Abstract
Prepulse inhibition (PPI) of acoustic startle and locomotor activity are both widely studied in the preclinical development of dopaminergic agents, including those acting at D3 dopamine receptors. In mice, the dopamine D3 receptor-preferential agonist pramipexole (PPX) alters locomotor activity in a biphasic manner at doses that have no effect on PPI. The present study examined the time-course of PPX effects on locomotion and PPI in rats. In adult male Sprague–Dawley rats, PPX (0, 0.1, 0.3, 1.0 mg/kg) was injected prior to measurement of locomotor activity for 90 min in photobeam chambers. Based on disparate early vs. late effects of PPX on locomotion, the effects of PPX (0 vs. 0.3 mg/kg) on PPI were tested 20 and 80 min after injection. All doses of PPX decreased locomotor activity for 30 min compared to vehicle, and the higher doses stimulated hyperlocomotion later in the session; the late hyperlocomotion, but not the early hypolocomotion, was blocked by the D2-selective antagonist, L741626 (1.0 mg/kg sc). In contrast to its locomotor effects, PPX caused a similar reduction in PPI at 20 and 80 min after administration. These findings suggest both a temporal and pharmacological dissociation between PPX effects on locomotor activity and PPI; these two behavioral measures contribute non-redundant information to the investigation of D3-related behavioral pharmacology.
Keywords: Dopamine, Locomotor activity, Pramipexole, Prepulse inhibition, Startle
1. Introduction
The dopamine D3 receptor has been studied as a potential source of pathology or target for novel pharmacotherapeutics for several neuropsychiatric disorders, including schizophrenia, Tourette Syndrome, and drug addiction (c.f. Sokoloff et al., 2006; Weber et al., 2009a,b). Animal models can help elucidate the mechanisms by which activity at D3 receptors regulates behaviors of relevance to these disorders. Two common behavioral measures in animal models of neuropsychiatric disorders are prepulse inhibition of startle (PPI) and locomotor exploration.
We (Chang et al., 2010b; Swerdlow et al., 2009; Weber et al., 2008, 2009b) and others (Caine et al., 1995; Varty and Higgins, 1998; Zhang et al., 2007) have reported that D3-preferential agonists can reliably suppress PPI in rats. These effects are detected across multiple rat strains, using different stimulus modalities, in males and females, and after either systemic or intracerebral administration (Chang et al., 2010b; Weber et al., 2010a). In contrast to their effects in rats, many D3-preferential and mixed D2/D3 agonists have no effects on PPI in mice (Chang et al., 2010a; Ralph-Williams et al., 2003; Ralph and Caine, 2007), consistent with other reports that different dopamine receptor subtypes regulate PPI in rats vs. mice (Ralph et al., 1999). Genetic approaches using mice are therefore suboptimal for elucidating the molecular mechanisms underlying the D3 regulation of sensorimotor gating. For example, we reported previously that the D3-preferential agonist, pramipexole (PPX), disrupts PPI in rats but not in C57BL/6J mice, a common background strain knockout studies; in contrast, PPI is disrupted in both species by the mixed D1/D2 agonist, apomorphine (Breier et al., 2010; Caine et al., 1995; Caldwell et al., 2009; Chang et al., 2010a; Martin et al., 2008; Ralph-Williams et al., 2002, 2003; Russig et al., 2004; Semenova et al., 2008; Swerdlow et al., 2005, 2008a,b; Van den Buuse et al., 2005; Weber et al., 2008, 2009a; Weber and Swerdlow, 2008; Yee et al., 2004). Interestingly, in mice, PPX produces dose-dependent, biphasic changes in locomotor exploratory activity, consisting of an early locomotor suppression followed by a later, D2-dependent hyperactivity (Chang et al., 2010a).
Rather than pursue separate, parallel efforts to elucidate the biology of two different D3-mediated effects (on locomotor activity and PPI) in two different species (mouse and rat, respectively), we tested PPX effects on both locomotor activity and PPI in rats, with the goal of developing a simpler, single-species model for understanding D3-mediated effects on these behavioral measures.
2. Methods
2.1. Animals
Adult male Sprague–Dawley rats (n=40, 225–250 g; Harlan Laboratories, Livermore, CA) were housed 2–3 animals per cage and maintained on a reversed light/dark schedule with food and water available ad libitum. All testing occurred during the dark phase, and rats were handled within 2 days of arrival and allowed to acclimate for at least 7 days before behavioral testing. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 85-23, revised 1985) and were approved by the UCSD Animal Subjects Committee (protocol #S01221).
2.2. Drugs
PPX was obtained from Toronto Research Chemicals (North York, Ontario, Canada) and L741626 from Tocris (Ellisville, MO, USA). PPX (0, 0.1, 0.3, 1.0 mg/kg) was dissolved in saline and injected subcutaneously. L741626 (0, 1.0 mg/kg) was dissolved in 0.15% lactic acid/water (w/v) and pH was adjusted to ≥5 with NaOH; injection was subcutaneous. PPX was administered immediately before locomotor testing, and either 15 or 75 min before placement into startle chambers for PPI testing. For locomotor studies using D2 antagonist pretreatment, L741626 was injected 30 min before PPX injection and placement in activity chambers.
2.3. Locomotor testing
Locomotor activity was measured using wire-mesh photocell cages (22 × 35 × 15 cm) fitted with two parallel infrared beams 1 cm above the floor, perpendicular to the long axis of the cage. The total number of beam breaks and crossovers (sequential interruption of separate beams) was calculated for each 10 min interval during 90 min of testing; rats were not habituated to the test chamber before locomotor activity measurement began. To understand the behavioral basis for changes in photocell activity counts, animals were observed through a viewing window and assessed for the presence of specific behaviors (Fray et al., 1980) by an individual who was blind to their drug condition; any combination of behaviors could be present during each rating period. Animals were first tested (test day 1) with either vehicle or active dose of agonist, and then assigned to new dose groups balanced for previous PPX doses, for studies of antagonist/agonist combinations (test days 2 and 3), with 7–9 days between test days.
2.4. Prepulse inhibition testing
Startle chambers (San Diego Instruments, San Diego, CA, USA) consisted of a Plexiglas cylinder 8.2 cm in diameter resting on a 12.5 × 25.5 cm Plexiglas frame within a ventilated enclosure, housed in a sound-attenuated room. Noise bursts were presented from a speaker mounted 24 cm above the cylinder, and a piezoelectric accelerometer mounted below the Plexiglas frame detected and transduced motions from within the cylinder. SR-LAB microcomputer and interface assembly controlled stimulus delivery and digitized (0–4095), rectified, and recorded stabilimeter readings. One hundred 1-ms readings were collected beginning at stimulus onset and averaged to yield the startle amplitude.
PPI effects were tested in a separate group of rats. Before drug testing, rats were exposed to a short “matching” session in startle chambers, which consisted of a 5 min acclimation period with 70 dB (A) background noise and then 17 PULSE-ALONE trials (40 ms–120 dB (A) noise bursts) interspersed with 3 PREPULSE+PULSE trials in which PULSE-ALONE was preceded 100 ms (onset-to-onset) by a 20 ms noise burst, 12 dB above background. The average %PPI from this session was used to assign rats to drug/dose groups with matched baseline PPI.
Based on findings from locomotor studies, PPI studies utilized pretreatment time as a between-subject factor, and vehicle vs. drug as a within-subject factor, with balanced dose orders. Test days were 5 days apart. 15 or 75 min after PPX (0, 0.3 mg/kg) injection, rats were placed into startle chambers for a 5 min acclimation period with a 70 dB(A) background noise. Active trials were presented in pseudorandom order and included: (1) PULSE-ALONE (40 ms–120 dB(A) noise burst); (2–4) PREPULSE+PULSE (PULSE-ALONE preceded 100 ms (onset-to-onset) by a 20 ms noise burst either 5, 10, or 15 dB above background). Interspersed between active trials were NOSTIM trials in which no stimulus was presented but activity was recorded. Average ITI between active trials was 15 s. Session duration was 18.5 min, including the acclimation period.
2.5. Data analysis
For locomotor data, photocell beam break and crossover counts were analyzed by ANOVA. Comparable results were found for both beam breaks and crossovers, so crossovers are presented to avoid redundancy. Data are reported as group mean ± SEM of total crossovers for each interval. On Day 1 of locomotor testing, PPX dose was a between-subject factor. For Days 2 and 3, L741626 pretreatment was the between-subject factor and PPX was a within-subject factor.
Behavioral ratings were scored as ‘present’ or ‘absent’. Analyses for behavioral rating data were based on those described in Fray et al. (1980). Briefly, the percentage of rats within each dose and interval displaying behaviors from each category was entered into a contingency table, which was then assessed for heterogeneity using a likelihood ratio method, called the ‘information statistic’ (Kullback, 1968; Robbins, 1977), which is analogous to the χ2 but is not constrained by small cell frequencies. Calculation of 2Î is detailed in Fray et al. (1980).
PPI was defined as 100−[(startle amplitude on PREPULSE+PULSE trials / startle amplitude on PULSE-ALONE trials) × 100], and was analyzed by mixed design ANOVAs. Data was inspected for “non-responders”, defined by mean startle response to PULSE-ALONE trials of less than 10. There was one non-responder on the second day of testing, who was eliminated from all analyses. Repeated measures ANOVAs were used to assess responses to PULSE-ALONE, PREPULSE + PULSE, or NOSTIM trials.
For all photocell and PPI studies, relevant post-hoc comparisons were conducted using Fisher's PLSD and one-factor ANOVA tests, and alpha was set at 0.05.
3. Results
3.1. PPX effects on locomotor activity
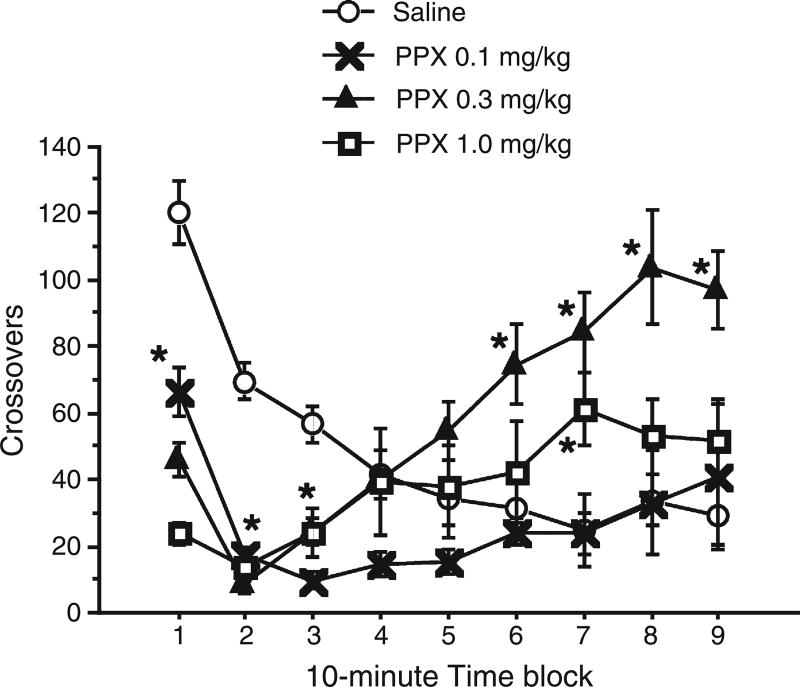
Rats exhibited a dose-dependent, biphasic pattern of PPX-induced locomotor changes that was strikingly similar to that previously observed in mice (Chang et al., 2010a). Repeated measures ANOVA for crossovers revealed significant effects of PPX dose (F = 5.23, df 3,20; p < 0.01) and interval (F = 9.75, df 8,160; p < 0.0001), and a PPX × interval interaction (F = 8.83, df 24,160; p < 0.0001; Fig. 1). Post hoc testing showed that crossovers for all active doses were significantly different from the vehicle-treated group during the first 30 min of testing, and that the 0.3 mg/kg PPX group had significantly increased crossovers during the last 40 min of testing. Inspection of the data (Fig. 1) suggested that there was also an increase in crossovers in the PPX 1.0 mg/kg group, but this effect was only statistically significant during Interval 7.
Fig. 1.
Dose–response effects of PPX on locomotor activity in rats. PPX (0, 0.1, 0.3, 1.0 mg/kg) was injected immediately before animals were placed into photobeam chambers. Crossovers were measured in 10-minute intervals across 90 min. Locomotor activity was significantly reduced compared to vehicle-treated rats in all active PPX dose-groups during the first 30 min. Rats treated with 0.3 mg/kg demonstrated increased locomotor activity in the last 40 min; hyperlocomotion in the 1.0 mg/kg dose group was only significant during the 60–70 min interval. *p<0.05, n = 6 rats/dose group.
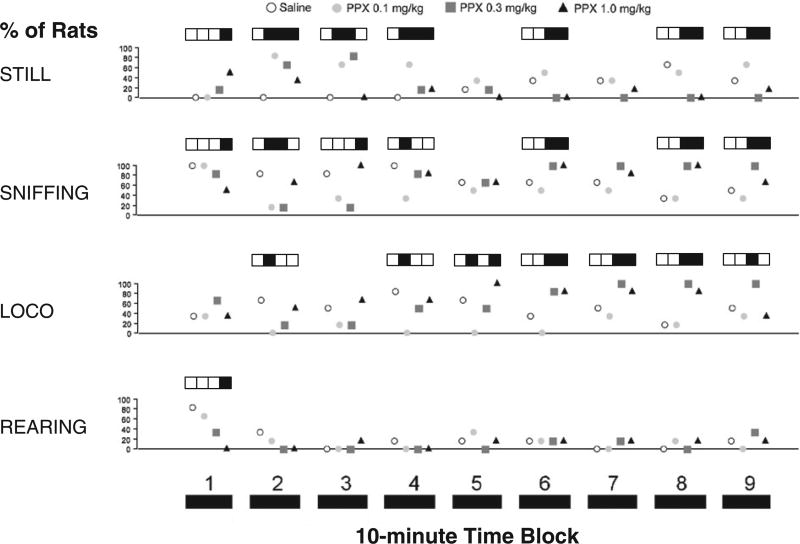
Understanding the basis for group differences in photocell counts requires information about the types of behaviors being exhibited by the rats. For example, reduced photocell counts can result from either sedation or intense stereotypy. Data (Fig. 2) revealed that the early PPX-induced hypoactivity was not accompanied by any stereotyped behaviors; behaviors during later periods of elevated photocell activity included exploratory locomotion, rearing, and sniffing.
Fig. 2.
PPX dose–response effects on behavioral ratings during locomotor activity testing. Animals were observed briefly during each 10-min interval of locomotor testing for the presence of behaviors (as described in Fray et al. (1980)). Data points represent the percentage of rats from that dose group that exhibited behavior from each category. Boxes above data points indicate statistical significance (p < 0.05) within each interval of that particular dose group compared to lower doses. Absence of boxes indicates a lack of overall significance for that interval. A change in box color from white to black or from black to white signifies a statistical difference between corresponding dose groups; no color change indicates no statistical difference. For example, in Interval 3 of SNIFFING, 0.1 mg/kg PPX is statistically different from 0 mg/kg PPX. 0.3 mg/kg PPX is not different from 0.1, but 1.0 mg/kg is different from 0.1 and 0.3 combined. Intense stereotyped behaviors – gnawing, licking, head down, and grooming – were not observed in more than one rat per dose, at any interval. n = 6 rats/dose group.
3.2. Effect of D2 blockade on PPX-induced locomotor activity
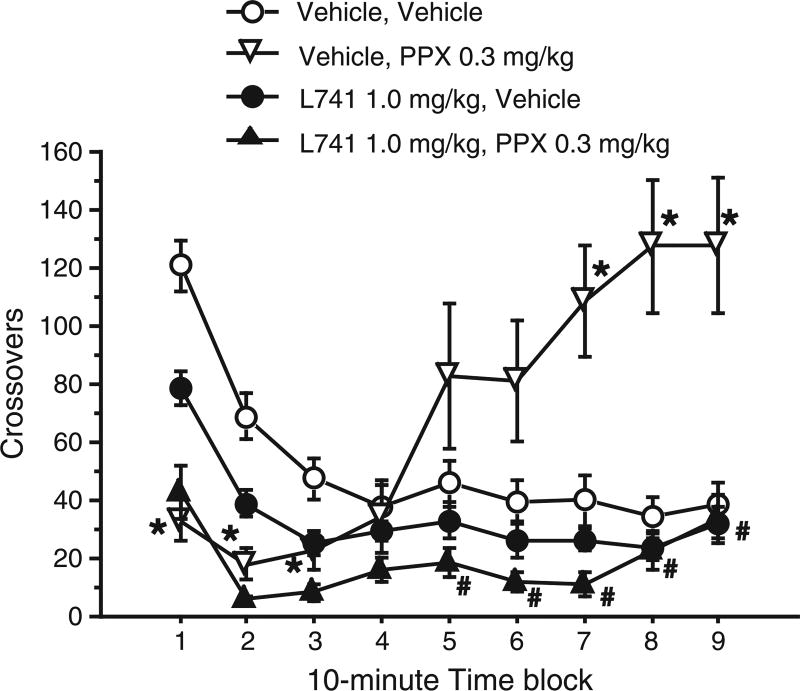
Animals were assigned to new dose groups, and tested for locomotor activity with L741626 pretreatment (0, 1.0 mg/kg) before PPX injection (0, 0.3 mg/kg). This dose of L741626 was chosen based on our experience that it is “D2-selective”, e.g. opposes the effects of D2 agonists but not PPX on PPI in SD rats (Weber et al., 2009b, 2010b); the dose of PPX was chosen so that we could assess the impact of D2 blockade on both PPX-induced hypo- and hyperactivity (Fig. 3). Repeated measures ANOVA revealed significant effects of L741626 pretreatment (F = 23.38, df 1,14; p < 0.0005) and interval (F = 18.05, df 8,112; p < 0.0001), and significant interactions of L741626 pretreatment × interval (F = 7.30, df 8,112; p < 0.0001), PPX × interval (F = 22.11, df 8,112; p < 0.0001), and L741626 × PPX × interval (F = 10.83, df 8,112; p < 0.0001). Similar to what was detected on test day 1 (Fig. 1), among rats receiving the vehicle pretreatment, 0.3 mg/kg PPX led to an early hypoactivity during the first 30 min, followed by significant hyperactivity during the last 30 min of testing. Pretreatment with the active dose of L741626 (1.0 mg/kg) did not affect the early PPX-induced hypoactivity, but completely blocked the late PPX-induced hyperactivity (see Fig. 3 for specific post-hoc comparisons).
Fig. 3.
Effect of L741626 on biphasic locomotor response to PPX. L741626 (0, 1.0 mg/kg) was injected 30 min prior to PPX (0, 0.3 mg/kg) and placement of animals into photobeam chambers. As in Fig. 1, PPX produced decreased and then increase locomotor activity compared to vehicle. The late hyperlocomotion effect was blocked by L741626 pretreatment, while early hypolocomotion was unaffected. *p < 0.05 for vehicle vs. PPX in animals that did not receive active pretreatment (open circle vs. open triangle). #p < 0.05 for Vehicle vs. L741626 pretreatment in animals that received PPX injection (open triangle vs. closed triangle).
3.3. PPX effects on PPI
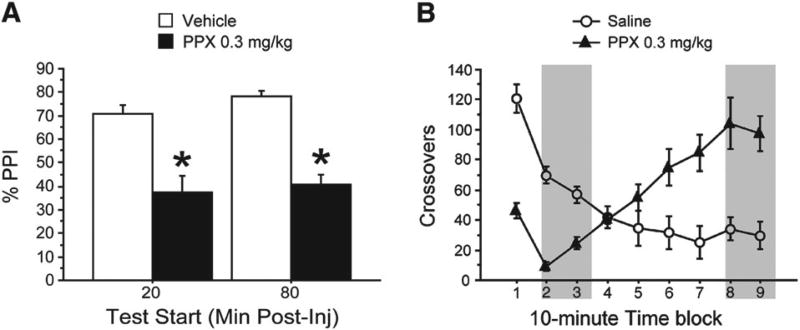
The effects of PPX on PPI were tested using both the dose (0.3 mg/kg) and time points (20 and 80 min post-injection) associated with the biphasic effects of PPX on locomotor activity. In contrast to its effects on locomotor activity, PPX-induced changes in PPI were constant rather than biphasic (Fig. 4A). Repeated measures ANOVA revealed significant effects of PPX dose (F = 54.96, df 1,13; p < 0.0001) and prepulse intensity (F = 15.78, df 2,26; p < 0.0001), but no effect of pretreatment time (F < 1). There were no significant 2-way interactions or meaningful 3-way interactions; when collapsed across intensities, there was a significant effect of PPX on PPI at both 20 and 80 min pretreatment times, and PPI did not differ between the two PPX-treated groups. PPX also had a significant effect on startle magnitude (F = 6.71, df 1,13; p < 0.03) (but there was no effect of pretreatment time (F < 1) on startle, and no significant interactions (mean(SEM) for 20 min: Vehicle = 161.61(27.69), PPX = 93.58 (19.29); for 80 min: Vehicle = 203.86(56.29), PPX = 98.96(16.19)). Splitting the 20 and 80 min pretreatment groups at the median for PPX-induced startle suppression (startle magnitude on vehicle treatment day minus startle on PPX treatment day), there was no significant interaction between low or high startle suppression and PPX, and no other meaningful interactions with startle suppression. Although PPX appeared to slightly increase NOSTIM activity, this effect did not reach statistical significance (main effects of PPX and time, and interactions all NS; mean(SEM) for 20 min: Vehicle = 0.09(0.07), PPX = 0.22(0.12); for 80 min: Vehicle = 0(0), PPX = 0.23(0.18)).
Fig. 4.
Effects of PPX 0.3 mg/kg on PPI, at time points corresponding to hypo- and hyper-locomotion effects. A different set of rats was tested with the key dose of PPX with either a 15 or 75 min pretreatment time before PPI testing. [A]: Effects on %PPI. Data are collapsed across prepulse intensities. p < 0.006 for vehicle vs. PPX at each pretreatment time. [B]: Locomotor activity data for this PPX dose as shown in Fig. 1. Gray boxes indicate the post-injection intervals corresponding to these PPI test sessions.
4. Discussion
Taken together with our previous findings using a similar testing paradigm in mice, the present findings support several conclusions. First, the impact of PPX on locomotor activity in SD rats is strikingly similar to that in C57BL/6J mice (Chang et al., 2010a), in terms of dose sensitivity and time course, including the elicitation of biphasic hypo- and hyperlocomotion. While this does not prove that similar underlying mechanisms mediate these PPX effects across species, it demonstrates that some mechanisms for both PPX-mediated reductions and increases in exploratory behavior exist in both mice and rats, that exhibit nearly-identical dose- and time-sensitivities. Second, in rats and mice, the late PPX-induced hyperactivity is blocked by L741626, suggesting that it is a D2-dependent drug effect in both species. Third, in both species, the early PPX-induced hypoactivity is not antagonized – and is perhaps potentiated – by D2 blockade. This pattern would be consistent with a D3-mediated stimulation of presynaptic receptors, resulting in a reduction of DA release. While many other mechanisms might be involved, such an effect of presynaptic D3 activation might be expected to produce the observed patterns: it would not be antagonized by D2 blockade, and might add to or synergize with the effects with L741626 (although, as in the present study, activity “floor effects” during this early PPX-induced hypoactivity might complicate the detection of such additive or synergistic effects). Direct evidence for such an autoreceptor effect is not compelling; in fact, microdialysis findings suggest that striatal DA release is not altered during the first two hours after PPX administration (Lagos et al., 1998). Also arguing against an exclusive role of D3 autoreceptors in this early hypoactivity, at least in mice, is our finding that PPX-induced hypoactivity is present – though diminished and shorter-lived – in mutant mice lacking D3 receptors (Richtand et al., in press).
Across previous reports of PPX effects on locomotor activity in rats, the same dose-range of PPX has yielded very different dose–response properties (Kitagawa et al., 2009; Lagos et al., 1998; Maj et al., 1997; Svensson et al., 1994a). Specifically, a “U-shaped” dose–response curve, characterized by decreased locomotion at lower doses and increased locomotion at higher doses, is reported when testing is conducted 1–2.5 h after PPX injection (Lagos et al., 1998; Maj et al., 1997), while hypolocomotion at all doses is seen with shorter wait times of 30 min or less (Kitagawa et al., 2009; Lagos et al., 1998; Svensson et al., 1994a). One report describes a similar, time-dependent biphasic effect with 0.5 mg/kg PPX eliciting decreased locomotion at 0–30 min post-injection and increased locomotion at 120–150 min post-injection (Lagos et al., 1998). The present results confirm that different time intervals between PPX administration and activity measurements capture mechanistically different effects of PPX: D2-independent early hypo-locomotion and D2-dependent later hyper-locomotor effects of a single dose of PPX were detected via an extended measurement period, divided into shorter measurement intervals. Conceivably, U-shaped dose–response effects of other D3-preferential agonists might also reflect a temporal conflation of disparate receptor mechanisms (Ahlenius and Salmi, 1994; Collins et al., 2007; Khroyan et al., 1997; Millan et al., 2004; Pugsley et al., 1995; Svensson et al., 1994b).
Having detected apparently multi-mechanism, biphasic locomotor effects of PPX, it was next possible to ask whether the PPI-disruptive effects of PPX exhibited such a dynamic profile in rats; this was not possible in mice, due to their complete insensitivity to PPX-induced PPI changes (Chang et al., 2010a). Most PPX effects on PPI have been studied 15–45 min post-injection (Chang et al., 2010b; Swerdlow et al., 2009; Weber et al., 2008, 2009b). In the present study, we determined that the PPI-disruptive effects of PPX were identical, whether tested 20 min after injection, coinciding with the D2-insensitive PPX-induced hypoactivity, or 80 min after injection, when PPX produced a D2-dependent hyperactivity. Thus, there is no apparent time-sensitive “shift” in the systems mediating the PPI-disruptive effects of PPX, and by extension, these PPI-disruptive effects appear to be independent of at least one (and perhaps both) of the mechanisms regulating PPX-induced changes in locomotor activity. That the early PPI-disruptive effects of PPX are relatively insensitive to L741626 (and are certainly not additive or synergistic with such effects) (Weber et al., 2009b), suggests that they are neither D2-dependent, nor do they reflect DA-suppressive effects that might result from activation of D3 autoreceptors. Sensitivity of the late PPI-disruptive effects of PPX to L741626 has not yet been tested; because no obvious temporal shift was detected in PPX effects on PPI or other startle measures, there is no clear reason to suspect a greater sensitivity to D2 blockade in the late vs. early PPI-disruptive effects of PPX.
In summary, the effects of PPX on locomotor activity in rats – as with those previously detected in mice – are most easily explained by an early hypodopaminergic response that includes (but is not exclusively mediated by) a participation of D3 autoreceptors, and a late hyperdopaminergic response reflecting the activation of D2 receptors. In contrast, the species-specific PPI-disruptive effects of PPX appear to be distinct from at least one and perhaps both of these mechanisms. Consequently, these two behavioral measures contribute non-redundant information about the neurobiological effects of D3 receptor activation, and its potential contributions to the etiology or treatment of brain disorders.
Acknowledgments
The authors gratefully acknowledge the assistance of Maria Bongiovanni in manuscript preparation, and Megan Mizera for her expert technical assistance. Research was supported by MH087109 (WLC), MH59803 (NRS), MH068366 (NRS), and MH042228 (NRS).
References
- Ahlenius S, Salmi P. Behavioral and biochemical effects of the dopamine D3 receptor-selective ligand, 7-OH-DPAT, in the normal and the reserpine-treated rat. Eur J Pharmacol. 1994;260(2–3):177–81. doi: 10.1016/0014-2999(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Breier MR, Lewis B, Shoemaker JM, Light GA, Swerdlow NR. Sensory and sensorimotor gating-disruptive effects of apomorphine in Sprague Dawley and Long Evans rats. Behav Brain Res. 2010;208(2):560–5. doi: 10.1016/j.bbr.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Effects of D3/D2 dopamine receptor agonists and antagonists on prepulse inhibition of acoustic startle in the rat. Neuropsychopharmacology. 1995;12:139–45. doi: 10.1016/0893-133X(94)00071-7. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS., III Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14:190–6. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- Chang WL, Geyer MA, Buell MR, Weber M, Swerdlow NR. The effects of pramipexole on prepulse inhibition and locomotor activity in C57BL/6J mice. Behav Pharmacol. 2010a;21:135–43. doi: 10.1097/FBP.0b013e328337be7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WL, Swerdlow NR, Breier MR, Thangaraj N, Weber M. Parametric approaches towards understanding the effects of the preferential D3 receptor agonist pramipexole on prepulse inhibition in rats. Pharmacol Biochem Behav. 2010b;95:473–8. doi: 10.1016/j.pbb.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, et al. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–70. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iversen SD. An observational method for quantifying the behavioural effects of dopamine agonists: contrasting effects of d-amphetamine and apomorphine. Psychopharmacology (Berl) 1980;69(3):253–9. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Fuchs RA, Baker DA, Neisewander JL. Effects of D3-preferring agonists 7-OH-PIPAT and PD128,907 on motor behaviors and place conditioning. Behav Pharmacol. 1997;8:65–74. [PubMed] [Google Scholar]
- Kitagawa K, Kitamura Y, Miyazaki T, Miyaoka J, Kawasaki H, Asanuma M, et al. Effects of pramipexole on the duration of immobility during the forced swim test in normal and ACTH-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:59–66. doi: 10.1007/s00210-009-0405-0. [DOI] [PubMed] [Google Scholar]
- Kullback S. Information theory and statistics. New York: Dover; 1968. [Google Scholar]
- Lagos P, Scorza C, Monti JM, Jantos H, Reyes-Parada M, Silveira R, et al. Effects of the D3 preferring dopamine agonist pramipexole on sleep and waking, locomotor activity and striatal dopamine release in rats. Eur Neuropsychopharmacol. 1998;8(2):113–20. doi: 10.1016/s0924-977x(97)00054-0. [DOI] [PubMed] [Google Scholar]
- Maj J, Rogoz Z, Skuza G, Kolodziejczyk K. The behavioural effects of pramipexole, a novel dopamine receptor agonist. Eur J Pharmacol. 1997;324:31–7. doi: 10.1016/s0014-2999(97)00066-6. [DOI] [PubMed] [Google Scholar]
- Martin S, Markus MA, Morris BJ, Davisson RL, Lawrence AJ, van den Buuse M. Does angiotensin interact with dopaminergic mechanisms in the brain to modulate prepulse inhibition in mice? Neuropharmacology. 2008;54:399–404. doi: 10.1016/j.neuropharm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M. The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology (Berl) 2004;174(3):341–57. doi: 10.1007/s00213-003-1770-x. [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, Mackenzie RG, Shih YH, Damsma G, et al. Neurochemical and functional characterization of the preferentially selective dopamine D3-agonist PD 128907. J Pharmacol Exp Ther. 1995;275:1355–66. [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Effects of selective dopamine D1-like and D2-like agonists on prepulse inhibition of startle in inbred C3H/HeJ, SPRET/EiJ, and CAST/EiJ mice. Psychopharmacology (Berl) 2007;191:731–9. doi: 10.1007/s00213-006-0511-3. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–33. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–11. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–18. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Chang WL, Ahlbrand R, Swerdlow NR. The effects of pramipexole on locomotor activity in D3 mutant mice. Soc Neurosci. in press. [Google Scholar]
- Robbins TW. A critique of the methods available for the measurement of spontaneous motor activity. In: Iverson L, Iversen S, Snyder S, editors. Handbook of psychopharmacology. Vol. 7. New York: Plenum; 1977. pp. 37–82. [Google Scholar]
- Russig H, Spooren W, Durkin S, Feldon J, Yee BK. Apomorphine-induced disruption of prepulse inhibition that can be normalised by systemic haloperidol is insensitive to clozapine pretreatment. Psychopharmacology (Berl) 2004;175:143–7. doi: 10.1007/s00213-004-1810-1. [DOI] [PubMed] [Google Scholar]
- Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB. Inactivation of the 5-HT(7) receptor partially blocks phencyclidine-induced disruption of prepulse inhibition. Biol Psychiatry. 2008;63:98–105. doi: 10.1016/j.biopsych.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Svensson K, Carlsson A, Huff RM, Kling-Petersen T, Waters N. Behavioral and neurochemical data suggest functional differences between dopamine D2 and D3 receptors. Eur J Pharmacol. 1994a;263:235–43. doi: 10.1016/0014-2999(94)90718-8. [DOI] [PubMed] [Google Scholar]
- Svensson K, Carlsson A, Waters N. Locomotor inhibition by the D3-ligand R-(+)-7-OH-DPAT is independent of changes in dopamine release. J Neural Transm. 1994b;95:71–4. doi: 10.1007/BF01283032. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Kuczenski R, Goins JC, Crain SK, Ma LT, Bongiovanni MJ, et al. Neurochemical analysis of rat strain differences in the startle gating-disruptive effects of dopamine agonists. Pharmacol Biochem Behav. 2005;80:203–11. doi: 10.1016/j.pbb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Breier M, Mora AB, Ko D, Shoemaker JM. A novel rat strain with enhanced sensitivity to the effects of dopamine agonists on startle gating. Pharmacol Biochem Behav. 2008a;88(3):280–90. doi: 10.1016/j.pbb.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008b;199:331–88. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Lelham SA, Sutherland Owens AN, Chang WL, Sassen SDT, Talledo JA. Pramipexole effects on startle gating in rats and normal men. Psychopharmacology (Berl) 2009;205:689–98. doi: 10.1007/s00213-009-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Buuse M, Martin S, Brosda J, Leck KJ, Matthaei KI, Hendry I. Enhanced effect of dopaminergic stimulation on prepulse inhibition in mice deficient in the alpha subunit of G(z) Psychopharmacology (Berl) 2005;183:358–67. doi: 10.1007/s00213-005-0181-6. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Dopamine agonist-induced hypothermia and disruption of prepulse inhibition: evidence for a role of D3 receptors? Behav Pharmacol. 1998;9:445–55. doi: 10.1097/00008877-199809000-00008. [DOI] [PubMed] [Google Scholar]
- Weber M, Swerdlow NR. Rat strain differences in startle gating-disruptive effects of apomorphine occur with both acoustic and visual prepulses. Pharmacol Biochem Behav. 2008;88:306–11. doi: 10.1016/j.pbb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chang WL, Breier M, Ko D, Swerdlow NR. Heritable strain differences in sensitivity to the startle gating-disruptive effects of D2 but not D3 receptor stimulation. Behav Pharmacol. 2008;19:786–95. doi: 10.1097/FBP.0b013e32831c3b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Breier M, Ko D, Thangaraj N, Marzan DE, Swerdlow NR. Evaluating the antipsychotic profile of the preferential PDE10A inhibitor, papaverine. Psychopharmacology (Berl) 2009a;203(4):723–35. doi: 10.1007/s00213-008-1419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chang WL, Durbin JP, Park PE, Luedtke RR, Mach RH, et al. Using prepulse inhibition to detect functional D3 receptor antagonism: effects of WC10 and WC44. Pharmacol Biochem Behav. 2009b;93:141–7. doi: 10.1016/j.pbb.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chang WL, Breier MR, Miller EJ, Thangaraj N, Yang AC, et al. Pramipexole infusion into the nucleus accumbens disrupts prepulse inhibition in rats. Biol Psychiatry. 2010a;67(9):73S. [Google Scholar]
- Weber M, Chang WL, Breier MR, Yang A, Millan MJ, Swerdlow NR. The effects of the dopamine D2 agonist sumanirole on prepulse inhibition in rats. Eur Neuropsychopharmacol. 2010b;20(6):421–5. doi: 10.1016/j.euroneuro.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Russig H, Feldon J. Apomorphine-induced prepulse inhibition disruption is associated with a paradoxical enhancement of prepulse stimulus reactivity. Neuropsychopharmacology. 2004;29:240–8. doi: 10.1038/sj.npp.1300323. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ballard ME, Unger LV, Haupt A, Gross G, Decker MW, et al. Effects of antipsychotics and selective D3 antagonists on PPI deficits induced by PD 128907 and apomorphine. Behav Brain Res. 2007;182:1–11. doi: 10.1016/j.bbr.2007.04.021. [DOI] [PubMed] [Google Scholar]