Abstract
Background
Allogeneic hematopoietic stem cell transplantation (allo-HCT) remains the only potentially curative treatment option for relapsed follicular lymphoma (FL), yet questions remain on the optimal timing. We have analysed long-term outcomes and associated factors among recipients of allo-HCT with FL.
Patients and Methods
Patients with relapsed FL who received an allo-HCT from 2001 to 2011 from an HLA-matched donor were included. Outcome analyses for overall (OS) and progression-free survival (PFS), transplant related mortality (TRM) and disease relapse/progression were calculated. Multivariate analysis was performed to determine factors associated with outcomes and a prognostic score for treatment failure was developed in a subset analysis of patients.
Results
1,567 patients with relapsed FL were included; median follow up was of 55 months. Five-year probabilities of OS and PFS were 61% and 52%. Five-year cumulative incidences of disease progression/relapse and TRM were 29% and 19%. Chemoresistant disease, older age, heavily pre-treated patients, poor performance status and myeloablative protocols were predictors for worse survival. Prognostic score using age, lines of prior therapy, disease status and performance status stratified three groups with 5-year PFS of 68%, 53% and 46% and 5-year OS of 80%, 62% and 50% for low, intermediate and high risks respectively.
Conclusions
Allo-HCT should be considered in patients with relapsed FL and available HLA matched donors. Outcomes are better in earlier phases of the disease and reduced intensity conditionings should be preferred. The prognostic score presented here can assist in counselling patients and deciding the timing to proceed to transplant.
Keywords: Follicular Lymphoma, Allogeneic Hematopoietic Stem Cell Transplantation, Prognostic Risk Score, Unrelated Donors, Reduced Intensity Conditioning Protocols
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) remains the only potentially curative therapy for patients with relapsed follicular lymphoma (FL) in spite of the introduction of novel agents. Allo-HCT is often associated with lower disease relapse compared to autologous transplant (auto-HCT), but transplant related mortality (TRM) offsets this benefit.1 Retrospective analyses2-4 that compared auto-HCT vs allo-HCT as first transplant indicate that long-term disease control might favour allo-HCT because of a higher relapse risk in the autografted population not compensated by the well described higher TRM of the allogeneic procedure. Advances in supportive care, use of reduced intensity conditioning regimens (RIC) and better unrelated donor selection have resulted in an increasing use of allo-HCT in FL. The use of RIC 5-7 has yet to be shown superior to myeloablative regimens (MAC),8 however it allows patients who are otherwise not candidates for allo-HCT to undergo this procedure. Further expansion in the number of candidates for allo-HCT is limited by the availability of HLA-matched donors. Unrelated donor allo-HCT (URD-HCT) in lymphomas is also associated with long-term disease control. Recipients of URD-HCT often have more advanced disease at time of transplantation.9,10 In spite of all that, timing of transplantation and the optimal patient population still remain to be defined; moreover, prognostic models able to predict allo-HCT outcomes for this histology are not currently available.
In order to better ascertain the role of allo-HCT in patients with relapsed FL, we have conducted a retrospective analysis that includes the largest cohort of patients ever studied from both international registries, the European Blood and Marrow Transplantation (EBMT) and the Center for International Bone Marrow Transplantation Research (CIBMTR); a prognostic score for treatment failure has also been developed in order to guide clinical decisions in our daily medical practice.
Patient and Methods
Data Sources
The study was performed through collaboration between EBMT and CIBMTR lymphoma disease committees. EBMT is a voluntary organization comprising 640 transplant centres mainly from Europe. Accreditation as a member centre requires submission of minimal essential data (MED-A form) from all consecutive patients to a central registry. Since 1996, accredited EBMT centres are subject to on-site audits. Informed consent was obtained locally according to regulations applicable at the time of transplantation. Since January 2003, all transplant centres have been required to obtain written informed consent prior to data registration following the Helsinki Declaration 1975. CIBMTR is a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive HCT to a Statistical Center located at the Medical College of Wisconsin in Milwaukee and at the National Marrow Program Coordinating Center in Minneapolis. The CIBMTR collects data at two levels, transplant essential data in all patients and more comprehensive data (CRF) in a subset of patients. The CIBMTR-CRF dataset was chosen to be used in the study in order to have more disease specific information for analysis. Among 1112 patients who fulfilled eligibility for this study from the CIBMTR, 452 patients reported in CRF were included in this study.
Patient Eligibility
Patients with relapsed FL who received an HLA-matched sibling or URD-HCT from January/2001 to December/2011 and reported to the EBMT or CIBMTR-CRF were included. Patients with transformed FL, umbilical cord blood, haploidentical stem cell transplantation or ex vivo T-cell depleted grafts and those where a tandem HCT was pre-planned were excluded.
Outcomes and Definitions
Histology was based on data reported to both registries. Conditioning regimens were defined as MAC or RIC according to previously established definitions.11 Disease response was evaluated following Cheson's criteria.12 Relapse or progression was considered to be chemosensitive if at least partial remission was achieved following the last course of chemotherapy before allo-HCT.
Grades II-IV acute GVHD (aGVHD) were defined according to standard criteria.13 Chronic GVHD (cGVHD) was determined by the treating physician. Performance status (PS) was defined according to the Karnofsky score criteria (Karnofsky PS).
In the URD-HCT group, donor-recipient pairs in the well-matched category according to Weisdorf et al14 were selected, which include high-resolution matching at HLA-A, -B, -C and -DRB1 (8/8).
The primary outcomes after allo-HCT included: incidences of acute and chronic GVHD; transplant-related mortality (TRM), defined as any death within the first 28 days of transplant or any death occurring after day 28 in the absence of overt disease progression; relapse/progression, the events were defined as progression or recurrence of FL; progression-free survival (PFS), defined as survival without recurrence or tumour progression; and overall survival (OS), were death of any cause is an event.
Statistical Analysis
Probabilities of PFS and OS were calculated using the Kaplan‐Meier estimator. Values for relapse/progression, TRM and GVHD were generated using cumulative incidence (CI) estimates to account for competing risks. The EBMT and CIBMTR cohorts were combined for all the analyses after confirming that all survival outcomes, TRM and disease progression were not statistically different.
Multivariate analyses using Cox proportional hazards models for overall mortality and treatment failure (1-PFS) were built using a forward stepwise approach. Among the 1,567 patients included in the primary analysis, after exclusion of patients without information on disease progression/relapse or death, 1,523 patients were tested in the multivariate analysis. Covariates analysed were: data source (CIBMTR vs EBMT), age, gender, Karnofsky PS at transplant (<80% vs ≥80%), disease stage at diagnosis (I/II vs III/IV), numbers of previous chemotherapy regimens (1-2 vs 3-4 vs ≥ 5 lines), prior auto-HCT, disease status at transplant [≥complete remission (CR2) vs Relapse vs Never in CR vs never in CR resistant vs unknown status], prior exposure to rituximab, intensity of the conditioning regimen (MAC vs RIC), donor type (HLA matched sibling vs URD), year of transplant and use of antithymocyte globulin (ATG) or alemtuzumab (yes vs no). Variables significantly associated with each outcome event were included as covariate factors in the subsequent comparisons. Proportionality assumptions were tested by adding a time-dependent variable.
The development of a model for prediction of treatment failure (1-PFS) was based on final Cox model and included a dataset with complete data on all significant covariates, all missing or unknown status were excluded. Cox regression analysis was repeated in this subset cohort and the covariates that maintained the association with the outcome were kept in the model. The cohort included 573 patients with complete information on all significant covariates: age (continuous), number of prior lines of chemotherapy (<3, 3-4 vs ≥5), disease status at time of allo-HCT (chemotherapy resistance) and Karnofsky PS (<80% vs ≥80%). The score was computed according to the magnitude of the effect between each significant covariate and treatment failure and three risk levels were developed: low, intermediate and high risk. Final probabilities by three risk groups were calculated by Kaplan-Meier estimate for OS and PFS, and cumulative incidence function for TRM and relapse/progression.
Results
Patients
Patient characteristics are shown in Table 1.
Table 1. Characteristics of patients who underwent HLA identical sibling allogeneic transplant or HLA matched unrelated donor allogeneic transplant for follicular lymphoma from 2001-2011, by registry.
| Total N (%) | CIBMTR N (%) | EBMT N (%) | P-value | |
|---|---|---|---|---|
| Number of patients | 1567 | 452 | 1115 | |
| Patient age at transplant, years | 0.062 | |||
| Median | 51 (21-74) | 50 (21-74) | 51 (24-72) | |
| 21-30 | 24 (2) | 12 (3) | 12 (1) | |
| 31-40 | 204 (13) | 67 (15) | 137 (12) | |
| 41-50 | 586 (37) | 155 (34) | 431 (39) | |
| 51-60 | 592 (38) | 175 (39) | 417 (37) | |
| ≥61 | 161 (10) | 43 (10) | 118 (11) | |
| Gender | 0.064 | |||
| Male | 935 (60) | 286 (63) | 649 (58) | |
| Female | 632 (40) | 166 (37) | 466 (42) | |
| Karnofsky score | <0.001 | |||
| <80 | 56 (4) | 35 (8) | 21 (2) | |
| ≥80 | 1005 (64) | 392 (87) | 613 (55) | |
| Missing | 506 (32) | 25 (6) | 481 (43) | |
| Disease-related | ||||
| Histology | <0.001 | |||
| Grade 1 | 455 (29) | 187 (41) | 268 (24) | |
| Grade 2 | 389 (25) | 163 (36) | 226 (20) | |
| Grade 3 | 319 (20) | 84 (19) | 235 (21) | |
| Unknown grade | 404 (26) | 18 (4) | 386 (35) | |
| Number of prior chemotherapy regimens | <0.001 | |||
| 1-2 | 291 (19) | 102 (23) | 189 (17) | |
| 3-4 | 397 (25) | 225 (50) | 172 (15) | |
| ≥5 | 172 (11) | 97 (21) | 75 (7) | |
| Missing number of previous regimens | 707 (45) | 28 (6) | 679 (61) | |
| Previous ASCT | 456 (29) | 53 (12) | 403 (36) | <0.001 |
| Interval ASCT-allo-HCT, median (range), months | 26 (1-249) | 19 (1-101) | 27 (6-249) | 0.001 |
| Disease stage at diagnosis | <0.001 | |||
| I-II | 121 (8) | 61 (13) | 60 (5) | |
| III-IV | 816 (52) | 359 (79) | 457 (41) | |
| Not available | 630 (40) | 32 (7) | 598 (54) | |
| Disease status at HCT | <0.001 | |||
| PIF sensitive | 63 (4) | 63 (14) | 0 | |
| PIF resistant | 138 (9) | 43 (10) | 95 (9) | |
| REL sensitive | 457 (29) | 139 (31) | 318 (29) | |
| REL resistant | 73 (5) | 70 (15) | 3 (<1) | |
| CR2+ | 452 (29) | 89 (20) | 363 (33) | |
| REL untreated/unknown | 315 (20) | 16 (4) | 299 (27) | |
| PIF unknown | 3 (<1) | 2 (<1) | 1 (<1) | |
| Not available | 66 (4) | 30 (7) | 36 (3) | |
| Response to therapy immediately prior to HCT | <0.001 | |||
| Sensitive | 1192 (76) | 307 (68) | 885 (79) | |
| Resistant | 258 (16) | 113 (25) | 145 (13) | |
| Unknown | 117 (7) | 32 (7) | 85 (8) | |
| Transplant-related | ||||
| Interval diagnosis to allo-HCT, median (range), months | 47 (3-363) | 42 (3-352) | 51 (4-363) | <0.001 |
| Rituximab at conditioning | 154 (10) | 92 (20) | 62 (6) | <0.001 |
| Conditioning regimens | <0.001 | |||
| Myeloablative (MA) | 365 (23) | 145 (32) | 220 (20) | |
| RIC/NMA | 1202 (77) | 307 (68) | 895 (80) | |
| Conditioning regimens | <0.001 | |||
| MAC | ||||
| TBI+Cy | 155 (10) | 79 (17) | 76 (7) | |
| BEAM ± others | 56 (4) | 4 (1) | 52 (5) | |
| Bu ± others | 51 (3) | 32 (7) | 19 (2) | |
| Bu+Cy | 40 (3) | 23 (5) | 17 (2) | |
| Fludarabine ± others | 16 (1) | 0 | 16 (1) | |
| TBI+Fludarabine | 8 (1) | 0 | 8 (1) | |
| Others | 39 (2) | 7 (2) | 32 (3) | |
| RIC | ||||
| Fludarabine+Melphalan | 303 (19) | 46 (10) | 257 (23) | |
| Bu ± Fludarabine | 196 (13) | 69 (15) | 127 (11) | |
| Cy+Thiotepa | 37 (2) | 0 | 37 (3) | |
| Melphalan+Cy | 19 (1) | 11 (2) | 8 (1) | |
| CCNU ± others | 11 (1) | 3 (1) | 8 (1) | |
| TBI+Cy | 10 (1) | 9 (2) | 1 (<1) | |
| Others | 29 (2) | 10 (2) | 19 (2) | |
| NMA | ||||
| TBI+Fludarabine | 226 (14) | 40 (9) | 186 (17) | |
| Fludarabine+Cy | 216 (14) | 113 (25) | 103 (9) | |
| TBI +/- Cy | 33 (2) | 4 (1) | 29 (3) | |
| Others | 122 (8) | 2 (<1) | 120 (11) | |
| Donor type | <0.001 | |||
| HLA-matched siblings | 1148 (73) | 285 (63) | 863 (77) | |
| Unrelated well matched | 419 (27) | 167 (37) | 252 (23) | |
| D-R CMV status | <0.001 | |||
| Any Positive | 885 (56) | 302 (67) | 583 (52) | |
| All negative | 381 (24) | 137 (30) | 244 (22) | |
| Missing | 301 (19) | 13 (3) | 288 (26) | |
| D-R sex match | 0.427 | |||
| M-M & F-F | 847 (54) | 248 (55) | 599 (54) | |
| M-F | 338 (22) | 86 (19) | 252 (23) | |
| F-M | 365 (23) | 113 (25) | 252 (23) | |
| Missing | 17 (1) | 5 (1) | 12 (1) | |
| Source of stem cells | 0.767 | |||
| Bone marrow | 154 (10) | 46 (10) | 108 (10) | |
| Peripheral blood | 1413 (90) | 406 (90) | 1007 (90) | |
| Year of transplant | <0.001 | |||
| 2001-2003 | 380 (24) | 150 (33) | 230 (21) | |
| 2004-2006 | 411 (26) | 123 (27) | 288 (26) | |
| 2007-2009 | 499 (32) | 135 (30) | 364 (33) | |
| 2010-2011 | 277 (18) | 44 (10) | 233 (21) | |
| ATG-Campath | <0.001 | |||
| ATG alone | 4 (1) | 0 | 4 (<1) | |
| Campath alone | 248 (16) | 60 (13) | 188 (17) | |
| ATG+Campath | 229 (14) | 29 (6) | 200 (18) | |
| None | 352 (22) | 352 (78) | 0 | |
| Missing | 734 (47) | 11 (2) | 723 (65) | |
| GVHD prophylaxis | <0.001 | |||
| CNI+MTX | 360 (23) | 61 (13) | 299 (27) | |
| CNI+MMF | 318 (20) | 42 (9) | 276 (25) | |
| CNI± other | 608 (39) | 334 (74) | 274 (24) | |
| Others/missing | 281 (18) | 15 (3) | 266 (24) | |
| Follow-up of survivors, median (range), months | 55 (3-160) | 58 (3-130) | 54 (3-160) |
EBMT = European Group for Blood and Marrow Transplantation; CIBMTR = Center for International Blood and Bone Marrow Transplant Research; ASCT = Autologous stem Cell Transplantation; Allo-HCT = Allogeneic Hematopoietic Transplantation; BuCy = Busulfan Cyclophosphamide; BEAM = BCNU, etoposide, cytarabine, melphalan; CBV = Cyclophosphamide, BCNU, etoposide; TBI = Total Body Irradiation; RIC = reduced intensity conditioning; NST = non-myeloablative transplant; Mel = Melphalan; M = Male; F = Female; CMV = Cytomegalovirus; GVHD = graft versus host disease; MTX = methotrexate; CsA = cyclosporine; MMF = Mycophenolatemofetil; FK506 = tacrolimus.
Other GVHD prophylaxis: MTX alone (n=14); MMF alone (n=13) and not specified (n=254)
Transplant Related Mortality
Three- and five-year cumulative incidence of TRM were 25% (95% CI, 23-27%) and 29% (95% CI, 26-31%), respectively (Figure 1). Age, chemoresistant disease, heavily pre-treated patients, low Karnofsky PS and the use of MAC regimens were associated to a higher TRM in the multivariate analysis (Table 2, Supplemental Materials Figure A).
Figure 1.
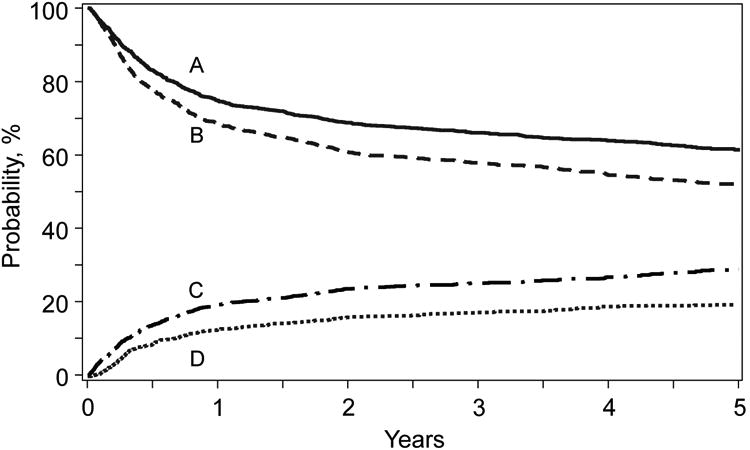
Probabilities for overall survival (A) and progression free survival (B) and cumulative incidences for transplant related mortality (C) and disease progression/relapse (D) for patients with FL who received an allogeneic hematopoietic cell transplantation from 2001-2011.
Table 2.
Multivariate analysis of patients receiving hematopoietic cell transplantation for follicular lymphoma from 2001-2011.
| Outcome/Factors | N | RR (95% CI) | P-value |
|---|---|---|---|
|
| |||
| Progression/Relapse | |||
|
| |||
| Histologic Grade | |||
| Grade 1 | 444 | 1.00 | |
| Grade 2 | 383 | 1.32 (0.95 – 1.84) | 0.101 |
| Grade 3 | 308 | 1.63 (1.16 – 2.28) | 0.004 |
| Missing | 388 | 1.20 (0.85 – 1.68) | 0.294 |
|
| |||
| Disease status at HCT | |||
| Chemosensitive | 1.158 | 1.00 | |
| Chemorefractory | 253 | 1.46 (1.07 – 1.97) | 0.015 |
| Missing | 112 | 1.23 (0.79 – 1.93) | 0.362 |
|
| |||
| Transplant Related Mortality | |||
|
| |||
| Age (Continuous) | 1.523 | 1.04 (1.02 – 1.05) | < 0.0001 |
|
| |||
| N. of prior chemotherapy lines | |||
| 1-2 | 285 | 1.00 | 0.008 |
| 3-4 | 390 | 1.56 (1.12 – 2.17) | |
| >=5 | 165 | 2.53 (1.77 – 3.62) | <0.0001 |
| Missing | 683 | 1.84 (1.35 – 2.51) | 0.0001 |
|
| |||
| KPS | |||
| >= 80 | 986 | 1.00 | |
| < 80 | 54 | 2.05 (1.32 – 3.19) | 0.001 |
| Missing | 483 | 0.94 (0.76 – 1.16) | 0.556 |
|
| |||
| Conditioning regimen | |||
| RIC/NMA | 1.168 | 1.00 | - |
| MAC | 355 | 1.49 (1.18 – 1.87) | 0.0007 |
|
| |||
| Disease status at HSCT | |||
| Chemosensitive | 1.158 | 1.00 | |
| Chemoresistant | 253 | 1.61 (1.28 – 2.03) | <0.0001 |
| Missing | 112 | 1.14 (0.78 – 1.66) | 0.510 |
|
| |||
| Progression free survival | |||
|
| |||
| Histology | |||
| Grade 1 | 444 | 1.00 | |
| Grade 2 | 383 | 1.15 (0.94 – 1.42) | 0.177 |
| Grade 3 | 308 | 1.42 (1.15 – 1.76) | 0.001 |
| Missing | 388 | 1.17 (0.94 – 1.44) | 0.154 |
|
| |||
| N. of prior chemotherapy lines | |||
| 1-2 | 285 | 1.00 | |
| 3-4 | 390 | 1.33 (1.04 – 1.70) | 0.022 |
| >=5 | 165 | 1.93 (1.46 – 2.55) | < 0.0001 |
| Missing | 683 | 1.56 (1.24 – 1.95) | 0.0001 |
|
| |||
| KPS | |||
| >= 80 | 986 | 1.00 | |
| < 80 | 54 | 1.78 (1.23 – 2.58) | 0.002 |
| Missing | 483 | 0.96 (0.81 – 1.14) | 0.634 |
|
| |||
| Disease status at HSCT | |||
| Chemosensitive | 1.158 | 1.00 | |
| Chemoresistant | 253 | 1.54 (1.28 – 1.86) | <0.0001 |
| Missing | 112 | 1.15 (0.86 – 1.54) | 0.336 |
|
| |||
| Conditioning regimen | |||
| RIC/NMA | 1.168 | 1.00 | |
| MAC | 355 | 1.36 (1.14 – 1.63) | 0.0008 |
|
| |||
| Overall Survival | |||
|
| |||
| Age (Continuous) | 1.523 | 1.03 (1.02 – 1.04) | > 0.0001 |
|
| |||
| Histology | |||
| Grade 1 | 444 | 1.00 | |
| Grade 2 | 383 | 1.17 (0.93 – 1.48) | 0.180 |
| Grade 3 | 308 | 1.44 (1.13 – 1.83) | 0.003 |
| Missing | 388 | 1.29 (1.01 – 1.63) | 0.039 |
|
| |||
| N. of prior chemotherapy lines | |||
| 1-2 | 285 | 1.00 | |
| 3-4 | 390 | 1.48 (1.11 – 1.97) | 0.007 |
| >=5 | 165 | 2.41 (1.77 – 3.30) | < 0.0001 |
| Missing | 683 | 1.73 (1.33 – 2.26) | < 0.0001 |
|
| |||
| KPS | |||
| >= 80 | 986 | 1.00 | |
| < 80 | 54 | 2.23 (1.52 – 3.25) | < 0.0001 |
| Missing | 483 | 0.89 (0.73 – 1.07) | 0.219 |
|
| |||
| Disease status at HCT | |||
| Chemosensitive | 1.158 | 1.00 | <0.0001 |
| Chemoresistant | 253 | 1.59 (1.30 – 1.95) | 0.227 |
| Missing | 112 | 1.20 (0.86 – 1.67) | |
|
| |||
| Conditioning regimen | |||
| RIC/NMA | 1.168 | 1.00 | |
| MAC | 355 | 1.42 (1.16 – 1.73) | 0.0006 |
RR = Relative risk; CI = Confidence interval; HCT = Stem cell transplantation; KPS. Karnofsky performance score; ASCT = Autologous stem cell transplantation; RIC = Reduced intensity conditioning; NMA = Non-myeloablative conditioning regimen; MAC = Myeloablative conditioning regimen.
Relapse/Progression
Three- and five year cumulative incidence of progression/relapse were 17% (95% CI, 15-19%) and 19% (95% CI, 17-22%), respectively (Figure 1). Chemoresistant disease and grade 3 histology were associated to a significantly higher relapse rate after the procedure (Table 2).
Progression Free Survival
Adjusted three- and five year probabilities of PFS were 58% (95% CI, 55-60%) and 52% (95% CI, 49-55%), respectively (Figure 1). Age, grade 3 histology, chemorefractory disease, number of prior lines of therapy before transplant, inadequate Karnofsky PS and MAC protocols were independent adverse prognostic factors (Table 2, Supplemental Materials Figure B).
Overall Survival
Adjusted three- and five-year probabilities of OS were 66% (95% CI, 64-68%) and 61% (95% CI, 59-64%, p = 0.13), respectively (Figure 1). Grade 3 histology, age at transplantation, chemotherapy burden before transplantation, chemorefractory disease, poor PS and the use of MAC protocols were adverse prognostic factors (Table 2, Supplemental Materials Figure C).
GVHD
Cumulative incidence of grade 2-4 aGVHD at day 100 was 20% (95% CI, 18-22%). Corresponding incidence for cGVHD at 1 year was 45% (95% CI, 42-48%). Cumulative incidence of both acute and cGVHD was higher in the CIBMTR cohort in comparison to the EBMT cohort in the univariate analysis (Supplemental Table A).
Risk Score
The population used to generate the score was a subset of all patients with complete information on all significant variables tested in multivariate analyses. In order to assess the representativeness of this subset, the cohort (N=573) was compared to the excluded population and there were no differences in OS (N=994, p=0.43) or PFS (N=950, p=0.13).
The proposed risk score used the model for treatment failure (1-PFS) included the following covariates: age at HCT, number of prior chemotherapy lines, chemotherapy sensitivity and Karnofsky PS. The score was computed using the formula in Table 3 and defined three distinct groups: 1) low risk (n=190) score range from 0.03-0.069 (mean=0.47), intermediate risk (n=191) score range from 0.70-1.05 (mean=0.87) and 3) high risk (n=192) score range from 1.06-2.29 (mean=1.36). The hazard ratio for treatment failure of patients with intermediate risk was 1.72 (95% CI, 1.24 to 2.43, p=0.0014) compared to patients with low risk; and patients with high risk was 1.40 (95% CI, 1.05-1.85, p=0.019) compared to intermediate risk. Corresponding hazard ratios for overall mortality were 2.10 (95% CI, 1.41-3.14, p=0.0003) and 1.66 (95% CI, 1.22-2.25, p=0.001); for TRM were 2.56 (95% CI, 1.57-4.16, p=0.002) and 1.74 (95% CI, 1.23-2.44, p=0.0015); and for disease progression/relapse were 1.14 (95% CI, 0.70-1.84, p=0.60) and 0.84 (95% CI, 0.50-1.41, p=0.51) (Figure 2A-D).
Table 3.
Prognostic score for treatment failure among patients with FL undergoing allogeneic HCT.
| Risk | β (Parameter) | p-value |
|---|---|---|
| Age (increment of 1 year) | 0.0160 | 0.0271 |
| Number of prior lines of therapy = 3-4 | 0.3592 | 0.0243 |
| Number of prior lines of therapy = ≥ 5 | 0.7013 | <0.0001 |
| Performance score (KPS) = <80% | 0.4962 | 0.0213 |
| Disease Status = Chemoresistant | 0.5134 | 0.0002 |
| Score = Age*0.0160 + (>5 prior lines of therapy)*0.7013 + (Chemoresistant)*0.5134 + (KPS<80%)*0.4962 + (3-4 prior lines of therapy) *0.3592 with range of (0.0331 – 2.2901) | ||
| Risk Level | Score | |
| Low Risk | <0.70 | |
| Intermediate Risk | 0.70-1.05 | |
| High Risk | >1.05 | |
Figure 2.
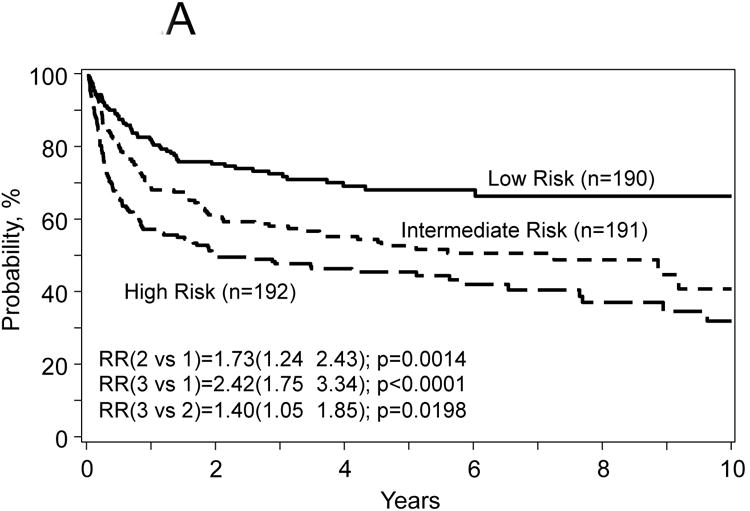
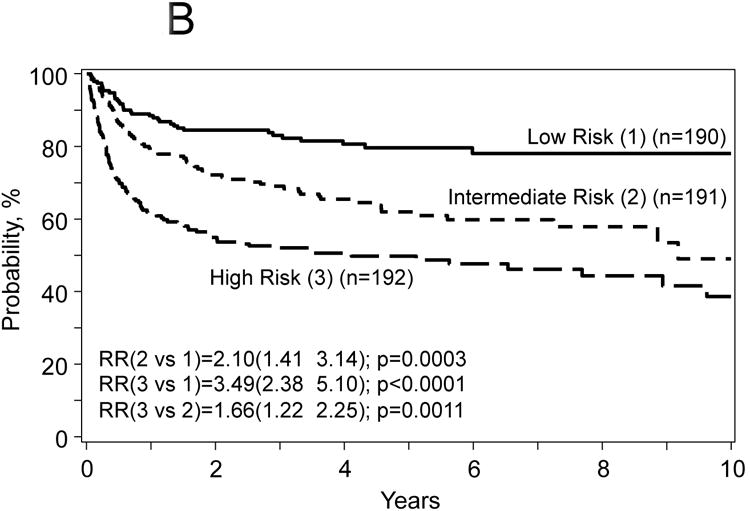
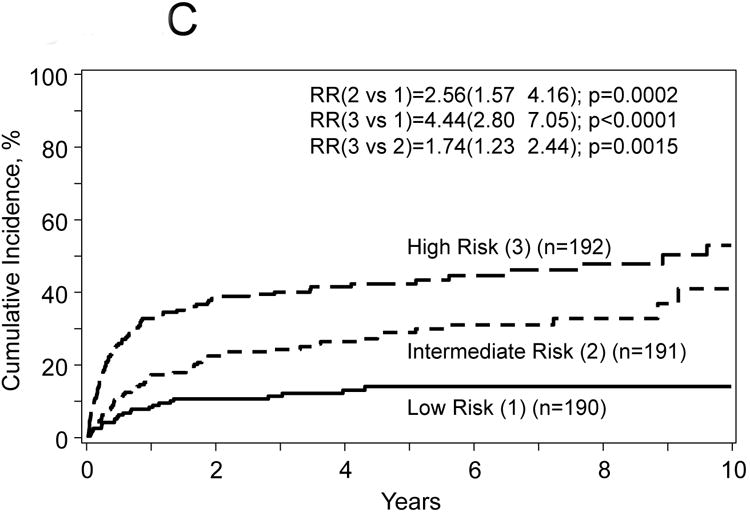
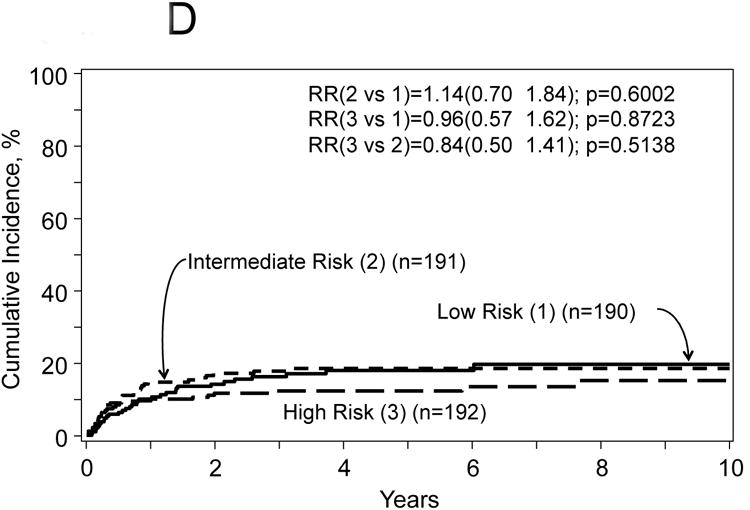
Probabilities for progression free survival (A) and overall survival (B), transplant related mortality (TRM) (C) and disease progression/relapse (D) according to prognostic score.
Discussion
The present analysis combining the EBMT and the CIBMTR experiences for a period of 10 years represents the largest study published to date that evaluates the long-term outcome of heavily pretreated FL patients receiving an allo-HCT. The therapeutic landscape of relapsed FL is undergoing rapid evolution with the development of several novel agents including phosphoinositide 3-kinase inhibitors,15 Bruton's tyrosine kinase inhibitors,16 and ABT-199 (NCT02187861).17 Although auto-HSCT as consolidation therapy at first chemosensitive relapse/progression is able to provide long-term durable remissions in the rituximab era, a significant proportion of patients will eventually relapse.18 Although allo-HCT is the only treatment strategy demonstrated to be curative in relapsed FL patients, its precise role and timing with the progressive incorporation of all these agents in clinical practice in the coming years needs to be reevaluated.
There are significant differences in clinical practice between European and US transplant centres; the more frequent use of MUD in the CIBMTR setting might account for the higher incidence of both acute and cGVHD in this cohort of patients. In spite of that, the four major post-transplant outcomes were super imposable between both registries (Supplemental Table A). Adjusted 5-year probabilities for PFS were 52% and 52% and for OS of 62% and 61% for EBMT- and CIBMTR-reported patients, respectively. These results are in line with what has already been published.19-24
Long-term outcome of patients allografted from well-matched URD is comparable to that of HLA-matched sibling. A review from the literature gives somewhat conflicting results; while the retrospective EBMT analysis 22 does not show any significant difference between the two donor sources, both the UK study 25 as well as the FHCRC experience 21 indicate that URDs are associated with a poorer outcome due to a higher TRM. Number of HLA mismatches may account for these differences.
Of note, the disease control achieved in this high-risk group of patients was impressive with a relapse rate of 19% at 5-years. Later relapses may yet occur, but the plateau observed in the relapse curve in this and other studies9,19,20,23-27 is suggestive that these procedures are a curative treatment. Chemorefractoriness as well as initial diagnosis being grade 3 FL were associated to an increased relapse / progression rate after the procedure. Reason for the later might have been the inclusion of grade 3b FL patients in this series. Long-term outcome is worse in those patients that had received more lines of therapy, with chemoresistant disease, poor KS status at the time of transplant and in whom a MAC protocols were used. These results are also in line with what has been published before19-23 and indicate that patients with relapsed FL should be allografted earlier in the course of the disease. The intensity of the conditioning regimen is still a matter of debate. While in a prior CIBMTR analysis,8 RIC protocols were associated to a higher relapse rate and a lower PFS, in our analysis, MAC protocols are associated to a higher TRM and a lower PFS and OS. In FL and in light of the low relapse rate seen, one might favour less intense conditioning protocols allowing disease cure on the basis of the clinically beneficial GVL effect.
Toxicity of allo-HCT remains significant with one-quarter of patients dying from a transplant-related complication at 3 years. The TRM rate in this study appears comparable with that reported in other studies.19,20,23,25 Strategies to further improve the prevention and management of allo-HCT-related complications are clearly required. The use of the transplant-related comorbidity index may help to identify patients where the risk of the allo-HCT is excessive and that alternative therapies may be more appropriate.28
Finally and most importantly, the large number of patients included in the study has allowed us to construct a score that identifies clearly distinct groups of patients in terms of long-term outcome. It is always challenging to assess the procedure-related risk of a given patient before an allo-HCT taking into consideration the existence of other effective and less toxic treatment options in this histology; there are no prognostic indexes validated in this setting. The proposed score includes age, number of prior chemotherapy lines, chemotherapy sensitivity and performance score into a 3-level risk model. Although not all these factors are modifiable, the score may assist in the clinical decision to offer an allo-HCT to a given patient. Of note, allo-HCT is able to achieve an OS of 80% and a PFS higher than 60% with a 10% TRM in the low risk group of patients; these results compare favorably with those of other non-transplant therapeutic strategies. The efficacy of allo-HCT and its place in the treatment armamentarium in relation to more conventional salvage therapies and to targeted therapy remains to be elucidated. Strategies employing such agents as a bridge to transplant or alternatively as a maintenance therapy following transplantation also require further studies.
Our analysis has important limitations. During the era of this analysis EBMT/CIBMTR case reports forms did not distinguish between grade 3a vs. 3b histologies, but considering that fact that grade 3b is a rare histology, we anticipate grade 3b numbers to be low in the current dataset. But using the current dataset we cannot predict if outcomes of grade 3a FL are different from grade 3b. We excluded transformed histologies from this analysis, as determined by reporting center (based on either histological or clinical grounds). However, we cannot exclude the possibility that a few transformed cases, not recognized by reporting center might have been included in current analysis. Since this analysis included cases reported to CIBMTR at TED level and EBMT at Med-A level (where elaborate pre and post transplant therapy data are not collected) we are not able to assess the impact of pre-transplant therapies, post alloHCT maintenance/consolidation treatments or chimerism kinetics on HCT outcomes. The fact that the EBMT and CIBMTR do not collect data on patients not undergoing HCT, means cost-effectiveness type analysis are not possible utilizing registry datasets.
In conclusion, the present analysis albeit retrospective in nature indicates an excellent long-term outcome of patients with relapsed FL being treated with allo-HCT, especially for those patients belonging to the low risk group. If allo-HCT is considered a treatment option and no HLA-matched sibling donor is available, an URD search should be started in a timely manner, it has to be considered earlier in the course of the disease, before the patient fails multiple lines of chemotherapy, his performance status deteriorates and the disease demonstrates to be chemorefractory. MAC protocols should be avoided due to the excess of toxicity.
Supplementary Material
Key Message.
Allogeneic stem cell transplantation (allo-HCT) from HLA matched donors is a curative therapy for patients with follicular lymphoma. Allo-HCT should be considered early in the course of the disease and myeloablative conditioning protocols should be avoided. A prognostic score based on easy to collect clinical data can be used to optimize the candidate and best timing for the procedure.
Acknowledgments
CIBMTR Support List: The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children's Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick's Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Funding Sources: None.
Footnotes
Conflicts of Interest: The authors of the manuscript declare no conflicts of interest.
Author Contribution: A. Sureda: Member of the European Group for Blood and Marrow Transplantation, designed the research, assisted with data interpretation and data review/approval, wrote first draft of the manuscript and contributed to clinical review. MJ. Zhang: Member of the Centre for International Blood and Marrow Transplant Research, assisted with data interpretation and data review/approval and approved the final version of the manuscript. P. Dreger: Member of the European Group for Blood and Marrow Transplantation, contributed to the design of the research, assisted with data interpretation and data review/approval, contributed to clinical review and approved the last version of the manuscript. J. Carreras: Member of the Centre for International Blood and Marrow Transplant Research, assisted with data interpretation and data review/approval and approved the final version of the manuscript. T. Fenske: Member of the Centre for International Blood and Marrow Transplant Research, contributed to the design of the research, assisted with data interpretation and data review/approval, contributed to clinical review and approved the last version of the manuscript. H. Finel: Member of the European Group for Blood and Marrow Transplantation, contributed to data collection and review and approved the final version of the manuscript. H. Schouten: Member of the European Group for Blood and Marrow Transplantation, designed the research, assisted with data interpretation and data review/approval, wrote first draft of the manuscript and contributed to clinical review. S. Montoto: Member of the European Group for Blood and Marrow Transplantation, contributed to the design of the research, assisted with data interpretation and data review/approval, contributed to clinical review and approved the last version of the manuscript. S. Robinson: Member of the European Group for Blood and Marrow Transplantation, contributed to the design of the research, assisted with data interpretation and data review/approval, contributed to clinical review and approved the last version of the manuscript. S.M. Smith: Member of the Centre for International Blood and Marrow Transplant Research, contributed to the design of the research, assisted with data interpretation and data review/approval, contributed to clinical review and approved the last version of the manuscript. A. Boumendil: Member of the European Group for Blood and Marrow Transplantation, assisted with data interpretation and data review/approval and approved the final version of the manuscript. M. Hamadani: Member of the Centre for International Blood and Marrow Transplant Research, contributed to the design of the research, assisted with data interpretation and data review/approval, contributed to clinical review and approved the last version of the manuscript. Marcelo Pasquini: Member of the Centre for International Blood and Marrow Transplant Research, designed the research, assisted with data interpretation and data review/approval, wrote first draft of the manuscript and contributed to clinical review.
References
- 1.van Besien K, Loberiza FR, Jr, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 2.Robinson S, Canals C, Luang JJ, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013;48:1409–1414. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- 3.Klyuchnikov E, Bacher U, Kreger NM, et al. Reduced-Intensity Allografting as First Transplantation Approach in Relapsed/Refractory Grades One and Two Follicular Lymphoma Provides Improved Outcomes in Long-Term Survivors. Biol Blood Marrow Transplant. 2015;21:2091–2099. doi: 10.1016/j.bbmt.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klyuchnikov E, Bacher U, Ahn KW, et al. Long-term survival outcomes of reduced-intensity allogeneic or autologous transplantation in relapsed grade 3 follicular lymphoma. Bone Marrow Transplant. 2016;51:58–66. doi: 10.1038/bmt.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulkner RD, Craddock C, Byrne JL, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103:428–434. doi: 10.1182/blood-2003-05-1406. [DOI] [PubMed] [Google Scholar]
- 6.Khouri IF, Champlin RE. Nonmyeloablative stem cell transplantation for lymphoma. Semin Oncol. 2004;31:22–26. doi: 10.1053/j.seminoncol.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 8.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Besien K, Carreras J, Bierman PJ, et al. Unrelated donor hematopoietic cell transplantation for non-hodgkin lymphoma: long-term outcomes. Biol Blood Marrow Transplant. 2009;15:554–563. doi: 10.1016/j.bbmt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devetten MP, Hari PN, Carreras J, et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15:109–117. doi: 10.1016/j.bbmt.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-Matching for Retrospective Analysis of Unrelated Donor Transplantation: Revised Definitions to Predict Survival. Biol Blood Marrow Transpl. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal AK, Kahl BS, de Vos S, et al. PI3Kd inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20- positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15:1019–1026. doi: 10.1016/S1470-2045(14)70311-0. [DOI] [PubMed] [Google Scholar]
- 17.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 18.Pettengell R, Schmitz N, Gisselbrecht C, et al. Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: a prospective randomized trial from the Lymphoma Working Party of the EBMT. J Clin Oncol. 2013;31:1624–1630. doi: 10.1200/JCO.2012.47.1862. [DOI] [PubMed] [Google Scholar]
- 19.Vigouroux S, Michallet M, Porcher R, et al. Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC) Haematologica. 2007;92:627–634. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 20.Piñana JL, Martino R, Gayoso J, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long term follow-up from two prospective clinical trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 22.Robinson S, Boumendil A, Finel H, et al. Reduced intensity allogeneic stem cell transplantation for follicular lymphoma relapsing after an autologous transplant achieves durable long-term disease control: an analysis from the Lymphoma Working Party of the EBMT. Ann Oncol. 2016;27:1088–1094. doi: 10.1093/annonc/mdw124. [DOI] [PubMed] [Google Scholar]
- 23.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laport GG, Wu J, Logan B, et al. Reduced-Intensity Conditioning with Fludarabine, Cyclophosphamide, and High-Dose Rituximab for Allogeneic Hematopoietic Cell Transplantation for Follicular Lymphoma: A Phase Two Multicenter Trial from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2016;22:1440–1448. doi: 10.1016/j.bbmt.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson KJ, Morris EC, Milligan D, et al. T-Cell-Depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28:3695–3700. doi: 10.1200/JCO.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 26.Van Besien K, Sobocinsky K, Rowlings P, et al. Allogeneic bone marrow transplantation for low grade lymphoma. Blood. 1998;92:1832–1836. [PubMed] [Google Scholar]
- 27.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 28.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)- specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


