Abstract
Medical expansion has become a prominent dynamic in today’s societies as the biomedical model becomes increasingly dominant in the explanation of health, illness, and other human problems and behavior. Medical expansion is multidimensional and represented by expansions in three major components of the healthcare system: increasing medical investment, medical professionalization/specialization, and the relative size of the pharmaceutical industry. Using Organisation for Economic Co-operation and Development health data and World Development Indicators 1981 to 2007, we find medical investment and medical professionalization/specialization significantly improve all three measures of life expectancy and decrease mortality rate even after controlling for endogeneity problems. In contrast, an expanded pharmaceutical industry is negatively associated with female life expectancy at age 65 and positively associated with the all-cause mortality rate. It further compromises the beneficial effect of medical professionalization/specialization on population health. In general, medical professionalization/specialization and gross domestic product per capita have similar and stronger effects than medical investment.
Keywords: expanded pharmaceutical industry, medical expansion, medical investment, medical professionalization/specialization, population health
Over the past 50 years, medical explanations have become increasingly dominant in discourses on health, illness, and other human problems. As Clarke et al. (2003:161) state, the growth of medical jurisdiction is “one of the most potent transformations of the last half of the twentieth century in the West.” And the growth of medical jurisdiction continues in the twenty-first century. The biomedical model of health and illness tends to marginalize social origins of disease (Waitzkin and Britt 1989) and “define[s] health problems as the result of individual failures of biology, hygiene, and behavior, with the implicit or explicit belief that the primary strategy for addressing these problems is through biomedical treatments delivered to individuals by physicians and other providers” (Lantz, Lichtenstein, and Pollack 2007:1254). As this biomedical model won legitimacy (or “a near-religious faith”; House 2015:30), it fueled the substantial expansion in medicine in the last half century.
The Western world has observed skyrocketing healthcare costs, explosive growth in the number of hospitals and health facilities, a burgeoning and increasingly specialized medical workforce, expansion of the pharmaceutical industry, and the extension of medical treatments to non medical problems. Some examples of this medical expansion come from the United States, where total health expenditures as a percentage of gross domestic product (GDP) increased from 5.1% in 1960 to 17.1% in 2014; Australia, where medical workforce employment increased from 12.5 per 1,000 persons in 1960 to 36.7 per 1,000 persons in 2006; the United Kingdom, where the number of doctors increased from .8 per 1,000 persons in 1960 to 2.7 per 1,000 persons in 2009; and Switzerland, where the number of medical specialists increased from .5 per 1,000 persons in 1960 to 2.7 per 1,000 persons in 2006 (Organisation for Economic and Co-operation and Development [OECD] 2014).
How much has population health among developed countries benefited from this massive medical expansion? This question is important given the continuous expansion of medicine. Whether society can or should afford healthcare for everyone has become a focus of intense political debate in the United States and other countries. But many sociology and public health studies have reported marginal improvements in population health from healthcare expenditures and argued for focusing on social and economic determinants of health instead (House 2015; Schoeni et al. 2008). Current studies, however, have not yet systematically investigated the effect of medical expansion, as represented by growth in several dimensions of healthcare, on population health. In this study, we characterize the extent of medical expansion by measuring three major components of healthcare systems at the national level: investment in medical infrastructure, the size and specialization of the medical workforce, and the size of the pharmaceutical industry. Then we test how the three dimensions of medical expansion are related to population health in 30 OECD countries between 1981 and 2007 in the context of recent stages of the epidemiological transition. We also compare the relative importance of medical expansion and socioeconomic development on life expectancy and all-cause mortality.
BACKGROUND
Biomedical Model and Medical Expansion
The increasingly dominant role of the biomedical model in health, illness, and other human problems generated expansions in three major components of healthcare systems: infrastructure, which is medical investment; the gatekeeper role played by the medical profession; and prescription drugs. First, the biomedical model drives medical investment by stressing the importance of healthcare, health services, and medical advances in improving population health. This creates societies with a propensity to aggressively screen for disease, treat less serious conditions, and invest heavily in the development of new medical technologies.
Second, the biomedical model promotes medical professionalization and specialization. It empowers medical professionals (e.g., Freidson 1968) and encourages specialization as a result of “the burst of new knowledge flowing from the dramatic rise and productivity of biomedical research since World War II, the array of technology deriving from those advances, and a widespread desire among physicians for related expertise” (Barondess 2000:1300). Since the turn of the twentieth century, physicians have energetically pursued specialization to attain greater prestige, advance clinical skills, pursue more interesting work, and increase their incomes.
Third, the biomedical model enlarges pharmaceutical markets by giving several stakeholders the legitimacy to become increasingly involved in the medical market. On the “supply” side of medical markets, pharmaceutical and biotechnology companies increase their influence on physicians through direct-to-physician advertising. On the “demand” side, consumers increasingly demand medical solutions because their tolerance for mild symptoms and benign problems has decreased, spurring a “progressive medicalization of physical distress in which uncomfortable body states and isolated symptoms are reclassified as diseases” (Barsky and Borus 1995:1931).
Expansion of medicine in these three domains is supported by empirical data. For example, in the United States, between 1960 and 2007, total health expenditures per capita increased by a factor of 3, and total health expenditures as a percentage of GDP increased by a factor of 46. Total health employment density increased by a factor of 5, and total health employment as a percentage of total employment increased by a factor of 4. In the United Kingdom, practicing physician density increased by a factor of 3 between 1960 and 2007. Practicing specialist density increased by a factor of 2 from 1987 to 2007. Also in the United Kingdom, pharmaceutical production per capita increased by a factor of 4.5 from 1980 to 2007. Pharmaceutical sales per capita increased by a factor of 6 from 1984 to 2007.1
Despite this massive medical expansion, research has not systematically investigated whether this expansion improved population health among developed countries in the last several decades. That is the focus of this study. Two macrolevel theories offer very different perspectives on the role of medical advances for population health in developed societies: epidemiologic transition theory and the McKeown thesis.
Epidemiologic Transition Theory and the McKeown Thesis
Epidemiologic transition theory, first formulated by Omran (1971) and further developed by Olshansky and Ault (1986), explains the shift in mortality-related disease patterns in human history. It posits four stages through which advanced societies evolve, starting with the age of pestilence and infectious diseases that characterizes most of human history, followed by the age of receding pandemics around the middle of the nineteenth century in developed countries, and advancing to the age of degenerative and man-made diseases (e.g., cardiovascular disease) in the early twentieth century. Beginning in the late 1960s, the United States and other developed nations began to experience unexpectedly rapid declines in mortality rates for major degenerative diseases (e.g., heart disease, cancer, and stroke). Olshansky and Ault (1986) label this fourth stage of the epidemiologic transition the age of delayed degenerative diseases. Epidemiologic transition theory claims that mortality shifts in the first and second stages were primarily driven by socioeconomic factors (including living standards, hygiene, and nutrition) and owed little to medical measures. Hygiene and nutrition are posited as socioeconomic factors rather than medical factors because their improvement in Western societies was a by-product of social change and public health initiatives rather than a result of medical design (Omran 1971). In contrast, mortality shifts in the twentieth century resulted largely from medical advances, including sophisticated diagnostic tests, surgical interventions, and medications to manage (but not cure) chronic illnesses.
Epidemiologic transition theory has been well received in demography and epidemiology. The stages of this theory consistently receive strong empirical support. Historical analyses document the stages in developed countries, which generally entered the stage of delayed degenerative diseases by the middle of the twentieth century (e.g., Cutler and Miller 2005). Recent studies describe the transitions of developing countries to the third and, occasionally, fourth stages (e.g., Karar, Alam, and Streatfield 2009). Stages of the epidemiological transition also are the framework used in the World Health Organization’s periodic Global Burden of Disease studies (e.g., Murray et al. 2013).
Despite general support for epidemiologic transition stages, consensus is lacking for the mechanisms producing those stages. In particular, demographic historian Thomas McKeown rejected the hypothesis that medical advances are responsible for the mortality shifts in causes of death and increased life expectancy in the third stage. He posited that the mortality decline and population growth in the industrialized world from the late 1700s to the present are mainly due to material advances associated with improved diet and nutrition rather than medical interventions (McKeown 1976; McKeown, Record, and Turner 1975). Thus, McKeown’s thesis is that broad socioeconomic conditions are the root causes of improved population health throughout history. In contrast, medical techniques, vaccines, the sanitation movement, and other medical and public health interventions are claimed to play only a marginal role in the mortality decline in developed nations. McKeown’s thesis is in conflict with epidemiologic transition theory, which argues that mortality shifts in the twentieth century mainly resulted from medical progress.
One body of research supports McKeown’s hypothesis that socioeconomic conditions remain strongly associated with population health (Gravelle and Backhouse 1987; Lantz et al. 2007; Uyanga 1990). Link and Phelan (2002) argue that socioeconomic development is a societal-level indicator of social conditions as fundamental causes of diseases at all stages of development. In another body of research, however, scholars emphasize that since the 1970s, medical advances and increased health-care spending have been strongly associated with declines in all-cause and disease-specific mortality (especially cardiovascular disease; Colgrove 2002; Cremieux, Quellette, and Pilon 1999). Unfortunately, these research traditions do not adjudicate the relative importance of socioeconomic conditions and medical advances for mortality declines in the third and fourth stages of the epidemiologic transition. Studies of the associations between socioeconomic development and mortality fail to include indicators of medical advances. Conversely, with the exception of studies of healthcare spending, studies of the relationships between other forms of medical expansion and mortality shifts typically fail to control for measures of socioeconomic development. To our knowledge, this is the first study to examine the relationships between multiple measures of socioeconomic development and medical expansion and multiple measures of mortality/longevity, using data from a sizeable number of countries in the third and fourth stages of the epidemiologic transition.
The discussion above leads to two hypotheses:
Hypothesis 1: If epidemiologic transition theory is correct, medical expansion should have had a stronger effect in reducing mortality and increasing longevity than socioeconomic development in recent decades among developed countries.
Hypothesis 2: If McKeown’s thesis is correct, medical expansion should have had a weaker effect in reducing mortality and increasing longevity than socioeconomic development in recent decades among developed countries.
In the following sections, we review empirical studies of the relationships between the three dimensions of medical expansion and population health.
Medical Investment and Health
Prior studies of the relationship between health expenditures and health outcomes rely on either a time series of health outcome data for an individual health system or cross-sectional comparisons (especially cross-national comparisons) of different health systems. This line of research is based on an overall health production function model and has produced mixed results. This research tradition can be traced back to Cochrane, Leger and Moore (1978), who examined the relationships among mortality rates, gross national product, and consumption of inputs such as healthcare provision among 18 developed countries. They found that health inputs were not associated with mortality rates. Nixon and Ulmann (2006) found that health-care expenditures made only a marginal contribution to improvements in life expectancy in 15 European Union countries over the period 1980 through 1995, after controlling socioeconomic factors. The poorer health of the U.S. population compared to the populations of other wealthy and even some developing countries, despite much higher healthcare expenditures, also casts doubt on the contribution of medical investment to population health (House 2015).
However, other research has found that healthcare spending is significantly associated with health outcomes. For example, Cremieux et al. (1999) found that increases in infant mortality and decreases in life expectancy were significantly related to lower health-care spending in 10 Canadian provinces during the period 1978 through 1992. Endogeneity is a possible explanation for these mixed results. Health expenditures may increase as a result of poorer population health. Failure to account for this endogeneity will bias the coefficients of endogenous variables (Gravelle and Backhouse 1987; Martin, Rice, and Smith 2008). The endogeneity problem is especially acute in cross-sectional studies.
The inconsistent research findings and methodological problems of some previous studies do not lead to a clear hypothesis. On the basis of epidemiologic transition theory and the biomedical model, however, we hypothesize the following:
Hypothesis 3: Medical investment should have a significant positive relationship with population health after controlling for endogeneity.
Medical Professionalization/Specialization and Health
Recent research reports diverse results concerning the impact of medical professionalization/specialization on health. Some research compares the quality of treatment by specialists and generalists. For example, Grilli et al. (1998) reviewed 47 studies concerning the association between quality-of-care indicators for cancer patients and clinician/center degree of specialization. These studies generally support the view that specialization has a positive impact on processes and outcomes of care. But Chen and colleagues (2000) studied one-year mortality among a national sample of older adults with acute myocardial infarction (AMI) and found no significant differences in the outcomes of care provided by cardiologists and family physicians after controlling for other factors (e.g., comorbidity and use of professional guidelines), although specialists may see sicker patients. Some research even found that patients receiving care from specialists for conditions outside their areas of expertise have higher mortality rates for comorbid conditions including AMI and congestive heart failure (Weingarten et al. 2002).
Other research focused on the geographic distribution of specialists and found that specialist-to-population ratios bear little relationship to health outcomes. For example, Starfield et al. (2005) examined the relationship between mortality rates and specialist supply in U.S. counties. Examining 99.9% of all U.S. counties for the period 1996 to 2000, they found that the higher the specialist-to-population ratios, the higher the rates of total mortality and cancer mortality, although this relationship disappeared after sociodemographic variables were controlled. Shi (1994) found that higher specialist-to-population ratios were associated with higher age-adjusted mortality, mortality from heart disease and cancer, neonatal mortality, life expectancy, and low birth weight ratios at the state level in the United States.
These studies suggest that the fragmentation of patient care and diffusion of responsibility caused by specialization in the modern medical system may have negative implications for health because specialists may be reluctant to view comorbid illnesses as outside their areas of expertise, which may delay patients from seeing appropriate specialists for these diseases (Hashem, Chi, and Friedman 2003). Nonetheless, it is hard to dismiss the possibility of positive impacts of medical professionalization/specialization on health. Moreover, these two lines of studies do not take into account social conditions, which may mask the effect of specialization. For example, it is possible that geographic areas with higher rates of specific illnesses may attract or actively recruit specialists, or patients may move to areas with more experts in their diseases, which then yields a spurious relationship between geographic concentration of specialists and negative health outcomes. Therefore, to examine the impact of medical professionalization/specialization on health, selection effect—or endogeneity—must be controlled. The vast majority of studies of the relationships between professionalization/specialization and population health also are based on U.S. samples. Virtually nothing is known about this relationship across countries.
Despite the preponderance of previous findings, in line with epidemiologic transition theory and the biomedical model more generally, our fourth hypothesis is as follows:
Hypothesis 4: Medical professionalization/specialization should have a significant positive relationship with population health after controlling for endogeneity.
The Expanded Pharmaceutical Industry and Health
Current literature reveals mixed results on the relationships between an expanded pharmaceutical industry and population health. In a series of both cross-sectional and longitudinal studies, Lichtenberg (2005) found that new drug launches and more drug prescriptions are associated with reduced mortality and increased life expectancy in both developed and developing countries.
Recent U.S. research, however, documents the substantial risks to health posed by prescription drugs (Light 2010). Of particular concern have been dramatic increases in prescription drug deaths in the past decade. According to the Food and Drug Administration (FDA; 2015), there was a 123% per capita increase in deaths from prescription drug overdoses between 2006 and 2014. Serious but nonfatal prescription drug overdose outcomes (e.g., hospitalization, brain damage) increased from 264,227 in 2006 to 807,270 in 2014. A large majority of these deaths and serious health problems results from opioid overdoses. A substantial volume of research links adverse drug effects to flawed or incomplete data provided to the FDA by pharmaceutical companies (e.g., Light 2010) and to lack of information about the long-term effects of approved medications (e.g., Schuster, Laggner, and Langer 2005). The increased number of deaths and adverse effects resulting from opioids, however, largely reflect prescribing practices rather than toxicity (Centers for Disease Control and Prevention 2012). Although the vast majority of research on adverse drug reactions, including death, is based on U.S. samples, Bouvy, De Bruin, and Koopmanschap (2015) reviewed 47 European studies of adverse drug reactions. All the studies were based on data from specific healthcare facilities rather than population data, precluding comparisons with U.S. data. Nonetheless, rates of adverse drug reactions were sufficiently high to raise concern.
Despite strong evidence that prescription drugs are a risk for deaths and other serious health problems, this does not mean that the net effect of medications on population health is negative. If the influx of new prescription drugs saves more lives than it shortens, the overall relationship between expanding use of drugs and population health should be positive. Thus, in line with epidemiologic transition theory and the biomedical model, our fifth hypothesis is as follows:
Hypothesis 5: The expanded pharmaceutical industry should have a positive relationship with population health.
Pharmaceutical companies have become increasingly involved in directing medical care and have surpassed medical professionals as new agents of medical expansion after the “golden age of doctoring” (McKinlay and Marceau 2002; see also Conrad 2005). In the past two decades, physicians’ autonomy and independence have been challenged by insurers and pharmaceutical companies due to the introduction of managed care, the bureaucratization (or corporatization) of doctoring, and the expansion of medical markets. If medical professionalization/specialization and the pharmaceutical industry increase together over time and if the net effects of pharmaceutical expansion are negative, as some research suggests, the expanded pharmaceutical industry may compromise the beneficial effect of medical professionalization/specialization on population health. Several underlying mechanisms may contribute to this. Light, Lexichin, and Darrow (2013) report a trend for pharmaceutical companies to develop large numbers of new drugs with few clinical advantages over existing ones, rather than tackling diseases for which existing drugs are absent or of limited effectiveness. The medical professions also have become more commercialized. For example, pharmaceutical companies increasingly urge clinicians to prescribe drugs for conditions other than those for which they are approved and promote off-label or unapproved uses (Light 2010). This potential for countervailing effects leads to our sixth hypothesis:
Hypothesis 6: The expanded pharmaceutical industry may compromise the beneficial effect of medical professionalization/specialization on population health.
In this paper, data from 30 OECD countries are used to examine the relationships between three dimensions of medical expansion—medical investment, medical professionalization/specialization, and pharmaceutical expansion—and four indicators of population health. We focus on life expectancy and mortality because deaths are measured more accurately and comparably across countries than measures of specific illnesses. Life expectancy for men and women at age 65 are examined separately because of recent evidence, based on data from 27 OECD countries, that in all but two of the countries, increases in health expenditures were associated with greater life expectancy improvements for men than for women (Barthold et al. 2014). Life expectancy at age 65 is examined because the third and fourth stages of the epidemiologic transition are characterized by increased rates of chronic and degenerative illnesses, which, along with mortality, are concentrated at advance ages.
METHOD
Data and Variables
In this study, we examine how medical expansion affects population health net of exogenous national characteristics (economic, social, demographic) in 30 OECD countries between 1981 and 2007. We primarily used OECD health data (http://www.oecd.org/els/health-systems/health-data.htm), which are panel data for the 30 countries with 27 data points between 1981 and 2007. We did not use data prior to 1981 due to severe missing-data problems. We did not include countries that joined OECD in the 2000s (Chile, Estonia, Israel, Latvia, and Slovenia) because they have very limited data before 2000. Some variables also were from the World Development Indicators (WDI; http://data.worldbank.org/data-catalog/world-development-indicators).
Table 1 describes the main outcome, explanatory, and control variables used in this study and their data sources. The outcome variables were life expectancy at birth, female life expectancy at age 65, male life expectancy at age 65, and the age-standardized all-cause mortality rate.
Table 1.
Variables and Data Sources.
| Variable | Data | Mean | Standard Deviation |
|---|---|---|---|
| Outcome variables | |||
| Life expectancy at birth | OECD health data | 75.7 | 3.5 |
| All causes mortality rate per 100,000 | OECD health data | 791.5 | 236.9 |
| Female life expectancy at age 65 | OECD health data | 18.4 | 1.8 |
| Male life expectancy at age 65 | OECD health data | 14.8 | 1.7 |
| Key explanatory variables (medical expansion) | |||
| Medical investment | |||
| Healthcare spending per capita (dollars) | OECD health data | 1,486 | 1,232 |
| Proportion of GDP on healthcare | OECD health data | 7.4 | 1.9 |
| Total health employment: density per 1000 population (head counts) | OECD health data | 22.7 | 11.1 |
| Total health employment: % of total employment (head counts) | OECD health data | 5.2 | 2.3 |
| Medical professionalization/specialization | |||
| Ratio of specialists to generalists | OECD health data | 2.2 | 2.3 |
| Practicing physicians density per 1000 | OECD health data | 2.5 | 0.8 |
| Practicing specialists density per 1000 | OECD health data | 1.3 | 0.6 |
| Expanded pharmaceutical industry | |||
| Pharmaceutical production per capita (dollars) | OECD health data | 248 | 378 |
| Pharmaceutical sales per capita (dollars) | OECD health data | 179 | 151 |
| Pharmaceutical value added per capita (dollars) | OECD health data | 101 | 134 |
| Expenditures on pharmaceutical industry R&D per capita (dollars) | OECD health data | 21 | 36 |
| Control variables | |||
| GDP per capita (dollars) | WDI | 23,253 | 9,672 |
| % Women in labor force | OECD health data | 41 | 5.4 |
| Government consumption expenditure (% of GDP) | OECD health data | 18.7 | 4.4 |
| Household consumption expenditure (% of GDP) | OECD health data | 56.6 | 7.2 |
| Foreign direct investment, net inflows (% of GDP) | WDI | 4.2 | 13.7 |
| Log of industry value added (dollars) | WDI | 24.7 | 1.6 |
| Gross fixed capital formation per capita (dollars) | WDI | 3,940 | 1,951 |
| Proportion of total population in urban areas | WDI | 72.5 | 11.6 |
Note: All data are from 1981 to 2007. GDP = gross domestic product; OECD = Organisation for Economic Cooperation and Development; R&D = research and development; WDI = World Development Index (published by the World Bank Group).
The key explanatory variables were the three dimensions of medical expansion.
Indicators of medical investment included healthcare spending per capita,2 the proportion of GDP spent on healthcare, the density of health employment, and health employment as a percentage of total employment. Indicators of medical professionalization/specialization included the density of physicians, the density of specialists, and the ratio of specialists to generalists. Indicators of the size of the pharmaceutical industry as a part of a society’s economy included pharmaceutical production per capita, pharmaceutical sales per capita, pharmaceutical industry value added per capita (i.e., GDP in pharmaceutical industry), and expenditures on pharmaceutical industry research and development (R&D) per capita. The key explanatory variables and control variables that involve units of currency were all denominated in U.S. dollars and adjusted for inflation.
Control variables included GDP per capita, which is a general measure of the level of socioeconomic development and is strongly related to health outcomes. Household consumption expenditure as a percentage of GDP is a measure of consumer spending on goods and services. Government consumption expenditure as a percentage of GDP sheds light on the involvement of governments in providing goods and services for the direct needs of the population. The percentage of women in the labor force is an indicator of gender equality and may be positively associated with both men’s and women’s health. The proportion of the total population in urban areas is an indicator of the extent of urbanization of a society.
Multiple Imputation and Identifying the Latent Variables
In the OECD health data and WDI, most macrolevel indicators had missing data for approximately 25% of observations. Therefore, we applied multiple imputation to create five “complete” data sets with no missing values to avoid the biases and inefficiencies caused by listwise deletion, single imputation, and best-guess imputation.3 Instead of using commonly used multiple imputation methods, such as the regression method, the propensity score method, and the Markov chain Monte Carlo method, we used the bootstrapped-based expectation maximization algorithm for multiple imputation developed by Honaker and King (2010), which is best suited for time-series cross-sectional data (see Appendix A in the online version of the article for more details).
After producing the five data sets, we applied exploratory factor analysis (EFA) to the 11 medical expansion indicators in each data set, calculated the five sets of factor scores for each domain, and then obtained the average factor score for each domain. Table 2 shows the results from the EFA without specifying the number of factors. As can be seen from the rotated factor loadings, EFA suggests that the three-factor solution is best. In addition, each indicator loads primarily on one factor. We name factor 1 “expanded pharmaceutical industry,” factor 2 “medical investment,” and factor 3 “medical professionalization/specialization.” These three factors are not highly correlated and measure distinct dimensions of medical expansion.4 These results suggest that our conceptualization of the three medical expansion domains has empirical support.
Table 2.
Rotated Factor Loadings (Pattern Matrix) and Unique Variances.
| Variable | Factor 1 | Factor 2 | Factor 3 | Uniqueness |
|---|---|---|---|---|
| Health employment density | .109 | .938 | .050 | .106 |
| Health employment as % of total employment | .067 | .918 | .050 | .150 |
| healthcare spending per capita | .612 | .668 | .142 | .159 |
| Proportion of GDP on healthcare | .380 | .768 | .224 | .216 |
| Practicing physicians density per 1,000 | .287 | .359 | .724 | .265 |
| Practicing specialists density per 1,000 | .153 | .190 | .906 | .120 |
| Ratio of specialists to generalists | −.210 | −.227 | .743 | .352 |
| Pharmaceutical production per capita | .931 | .180 | .038 | .099 |
| Pharmaceutical sales per capita | .628 | .541 | .252 | .250 |
| Pharmaceutical value added per capita | .926 | .105 | −.003 | .131 |
| Expenditure on pharmaceutical industry R&D per capita | .787 | .133 | .204 | .321 |
Source: Organisation for Economic Co-operation and Development health data and World Development Index, 1981 to 2007.
Note: GDP = gross domestic product; R&D = research and development.
Methods of Analysis
Estimates from cross-national studies may be biased because of unobserved heterogeneity among nations. Unobserved heterogeneity is not a problem unless it has an effect on the explanatory and outcome variables in the model. There is potential for bias in estimating the causal effects of medical expansion on health due to the possibility of “medical expansion endogeneity,” because medical expansion is affected by other variables, including observed and unobserved nation-specific characteristics. Failure to control for unobserved national differences will bias estimates of the effect of medical expansion on health.
Three aspects of these analyses help to mitigate this endogeneity problem. First, we controlled for a set of observed variables that may impact medical expansion as listed in Table 1. Second, we employed a panel data fixed-effects model to control for unobserved time-invariant nation-specific factors, which helps mitigate the unobserved heterogeneity problem. The structural equation for the fixed effects model can be specified in the following form:
| (1) |
where i = 1, …, N; t = 1, …, T; Yit is country i’s population health indicator at time t; Iit, Sit, and Pit are country i’s medical investment, medical professionalization/specialization, and expanded pharmaceutical industry at time t; Wkit is a set of time-varying variables; Zpi is a set of time-constant variables; θi is an unobserved unit-specific effect (i.e., unobserved heterogeneity); and εit is the time-varying error term (i.e., idiosyncratic error).
We used the fixed effects model to purge Zpi and the unobserved unit heterogeneity θi by subtracting the within-unit average from each observation on that unit. Although the fixed-effects model cannot completely solve the endogeneity problem, it is superior to many previous cross-national studies of health that used simple regression models with minimal statistical controls. Before estimating the fixed-effects model, we applied the Hausman test to determine whether the unobserved unit effects are uncorrelated with the explanatory variables (Wooldridge 2002). This assumption failed, which implies unobserved nation-specific factors are correlated with explanatory variables, so we used fixed-effects models to remove the unobserved heterogeneity. Fixed-effects models assume that the time-varying idiosyncratic errors are mean independent of the explanatory variables. We again used the Hausman test to test this assumption (Wooldridge 2002). If this strict exogeneity assumption holds, fixed-effects models provide unbiased and consistent estimates. When this assumption is violated, the inconsistency in fixed-effect estimators will decrease as t increases and may be very small for large t (Halaby 2004; Wooldridge 2002). The data set used in this study had 27 periods, which is much larger than in many panel data analyses. If the idiosyncratic errors are serially correlated, robust variance estimators can be used to obtain valid standard errors.
Third, we used dynamic panel models to control for omitted or unobserved time-varying explanatory variables by including the lagged endogenous variable Yit–1 in the model. This model handles the potential bias in the estimates of explanatory variables caused by the unobserved effect from Yit–1 to them. Adding the lagged dependent variable (Yit–1) on the right side of the equation, however, will induce more bias in the coefficient estimates of the explanatory variables (Halaby 2004). To solve this problem, we use Arellano and Bond’s (1991) generalized method of moments (GMM) estimator to estimate the model, which includes not only the lagged levels of the endogenous variables but also the lagged levels and differences of predetermined and/or strictly exogenous variables as instruments. First-difference operator is applied to remove time-invariant unobserved heterogeneity. This estimator, however, assumes the idiosyncratic errors are serially uncorrelated.
RESULTS
Time Trends of Medical Expansion
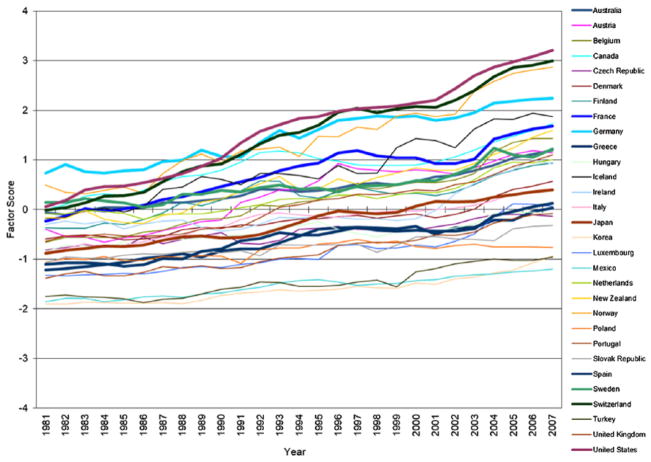
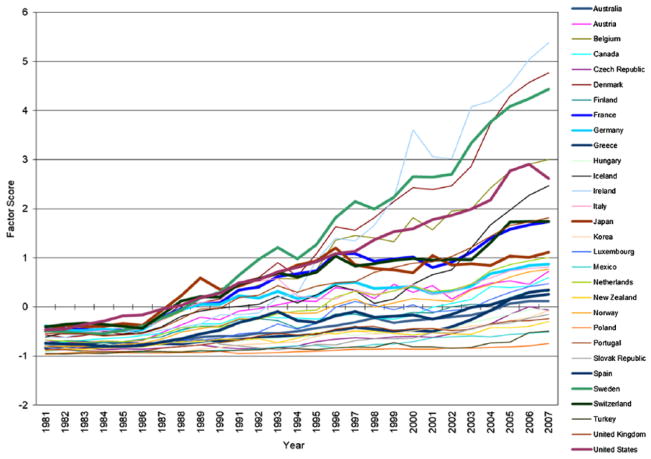
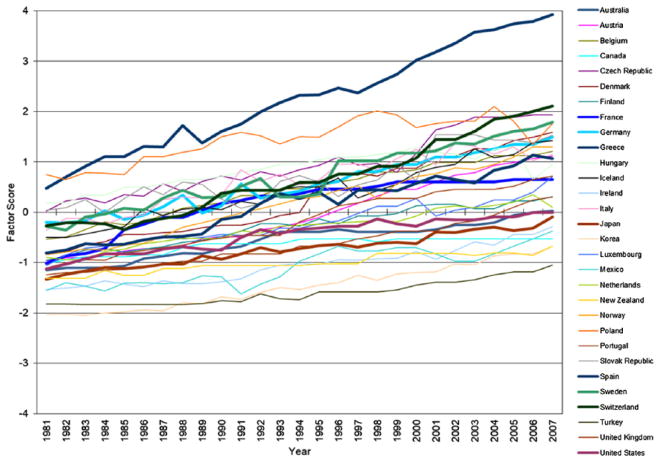
Figures 1 through 3 plot trends in the factor scores of three domains of medical expansion in 30 OECD countries from 1981 to 2007. Consistent with temporal trends described in the literature review, all three domains of medical expansion increased substantially over time, especially expanded pharmaceutical industry. Moreover, heterogeneity in rates of growth across countries leads to increasing differences in the levels of medical expansion among these countries. It is important to note that although the three dimensions of medical expansion are correlated, trajectories of expansion for individual countries vary substantially in their rank orders across the three dimensions. The United States, for example, exhibits the steepest trajectory of medical investment, a steep trajectory for pharmaceutical expansion, and one of the flattest trajectories for professionalization/specialization.
Figure 1.
Exploratory Factor Analysis: Trends in Medical Investment in Organisation for Economic Co-operation and Development Countries, 1981 to 2007.
Figure 3.
Exploratory Factor Analysis: Trends in Expanded Pharmaceutical Industry in Organisation for Economic Co-operation and Development Countries, 1981 to 2007.
The Impact of Increasing Medical Expansion on Population Health
We applied the Hausman test to determine whether the idiosyncratic errors are mean independent of the explanatory variables (Wooldridge 2002). The robust Wald test statistic suggested no correlation between time-varying disturbance and the medical expansion variables in four equations with different outcome variables for each set of imputed data.5 Therefore, the fixed-effects models provide unbiased and consistent estimates for the medical expansion variables. Application of a serial correlation test (Wooldridge 2002) suggested that the idiosyncratic errors are serially correlated. Therefore, robust covariance matrix estimators were used to obtain valid standard errors.
Table 3 presents the standardized coefficients from the fixed-effects models for the four population health outcomes as predicted by the three domains of medical expansion and other national characteristics. Medical investment and medical professionalization/specialization have significant positive associations with all three measures of life expectancy and a negative association with the mortality rate. Therefore, Hypotheses 3 and 4 are supported. In contrast, expansion of the pharmaceutical industry is negatively related to female life expectancy at age 65 and positively associated with all-cause mortality. Thus, Hypothesis 5 is not supported.
Table 3.
Standardized Coefficients for Fixed-effects Models of Life Expectancy at Birth, Female Life Expectancy at Age 65, Male Life Expectancy at Age 65, and All-cause Mortality Rate on Medical Expansion and Other National Predictors in Multiple Imputed Data.
| Variable | Life Expectancy at Birth | Female Life Expectancy at Age 65 | Male Life Expectancy at Age 65 | All-cause Mortality Rate |
|---|---|---|---|---|
| Medical expansion | ||||
| Medical investment | .142** | .196*** | .444*** | −.190*** |
| (3.00) | (3.96) | (9.74) | (−3.82) | |
| Expanded pharmaceutical industry | −.027 | −.072** | .006 | .054* |
| (−1.20) | (−2.92) | (.25) | (2.11) | |
| Medical professionalization/specialization | .363*** | .352*** | .261*** | −.331*** |
| (10.03) | (9.30) | (7.51) | (−7.46) | |
| GDP per capita | .284*** | .444*** | .501*** | −.334*** |
| (6.94) | (10.26) | (12.79) | (−7.57) | |
| % of women in labor force | .054** | .140*** | .105*** | −.131** |
| (1.47) | (4.14) | (3.34) | (−3.26) | |
| General government consumption expenditure (% of GDP) | −.084** | .018 | −.107** | .113** |
| (−2.58) | (.52) | (−3.16) | (3.01) | |
| Household consumption expenditure (% of GDP) | .092** | .042 | .104*** | −.018 |
| (2.65) | (1.25) | (3.35) | (−.46) | |
| Foreign direct investment, net inflows (% of GDP) | .003 | .002 | −.003 | −.017 |
| (.23) | (.15) | (−0.18) | (−1.22) | |
| Industry value added | .235 | .598 | .209 | −.235 |
| (2.13) | (4.39) | (1.85) | (−2.18) | |
| Gross capital formation | −.226 | −.428 | −.216 | .240 |
| (−2.67) | (−4.32) | (−2.46) | (2.76) | |
| % of total population in urban areas | .973*** | .200*** | .254*** | −.640*** |
| (19.83) | (3.84) | (5.20) | (−8.75) | |
Source: Organisation for Economic Co-operation and Development health data and World Development Index, 1981 to 2007.
Note: t statistics in parentheses. GDP = gross domestic product.
p < .05,
p < .01,
p < .001.
Several control variables are significantly related to the health indicators. GDP per capita, percentage of women in the labor force, and percentage of total population in urban areas are positively associated with the three measures of life expectancy and negatively associated with the all-cause mortality rate. General government consumption expenditure as percentage of GDP is negatively associated with life expectancy at birth and male life expectancy at age 65 and positively associated with the all-cause mortality rate. Household consumption expenditure as percentage of GDP has a significant positive impact on life expectancy at birth and male life expectancy at age 65.
Because the coefficients in Table 3 are standardized, we can compare the relative importance of medical expansion and socioeconomic development (especially GDP per capita) on the four outcomes. Across these four outcomes, GDP per capita and medical professionalization/specialization have comparable beneficial effects and are more important than medical investment, except for male life expectancy at age 65, where GDP per capita is the most important, followed by medical investment and then medical professionalization/specialization. Urbanization has a larger impact on life expectancy at birth and all-cause mortality than other predictors. This comparison suggests that even in the fourth stage of the epidemiologic transition in OECD countries, socioeconomic development is a very important factor for longevity and mortality. Medical professionalization/specialization is also very important and even surpasses GDP per capita in its impact on life expectancy at birth. Medical investment is less important but still significant. These findings partially support Hypotheses 1 and 2.
Table 3 also raises the question of the causal order between expanded pharmaceutical industry and the outcomes of female life expectancy at age 65 and all-cause mortality. That is, does lower female life expectancy at age 65 or higher all-cause mortality lead to expansion of the pharmaceutical industry—rather than the reverse? The rationale for reverse causality is that countries with worse health may have higher need for drugs. To test this possibility, we regressed the relative size of the pharmaceutical industry on contemporaneous female life expectancy at age 65 and all-cause mortality rate (Model 1) and one-year lagged values of these two variables (Model 2). As shown in Table 4, the results suggest no significant feedback from contemporaneous and lagged female life expectancy at age 65 and from contemporaneous all-cause mortality rate to expanded pharmaceutical industry. The only exception is the lagged all-cause mortality rate in Model 2, which is negatively related to expanded pharmaceutical industry. That is, higher all-cause mortality rate is associated with a smaller pharmaceutical industry. Overall, reverse causality cannot explain the net harmful impact of expanded pharmaceutical industry.
Table 4.
Unstandardized Coefficients for Fixed-effects Models of Expanded Pharmaceutical Industry on (Lagged) Female Life Expectancy at Age 65 and (Lagged) All-cause Mortality Rate in Multiple Imputed Data.
| Variable | Model 1 | Model 2 |
|---|---|---|
| Female life expectancy at age 65 | .026 | |
| (.52) | ||
| All-cause mortality rate | −.0008 | |
| (−1.71) | ||
| Lagged female life expectancy at age 65 | −.005 | |
| (−.09) | ||
| Lagged all-cause mortality rate | −.001* | |
| (−2.16) | ||
| GDP per capita | .000*** | .000*** |
| (6.97) | (7.26) | |
| % of women in labor force | .036** | .038** |
| (3.07) | (3.19) | |
| General government consumption expenditure (% of GDP) | .004 | .005 |
| (.30) | (.42) | |
| Household consumption expenditure (% of GDP) | −.023** | −.023** |
| (−2.79) | (−2.75) | |
| Foreign direct investment, net inflows (% of GDP) | .040*** | .040*** |
| (6.07) | (6.01) | |
| Industry value added | .000 | .000 |
| (.67) | (.77) | |
| Gross capital formation | .000 | .000 |
| (1.46) | (1.38) | |
| % of total population in urban areas | −.024** | −.025** |
| (−2.62) | (−2.76) |
Source: Organisation for Economic Co-operation health data and Development and World Development Index, 1981 to 2007.
Note: t statistics in parentheses. GDP = gross domestic product.
p < .05,
p < .01,
p < .001.
Next, we investigate what specific indicators of medical professionalization/specialization and expanded pharmaceutical industry are associated with these population health outcomes. Table 5 shows that among medical professionalization/specialization indicators, densities of practicing physicians and specialists are positively associated with health outcomes, but ratio of specialists to generalists is not. Among expanded pharmaceutical industry indicators, as shown in Table 6, only pharmaceutical sales per capita and expenditures on pharmaceutical industry R&D per capita are significantly and negatively associated with health outcomes.
Table 5.
Unstandardized Coefficients for Fixed-effects Models of Life Expectancy at Birth, Female Life Expectancy at Age 65, Male Life Expectancy at Age 65, and All-cause Mortality Rate on Three Indicators of Medical Professionalization/Specialization and Other National Predictors in Multiple Imputed Data.
| Variable | Life Expectancy at Birth | Female Life Expectancy at Age 65 | Male Life Expectancy at Age 65 | All-cause Mortality Rate |
|---|---|---|---|---|
| Model 1 | ||||
| Practicing physicians density per 1,000 | 1.665*** | .864*** | .560*** | −81.480*** |
| (8.53) | (8.36) | (6.34) | (−7.63) | |
| Model 2 | ||||
| Practicing specialists density per 1,000 | 1.258*** | .611*** | .422*** | −63.361*** |
| (7.63) | (6.03) | (5.11) | (−5.80) | |
| Model 3 | ||||
| Ratio of specialists to generalists | .090 | .045 | .043 | −3.637 |
| (1.24) | (1.35) | (1.16) | (−1.01) | |
Note: t statistics in parentheses. Every model controls for medical investment; expanded pharmaceutical industry; gross domestic product (GDP) per capita; percentage of women in labor force; general government consumption expenditure (percentage of GDP); household consumption expenditure (percentage of GDP); foreign direct investment, net inflows (percentage of GDP); industry value added; gross capital formation; and percentage of total population in urban areas.
p < .05,
p < .01,
p < .001.
Table 6.
Unstandardized Coefficients for Fixed-effects Models of Life Expectancy at Birth, Female Life Expectancy at Age 65, Male Life Expectancy at Age 65, and All-cause Mortality Rate on Four Indicators of Expanded Pharmaceutical Industry and Other National Predictors in Multiple Imputed Data.
| Variable | Life Expectancy at Birth | Female Life Expectancy at Age 65 | Male Life Expectancy at Age 65 | All-cause Mortality Rate |
|---|---|---|---|---|
| Model 1 | ||||
| Pharmaceutical sales per capita (dollars) | −.002** | −.001* | −.000 | .151** |
| (−2.93) | (−2.28) | (−.59) | (3.06) | |
| Model 2 | ||||
| Pharmaceutical production per capita (dollars) | −.000 | −.0004† | −.000 | .036† |
| (−1.18) | (−1.88) | (−.04) | (1.67) | |
| Model 3 | ||||
| Pharmaceutical value added per capita (dollars) | .000 | −.001† | −.000 | .019 |
| (.26) | (−1.74) | (−.34) | (.48) | |
| Model 4 | ||||
| Expenditures on pharmaceutical industry R&D per capita (dollars) | −.002 | −.004** | .001 | .247* |
| (−.86) | (−3.15) | (1.40) | (1.99) | |
Source: Organisation for Economic Co-operation and Development health data and World Development Index, 1981 to 2007.
Note: t statistics in parentheses. Every model controls for medical investment; expanded pharmaceutical industry; gross domestic product (GDP) per capita; percentage of women in labor force; general government consumption expenditure (percentage of GDP); household consumption expenditure (percentage of GDP); foreign direct investment, net inflows (percentage of GDP); industry value added; gross capital formation; and percentage of total population in urban areas.
p < .1,
p < .05,
p <.01,
p < .001.
Sensitivity analysis
We further checked whether the findings are sensitive to inclusion/exclusion of any specific country by rerunning the analyses 30 times, dropping a different country each time. Overall, results are remarkably similar (tables available upon request), which suggests that the patterns found in this study do not hinge on any particular nation. We also used GMM models to control for selection due to omitted or unobserved time-varying errors. But this model was not as good as the static fixed-effects model used above for two reasons: first, the strict exogeneity assumption holds, so the dynamic panel model is not necessary; second, the idiosyncratic errors are serially correlated, so the assumption of the GMM model is violated. The GMM models yield results that are similar to those for the static fixed-effects models (see Appendix B), but findings from the GMM models are less stable in terms of statistical significance and some control variables. This suggests that this model provides less efficient estimates than static fixed-effects models. We also used generalized estimating equation (GEE) estimators to adjust for the correlations among repeated measures. Under the assumption of missing completely at random, GEE can provide consistent parameter estimation even if the correlation structure is misspecified (Liang and Zeger 1986). Findings from the GEE method concerning medical expansion indicators are similar but differ for some control variables. But GEE is not a favorable estimator here because it ceases to provide consistent estimations if any of the covariates are endogenous (Crouchley and Davies 1999). The Hausman test suggests the covariates are correlated with the unobserved unit effects, which results in inconsistent parameter estimations. Moreover, the GEE method is based on large sample theory or asymptotic properties of regression parameter estimators (Lee et al. 2007), but this data set includes only 30 countries.
Does an Expanded Pharmaceutical Industry Compromise the Beneficial Impact of Medical Professionalization/Specialization on Health?
The results in Table 3 suggest that an expanded pharmaceutical industry has a negative impact on female life expectancy at age 65 and a positive impact on all-cause mortality. We next explore whether an expanded pharmaceutical industry compromises the beneficial impact of medical professionalization/specialization on health. We created an interaction term between medical professionalization/specialization and expanded pharmaceutical industry in the fixed-effects models. As shown in Table 7, the interaction terms are negative for life expectancy at birth and female life expectancy at age 65. This means that as the pharmaceutical industry expands, the beneficial impact of medical professionalization/specialization on these two outcomes decreases. The positive interaction term for all-cause mortality rate means the negative impact of medical professionalization/specialization on mortality is compromised as the pharmaceutical industry expands. These results support Hypothesis 6.
Table 7.
Unstandardized Coefficients for Fixed-effects Models of Life Expectancy at Birth, Female Life Expectancy at Age 65, Male Life Expectancy at Age 65, and All-cause Mortality Rate on the Interaction between Medical Professionalization/Specialization and Expanded Pharmaceutical Industry and Other Covariates in Multiple Imputed Data.
| Variable | Life Expectancy at Birth | Female Life Expectancy at Age 65 | Male Life Expectancy at Age 65 | All-cause Mortality Rate |
|---|---|---|---|---|
| Expanded pharmaceutical industry | −.011 | −.070 | .010 | 1.354 |
| (−.13) | (−1.37) | (.24) | (.26) | |
| Medical professionalization/specialization | 1.324*** | .682*** | .435*** | −68.400*** |
| (10.07) | (9.74) | (7.33) | (−7.84) | |
| Professionalization × pharmaceutical industry | −.106* | −.077** | −.001 | 11.102*** |
| (−1.97) | (−2.61) | (−.04) | (3.28) |
Source: Organisation for Economic Co-operation and Development health data and World Development Index, 1981 to 2007.
Note: t statistics in parentheses. Control variables are medical investment; gross domestic product (GDP) per capita; percentage of women in labor force; general government consumption expenditure (percentage of GDP); household consumption expenditure (percentage of GDP); foreign direct investment, net inflows (percentage of GDP); industry value added; gross capital formation; and percentage of total population in urban areas.
p < .05,
p < .01,
p < .001.
DISCUSSION
Medical expansion has become a prominent dynamic in contemporary societies as the biomedical model has become increasingly dominant in the explanation of health, illness, and other human problems. Medical expansion is multidimensional and is represented here by expansions in three major components of the healthcare system: medical investment, medical professionalization/specialization, and the relative size of the pharmaceutical industry. Using OECD health data, we investigated how medical expansion is related to four important indicators of population health and whether the various dimensions of medical expansion vary in importance for health outcomes in 30 OECD countries. The findings also speak to conflicting expectations about the role of medical advances in population health in the context of recent stages of the epidemiologic transition.
For the 30 OECD countries, medical investment and medical professionalization/specialization have significant positive impacts on all three measures of life expectancy and a negative impact on the mortality rate after controlling for endogeneity problems. These findings suggest that the mixed results in prior studies are probably due to endogeneity issues. If the endogeneity problems can be mitigated, as in this study by using fixed-effects models, the positive effects of medical investment and medical professionalization/specialization on population health may emerge. Another problem related to medical professionalization/specialization in prior studies is the use of local, rather than national, data. Local-level analysis is more likely to be affected by patients’ health-seeking behaviors and demand-driven recruitment of specialists. In contrast, even after controlling for endogeneity, an expanded pharmaceutical industry is negatively associated with female life expectancy at age 65 and positively associated with the all-cause mortality rate. It further compromises the beneficial effect of medical professionalization/specialization on population health. In general, GDP per capita and medical professionalization/specialization have similar and stronger effects than medical investment. These findings support McKeown’s thesis that socioeconomic development is the most important factor for morbidity and mortality but do not support his hypothesis that medical expansion has relatively little effect. The findings also suggest that the prediction of epidemiologic transition theory that medical advances play the major role for improved population health in the fourth stage of epidemiologic transition should be modified. Socioeconomic development remains very important for population health even for the most advanced countries.
An expanded pharmaceutical industry has detrimental effects on some population health outcomes—findings that could not be explained by reverse causality. There are several possible explanations for this detrimental effect. First, many drugs have side effects. The toxic side effects or misuse of prescription drugs now makes prescription medications a significant and increasing cause of death in the United States, especially for white men and women in midlife (Case and Deaton 2016), and perhaps in other societies as well. Second, multiple prescriptions can generate drug interactions that may harm health, a pattern more likely in societies with aging populations and with large and complex pharmaceutical industries. Third, there are hidden pharmaceutical pollutants in water (Kluger 2010). Millions of drugs’ chemical residuals pass through our bodies and into the sewage system, posing a potential danger for population health in the future. Overall, in this age of biomedicine, the formal and informal norms of medical practice encourage widespread use of prescription medications, expansion of drug use through off-label prescribing practices, and efforts to meet consumer demand for relief of bodily experiences not previously viewed as symptoms of disease. Fueling all of these possible explanations for the negative relationships between pharmaceutical expansion and population health is the pharmaceutical industry’s marketing strategies, including direct marketing to physicians, encouraging off-label prescribing practices, and lobbying efforts for less stringent requirements for FDA approval (and that of equivalent agencies in other countries).
Our results also suggest that societies with higher percentages of women in the labor force have higher life expectancy and lower all-cause mortality rates. If we interpret this variable as an indicator of gender equality, this finding suggests that gender equality is beneficial to population health—a pattern also observed in developing countries in terms of infant mortality and children’s nutritional status (Heaton 2015). We also find that larger percentages of GDP spent on general government consumption expenditures are associated with lower life expectancy at birth, lower male life expectancy at age 65, and higher all-cause mortality rates. These negative associations may result from omitted-variable bias because unemployment, deindustrialization, and the percentage of the population over 65 years old are strong predictors of government spending (e.g., Brady, Beckfield, and Seeleib-Kaiser 2005), and all these factors are associated with poor population health. In addition, we find that a higher percentage of the total population in urban areas has positive impacts on all four indicators of population health.
While this study has opened new pathways for empirical studies of the effects of medical expansion, it has several limitations. First, medical expansion may be related to health outcomes other than longevity/survival, and those relationships may differ in effect sizes and statistical significance from those reported here. Second, there may be better indicators for the three dimensions of medical expansion, especially for expansion of the pharmaceutical industry. As shown in Tables 5 and 6, not all indicators of the dimensions of medical expansion are significantly related to the health outcomes. Third, this sample focuses on 30 relatively advanced countries. Therefore, the findings here may not generalize straightforwardly to other countries, especially developing countries.
Our findings also suggest important priorities for future research on medical expansion and population health. The most important issue for additional inquiry is estimation of multilevel models that incorporate both country-level and individual-level predictors of the health outcomes. As documented in a broad body of research, individual-level variables also are associated with mortality and survival. With regard to pharmaceutical expansion, for example, individual propensities to seek prescription drugs, purchase drugs illicitly, and comply with medication instructions may contribute to the negative relationship between pharmaceutical expansion and population health. Inclusion of individual-level variables also will permit modeling individual selection effects that contribute to potential endogeneity in the relationships between medical expansion and health outcomes.
There also are priority issues for research to better understand the relationships of each dimension of medical expansion and population health. With regard to medical investment, a large number of studies report that the health of the American population is worse than that of the populations of other affluent democracies, despite the fact that the percentage of GDP spent on healthcare in the United States is essentially double that spent in those countries (e.g., Fuchs 2013; Institute of Medicine 2013). And many researchers claim that the ever-increasing percentage of GDP spent on healthcare cannot be sustained in the future (e.g., House 2015). Despite concerns about the sustainability of health-care expenditures in the United States and, to some extent, other developed countries, our findings indicate that medical investment is a significant positive predictor of population health. One important issue for future research is examining whether some kinds of healthcare expenditures have higher payoff for population health than others. For example, compared to other OECD countries, the United States has significantly fewer practicing physicians and hospital beds per capita (Fuchs 2013) but two to three times the number of magnetic resonance imaging and computerized tomography scanners. Williame and DuMont (2015) estimate that between 1981 and 2012, new medical technology and prescription drugs accounted for, on average, 50% of the expanding healthcare expenditures in OECD countries. There are substantial differences across countries, however, and the United States adopts new technology and launches new drugs more quickly than other members of the Organization of the Petroleum Exporting Countries (Kanavos et al. 2013). Other types of healthcare expenditures may have implications for population health. For example, the United States is unique among OECD countries in the proportion of healthcare expenditures paid from public versus private funds. In 2012, 48% of healthcare expenditures in the United States were publicly financed, compared to the average of 72% in other OECD countries (OECD 2014). Numerous scholars hypothesize that a single-payer, publicly financed healthcare system improves population health (e.g., Weisbart 2012), but research evidence is lacking.
Our findings suggest that the dimension of medical expansion with the strongest positive relationship to population health is medical professionalization/specialization. As noted above, however, U.S. research consistently fails to support this conclusion. Additional research is needed to understand the reasons for this apparent discrepancy. Research also is needed to better understand the negative relationships observed between pharmaceutical expansion and population health. Identifying the extent to which these relationships reflect problems with drugs per se (e.g., toxicity, high risk of interactions with other medications) versus the prescribing practices of physicians will be critical to reducing the risks that prescription medicines pose to population health.
In conclusion, this study suggests that the medical expansion occurring in OECD countries since the 1980s has both positive and negative relationships with population health. The results also help to clarify the apparent contradictions between epidemiologic transition theory and the McKeown hypothesis. Social and economic factors continue to be critically important for population health; their significance for population health has not been substantially reduced in later stages of the epidemiologic transition. But medical expansion, especially healthcare spending and professionalization/specialization of healthcare providers, also has positive consequences for population health, although medical expansion also has the potential to pose health risks.
Supplementary Material
Figure 2.
Exploratory Factor Analysis: Trends in Medical Professionalization/Specialization in Organisation for Economic Co-operation and Development Countries, 1981 to 2007.
Acknowledgments
We thank David Brady, Allan Horwitz, Lisa Keister, Kenneth Land, Bruce Link, and Sigrun Olafsdottir for useful comments.
Biographies
Hui Zheng is an associate professor of sociology at The Ohio State University. His work encompasses three interconnected areas: social and policy determinants of health and dynamics of health disparities; population heterogeneity and dynamics of obesity, aging, and mortality; and medical expansion and population health. His current work addresses (1) the effect of selection bias in the process of health production, aging, obesity, health disparities, and life expectancy; (2) the recent trend in mortality and health disparities in the United States; (3) the effect of sex ratio and marriage market on health; and (4) work place and health.
Linda K. George is Arts and Sciences Professor of Sociology at Duke University. Her research interests include racial-ethnic health disparities, application of life course theory to health and well-being, and the effects of medical technology on population health. Her career contribution awards include the Kleemeier Award of the Gerontological Society of America, the Matilda White Riley Award of the American Sociological Association for distinguished scholarship on aging and the life course, and the Leonard I. Pearlin Award of the American Sociological Association for distinguished scholarship on the sociology of mental health.
Appendix B
Unstandardized Coefficients for GMM Models of Life Expectancy at Birth, Female Life Expectancy at Age 65, Male Life Expectancy at Age 65, and All-cause Mortality Rate on Medical Expansion and Other National Predictors in Multiple Imputed Data (t Statistics in Parentheses)
| Life Expectancy at Birth | Female Life Expectancy at Age 65 | Male Life Expectancy at Age 65 | All-cause Mortality Rate | |
|---|---|---|---|---|
| Lagged dependent variable | .867*** | .794*** | .772*** | .746*** |
| (39.91) | (25.52) | (24.90) | (22.82) | |
| Medical Expansion | ||||
| Medical investment | .204** | .164* | .326*** | −24.127* |
| (2.35) | (1.96) | (4.63) | (−2.48) | |
| Expanded pharmaceutical industry | .009 | −.050 | −.061* | 7.989* |
| (.24) | (−1.32) | (−1.97) | (1.96) | |
| Medical professionalization/specialization | .081 | .087 | .033 | −17.047** |
| (1.33) | (1.53) | (.75) | (−2.71) | |
| GDP per capita | .000*** | .000*** | .000*** | −.001 |
| (3.51) | (3.98) | (5.78) | (−1.74) | |
| % of women in labor force | .000 | .000 | .001 | −1.521 |
| (−.01) | (−.03) | (.09) | (−1.70) | |
| General government consumption expenditure (% of GDP) | −.009 | −.000 | −.002 | 2.131 |
| (−.90) | (−.05) | (−.22) | (1.91) | |
| Household consumption expenditure (% of GDP) | .010 | .008 | .007 | .242 |
| (1.61) | (1.26) | (1.51) | (.40) | |
| Foreign direct investment, net inflows (% of GDP) | −.002 | −.002 | .001 | −.079 |
| (−.49) | (−.64) | (.29) | (−.21) | |
| Industry value added | .000 | .000 | .000 | .000 |
| (−.12) | (.58) | (.30) | (.70) | |
| Gross capital formation | .000 | .000 | .000 | .000 |
| (−.35) | (.55) | (−.60) | (−.49) | |
| % of total population in urban areas | .026* | .001 | −.009 | −2.097* |
| (2.04) | (.07) | (−.99) | (−2.02) | |
Source: Organisation for Economic Co-operation health data and Development and World Development Index, 1981–2007.
p < .05,
p <.01,
p < .001.
Footnotes
All the numbers in this paragraph were calculated by the authors based on Organisation for Economic Co-operation and Development health data.
It includes all expenditures directly related to medical care: curative and rehabilitative care, long-term care, ancillary services (e.g., laboratory, imaging, and patient transportation), medical goods, preventive care, governance, and health system and financing administration.
We also created 10 imputed data sets, and findings were generally similar.
Corr (factor 1, factor 2) = .5733; Corr (factor 1, factor 3) = .2949; Corr (factor 2, factor 3) = .3244.
For example, in the first imputed data, F(3, 711) = 1.52 and Prob > F =.209 for the life-expectancy-at-birth equation, F(3, 711) = 1.85 and Prob > F = .137 for the female-life-expectancy-at-age-65 equation, F(3, 711) = .59 and Prob > F = .624 for the male-life-expectancy-at-age-65 equation, and F(3, 711) = 1.52 and Prob > F = .209 for the mortality equation.
Appendix A is available in the online version of the article.
An earlier version of this paper was presented at the Annual Meeting of the American Sociological Association.
References
- Arellano Manuel, Bond Stephen R. Some Tests of Specification for Panel Data: Monte Carlo Evidence and an Application to Employment Equations. Review of Economic Studies. 1991;58(2):277–97. [Google Scholar]
- Barondess Jeremiah A. Specialization and the Physician Workforce: Drivers and Determinants. Journal of American Medical Association. 2000;284(10):1299–301. doi: 10.1001/jama.284.10.1299. [DOI] [PubMed] [Google Scholar]
- Barsky Arthur J, Borus Jonathan F. Somatization and Medicalization in the Era of Managed Care. Journal of the American Medical Association. 1995;274:1931–34. [PubMed] [Google Scholar]
- Barthold Douglas, Nandi Arijit, Mendoza-Rodriguez Jose M, Heymann Jody. Analyzing Whether Countries Are Equally Efficient at Improving Longevity for Men and Women. American Journal of Public Health. 2014;104(11):216–69. doi: 10.2105/AJPH.2013.301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvy Jacoline C, DeBruin Marie L, Koopmanschap Marc A. Epidemiology of Adverse Drug Reactions in Europe: A Review of Observational Studies. Drug Safety. 2015;38(5):437–53. doi: 10.1007/s40264-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady David, Beckfield Jason, Seeleib-Kaiser Martin. Economic Globalization and the Welfare State in Affluent Democracies, 1975–2001. American Sociological Review. 2005;70(6):921–48. [Google Scholar]
- Case Anne, Deaton Angus. Rising Morbidity and Mortality in Midlife among White Non-Hispanic Americans in the 21st Century. PNAS. 2016;112(49):15078–83. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC Grand Rounds: Prescription Drug Overdoses. A U.S. Epidemic. MMWR Morbidity and Mortality Weekly Report. 2012;61(01):10–13. [PubMed] [Google Scholar]
- Chen Jersey, Radford Martha J, Wang Yun, Krumholz Harlan M. Care and Outcomes of Elderly Patients with Acute Myocardial Infarction by Physician Specialty: The Effects of Comorbidity and Functional Limitations. American Journal of Medicine. 2000;108(6):460–69. doi: 10.1016/s0002-9343(00)00331-4. [DOI] [PubMed] [Google Scholar]
- Clarke Adele E, Shim Janet K, Mamo Laura, Fosket Jennifer R, Fishman Jennifer R. Biomedicalization: Technoscientific Transformations of Health, Illness, and U.S. Biomedicine. American Sociological Review. 2003;68(2):161–94. [Google Scholar]
- Cochrane A, Leger A, Moore SF. Health Service ‘Input’ and Mortality ‘Output’ in Developed Countries. Journal of Epidemiology and Community Health. 1978;32(3):200–205. doi: 10.1136/jech.32.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgrove James. The McKeown Thesis: A Historical Controversy and Its Enduring Influence. American Journal of Public Health. 2002;92(5):725–29. doi: 10.2105/ajph.92.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad Peter. The Shifting Engines of Medical Expansion. Journal of Health and Social Behavior. 2005;46(1):3–14. doi: 10.1177/002214650504600102. [DOI] [PubMed] [Google Scholar]
- Cremieux Pierre-Yves, Quellette Pierre, Pilon Caroline. Health Care Spending as Determinants of Health Outcomes. Health Economics. 1999;8(7):627–39. doi: 10.1002/(sici)1099-1050(199911)8:7<627::aid-hec474>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Crouchley R, Davies RB. A Comparison of Population Average and Random-effect Models for the Analysis of Longitudinal Count Data with Baseline Information. Journal of the Royal Statistical Society. 1999;162(3):331–47. [Google Scholar]
- Cutler David, Miller Grant. The Role of Public Health Improvements in Health Advances: The Twentieth-century United States. Demography. 2005;42(1):1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. FAERS Reporting by Patient Outcomes by Year. 2015 Retrieved August 8, 2016 ( https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070461.htm)
- Freidson Eliot. The Impurity of Professional Authority. In: Becker HS, Geer B, Riesman D, Weiss RS, editors. Institutions and the Person. Chicago: Aldine; 1968. pp. 25–34. [Google Scholar]
- Fuchs Victor R. How and Why US Health Care Differs from That in Other OECD Countries. JAMA. 2013;309(1):33–34. doi: 10.1001/jama.2012.125458. [DOI] [PubMed] [Google Scholar]
- Gravelle H, Backhouse M. International Cross-section Analysis of the Determination of Mortality. Social Science & Medicine. 1987;25(5):427–41. doi: 10.1016/0277-9536(87)90167-5. [DOI] [PubMed] [Google Scholar]
- Grilli R, Minozzi S, Tinazzi A, Labinaca R, Sheldon TA, Liberati A. Do Specialists Do It Better? The Impact of Specialization on the Processes and Outcomes of Care for Cancer Patients. Annals of Oncology. 1998;9(4):365–74. doi: 10.1023/a:1008201331167. [DOI] [PubMed] [Google Scholar]
- Halaby Charles N. Panel Models in Sociological Research: Theory into Practice. Annual Review of Sociology. 2004;30:507–44. [Google Scholar]
- Hashem Ahmad, Chi Michelene TH, Friedman Charles P. Medical Errors as a Result of Specialization. Journal of Biomedical Informatics. 2003;36(1/2):61–69. doi: 10.1016/s1532-0464(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Heaton Tim B. Are Improvements in Child Health Due to Increasing Status of Women in Developing Nations? Biodemography and Social Biology. 2015;61(3):252–65. doi: 10.1080/19485565.2015.1047487. [DOI] [PubMed] [Google Scholar]
- Honaker James, King Gary. What to Do about Missing Values in Time Series Cross-section Data. American Journal of Political Science. 2010;54(3):561–81. [Google Scholar]
- House James S. Beyond Obamacare: Life, Death, and Social Policy. New York: Russell Sage Foundation; 2015. [Google Scholar]
- Institute of Medicine. US Health in International Perspective: Shorter Lives, Poorer Health. Washington, DC: Author; 2013. [Google Scholar]
- Kanavos Panos, Ferrario Alessandra, Vandoros Sotiris, Anderson Gerard F. Higher US Branded Drug Prices and Spending Compared to Other Countries May Stem Partly from Quick Uptake of New Drugs. Health Affairs. 2013;32(4):753–61. doi: 10.1377/hlthaff.2012.0920. [DOI] [PubMed] [Google Scholar]
- Karar Z Ahsan, Alam N, Kim Streatfield P. Epidemiologic Transition in Rural Bangladesh, 1986–2006. Global Health Action. 2009 doi: 10.3402/gha.v2i0.1904. doi.org/10.3402/gha.v21.1904. [DOI] [PMC free article] [PubMed]
- Kluger Jeffrey. Flushed Away. Time Magazine. 2010 Retrieved April 1, 2010 ( http://content.time.com/time/specials/packages/article/0,28804,1976909_1976907_1976871,00.html) [PubMed]
- Lantz Paula M, Lichtenstein Richard L, Pollack Harold A. Health Policy Approaches to Population Health: The Limits of Medical expansion. Health Affairs. 2007;26(5):1253–57. doi: 10.1377/hlthaff.26.5.1253. [DOI] [PubMed] [Google Scholar]
- Lee Ji-Hyun, Herzog Thaddeus A, Meade Cathy D, Webb Monica S, Brandon Thomas H. The Use of GEE for Analyzing Longitudinal Binomial Data: A Primer Using Data from a Tobacco Intervention. Additive Behaviors. 2007;32(1):187–93. doi: 10.1016/j.addbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Liang Kung-Yee, Zeger Scott L. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- Lichtenberg Frank R. The Impact of New Drug Launches on Longevity: Evidence from Longitudinal, Disease-level Data from 52 Countries, 1982–2001. International Journal of Health Care Finance and Economics. 2005;5(1):47–73. doi: 10.1007/s10754-005-6601-7. [DOI] [PubMed] [Google Scholar]
- Light Donald W. Bearing the Risks of Prescription Drugs. In: Light DW, editor. The Risks of Prescription Drugs. New York: Columbia University Press; 2010. pp. 1–39. [Google Scholar]
- Light Donald W, Lexchin Joel, Darrow Jonathan J. Institutional Corruption of Pharmaceuticals and the Myth of Safe and Effective Drugs. Journal of Law and Medical Ethics. 2013;14(3):590–610. doi: 10.1111/jlme.12068. [DOI] [PubMed] [Google Scholar]
- Link Bruce G, Phelan Jo C. McKeown and the Idea that Social Conditions Are Fundamental Causes of Disease. American Journal of Public Health. 2002;92(5):730–32. doi: 10.2105/ajph.92.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Stephen, Rice Nigel, Smith Peter C. Does Health Care Spending Improve Health Outcomes? Evidence from English Programme Budgeting Data. Journal of Health Economics. 2008;27(4):826–42. doi: 10.1016/j.jhealeco.2007.12.002. [DOI] [PubMed] [Google Scholar]
- McKeown Thomas. The Role of Medicine: Dream, Mirage, or Nemesis? London: Nuffield Provincial Hospitals Trust; 1976. [Google Scholar]
- McKeown Thomas, Record RG, Turner RD. An Interpretation of the Decline in Mortality in England and Wales during the Twentieth Century. Population Studies. 1975;29(3):391–422. [PubMed] [Google Scholar]
- McKinlay John B, Marceau Lisa D. The End of the Golden Age of Doctoring. International Journal of Health Services. 2002;32(2):379–416. doi: 10.2190/JL1D-21BG-PK2N-J0KD. [DOI] [PubMed] [Google Scholar]
- Murray Christopher JL, Vos Theo, Lozano Rafael, et al. Disability-adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Nixon John, Ulmann Philippe. The Relationship between Health Care Expenditure and Health Outcomes. European Journal of Health Economics. 2006;7(1):7–18. doi: 10.1007/s10198-005-0336-8. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Development and Co-operation. Health Statistics 2014: How Does the United States Compare? 2014 Retrieved March 27, 2015 ( http://www.oecd.org/unitedstates/Briefing-Note-UNITED-STATES-2014.pdf)
- Olshansky S Jay, Brian Ault A. The Fourth Stage of the Epidemiologic Transition: The Age of Delayed Degenerative Diseases. Milbank Quarterly. 1986;64(3):355–91. [PubMed] [Google Scholar]
- Omran Abdel R. The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. Milbank Memorial Fund Quarterly. 1971;49(4):509–38. [PubMed] [Google Scholar]
- Schoeni Robert F, House James S, Kaplan George A, Pollack Harold. Making Americans Healthier: Social and Economic Policy as Health Policy. New York: Russell Sage Foundation; 2008. [Google Scholar]
- Schuster Daniela, Laggner Christian, Langer Thierry. Why Drugs Fail: A Study on Side Effects in New Chemical Entities. Current Pharmaceutical Design. 2005;11(27):3545–59. doi: 10.2174/138161205774414510. [DOI] [PubMed] [Google Scholar]
- Shi Leiyu. Primary Care, Specialty Care, and Life Chances. International Journal of Health Services. 1994;24(3):431–58. doi: 10.2190/BDUU-J0JD-BVEX-N90B. [DOI] [PubMed] [Google Scholar]
- Starfield Barbara, Shi Leiyu, Grover Atul, Macinko James. The Effects of Specialist Supply on Populations’ Health: Assessing the Evidence. Health Affairs. 2005;W5:97–107. doi: 10.1377/hlthaff.W5.97. [DOI] [PubMed] [Google Scholar]
- Waitzkin Howard, Britt Theron. A Critical Theory of Medical Discourse: How Patients and Health Professionals Deal with Social Problems. International Journal of Health Services. 1989;19(4):577–97. doi: 10.2190/L84U-N4MQ-9YAC-D4PP. [DOI] [PubMed] [Google Scholar]
- Weingartern Scott R, Lloyd Lynne, Chiou Chiun-Fang, Braunstein Glenn D. Do Subspecialists Working outside of Their Specialty Provide Less Efficient and Lower-quality Care to Hospitalized Patients Than Do Primary Care Physicians? Archives of Internal Medicine. 2002;162(5):527–32. doi: 10.1001/archinte.162.5.527. [DOI] [PubMed] [Google Scholar]
- Williame Peter, DuMont Michel. Machines That Go ‘Ping’: Medical Technology and Health Expenditures in OECD Countries. Health Economics. 2015;24(8):1027–41. doi: 10.1002/hec.3089. [DOI] [PubMed] [Google Scholar]
- Weisbart Ed. A Single-payer System Would Reduce US Health Care Costs. AMA Journal of Ethics. 2012;14(11):897–903. doi: 10.1001/virtualmentor.2012.14.11.oped1-1211. [DOI] [PubMed] [Google Scholar]
- Wooldridge Jeffrey M. Econometric Analysis of Cross Section and Panel Data. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- Uyanga Joseph. Economic Development Strategies: Maternal and Child Health. Social Science & Medicine. 1990;31(6):649–59. doi: 10.1016/0277-9536(90)90246-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.