. 1997;115:1267–1276. doi: 10.1104/pp.115.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Ossterhuis DM. Effect of water-deficit stress and genotypes on pinitol occurrence in soybean plants. Environ Exp Bot. 1997;37:147–152. [Google Scholar]
- Hanson AD, Hitz WD. Metabolic response of mesophytes to plant water deficits. Annu Rev Plant Physiol. 1982;33:163–203. [Google Scholar]
- Jacomini E, Bertani A, Mapelli S. Accumulation of polyethylene glycol 6000 and its effects on water content and carbohydrate level in water-stressed tomato plants. Can J Bot. 1988;66:970–973. [Google Scholar]
- Jennings DB, Ehrenshaft M, Pharr DM, Williamson JD. Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc Natl Acad Sci USA. 1998;95:15129–15133. doi: 10.1073/pnas.95.25.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F, Ludlow MM. Carbohydrate metabolism in drought-stressed leaves of pigeonpea (Cajanus cajan L.) J Exp Bot. 1993;44:1351–1359. [Google Scholar]
- Koster KL, Leopold AC. Sugars and dessication tolerance in seeds. Plant Physiol. 1988;88:829–832. doi: 10.1104/pp.88.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJJ, Krenz DC, Galvez AF, De Lumen BO. Galactinol synthase (GS): increased enzyme activity and levels of mRNA due to cold and desiccation. Plant Sci. 1998;134:11–20. [Google Scholar]
- Madore MA. Carbohydrate metabolism in photosynthetic and nonphotosynthetic tissues of variegated leaves of Coleus blumei Benth. Plant Physiol. 1990;93:617–622. doi: 10.1104/pp.93.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore MA, Mitchell DE, Boyd CM. Stachyose synthesis in source leaf tissues of the CAM plant Xerosicyos danguyi H. Humb Plant Physiol. 1988;87:588–591. doi: 10.1104/pp.87.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthen B, Popp M, Smirnoff N. Hydroxyl radical scavenging properties of cyclitols. Proc R Soc Edinb Sect B (Biol) 1994;102:269–272. [Google Scholar]
- Pelleschi S, Rocher JP, Prioul JL. Effects of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997;20:493–503. [Google Scholar]
- Pharr DM, Stoop JMH, Williamson JD, Studer Feusi ME, Massel MO, Conkling MA. The dual role of mannitol as osmoprotectant and photoassimilate in celery. Hortscience. 1995;30:1182–1188. [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- Smith PT, Kuo TM, Crawford CG. Purification and characterization of galactinol synthase from mature zucchini squash leaves. Plant Physiol. 1991;96:693–698. doi: 10.1104/pp.96.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ. Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science. 1993;259:508–510. doi: 10.1126/science.259.5094.508. [DOI] [PubMed] [Google Scholar]
- Turner NC. Techniques and experimental approaches for the measurement of plant water status. Plant Soil. 1981;58:339–366. [Google Scholar]
- Vernon DM, Bohnert HJ. A novel methyltransferase induced by osmotic stress in the facultative halophyte Mesembryanthemum crystallinum. EMBO J. 1992;11:2077–2085. doi: 10.1002/j.1460-2075.1992.tb05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon DM, Tarczynski MC, Jensen RG, Bohnert HJ. Cyclitol production in transgenic tobacco. Plant J. 1993;4:199–205. [Google Scholar]
- Volaire F, Thomas H. Effects of drought on water relations, mineral uptake, water-soluble carbohydrate accumulation and survival of two contrasting populations of cocksfoot (Dactylis glomerata L.) Ann Bot. 1995;75:513–524. [Google Scholar]
- Vu JCV, Baker JT, Pennanen AH, Allen LH, Jr, Bowes G, Boote KJ. Elevated CO2 and water deficit effects on photosynthesis, ribulose bisphosphate carboxylase-oxygenase, and carbohydrate metabolism in rice. Physiol Plant. 1998;103:327–339. [Google Scholar]
- Vyas SP, Kathju S, Garg BK, Lahiri AN. Performance and metabolic alterations in Sesamum indicum under different intensities of water stress. Ann Bot. 1985;56:323–332. [Google Scholar]
- Wanek W, Richter A. Biosynthesis and accumulation of d-ononitol in Vigna umbellata in response to drought stress. Physiol Plant. 1997;101:416–424. [Google Scholar]
- Westgate ME, Boyer JS. Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize [Zea mays L.] Planta. 1985;164:540–549. doi: 10.1007/BF00395973. [DOI] [PubMed] [Google Scholar]