Abstract
PD-1 is an inhibitory receptor induced in T cells by antigen stimulation and sustained PD-1 expression plays a key role in T cell dysfunction. Blocking PD-1 signaling rescues exhausted T cells and is an effective treatment for chronic infections and cancer. Nonetheless, combining PD-1 pathway blockade to therapeutic vaccination should further improve T cell rescue. PD-1 is induced shortly after T cell priming, but little is known about the role of PD-1 in the initiation of immune responses. In addition, the PD-1 pathway may also modulate humoral responses, since both B cells and Tfh cells express PD-1. Therefore, even though much progress has been achieved by manipulation of the PD-1 pathway to rescue exhausted T cells, this powerful immunotherapy could still be further exploited.
Introduction
Programmed cell death (PD)-1 (CD279) is an inhibitory receptor that belongs to the CD28/CTLA-4 family [1,2]. Activated T cells transiently express PD-1 but sustained PD-1 expression is associated with T cell dysfunction [3]. T cell exhaustion and the role of PD-1 in chronic infection were first described in mice during lymphocytic choriomeningitis virus (LCMV) infection and later shown to occur in several situations of antigen persistence in mice, non-human primates and humans [4]. Importantly, blockade of the PD-1 pathway restores function in exhausted T cells and was recently used to treat patients with advanced cancer with promising results [**5–7].
In this review we will briefly summarize the current understanding on the role of the PD-1 pathway in adaptive immunity. We will then discuss the potential applications for PD-1 pathway blockade to improve immune responses, both in prophylactic and therapeutic settings.
PD-1 and its ligands
PD-1 function has been best characterized in T cells. The cytoplasmic domain of PD-1 contains both an immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) [8]. PD-1 conveys negative signals through ITSM recruitment of SH2-domain containing tyrosine phosphatase (SHP2) that dampens TCR signaling [9–11] (Fig. 1). PD-1 expression can also be detected on B cells, NK and NKT cells, as well as on monocytes/macrophages and dendritic cells (DCs) [1,12]. However since no mechanism for PD-1 signaling without association with antigen receptors has been described, the role of PD-1 in non-lymphocytes still requires further study.
Figure 1. PD-1 inhibits TCR/CD28 signaling.
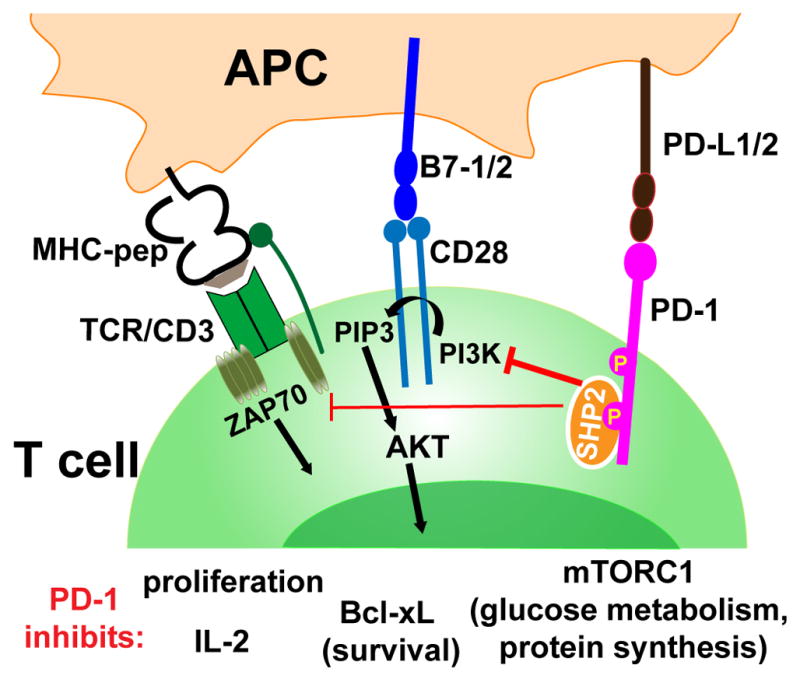
Upon engagement with PD-L1 or PD-L2, PD-1 is phosphorylated at both thyrosine containing motifs ITIM and ITSM. The phosphatase SHP2 (and possible SHP1 as well) is recruited to phosphorylated ITSM. SHP2 dephosphorylates phosphatidylinositol-3-kinase (PI3K) and ZAP70/CD3ζ, ultimately attenuating TCR/CD28 signaling and T cell activation [9–11].
PD-1 binds PD-L1 (also know as B7-H1 or CD274) or PD-L2 (also known as B7-DC or CD273). PD-L1 is widely and constitutively expressed on numerous cells such as T and B cells, DCs, macrophages and also in non-hematopoietic cells (e.g. endothelial cells and lymphoid stromal cells). PD-L2 expression is much more restricted: it is expressed on some B cell subsets [13] and can be inducibly expressed on DCs, monocytes and macrophages depending on the cytokine milieu [1,2]. PD-1 ligands have short cytoplasmic tails with no known signaling motifs. However there are some reports showing effects on PD-L1-expressing cells after interaction with PD-1, but how this reverse signaling takes place remains unsolved [2]. Of note, PD-L1 also binds B7-1 (CD80) and PD-L1/B7-1 interactions are reported to be of inhibitory nature [14,15].
PD-1 expression and regulation of T cell responses
PD-1 expression is induced by antigen receptor ligation [9,16]. TCR stimulation leads to nuclear translocation of NFAT, and NFATc1 (NFAT2) binds to the promoter region of the PD-1 gene inducing its transcription [17]. PD-1 is transiently expressed in viral-specific CD8 T cells after infection but once the infection is resolved and there is no more TCR signaling, PD-1 expression decreases [3,18]. Conversely, PD-1 expression is maintained during chronic infections. Sustained PD-1 expression is maintained primarily due to continuous TCR ligation [19,20]. TCR signals promote demethylation of regulatory regions of the PD-l locus. After prolonged antigen stimulation, the PD-1 locus fails to be re-methylated resulting in an open chromatin state poised for expression [18]. Additionally, T-bet binds upstream of the PD-1 gene and represses its transcription. And since persistent TCR stimulation downregulates T-bet, low T-bet expression is another mechanism that helps maintain PD-1 transcription [21]. Cytokines may also further enhance PD-1 expression [22,23].
High PD-1 expression is a hallmark of dysfunctional T cells found in chronic infections and cancer. Importantly, interfering with the PD-1 pathway rescues function in exhausted T cells [2,4]. It was first shown by our group that administration of anti-PD-L1 (or anti-PD-1) blocking antibodies to mice chronically infected with LCMV, increased the number and function of LCMV-specific CD8 T cells and promoted viral control [3]. In the same way, PD-1 blockade improved immunity in macaques chronically infected with simian immunodeficiency virus (SIV) [24]. Likewise, HIV-specific CD8 T cells express PD-1, which correlates with T cell dysfunction and disease progression. And in vitro blockade of the PD-1 pathway improves function of HIV-specific CD8 and CD4 T cells from chronically infected patients [25]. Similar observations were also described in hepatitis C virus infected patients [12,26]. And more recently, in a humanized mouse model of HIV infection, PD-L1 blockade dramatically suppressed HIV viremia and restored CD4 counts, even after treatment cessation [27]. Besides viral infections, PD-1 signaling plays a major role in other microbial infections [2], such as infection with the pathogenic fungus histoplasma capsulatum [28] and Plasmodium parasite during chronic malaria [*29]. Thus PD-1 plays a major role in T cell exhaustion during chronic infections and blockade of the PD-1 pathway can restore T cell function and promote pathogen control.
Dysfunctional CD8 T cells in various cancers also express PD-1 [30–32]. Recently two different antibodies that interfere with the PD-1 pathway have been used to successfully treat patients with advanced cancer. After treatment, durable anti-tumor responses were observed in patients with treatment-refractory melanoma, renal-cell cancer or non-small-cell lung cancer. Remarkably, when stratified, 36% of patients with PD-L1-expressing tumors had an objective response to PD-1 pathway blockade [**5–7]. The results from those clinical trials are extremely promising and subsequent studies involving more patients and the use of predictive biomarkes are highly anticipated.
PD-1 blockade to improve prophylactic vaccination
Interfering with the PD-1/PD-L1 pathway during the early stage of immune responses can result in improved T cell responses. In mice, PD-L1 blockade during acute herpes simplex virus (HSV)-1 infection increases the magnitude and polyfunctionality of effector HSV-specific CD8 responses and improves recall to secondary HSV infection [33]. In rhesus monkeys, blockade of PD-1 during immunization with adenovirus vector type 5 encoding SIV-Gag improves Gag-specific CD8 T cell responses [34].
The hypothesis is that without PD-L1/PD-1 interactions, antigen presenting cells (APCs) can provide stronger stimulation to T cells. This hypothesis is supported by experiments showing a significant enhancement of CD8 and CD4 T cell responses to LCMV infection when hematopoietic cells lack PD-L1 [35]. Costimulatory molecules act like a rheostat to modulate T cell activation: positive costimulatory molecules reduce the TCR signaling threshold necessary for T cell activation, whereas inhibitory molecules restrict T cell activation. So ultimately, T cell activation occurs when there are more positive than negative signals. As a result, blockade of the PD-1 pathway has more significant effects in promoting T cell activation during conditions of sub-optimal antigen presentation such as with low antigen dose or with weak or low numbers of APCs [36,37]. Therefore suboptimal immunization strategies would benefit the most from blockade of the PD-1 pathway.
Even though PD-1 pathway blockade is an attractive strategy to improve prophylactic vaccination, few studies have focused on the PD-1 pathway during early stages of T cell responses. And most importantly, mainly CD8 T cell responses have been assessed. Still, most effective vaccines rely on the development of neutralizing antibodies, and the role of the PD-1 pathway on B cells and CD4 T cell differentiation has been relatively neglected.
Among conventional CD4 T cells, follicular helper cells (Tfh) express the highest levels of PD-1. Tfh are key cells to provide B cell help and promote the germinal center (GC) reaction. Tfh cells are required for differentiation of long-lived plasma cells and production of high affinity antibodies [38,39] (Figure 2A). Although Tfh cells have been extensively studied in recent years, the role of PD-1 in CD4 T cell differentiation remains largely unexplored. The current thought is that Tfh cells express high levels of PD-1 due to continuous TCR triggering by interactions with cognate B cells. In Tfh cells, PD-1 most likely also reduces TCR signaling and duration of cognate interactions, however this has not been formally demonstrated. Interestingly, PD-1 may aid Tfh cells by limiting IL-2 production. IL-2 is deleterious to Tfh because it induces Blimp-1, which in turn antagonizes Bcl6, the Tfh master regulator [40,41]. It has been shown that PD-1 deficient Tfh cells have altered cytokine secretion, with decreased IL-21 production [13,42]. And mice deficient in PD-1 have dysfunctional Tfh cells that cannot appropriately select IgA B cells in germinal center of Peyer’s patches [42]. Thus PD-1 signaling contributes to CD4 T cell differentiation and function of Tfh cells.
Figure 2. Potential role of the PD-1 pathway in B cell responses.
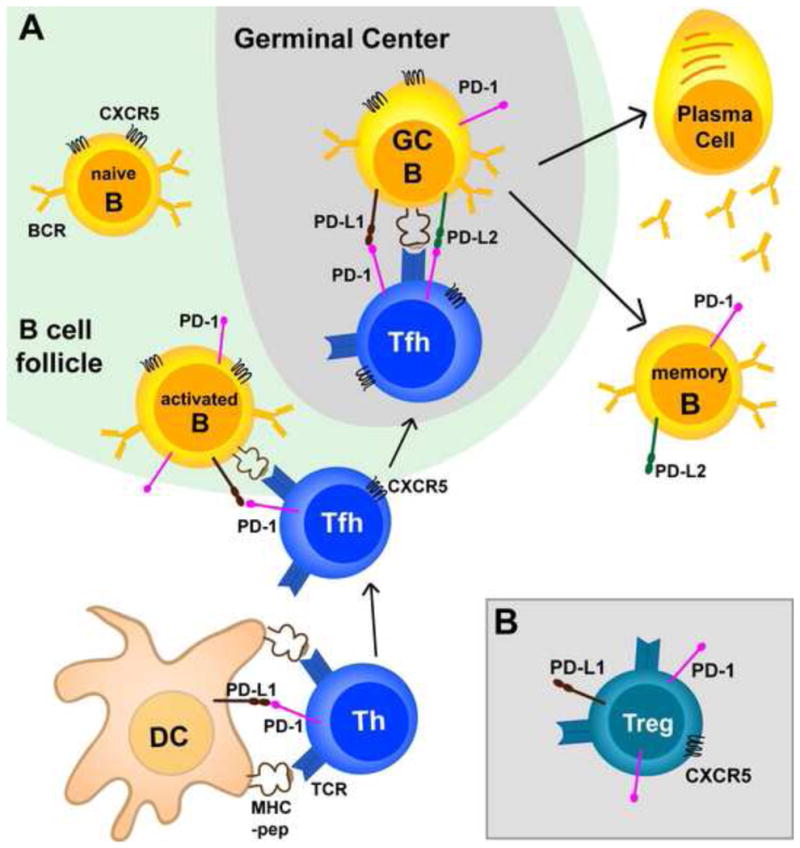
A. Naïve T cells interact with dendritic cells (DCs) presenting cognate antigen and initiate commitment to different CD4 T helper (Th) lineages. Upon activation all CD4 cells express PD-1, but PD-1 expression is maintained and further increase in CD4 T cells that interact with cognate B cells and fully commit to Tfh lineage. Tfh cells express CXCR5 and migrate to the germinal center (GC) where they select high affinity B cells by providing IL-21 and CD40L. GC B cells that receive T cell help survive and differentiate into memory and plasma cells. Reports have shown that B cells express PD-1 upon activation, and PD-1 expression is maintained in GC and memory B cells. In addition, it has been reported that GC and memory B cells express PD-L2. PD-L1 expression is ubiquitous and was shown in the figure only in situations where engagement with PD-1 has been demonstrated to occur [13,38–43] B.PD -1 is expressed by CXCR5+ Tregs that limit GC reactions, and PD-1 engagement suppresses Tregs [43,48].
The in vivo role of PD-1 signaling specifically in B cells also deserves further investigation. PD-1 is upregulated in activated B cells and germinal center B cells, and GC B cells also express PD-L2 and PD-L1 [13,16,43] (Figure 2A). Similar to T cells, PD-1 ligation inhibits B cell receptor (BCR) signaling by recruitment of the phosphatase SHP2 [8] and PD-1 deficient B cells have increased proliferation upon BCR ligation [44].
It was originally reported that PD-1 KO mice produce higher levels of antibodies after immunization with a T-cell independent antigen, but no differences are observed between wild type and PD-1 deficient mice after immunization with a T-cell dependent antigen [44]. On the contrary, it was more recently shown that mice deficient in components of the PD-1 pathway have diminished B cell responses to alum/protein T-cell dependent immunization. In this study, long-lived plasma cells were reduced when B cells lacked PD-L1 and/or PD-L2, or when T cells lacked PD-1 [13]. However, another study found increased germinal center responses (and Tfh cells) in PD-L1 deficient mice after infection with the parasite Schistosoma mansoni or immunization with protein in complete Freund’s adjuvant [45]. Different requirements for Tfh differentiation depending on the immunization protocol might explain these conflicting data. Alternatively, differences in gut flora on PD-1 deficient mice may also distinctively affect general immunoreactivity of different mouse colonies [42].
Importantly, experiments with mice deficient in components of the PD-1 pathway or with antibody blockade cannot clearly address the mechanisms whereby PD-1 signaling modulates humoral responses. Thus more complicated experiments, such as those performed by Good-Jacobson et al. [13], are necessary to address the intrinsic role of PD-1 in specific cell types. Additionally, it is important to consider that PD-1 pathway blockade may affect B cell responses in a different way whether the blockade happens at early (during Tfh differentiation and GC reaction) or late stage (memory) of immune responses.
Moreover, regulatory CD4 T cells (Tregs) express both PD-1 and PD-L1 and those molecules may play a role in Treg suppressive activity [46]. For example it has been shown that Tregs suppress autoreactive PD-1+ B cells through interactions with PD-1 ligands [47]. Importantly, some Tregs can express CXCR5 and suppress germinal center reactions [48] (Figure 2B). And it was recently reported that PD-1 inhibits CXCR5+ Tregs, suggesting that PD-1 pathway blockade may decrease humoral responses through enhancement of CXCR5+ Treg function [43].
In conclusion, the role of PD-1 pathway on humoral responses is complex and may depend on the model or disease as well as the stage of the response, and further studies need to be performed to clarify the mechanisms involved.
Additionally, the benefits of PD-1 pathway blockade during prophylactic vaccination would have to outweigh the therapy costs and also risks of potential autoimmune side effects. Hence, more studies have focused on blockade of the PD-1 pathway in a therapeutic setting, in situations of cancer or chronic infections.
PD-1 blockade to improve therapeutic vaccination
During chronic infections and cancer several immunosuppressive mechanisms are in place. Increased expression of immunosuppressive molecules (such as IL-10 and TGF-β), as well as increase recruitment and differentiation of regulatory T cells and myeloid-derived suppressor cells have all been shown to contribute to inhibition of T cell responses [49*]. In addition, besides PD-1, antigen-specific T cells co-express other inhibitory receptors such as Tim-3, LAG-3, 2B4 and CTLA-4, which maintain T cell exhaustion [50].
To overcome immunosuppression and elicit an effective immune response capable of controlling pathogens and tumors is not an easy task and will probably require combination therapies. For example, blockade of PD-1 signaling in combination with blockade of other inhibitory receptors has shown additive effects in different models of chronic infection and cancer [*29,51–54]. Likewise, in chronic LCMV infection, blockade of PD-L1 and IL-10 was more effective than either therapy alone at rescuing function of exhausted CD8 T cell responses and promoting viral control [55].
Another approach to reinvigorate the immune system consists of combining blockade of suppressive pathways to strategies that boost immune responses, such as therapeutic vaccination. Therapeutic vaccination is based on the assumption that introducing antigens in an immunogenic form can stimulate immune responses to clear infected or tumor cells. Strategies used in therapeutic vaccination to introduce antigen include: DCs, DNA, modified recombinant viruses, and peptides or proteins with adjuvants [49*].
However, therapeutic vaccination in cancer or chronic infections has so far only shown very modest results. Perhaps not surprisingly, given the number of immunosuppressive mechanisms that preserve immune escape of tumors and infected cells. Furthermore, even if therapeutic vaccination strategies would achieve effective activation of antigen-specific T cells, target cells would still need to be eliminated by PD-1 expressing T cells. Since PD-L1 is overexpressed during inflammation associated with chronic infections and tumors [56–59], only improving T cell responses, without manipulation of the PD-1 pathway may not be enough to successfully control infections or tumors.
Distinctively, blockade of the PD-1 pathway improves effector responses, not only by increasing T cell activation by APCs, but also by unleashing their ability to act upon target cells (Figure 3). To investigate the differential role of PD-L1 on hematopoietic cells and nonhematopoietic cells, Mueller et al. generated bone marrow chimera mice where either hematopoietic cells or nonhematopoietic cells were genetically deficient in PD-L1 expression. In this system, after chronic LCMV infection, CD8 T cells had a greater ability to control viral replication in mice where nonhematopoietic cells lacked PD-L1 [35]. Furthermore, PD-L1 expression on target cells has been shown to directly inhibit the lytic activity of cytotoxic CD8 T cells [57,60].
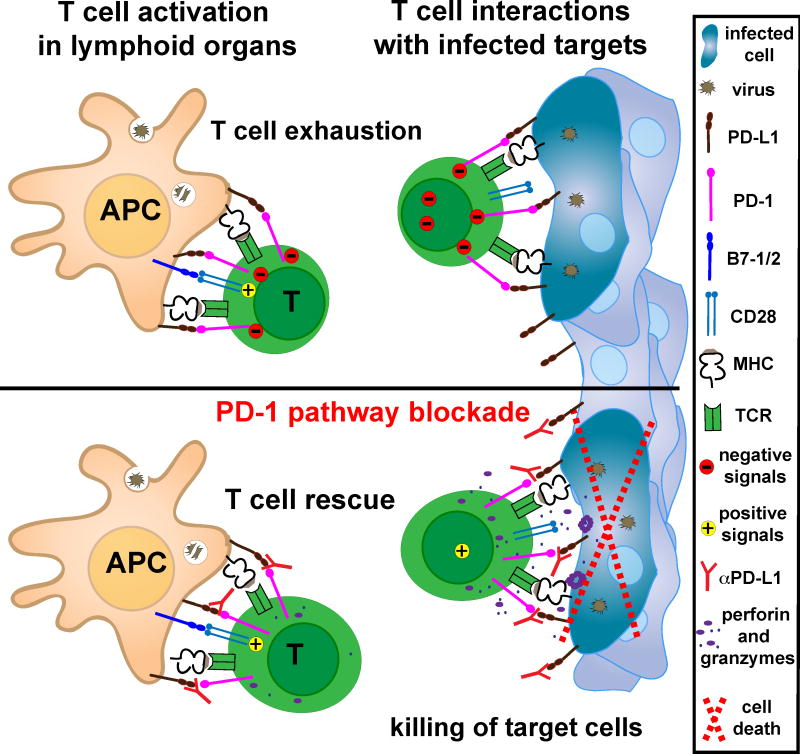
Figure 3. PD-1 pathway blockade promotes T cell activation and elimination of infected cells.
During chronic infections, antigen specific T cells are exhausted due to PD-1 inhibitory signals and lack of positive co-stimulation. PD-1 pathway blockade promotes T cell activation by shifting the balance of signals delivered by cognate APCs from suppressive to activating. When rescued T cells recognize antigen in the periphery, in the absence of PD-1 engagement, T cells can assume full effector function and eliminate target cells [35]. Similar mechanisms also occur in cancer patients.
Thus combining PD-1 blockade to therapeutic vaccination would improve T cell priming and control of tumors or infected cells. Confirming this conceptual idea, therapeutic vaccination, of LCMV chronically infected mice, with recombinant vaccinia virus expressing LCMV-GP33 peptide was highly effective to stimulate CD8 T cell responses and reduce viral load, when combined with anti-PD-L1 antibodies [61]. Likewise, PD-1 pathway blockade improved immunotherapy consisting of irradiated tumor cells secreting granulocyte macrophage colony stimulating factor (GM-CSF) in mouse models of melanoma and colon carcinoma [62]. And complete melanoma regression in mice was only achieved when therapeutic vaccination with adenovirus encoding tumor antigen and 4-1BB co-stimulation were combined with blockade of the PD-1 pathway [63].
Following this same direction, several clinical trials have been planned to test the safety and effects on tumors of PD-1 pathway blockade combined to therapeutic vaccination. For example, PD-1 pathway blockade will be given in conjunction to dendritic cell/tumor fusion vaccines in the treatment of renal cell carcinoma and multiple myeloma (NCT: NCT01441765, NCT01067287). Also, a combination of PD-1 pathway blockade with tumor peptides in oil-based adjuvants will be tested in advanced melanoma patients (NCT: NCT01176474, NCT01176461). The results from those clinical trials should further advance the use of combinatorial immunotherapy for the treatment of cancer patients.
Conclusions
Blockade of the PD-1 pathway has proved to be one of the most effective strategies to rescue function of exhausted T cells. The recent cancer clinical trials with PD-1 pathway blockade are very promising and should drive the use of this therapy into other clinical applications. The PD-1 pathway suppresses T cells responses in lymphoid organs but also in the periphery, since non-hematopoietic cells also express PD-L1. This important and unique feature of PD-1/PD-L1 interactions probably underlines the positive results observed in advanced cancer patients subjected to PD-1 blockade therapy. Although there is an understanding on the role of the PD-1 pathway in CD8 T cell exhaustion during chronic stimulation, much less is known about the early phase of immune responses or in other cell types. Thus, manipulation of the PD-1 pathway to improve prophylactic vaccination remains to be fully explored. Likewise a better understanding of the role of the PD-1 pathway on CD4 T cells and B cells is imperative to fully exploit this powerful therapy.
Highlights.
Blocking PD-1 signaling rescues exhausted T cells in chronic infections and cancer
PD-1 pathway blockade unleashes effector T cell functions on target cells
Combining PD-1 pathway blockade to therapeutic vaccination improves T cell rescue
PD-1 pathway may regulate humoral immunity, but mechanisms are not well established
Further studies on how PD-1 modulates initiation of immune responses are needed
Acknowledgments
This work was supported by grants from the National Institutes of Health R01 AI030048 and P01 A1080192 (RA) and by the Irvington Institute Fellowship Program of the Cancer Research Institute (AOK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 2.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 4.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, et al. Durable Cancer Regression Off-Treatment and Effective Reinduction Therapy with an Anti-PD-1 Antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. Those three papers describe the promising clinical results regarding the use of PD-1 pathway blockade for treatment of advanced cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 11.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson AM, Brown KE, Keir ME, Vanguri VK, Riella LV, Chandraker A, Sayegh MH, Blazar BR, Freeman GJ, Sharpe AH. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187:1097–1105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 17.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 23.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186:2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 24.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 26.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer BE, Neff CP, Lecureux J, Ehler A, Dsouza M, Remling-Mulder L, Korman AJ, Fontenot AP, Akkina R. In Vivo Blockade of the PD-1 Receptor Suppresses HIV-1 Viral Loads and Improves CD4+ T Cell Levels in Humanized Mice. J Immunol. 2013;190:211–219. doi: 10.4049/jimmunol.1201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazar-Molnar E, Gacser A, Freeman GJ, Almo SC, Nathenson SG, Nosanchuk JD. The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proc Natl Acad Sci U S A. 2008;105:2658–2663. doi: 10.1073/pnas.0711918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. This study shows that T cell exhaustion occurs during malaria. It provides a therapeutic strategy to control infection with combinatorial blockade of inhibitory receptors. Importantly, it also shows further evidence on the role of the PD-1 pathway in CD4 T cell exhaustion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 31.Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channappanavar R, Twardy BS, Suvas S. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One. 2012;7:e39757. doi: 10.1371/journal.pone.0039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, Sykes KJ, Guare JP, Miller MD, Olsen DB, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980–987. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- 35.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 38.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 39.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 43.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2012;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 45.Hams E, McCarron MJ, Amu S, Yagita H, Azuma M, Chen L, Fallon PG. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011;186:5648–5655. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 46.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gotot J, Gottschalk C, Leopold S, Knolle PA, Yagita H, Kurts C, Ludwig-Portugall I. Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo. Proc Natl Acad Sci U S A. 2012;109:10468–10473. doi: 10.1073/pnas.1201131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. This review describes the hurdles regarding the contribution of immunotherapy to cancer treatment and describes recent successful strategies along with novel promising directions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 57.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 59.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 60.Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209:2485–2499. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 63.McGray AJ, Bernard D, Hallett R, Kelly R, Jha M, Gregory C, Bassett JD, Hassell JA, Pare G, Wan Y, et al. Combined vaccination and immunostimulatory antibodies provides durable cure of murine melanoma and induces transcriptional changes associated with positive outcome in human melanoma patients. Oncoimmunology. 2012;1:419–431. doi: 10.4161/onci.19534. [DOI] [PMC free article] [PubMed] [Google Scholar]