Abstract
Aims
Standardized, population-based kinetics of C-peptide distribution and clearance are used to estimate insulin secretion from plasma C-peptide concentrations without direct measurement of C-peptide kinetics. We then compared the performance of population-based kinetics to directly measured C-peptide kinetics when used to calculate β-cell responsivity indices. To ensure that population-based kinetics apply to all conditions where β-cell function is measured, subjects were studied in the presence and absence of acute insulin resistance.
Materials and Methods
Somatostatin was used to inhibit endogenous insulin secretion in 56 nondiabetic subjects. Subsequently, a C-peptide bolus was administered and the changing concentrations used to calculate individual kinetic parameters of C-peptide clearance. In addition, they were studied on 2 occasions in random order using an oral glucose tolerance test (OGTT). On one occasion, free fatty acid (FFA) elevation to cause insulin resistance, was achieved by infusion of intralipid + heparin. Disposition Index (DI) was then estimated by the oral minimal model using either population-based or individual C-peptide kinetics.
Results
There were marked differences in the exchange parameters (k12 and k21) of the model describing C-peptide kinetics, but smaller differences in the fractional clearance, i.e. the irreversible loss from the accessible compartment (k01), obtained from population-based estimates compared to experimental measurement. Since it is predominantly influenced by k01, DI estimated using individual kinetics correlated well with those estimated using population-based kinetics.
Conclusions
These data support the use of population-based measures of C-peptide kinetics to estimate β-cell function during OGTT.
Keywords: C-peptide fractional clearance, hepatic insulin extraction, insulin secretion, insulin action, β-cell function, acute insulin resistance
Introduction
Defects in insulin secretion are central to the pathogenesis of type 2 diabetes, and are evident early in the development of impaired glucose tolerance and the subsequent transition to type 2 diabetes 1. As such, accurate and reproducible measurement of insulin secretion is central to understanding disorders of glucose metabolism as well as quantifying the effect of therapies or other interventions on β-cell function 2. The Disposition Index 2 expresses insulin secretion as a function of the prevailing insulin action and is accepted as a reliable measure of β-cell function 3 producing consistent results in response to different oral challenges 4.
Unfortunately, measurement of insulin concentrations before and after a stimulus to β-cell secretion does not accurately reflect insulin secretion into the portal vein, since insulin undergoes hepatic extraction prior to its appearance in the systemic circulation 5. Because of this, peripheral insulin concentrations reflect the net sum of two processes; insulin secretion and hepatic extraction 2. Indeed, there is evidence that the fraction of insulin extracted across the liver is altered by changes in β-cell function (measured using the disposition index 6), or by insulin secretory burst mass during euglycemia or hyperglycemia 7. Therefore, differences in hepatic insulin clearance introduce a systematic error in measures based on peripheral insulin concentrations when comparing groups with differing β-cell function 6.
C-peptide is co-secreted in an equimolar ratio with insulin and does not undergo hepatic extraction 8, therefore C-peptide concentrations in the peripheral circulation would theoretically be more representative of insulin secretion by the β-cell. However, the half-lives of insulin and C-peptide in the circulation differ dramatically so that C-peptide accumulates in the circulation compared to insulin 9. Since the circulating concentration of a hormone or substrate represents the balance between secretion and clearance, estimating the rate of insulin secretion from C-peptide concentrations requires knowledge of C-peptide clearance.
We 10 and others 11 have used measures of β-cell function that depend on the deconvolution of insulin secretion rates from C-peptide concentrations in the systemic circulation. These methods incorporate age-associated changes in C-peptide kinetics that were directly measured in an experiment undertaken by Van Cauter et al. in 200 subjects, including people with type 2 diabetes and obesity 12. Subsequently, these data were utilized to measure 24 hour profiles of insulin secretion in 36 subjects. Toffolo et al, validated this methodology 13 by directly comparing these standardized estimates to individually-measured C-peptide kinetics and the effect of these differences on the estimates of β-cell function during an intravenous glucose tolerance test (IVGTT). The authors concluded that the population-based parameters, as proposed by Van Cauter et al, allow for accurate estimates of β-cell function during an IVGTT in 7 subjects. The time course of insulin secretion in response to an intravenous challenge (Intravenous Glucose Tolerance Test – IVGTT) differs significantly from that of an oral challenge 14. Indeed measurement of β-cell responsivity in prediabetes with IVGTT and OGTT can produce discordant results 3. It is currently unknown if population and individually-assessed C-peptide kinetics provide comparable assessment of indices of β-cell function in response to an oral challenge because a validation comparable to that performed by Toffolo et al. 13 has not been undertaken. For a full discussion of the methodology please see 2.
As part of a series of experiments examining the pathogenesis of prediabetes and the potential role of insulin pulse frequency and amplitude, we measured sequential insulin and C-peptide concentrations after a bolus injection of both peptides in 56 individuals when endogenous insulin (and C-peptide) secretion was inhibited by somatostatin as part of a euglycemic, hyperinsulinemic clamp experiment 15. This enabled derivation of individual C-peptide kinetic parameters. We subsequently compared indices of β-cell function obtained using population-based kinetics to those resulting from the use of individually-derived kinetics in response to an oral challenge during basal conditions and during acute insulin resistance induced by Free Fatty Acid (FFA) elevation 10.
Materials and Methods
Subjects
The subjects in this study represent a subset of those who participated in a series of previously published experiments intended to examine the role of the diabetes-associated genotype at the TCF7L2 locus in the pathogenesis of prediabetes 10, 15. After approval from the Mayo Clinic Institutional Review Board, we identified suitable subjects who provided written, informed consent. At the time of screening, body composition was measured using dual-energy X-ray absorptiometry (iDXA scanner; GE, Wauwatosa, WI).
Experimental Design – Clamp Experiment
On one study day, subjects underwent a euglycemic, hyperinsulinemic clamp over a 375 minute period. The first part of the experiment (0 to 240 minutes) has been described previously 15. During this time an infusion of somatostatin (60ng/kg/min), glucagon (0.65ng/kg/min) and growth hormone (0.25ng/kg/min) was started and maintained for the duration of study. Insulin was also infused at 0.30mU/kg/min. Dextrose was infused to maintain glucose at ~5.5 mmol/l over the period of study. Arterialized venous blood samples were collected to allow measurement of hormone, tracer and substrate concentrations. At 255 min, a bolus of C-peptide (60pmol/kg) was administered over 1 minute and blood samples collected at 10 minute intervals over the subsequent 2 hours to enable individual calculation of C-peptide clearance.
Experimental Design – Oral Glucose Challenge in the presence (FFA) or absence (GLY) of acute insulin resistance
This experimental design has been described previously 10, 15. Briefly, subjects were initially studied on two occasions in random order, two weeks apart. On one occasion, subjects received an infusion of Intralipid and heparin to raise free fatty acid (FFA) concentrations while on the other occasion glycerol (GLY) was infused at a rate of 5μmol/kg/min (to match the amount of glycerol present in the Intralipid infused during the FFA study day). The infusions commenced three hours prior to challenge with a glucose drink (1g per kg body weight) and were continued until the end of study (six hours after the start of the meal). Blood samples were obtained at periodic intervals for hormone and substrate measurement over the course of the experiment.
Analytical techniques
Glucose concentrations were measured using a glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured using a chemiluminescence assay (Access Assay; Beckman, Chaska, MN). Plasma glucagon and C-peptide were measured by Radio-Immunoassay (Linco Research, St. Louis, MO).
Calculations
Data are presented as Means ± SEMs. We utilized a two-compartment model of C-peptide kinetics, where the relevant kinetic indices were estimated using anthropometric data as previously described 12. The population-based C-peptide kinetics were then used to estimate insulin secretion and subsequent β-cell responsivity during the oral challenges 12. Separately, observed C-peptide decay curves were used to estimate the kinetic parameters of C-peptide 16 in each individual after bolus injection when endogenous secretion is inhibited, using a Maximum A Posteriori (MAP) estimation 17, 18. The resulting parameters were then used to derive the β-cell responsivity indices during the oral glucose challenge (both on the GLY and FFA study day) using the oral C-peptide minimal model 19.
The oral C-peptide minimal model assumes that insulin secretion comprises static and dynamic components. The static component, proportional, through the parameter Фs, to the delayed glucose concentration, represents the provision of new insulin to the releasable pool. The dynamic component is proportional, through the parameter Фd, to the rate of increase of glucose concentrations. An index of total beta-cell responsivity to glucose (Ф) is then derived from both indices 20. Net insulin action (Si) was measured using the oral minimal model as previously described 21. Disposition indices (DI) were subsequently calculated by multiplying the relevant β-cell responsivity indices (Фd, Фs and Φ) by Si.
Statistics
Data are presented in the text as mean ± SEM. The primary analysis compared individual indices of β-cell responsivity obtained using either population-based or individually-derived C-peptide clearance. The between-group differences in indices of β-cell responsivity obtained using either population-based or individually-derived C-peptide kinetics were examined by a paired, two-tailed t-test or a signed-rank test as warranted by their distribution. In addition, to better understand intra-individual differences we calculated the percentage differences for kinetic parameters and Disposition Indices for each individual derived using individually measured or population-derived data using the formula: -
To characterize the sensitivity of individual variation in DI to each of the kinetic parameters (k01, k12 and k21) we identified the C-peptide minimal model three times for every individual. For each identification, we fixed two of the kinetic parameters to their individually-measured values and the third to its population value. We then calculated the sensitivity of DI to that parameter using the following formula: -
where kij represents one of the coefficients k01, k12 and k21.
Statistical analysis was performed in Primer 5 (GraphPad Software, San Diego, CA). Bland-Altman plots (Absolute difference vs. average values) were used to examine differences in indices calculated using the different methods of estimating C-peptide clearance. A multivariate analysis performed in JMP Pro 11 (SAS, Cary, NC) was used to examine the contribution of anthropometric characteristics to the absolute differences in indices ([Population based] – [Individualized kinetics]).. A p-value < 0.05 was considered statistically significant.
Results
Volunteer Characteristics
We studied 56 subjects (19 men and 37 women), with a mean age of 47 ± 2 years. The Body mass Index (BMI) was 27.8 ± 0.5 Kg/M2 and fasting glucose was 4.9 ± 0.1 mmol/l. Serum Creatinine was 0.83 ± 0.02 mg/dl.
C-peptide Concentrations after bolus injection (Figure 1)
Fig. 1.
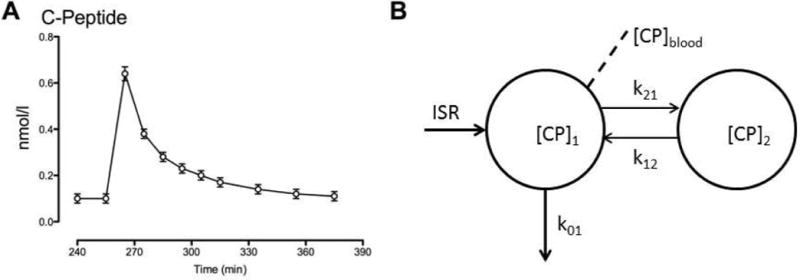
C-peptide concentrations after bolus injection in the presence of somatostatin to block endogenous insulin (and C-peptide) secretion (Panel A). Kinetics of C-peptide (Panel B) where [CP]1 and [CP]2 are C-peptide concentrations in the accessible and peripheral compartments respectively; k01, k12, k21 are kinetic parameters while ISR represents the insulin secretion rate and [CP]blood is the C-peptide concentration sampled from the peripheral circulation.
C-peptide concentrations (Panel A) rose from 0.10 ± 0.02 nmol/l at baseline to a peak concentration of 0.63 ± 0.02 nmol/l 10 minutes after intravenous injection. They subsequently declined to a nadir concentration of 0.11 ± 0.02 nmol/l at the end of the study.
Comparison of C-peptide kinetic parameters k01, k12 and k21 and indices of β-cell responsivity calculated from population data or from individual C-peptide clearance (Figure 2)
Fig. 2.
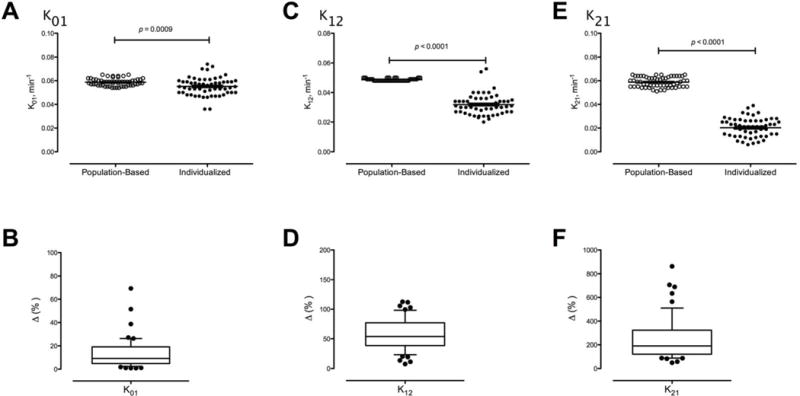
Comparison of C-peptide kinetic parameters k01, k12 andkK21 calculated from demographic data or from individual C-peptide kinetics shown as absolute values (Panels A, C and E). The absolute percentage inter-individual difference for each parameter is shown in the lower panels (Panels B, D and F).
The kinetic parameter k01 (Figure 1, Panel B), was only slightly, albeit significantly, higher when calculated from demographic data (Figure 2, Panel A – 0.059 ± 0.001 vs. 0.055 ± 0.001 min−1, p = 9.0 × 10−4). The mean % absolute difference was 13 ± 2% (Panel B). The kinetic parameter k12 (Figure 1, Panel B) was greater when calculated from demographic data (Panel C – 0.049 ± 0.001 vs. 0.032 ± 0.001 min−1, p < 1.0 × 10−4). The mean % absolute difference was much higher than that for k01 (Panel D, 58 ± 4%). k21 (Figure 1, Panel B) calculated from demographic data was even higher than those calculated from individual C-peptide clearance (Panel E – 0.058 ± 0.004 vs. 0.020 ± 0.008 min−1, p < 1.0 × 10−4). The mean % absolute difference between methods was the highest of the three kinetic parameters (Panel F, 242 ± 24%).
Correlation of DId, DIs and DI calculated using population-based and individually-measured C-peptide clearance during Glycerol infusion or FFA elevation (Figure 3)
Fig. 3.
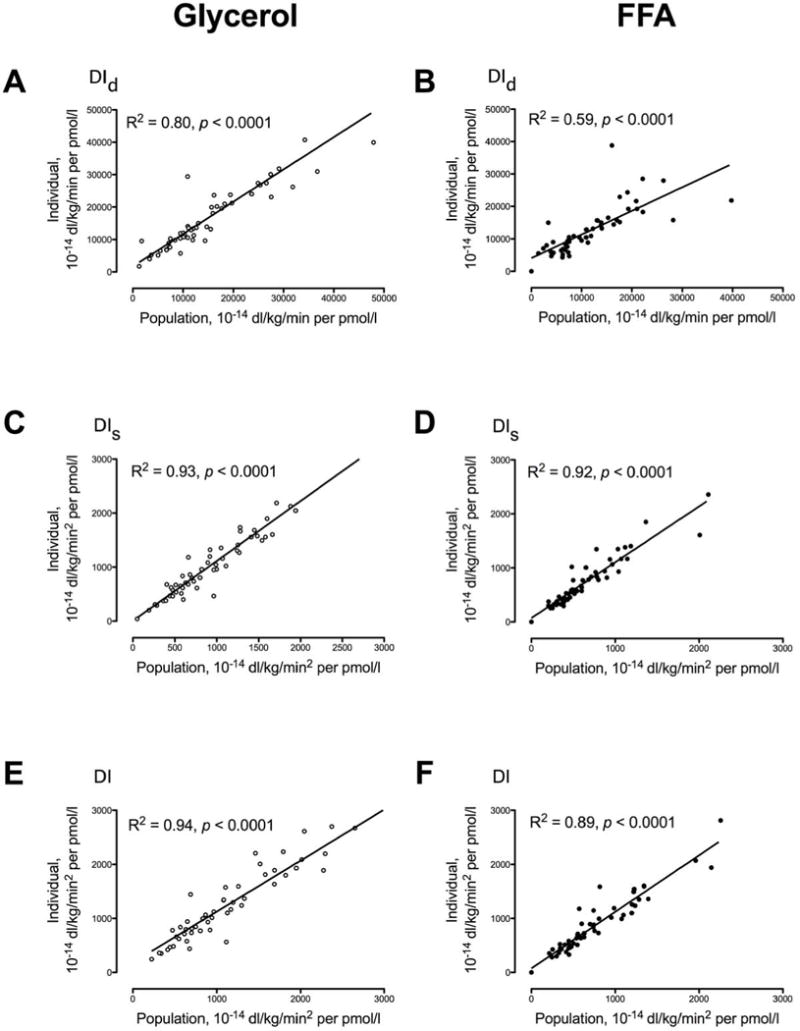
Relationship of DId (Panels A and B), DIs (Panels C and D) and DI (Panels E and F) calculated using anthropometric characteristics (Population) versus those calculated from experimental data for C-peptide kinetics (Individual) for each subject (open circles) in response to a 1g/Kg body weight glucose challenge with accompanying glycerol infusion or during an identical glucose challenge with free fatty acid (FFA) elevation (solid circles).
DId (Panels A and B), DIs (Panel C and D), and DI (Panel E and F) derived using population-based measures of C-peptide kinetics were closely correlated with those calculated using individually-determined rates of C-Peptide kinetics during both the GLY and FFA study day. The correlation for all indices of β-cell responsivity did not differ significantly between the GLY and FFA study days with the exception of DId (p = 0.02). There was no evidence of bias detected by Bland-Altman plots comparing the two methods. Moreover the absolute differences in indices ([Population based] – [Individualized kinetics]) could not be explained by age, sex or weight (data not shown).
Comparison of Intra-individual differences in DId, DIs and DI calculated using population-based and individually-measured C-peptide clearance during Glycerol infusion or FFA elevation (Figure 4)
Fig. 4.
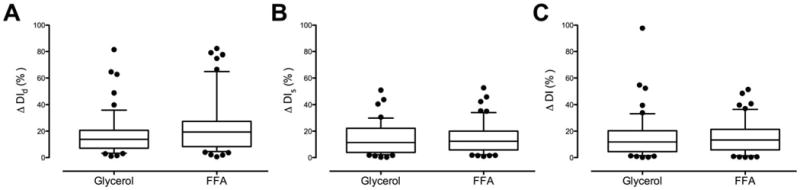
Comparison of absolute percentage inter-individual difference for DId (Panel A), DIs (Panel B) and DI (Panel C) using two estimates of C-peptide kinetics during the glycerol and FFA study days.
The % intra-individual difference in DId did not differ during the GLY and FFA study days respectively (17 ± 2 vs. 24 ± 3 %, p = 0.11 – Panel A). The same was true for DIs (15 ± 2 vs. 16 ± 2 %, p = 0.32 – Panel B) and DI (16 ± 2 vs. 16 ± 2 %, p = 0.38 – Panel C).
Sensitivity of DI to intra-individual differences in kinetic parameters of C-peptide clearance during Glycerol infusion or FFA elevation (Figure 5)
Fig. 5.
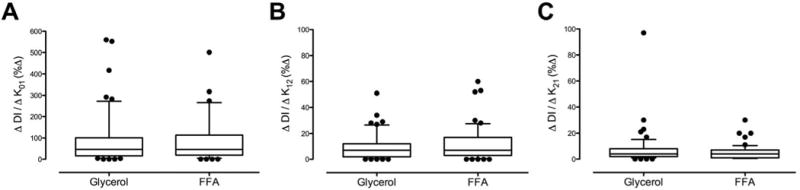
Sensitivity of DI to inter-individual differences in k01 (Panel A), k12 (Panel B) and k21 (Panel C) during the glycerol and FFA study days.
The sensitivity of DI to k01 did not differ between study days (92 ± 17 vs. 104 ± 17 %, p = 0.10 – Panel A) but was much higher than for the other kinetic parameters. The sensitivity of DI to k12 (9 ± 1 vs. 12 ± 2 %, p = 0.55 – Panel B) also did not differ between study days. The sensitivity of DI to k21 did not differ between study days and was even lower (7 ± 2 vs. 5 ± 1 %, p = 0.14 – Panel C).
Discussion
We report that, in general, indices of β-cell responsivity to oral glucose calculated using population-based kinetics correlated well with those derived using individually measured C- peptide kinetics. Taken together these data imply that utilization of population-based C-peptide kinetics enables reliable estimation of β-cell function in response to an oral challenge even in situations where insulin action is decreased acutely. This is because DI is relatively insensitive to errors in the estimation of k12 and k21 but sensitive to errors in the estimation of k01. This is to some extent expected as this parameter represents the fractional clearance rate of C-peptide. A large error in this parameter translates into a large error in insulin secretion rate and thus in the β-cell responsivity index (Φ) and DI 2. However, the differences between parameters calculated by either method are lowest for k01 explaining the good correlation of DI calculated using population kinetics with that calculated using individually measured kinetics.
Measurement of β-cell function using the oral C-peptide minimal model depends on of C-peptide kinetics. We 6, 22, 23 and others 24, 25, have utilized C-peptide kinetics data derived by Van Cauter et al 12 where C-peptide kinetic parameters were estimated from direct measurement in a group of 200 subjects of varying age, gender, obesity and glucose tolerance. Although measures of β-cell function using alternative methodology (independent of C-peptide concentrations) can correlate well with those dependent on these measures of C-peptide kinetics, direct comparison of functional indices obtained with or without individually-determined C-peptide kinetics has not been undertaken in the presence of acute change in insulin action during an oral glucose tolerance test. The latter is an important consideration given that insulin secretion in response to an intravenous glucose challenge (where the approach had been validated independently 13) differs significantly from that in response to an oral challenge 26, 27.
To ensure an ability to accurately detect insulin pulses in the hepatic venous circulation and in the systemic circulation (as part of a series of studies examining the pathogenesis of prediabetes), we estimated C-peptide kinetics in 56 subjects (whose body weight varied from 50 to 110kg) during a pancreatic clamp to ensure that C-peptide measurement was not confounded by endogenous β-cell secretion. These data provide an opportunity to compare oral minimal model-derived indices obtained using population-based C-peptide kinetics to those measured experimentally in each individual. Furthermore, prior participation in a paired experiment enabled comparison of the methodology in the presence (FFA) and absence (GLY) of acute insulin resistance 10.
We report that in this population of otherwise healthy adults, utilization of population-based C-peptide kinetics produces disposition indices that correlate well with those calculated using direct measures of C-peptide kinetics. There are small, but significant, differences in the kinetic parameters estimated by the different methods, with the population-based data exhibiting systematic overestimation compared to those calculated from individual C-peptide clearance. However, these data show that estimation of DI using the oral minimal model is more sensitive to the actual hormone and substrate concentrations used to measure β-cell responsivity than it is to these kinetic parameters in otherwise healthy humans – at least in those with intact renal function and demographic characteristics like those of the study population.
Total Disposition Index varied by 16 ± 2 % depending on whether directly-measured C-peptide kinetics or a population-based measure of C-peptide kinetics was used. This is similar to the difference in values of mean insulin secretion, reported as 10-12 % by Van Cauter et.al. depending on the methodology used to estimate C-peptide kinetics 12. In the presence of FFA elevation to cause insulin resistance, a similar variance was also observed. In light of this, it is reasonable to conclude that population-based measures of C-peptide kinetics performed adequately, when compared to individual measurement of C-peptide clearance. This is of considerable importance, given the increasing use of the oral minimal model for estimating β-cell function and the cost and inconvenience of measuring individual C-peptide kinetics in each subject 2. However, it is important to underline that, while on average the performance of the population kinetics is satisfactory, the error in individual DI estimation, especially in subjects with low DI, may be far higher than the mean difference of 16%.
While the results from this study are reassuring, it is important to recognize that the calculation of individual C-peptide kinetics was limited by the absence of frequent sampling immediately after the bolus injection of C-peptide – a constraint imposed by both immunoassay cost and blood volume limitations. In order to improve accuracy and precision of parameters and overcome this limitation, a MAP estimation has been used to obtain the individual- measures of C-peptide kinetics, since Bayesian estimation improves model performance in data-poor scenarios 17, 18. However, despite this limitation, indices of β-cell responsivity (and DI) were relatively unaffected by the method used to determine C-peptide kinetics.
The other potential limitation is that the cohort studied was recruited on the basis of subjects’ TCF7L2 genotype as previously described 10. This locus has been associated with type 2 diabetes 28, impaired insulin secretion 29 and impaired suppression of glucagon 10. However, we studied subjects with both the TT (diabetes-associated) and the CC (diabetes-protective) genotype at rs7903146 (in the TCF7L2 locus) and there was no evidence of an effect of genotype on C-peptide clearance (Data not shown). This concurs with an a priori absence of evidence that this locus alters C-peptide clearance, has effects on renal function or on hepatic and extra-hepatic insulin action 15. In this cohort, the genotype at rs7903146 in the TCF7L2 locus did not alter the relationship between indices calculated using individually-determined C-peptide clearance versus those using population-based data, on either study day (Data not shown).
Taken together, these data support the application of population-based measures of C-peptide kinetics to the measurement of β-cell function using the oral minimal model in non-diabetic subjects with normal renal function.
Acknowledgments
Grant Support: This work was supported by the National Institutes of Health grant numbers UL1 TR000135, R01 DK78646, and 5T32 DK007352-37.
Footnotes
Author contributions: R.T.V, A.S., M.S. researched data and ran the studies; F.P. & M.C.L. undertook mathematical modeling of insulin secretion and action; K.R.B. oversaw the statistical analysis; and C.D.M. supervised mathematical modeling of insulin secretion and action and contributed to discussion and reviewed/edited manuscript; R.A.R. contributed to discussion and reviewed/edited manuscript; C.C. contributed to discussion and reviewed/edited manuscript and A.V. designed the study, oversaw its conduct, researched data, wrote the manuscript. Dr. Adrian Vella is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: Dr. Vella has consulted for VTV therapeutics, XOMA, Sanofi-Aventis, Novartis and Bristol-Myers Squibb in the past 5 years. None of the other authors (Drs. Varghese, Laurenti, Piccinini, Dalla Man, Sharma, Shah, Bailey, Cobelli, Rizza) have relevant disclosures.
Conflict of Interest: No potential conflicts of interest.
References
- 1.Bock G, Dalla Man C, Campioni M, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55:3536–3549. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 2.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63:1203–1213. doi: 10.2337/db13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar SS, Vella A, Raymond RH, et al. Standardized Mixed-Meal Tolerance and Arginine Stimulation Tests Provide Reproducible and Complementary Measures of beta-Cell Function: Results From the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care. 2016;39:1602–1613. doi: 10.2337/dc15-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock G, Dalla Man C, Campioni M, et al. Effects of nonglucose nutrients on insulin secretion and action in people with pre-diabetes. Diabetes. 2007;56:1113–1119. doi: 10.2337/db06-1272. [DOI] [PubMed] [Google Scholar]
- 5.Peiris AN, Mueller RA, Smith GA, Struve MF, Kissebah AH. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. Journal of Clinical Investigation. 1986;78:1648–1657. doi: 10.1172/JCI112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sathananthan A, Man CD, Zinsmeister AR, et al. A concerted decline in insulin secretion and action occurs across the spectrum of fasting and postchallenge glucose concentrations. Clin Endocrinol (Oxf) 2012;76:212–219. doi: 10.1111/j.1365-2265.2011.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 8.Polonsky K, Jaspan J, Pugh W, et al. Metabolism of C-peptide in the dog. In vivo demonstration of the absence of hepatic extraction. J Clin Invest. 1983;72:1114–1123. doi: 10.1172/JCI111036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faber OK, Hagen C, Binder C, et al. Kinetics of human connecting peptide in normal and diabetic subjects. J Clin Invest. 1978;62:197–203. doi: 10.1172/JCI109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah M, Varghese RT, Miles JM, et al. TCF7L2 Genotype and alpha-Cell Function in Humans Without Diabetes. Diabetes. 2016;65:371–380. doi: 10.2337/db15-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muscelli E, Casolaro A, Gastaldelli A, et al. Mechanisms for the antihyperglycemic effect of sitagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1205. [DOI] [PubMed] [Google Scholar]
- 12.Cauter EV, Mestrez F, Sturis J, Polonsky KS. Estimation of Insulin Secretion Rates from C-Peptide Levels: Comparison of Individual and Standard Kinetic Parameters for C-Peptide Clearance. Diabetes. 1992;41:368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 13.Toffolo G, De Grandi F, Cobelli C. Estimation of beta-cell sensitivity from intravenous glucose tolerance test C-peptide data. Knowledge of the kinetics avoids errors in modeling the secretion. Diabetes. 1995;44:845–854. doi: 10.2337/diab.44.7.845. [DOI] [PubMed] [Google Scholar]
- 14.Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab. 2004;287:E371–385. doi: 10.1152/ajpendo.00139.2003. [DOI] [PubMed] [Google Scholar]
- 15.Varghese RT, Viegas I, Barosa C, et al. Diabetes-Associated Variation in TCF7L2 Is Not Associated With Hepatic or Extrahepatic Insulin Resistance. Diabetes. 2016;65:887–892. doi: 10.2337/db15-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toffolo G, Campioni M, Basu R, Rizza RA, Cobelli C. A minimal model of insulin secretion and kinetics to assess hepatic insulin extraction. Am J Physiol Endocrinol Metab. 2006;290:E169–E176. doi: 10.1152/ajpendo.00473.2004. [DOI] [PubMed] [Google Scholar]
- 17.Magni P, Bellazzi R, Sparacino G, Cobelli C. Bayesian identification of a population compartmental model of C-peptide kinetics. Annals of biomedical engineering. 2000;28:812–823. doi: 10.1114/1.1289459. [DOI] [PubMed] [Google Scholar]
- 18.Sparacino G, Tombolato C, Cobelli C. Maximum-likelihood versus maximum a posteriori parameter estimation of physiological system models: the C-peptide impulse response case study. IEEE transactions on bio-medical engineering. 2000;47:801–811. doi: 10.1109/10.844232. [DOI] [PubMed] [Google Scholar]
- 19.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50:150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 20.Cobelli C, Dalla Man C, Sparacino G, Magni L, De Nicolao G, Kovatchev BP. Diabetes: Models, Signals, and Control. IEEE Reviews in Biomedical Engineering. 2009;2:54–96. doi: 10.1109/RBME.2009.2036073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–643. doi: 10.1152/ajpendo.00319.2003. [DOI] [PubMed] [Google Scholar]
- 22.Dalla Man C, Bock G, Giesler PD, et al. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care. 2009;32:14–18. doi: 10.2337/dc08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalla Man C, Micheletto F, Sathananthan A, Rizza RA, Vella A, Cobelli C. A model of GLP-1 action on insulin secretion in nondiabetic subjects. Am J Physiol Endocrinol Metab. 2010;298:E1115–E1121. doi: 10.1152/ajpendo.00705.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. Journal of Clinical Endocrinology & Metabolism. 2010;95:872–878. doi: 10.1210/jc.2009-2054. [DOI] [PubMed] [Google Scholar]
- 25.Daniele G, Gaggini M, Comassi M, et al. Glucose Metabolism in High-Risk Subjects for Type 2 Diabetes Carrying the rs7903146 TCF7L2 Gene Variant. The Journal of clinical endocrinology and metabolism. 2015;100:E1160–1167. doi: 10.1210/jc.2015-1172. [DOI] [PubMed] [Google Scholar]
- 26.Campioni M, Toffolo G, Shuster LT, Service FJ, Rizza RA, Cobelli C. Incretin effect potentiates beta-cell responsivity to glucose as well as to its rate of change: OGTT and matched intravenous study. Am J Physiol Endocrinol Metab. 2007;292:E54–E60. doi: 10.1152/ajpendo.00033.2006. [DOI] [PubMed] [Google Scholar]
- 27.Caumo A, Bergman RN, Cobelli C. Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. J Clin Endocrinol Metab. 2000;85:4396–4402. doi: 10.1210/jcem.85.11.6982. [DOI] [PubMed] [Google Scholar]
- 28.Grant SFA, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 29.Lyssenko V, Lupi R, Marchetti P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007;117:2155–2163. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]


