Abstract
Pulmonary hypertension (PH) is a progressive disorder that causes significant morbidity and mortality despite existing therapies. PH pathogenesis is characterized by metabolic derangements that increase pulmonary artery smooth muscle cell (PASMC) proliferation and vascular remodeling. PH-associated decreases in peroxisome proliferator-activated receptor γ (PPARγ) stimulate PASMC proliferation, and PPARγ in coordination with PPARγ coactivator 1α (PGC1α) regulates mitochondrial gene expression and biogenesis. To further examine the impact of decreases in PPARγ expression on human PASMC (HPASMC) mitochondrial function, we hypothesized that depletion of either PPARγ or PGC1α perturbs mitochondrial structure and function to stimulate PASMC proliferation. To test this hypothesis, HPASMCs were exposed to hypoxia and treated pharmacologically with the PPARγ antagonist GW9662 or with siRNA against PPARγ or PGC1α for 72 hours. HPASMC proliferation (cell counting), target mRNA levels (qRT-PCR), target protein levels (Western blotting), mitochondria-derived H2O2 (confocal immunofluorescence), mitochondrial mass and fragmentation, and mitochondrial bioenergetic profiling were determined. Hypoxia or knockdown of either PPARγ or PGC1α increased HPASMC proliferation, enhanced mitochondria-derived H2O2, decreased mitochondrial mass, stimulated mitochondrial fragmentation, and impaired mitochondrial bioenergetics. Taken together, these findings provide novel evidence that loss of PPARγ diminishes PGC1α and stimulates derangements in mitochondrial structure and function that cause PASMC proliferation. Overexpression of PGC1α reversed hypoxia-induced HPASMC derangements. This study identifies additional mechanistic underpinnings of PH, and provides support for the notion of activating PPARγ as a novel therapeutic strategy in PH.
Keywords: HPASMC, hypoxia, mitochondria, PGC1α, PPARγ
Clinical Relevance
Pulmonary hypertension pathogenesis is characterized by metabolic derangements, increased pulmonary artery smooth muscle cell proliferation, and decreases in peroxisome proliferator-activated receptor γ (PPARγ). The current study provides novel evidence that either hypoxia-induced or siRNA-mediated knockdown of PPARγ causes decreases in PGC1α, a major regulator of mitochondrial biogenesis. Depletion of PPARγ is sufficient to increase human pulmonary artery smooth muscle cell proliferation, enhance mitochondria-derived H2O2 production, decrease mitochondrial mass, increase mitochondrial fragmentation, and impair mitochondrial bioenergetics. These findings reveal additional mechanisms linking diminished PPARγ expression to altered pulmonary vascular cell phenotypes with metabolic dysregulation, and provide support for strategies targeting PPARγ as a novel therapeutic approach in pulmonary hypertension.
Pulmonary hypertension (PH) causes significant morbidity and mortality (1). Complex mechanisms, including alterations in cellular metabolism, derangements in the production of vasoactive mediators, and enhanced proliferation of cells in the pulmonary vascular wall, contribute to PH pathogenesis (2). These alterations in vascular structure and function stimulate increases in pulmonary vascular remodeling and pulmonary vascular resistance, increasing right ventricular pressure (2, 3). Despite recent pharmacological advances in PH therapy that ameliorate imbalances in the production of vasodilating and vasoconstricting mediators, current therapeutic approaches largely fail to reverse the underlying derangements in metabolism and proliferation in cells of the pulmonary vasculature. These observations suggest that interventions that target and reverse metabolic and proliferative abnormalities in pulmonary vascular wall cells might be more effective for PH treatment.
Peroxisome proliferator-activated receptor γ (PPARγ), a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors, plays an important role in regulating a variety of fundamental cellular processes, including metabolism, proliferation, and inflammation (4). For example, PPARγ is expressed in pulmonary vascular endothelial cells and smooth muscle cells (SMCs), where it participates in the regulation of normal pulmonary vascular function (5). PPARγ expression is decreased in the vascular lesions of patients with idiopathic pulmonary arterial hypertension (PAH) (6), and in cellular studies, depletion of PPARγ was sufficient to promote development of the PH phenotype by upregulating cell cycle–related genes and angiogenesis-related genes in pulmonary artery endothelial cells (7), and to promote the proliferation of human pulmonary artery SMCs (HPASMCs) (8). In contrast, pharmacological PPARγ activation decreased PH in experimental models (9, 10) and favorably altered transcriptional and post-transcriptional pathways and the levels of vasoactive mediators involved in PH pathogenesis (9–11). PPARγ activation using the thiazolidinedione ligand rosiglitazone decreased pulmonary artery remodeling, muscularization of distal pulmonary arterioles, and SMC proliferation in rats subjected to chronic hypoxia (HYP) (12), and reversed right ventricular hypertrophy and PH in insulin-resistant, apolipoprotein E–deficient mice fed a high-fat diet (13). Combined with evidence that mice with vascular-targeted PPARγ deletion develop PH (8), these studies indicate that decreases in PPARγ contribute to PH pathogenesis. The current study seeks to further explore the role of PPARγ in the underlying metabolic derangements that contribute to SMC proliferation.
PPARγ activates the promoter of PPARγ coactivator 1α (PGC1α), a 795-amino-acid protein that coordinates nuclear and mitochondrial gene expression and also is a transcriptional coactivator of PPAR (14). In PH, metabolic derangements related to reductions in the number of mitochondria and fragmentation of existing mitochondria (15–17) underlie phenotypic changes in pulmonary vascular wall cells (18), such as SMC proliferation. In human and experimental PH, decreased expression of PGC1α contributes to mitochondrial fragmentation and proliferation, which are in part related to changes in mitochondrial number, structure, and function (19). PGC1α also contributes to the adaptive response of PASMCs to HYP (20). Collectively, these observations suggest that losses of PPARγ and PGC1α may mutually contribute to PASMC proliferation. The current study identifies novel roles for PPARγ in the regulation of SMC mitochondria that contribute to metabolic dysfunction and proliferation in pulmonary vascular wall cells.
This study also further defines the molecular mechanisms whereby decreases in PPARγ stimulate metabolic derangements that are associated with increased pulmonary vascular cell proliferation. We previously reported that HYP decreased PPARγ in vitro and in vivo (21). Our current findings demonstrate that HYP exposure also decreases PGC1α expression, attenuates expression of mitochondrial proteins, and increases SMC proliferation. In addition, they demonstrate that short interfering RNA (siRNA)–mediated depletion of either PPARγ or PGC1α is sufficient to stimulate mitochondria-derived H2O2 and alter mitochondrial metabolism and function, which induce SMC proliferation. PGC1α activation using adenovirus overexpression reversed these HYP-induced HPASMC derangements. Taken together, these findings identify PPARγ as a critical regulator of PASMC mitochondrial metabolism and proliferation, and illustrate mechanisms whereby decreases in this receptor could potentially contribute to PH pathogenesis.
Methods
In Vitro HPASMC Studies
HPASMCs were purchased from Lonza and Sci-Cell. HPASMC monolayers were grown at 37°C in a 5% CO2 atmosphere and cultured in complete SmGM-2 medium (Lonza) containing 5% fetal calf serum, growth factors, and antibiotics as previously described (22). Cells were cultured in a HYP chamber (1% O2; Biospherix) or cell culture incubator (normoxia [NOR], 21% O2) for 72 hours. HPASMC passages 2–9 were used for all experimental studies.
Knockdown or Overexpression of PPARγ and PGC1α
To knock down PPARγ in HPASMC, the PPARγ antagonist GW9662 (5–10 μM; Sigma-Aldrich) or PPARγ siRNA (100 nM; Dharmacon) was used as previously described (8). To attenuate PGC1α, PGC1α siRNA (10 nM; Integrated DNA Technologies Inc.) was used. An adenovirus overexpressing PGC1α, AdPGC1α (a gift from Dr. Russ Price, Emory University), was used. Briefly, cells (40–50% confluent) were incubated for 3 hours with Opti-MEM (Life Technologies), transfected with siRNA using Lipofectamine RNAi reagent (Life Technologies) or treated with AdPGC1α for 4 hours, and grown in complete growth media for 72 hours. These HPASMCs were compared with cells transfected with scrambled siRNA (SCR) or treated with an adenovirus expressing green fluorescent protein (AdGFP) as an experimental control.
HPASMC Proliferation
HPASMCs were cultured in 12-well plates in complete medium, followed by 72 hours of HYP or GW9662 treatment or siRNA transfection. HPASMCs (75–85% confluent) were trypsinized in 500 μl trypsin, and a 1:1 cell suspension dilution with trypan blue was performed. Cells were counted in triplicate using a hemocytometer and expressed as mean cell counts ± SEM relative to NOR, control DMSO vehicle, or SCR.
Analysis of mRNA, Protein, and Mitochondrial Alterations
Target mRNA levels were measured and quantified by qRT-PCR with specific human, mouse, and rat primer sequences as outlined in Table E1 in the data supplement. Target protein levels were assessed by Western blotting. HPASMC mitochondria number, volume, and H2O2 generation were determined by confocal microscopy. The mitochondrial bioenergetics were by measuring the oxygen consumption rate (OCR) (23) and expressed as mean ± SEM. See the data supplement for additional details.
In Vivo Rodent Models of PH
Male C57BL/6J mice (The Jackson Laboratory), 8–12 weeks old, were exposed to HYP (10% O2) or NOR (room air, 21% O2) for 3 weeks (24). Male Sprague-Dawley rats, 8 weeks old, were injected subcutaneously with SU5416, a vascular endothelial growth factor receptor 2 inhibitor (25). See the data supplement for further details. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Atlanta Veterans Affairs Medical Center, and all animal studies were performed in accordance with guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statistical Analysis
Data were analyzed using Student’s t test or two-way ANOVA followed by Tukey’s post hoc test in GraphPad Prism version 6 (GraphPad) to determine the significance of the treatment effects. Statistical significance was set at an α-value of P ≤ 0.05.
Results
HYP, PPARγ Knockdown, or PGC1α Knockdown Increases HPASMC Proliferation
In previous studies, exposure to HYP regimens sufficient to induce PH in experimental animals in vivo (24) or to stimulate proliferation in HPASMC in vitro (9, 21, 22) was shown to significantly decrease lung-tissue or cellular levels of PPARγ, respectively. Here, to further explore the relationships among PPARγ, PGC1α, and PASMC proliferation, we treated cells with HYP, GW9662, siPPARγ, or siPGC1α for 72 hours. HPASMC proliferation was measured by cell counting. We previously reported that HYP (21) or siPPARγ (8) treatment regimens depleted PPARγ levels by 50%. Figure 1 establishes that treatment with HYP (Figure 1A), GW9662 (Figure 1B), or siPPARγ (Figure 1C) comparably stimulated HPASMC proliferation by roughly 40%. This confirms our previous reports that HYP and loss of PPARγ increase HPASMC proliferation (8, 9). Treatment with siPGC1α decreased HPASMC PGC1α mRNA and protein levels by 42 ± 8.4% (Figure E1A) and 27 ± 3.3% (Figure E1B), respectively, and stimulated proliferation (Figure 1C). Although treatment with combined siPPARγ and siPGC1α also increased HPASMC proliferation, the incremental increase in proliferation caused by the combination of siRNAs was not significantly greater than that observed with each siRNA alone (Figure 1C). Collectively, these findings illustrate that loss of PPARγ activity alone (GW9662), loss of PPARγ protein and activity (by treatment with HYP or siPPARγ), or loss of PGC1α is sufficient to stimulate HPASMC proliferation.
Figure 1.
Hypoxia (HYP) and knockdown of PPARγ or PGC1α increases human pulmonary artery smooth muscle cell (HPASMC) proliferation. Cell counting was used to measure HPASMC proliferation. (A) HPASMCs were treated with HYP (1% O2) or NOR (21% O2) for 72 hours. (B) HPASMCs were treated with vehicle (DMSO) or the PPARγ antagonist GW9662 (10 μM) for 72 hours. (C) HPASMCs were transfected with scrambled siRNA control (SCR), 100 nM siPPARγ, 100 nM siPGC1α, or combined 100 nM siPPARγ and 100 nM siPGC1α for 72 hours. n = 4, *P < 0.05 versus NOR, DMSO, or SCR. NOR = normoxia; PGC1α = PPARγ coactivator 1α; PPARγ = peroxisome proliferator-activated receptor γ.
PPARγ or PGC1α Knockdown Induces Mitochondrial Derangements in HPASMCs
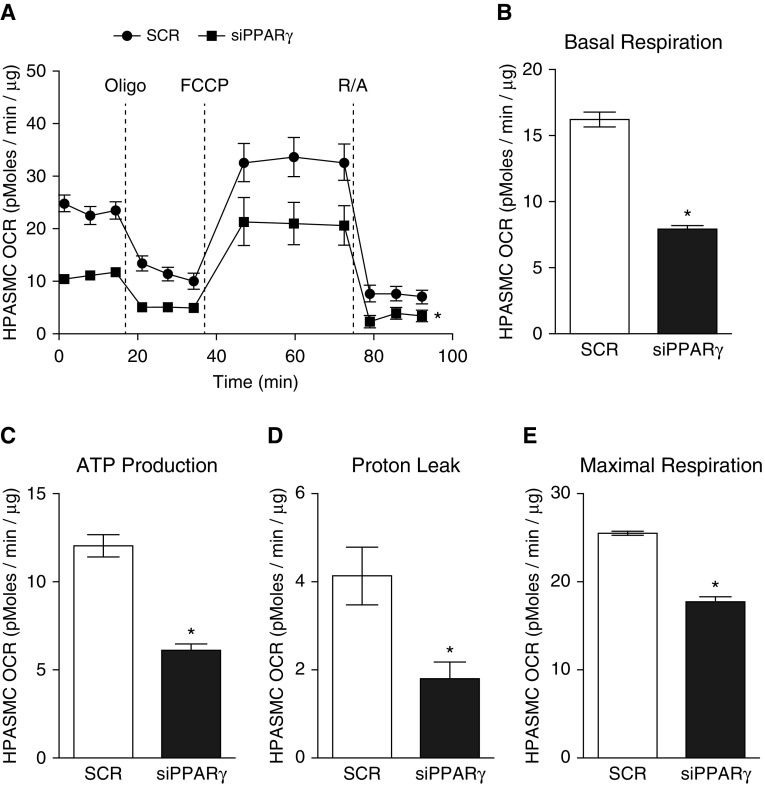
Because metabolic derangements play a significant role in the pathogenesis of pulmonary vascular cell proliferation in PH (18), and because PPARγ regulates both metabolism and PGC1α, a master regulator of mitochondrial biogenesis (26), we sought to further define the relationships between alterations in PPARγ and mitochondrial structure and function. As illustrated in Figure 2, treatment with siPPARγ for 72 hours enhanced mitochondria-derived H2O2 generation, as measured with MitoPY1 (Figures 2A and 2B). Further, siPPARγ decreased the mRNA levels of mitochondrial transcription factor A (TFAM; Figure 2C); the expression of mitochondrial-specific proteins, including heat shock protein family A member 9 (GRP75; Figure 2D) and voltage-dependent anion-selective channel (VDAC; Figure 2E); and mRNA levels of mitofusin-2 (MFN2; Figure 2F). These data, demonstrating decreases in mitochondrial mass, along with evidence of increased mitochondrial reactive oxygen species (ROS) generation (Figures 2A and 2B), suggested an increase in mitochondrial fission (27). Consistent with this, confocal microscopy of HPASMCs labeled with MitoTracker Orange (Thermo Fisher Scientific) demonstrated that siPPARγ decreased the overall volume of mitochondria but increased the number of labeled mitochondria per cell (Figure 2G). To further examine the functional correlates of these siPPARγ-induced alterations in mitochondrial structure, we determined the HPASMC mitochondrial bioenergetics. Treatment with siPPARγ decreased HPASMC OCR (Figure 3A) and diminished basal respiration (Figure 3B), ATP production (Figure 3C), proton leak (Figure 3D), and maximal respiration (Figure 3E).
Figure 2.
PPARγ knockdown induces mitochondrial H2O2 generation and attenuates mitochondrial mass in HPASMCs. HPASMCs were transfected with SCR control or 100 nM siPPARγ for 72 hours. (A) Representative confocal fluorescence images of treated HPASMC monolayers stained with DAPI, MitoPY1, and MitoTracker Red. Merged images confirm colocalization of H2O2 production with mitochondria labeling. Magnification ×90. (B) The fluorescence intensity of MitoPY1 is quantified in the bar graph. n = 6, *P < 0.05 versus SCR. (C) TFAM mRNA levels were measured by qRT-PCR (n = 5–6). (D and E) The protein levels of mitochondrial-specific GRP75 (D) and VDAC (E) were measured by Western blot analysis (n = 8). (F) The mRNA levels of the mitochondrial fusion gene MFN2 were measured by qRT-PCR. The mRNA and protein levels of the targets were normalized to housekeeping genes and proteins and expressed as mean ± SEM fold change relative to SCR. (G) Representative confocal fluorescence images of treated HPASMC monolayers stained with MitoTracker Orange. Magnification ×63. The fluorescence intensity of MitoTracker Orange and the number of mitochondria are quantified in the bar graphs. n = 6, *P < 0.05 versus SCR. GRP75 = heat shock protein family A member 9; MFN2 = mitofusin-2; RFU = relative fluorescence units; TFAM = mitochondrial transcription factor A; VDAC = voltage-dependent anion-selective channel.
Figure 3.
PPARγ knockdown attenuates mitochondrial bioenergetics in HPASMCs. HPASMCs were transfected with SCR control or 100 nM siPPARγ for 72 hours. (A) Mitochondrial bioenergetic profiling was performed with a Seahorse XF96 Extracellular Flux Analyzer, and the oxygen consumption rate (OCR) was recorded and normalized to protein levels. (B–E) OCR profiles were used to determine basal respiration (B), ATP production (C), proton leak (D), and maximal respiration (E). n = 6, *P < 0.05 versus SCR. FCCP = carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; Oligo = oligomycin; R/A = rotenone/antimycin A.
Loss of PPARγ Decreases PGC1α Expression In Vitro and In Vivo
PPARγ and PGC1α are mutually activating central metabolic regulators (14, 28). Because the PGC1α promoter is regulated by PPARγ, we sought to determine whether alterations in PPARγ expression modulate PGC1α levels. HPASMCs were treated with the PPARγ antagonist GW9662 or with siPPARγ for 72 hours. Compared with vehicle, GW9662 decreased the levels of PGC1α mRNA (Figure 4A) and protein (Figure 4B). Similarly, compared with SCR, siPPARγ decreased both PGC1α mRNA (Figure 4C) and protein (Figure 4D) levels. These data demonstrate that loss of PPARγ decreases PGC1α expression in HPASMCs.
Figure 4.
Loss of PPARγ attenuates PGC1α expression in HPASMCs. (A and B) HPASMCs were treated with vehicle (DMSO) or the PPARγ antagonist GW9662 (10 μM) for 72 hours. PGC1α mRNA (A) and protein (B) levels were measured by qRT-PCR and Western blot (Calbiochem) analysis, respectively. (C and D) HPASMCs were transfected with SCR control or 100 nM siPPARγ for 72 hours. PGC1α mRNA (C) and protein (D) levels were measured. The mRNA (n = 5–6) and protein (n = 8) levels of the targets were normalized to housekeeping genes and proteins and expressed as mean ± SEM fold change relative to control. *P < 0.05 versus DMSO or SCR. Readers may view the uncut gels for Figures 4B and 4D in the data supplement.
Because HYP was previously shown to downregulate PPARγ expression in HPASMCs in vitro (21) and in mouse lungs in vivo (9), stimulating PASMC proliferation and PH, respectively, we examined lung PGC1α in C57BL/6J mice exposed to HYP (10% O2) or NOR (21% O2) for 3 weeks. HYP decreased mouse lung PGC1α mRNA (Figure 5A) and protein levels (Figure 5B). Similarly, PGC1α mRNA (Figure 5C) and protein (Figure 5D) levels were depleted in HPASMC treated with HYP (1% O2) for 72 hours. Collectively, these data indicate that HYP decreases expression of both PPARγ and PGC1α in mouse lungs and HPASMCs.
Figure 5.
HYP decreases PGC1α expression in mouse lungs and HPASMCs. (A and B) Male C57BL/6J mice were exposed to HYP (10% O2) or NOR (room air, 21% O2) for 3 weeks. PGC1α mRNA (A) and protein (B) levels were measured by qRT-PCR and Western blot (Calbiochem) analysis, respectively. (C and D) HPASMCs were treated with HYP (1% O2) or NOR (21% O2) for 72 hours. PGC1α mRNA (C) and protein (D) levels were measured. The mRNA (n = 5–6) and protein (n = 8) levels of the targets were normalized to housekeeping genes and proteins and expressed as mean ± SEM fold change relative to NOR. *P < 0.05 versus NOR. Readers may view the uncut gels for Figure 5B in the data supplement.
Because depletion of PPARγ perturbed mitochondrial structure and function, and because PGC1α is a critical PPARγ cofactor in regulating mitochondrial mass and bioenergetics, we further examined the relationship between decreases in HPASMC PPARγ and PGC1α. HPASMCs were transfected with SCR or siPGC1α for 72 hours, which knocked down PGC1α mRNA (Figure E1A) and protein (Figure E1B) levels. Treatment with siPGC1α did not affect PPARγ mRNA levels (Figure E1C), suggesting that the effects of PPARγ are upstream of PGC1α in HPASMCs. Similar to the effects of induced knockdown of PPARγ, siPGC1α diminished TFAM mRNA levels (Figure E2A) and perturbed the HPASMC mitochondrial bioenergetic profile (Figures E2B–E2F). Taken together, these data demonstrate that decreases in either PPARγ or PGC1α are sufficient to alter HPASMC mitochondrial structure and function. Because decreases in PPARγ or PGC1α are sufficient to stimulate HPASMC proliferation, these data indicate that targeting the PPARγ-PGC1α axis is a promising therapeutic strategy for reversing metabolic and proliferative derangements in pulmonary vascular wall cells.
HYP Decreases Expression of Mitochondrial Proteins in HPASMCs
Because HYP downregulated PPARγ expression in HPASMCs in vitro (21) and siPPARγ induced HPASMC mitochondrial fragmentation (Figure 2), we sought to determine the effect of HYP on the expression of HPASMC mitochondrial proteins. Treatment with HYP caused decreases in HPASMC TFAM (Figure 6A), GRP75 (Figure 6B), and VDAC (Figure 6C) protein expression, comparable to those caused by siRNA-mediated PPARγ knockdown. To confirm these results in an in vivo experimental model of PH, we used a hypoxic SU5416 rat model of PH. Compared with the lungs isolated from normoxic SU5416 rats, the lungs from hypoxic SU5416 rats showed decreased mRNA levels of PGC1α (Figure E3A), TFAM (Figure E3B), GRP75 (Figure E3C), VDAC (Figure E3D), and MFN2 (Figure E3E). These data further support the postulate that HYP-induced loss of PPARγ mediates derangements in mitochondrial structure and function in HPASMC, and that these mitochondrial derangements are not specific to a single rodent model of PH.
Figure 6.
HYP decreases expression of mitochondrial proteins in HPASMCs. HPASMCs were treated with HYP (1% O2) or NOR (21% O2) for 72 hours. The protein levels of TFAM (A), GRP75 (B), and VDAC (C) were measured by Western blot analysis. The protein levels of the targets were normalized to housekeeping proteins and expressed as mean ± SEM fold change relative to NOR. n = 8, *P < 0.05 versus NOR. Readers may view the uncut gels for Figures 6A, 6B, and 6C in the data supplement.
Overexpression of PGC1α Reverses HYP-Induced HPASMC Proliferation
Because HYP decreased PGC1α expression in mouse lungs and HPASMCs (Figure 5), and knockdown of PGC1α increased HPASMC proliferation (Figure 1C), we sought to determine whether an adenovirus overexpressing PGC1α could reverse HYP-induced HPASMC derangements. The PGC1α overexpression adenovirus increased PGC1α mRNA and protein levels (Figures E4A and E4B) in a dose-dependent manner, but did not affect PPARγ mRNA and protein levels (Figures E4C and E4D). PGC1α adenoviral overexpression reversed HYP-induced HPASMC proliferation (Figure E5). Collectively, these data indicate the critical importance of PPARγ and PGC1α for HYP-induced HPASMC derangements.
Discussion
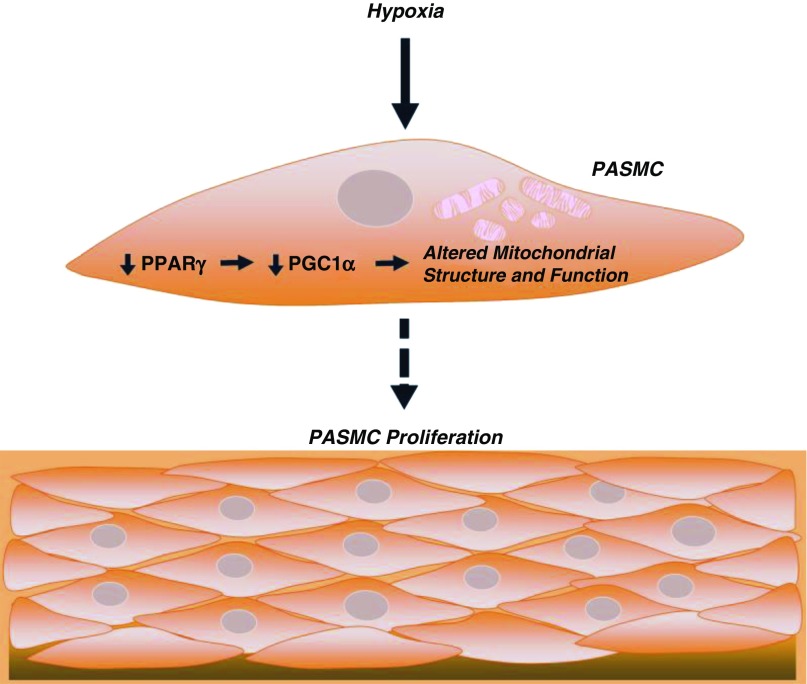
The pathogenesis of PH is complex and characterized by alterations in metabolism and proliferation in cells of the pulmonary vascular wall (2). Chronic HYP constitutes a common stimulus for experimental PH in rodent models (29). HYP promotes a variety of molecular and cellular changes in the lung, including decreases in PPARγ expression and activity (21). PPARγ expression is downregulated in the lungs of rodents with HYP-induced PH in vivo (24, 30) and in HPASMCs exposed to HYP in vitro (21). PPARγ downregulation in these models is comparable to that reported in the vascular lesions (6) of patients with PAH (31). Further, SMC-targeted PPARγ deletion is sufficient to cause PH in mice (8). Taken together, these observations suggest that decreases in PPARγ represent a common pathogenic factor in PH. The precise mechanisms by which reductions in PPARγ promote PASMC proliferation and pulmonary vascular remodeling continue to be defined. The current study further clarifies molecular pathways linking decreases in PPARγ to alterations in cell metabolism and proliferation by showing that HYP or decreases in PPARγ in HPASMCs deplete PGC1α and cause derangements in mitochondrial structure and function that stimulate HPASMC proliferation (Figure 7). These novel results identify PPARγ and PGC1α as potential targets in PH pathogenesis and therapy.
Figure 7.
Summary schematic. HYP decreases PPARγ expression, leading to diminished PGC1α expression. Loss of PPARγ and the subsequent loss of PGC1α alter mitochondrial structure through decreased expression of TFAM, GRP75, VDAC, and MFN2, and impair mitochondrial function and bioenergetics. These mitochondrial derangements may contribute to the proliferation of HPASMCs observed under chronic hypoxic conditions.
Current evidence indicates that metabolic derangements (18), including decreased mitochondrial numbers, mitochondrial fragmentation (15–17), and a shift toward glycolysis-derived ATP production, contribute to pulmonary vascular wall cell phenotypic changes that cause pulmonary vascular remodeling. PPARγ is a central metabolic regulator that activates PGC1α, which coordinates mitochondrial gene expression and in turn activates PPAR. Our data demonstrate that HYP decreases PPARγ and PGC1α, and that decreases in PPARγ are sufficient to diminish PGC1α expression (Figures 4 and 5). Our findings further demonstrate that loss of PPARγ or PGC1α is sufficient to stimulate HPASMC proliferation (Figure 1). PGC1α stimulates mitochondrial biogenesis (32) via activation of nuclear respiratory factor 1, which increases expression of TFAM (33) and nuclear respiratory factor 2 (32). TFAM is a mitochondrial-specific transcription factor that is required for maintenance of mitochondrial DNA copy number and the integrity of respiration (33). Depletion of PPARγ (Figure 2) or PGC1α (Figure E2) by siRNA or HYP (Figure 6) decreased expression of the mitochondrial-specific proteins TFAM, GRP75, and VDAC in HPASMCs, and these same targets were decreased in lung tissue from HYP-exposed, SU5416-treated rats (Figure E3). Further, activation of PPARγ (34) or PGC1α overexpression (Figure E5) attenuated HYP-induced HPASMC proliferation, supporting the importance of PPARγ and PGC1α in HYP-induced HPASMC derangements. VDAC is located on the outer membrane of the mitochondria and forms the permeability transition pore complex, which is responsible for the release of mitochondrial products that trigger apoptosis (35). GRP75 interacts with VDAC and modulates its channel properties (36). Therefore, PPARγ or PGC1α depletion-mediated decreases in GRP75 and VDAC expression may promote HPASMC proliferation (Figure 1) by inhibiting apoptosis. Consistent with this postulate, siRNA-mediated PPARγ knockdown decreased HPASMC apoptosis (caspase-3 activity) (34). siPPARγ-mediated decreases in MFN2 mRNA levels, coupled with decreases in mitochondrial volume and increased numbers of mitochondria per HPASMC (Figure 2), also suggest fragmentation of existing mitochondria, which may contribute to the impaired mitochondrial bioenergetics shown in Figure 3.
Loss of PPARγ (Figure 3A) or PGC1α (Figure E2B) decreased the overall mitochondrial bioenergetic profile in HPASMCs and suppressed basal OCR, proton leak, ATP production, and maximal respiration, consistent with broad derangements in mitochondrial function. Our results indicate that these alterations in mitochondrial function, secondary to depletion of PPARγ or PGC1α, are related in part to (1) decreased mitochondrial biogenesis, as evidenced by decreases in PGC1α (Figures 4C and 4D), TFAM, GRP75, and VDAC (Figures 2C–2E), and (2) increased mitochondrial fission, as evidenced by decreases in MFN2 and mitochondrion volume and increases in the number of mitochondria per cell (Figures 2F and 2G). In a previous study, SMCs isolated from the pulmonary vessels of rats with monocrotaline-induced PH showed decreases in mitochondrial function due to loss of complex I assembly, decreased activities of complexes II and III, and increased levels of mitochondrial-derived ROS, membrane potential, and glycolysis (37). Our findings extend those observations by demonstrating that decreases in PPARγ are sufficient to increase mitochondrial-derived ROS generation (Figure 2) and decrease mitochondrial function (Figure 3). Ongoing studies will further characterize the mitochondrial derangements caused by loss of PPARγ by defining the uncoupling protein activity, mitochondrial membrane potential, and integrity of the electron transport chain.
Several limitations in our study merit additional consideration. First, it is well recognized that the mouse model of HYP-induced PH used in our studies fails to recapitulate many characteristics of PH in humans (29). Determining the relevance of these pathways to human PH will require additional studies employing cells or tissues isolated from PH patients. However, because PPARγ is also decreased in the lungs and pulmonary vascular cells of patients with PAH (6), and because decreases in PPARγ are sufficient to induce PH in experimental animals (38), our findings suggest that decreases in PPARγ may represent a common pathogenic stimulus in PH regardless of etiology or species. Second, although our in vivo HYP studies in mice suggest that pathways defined in hypoxic HPASMCs in vitro are also seen in vivo, our data do not define which specific pulmonary vascular cells sustain HYP-induced depletion of PPARγ and PGC1α in vivo. Therefore, the effects of alterations in PPARγ or PGC1α in other cell types in the vascular wall or in circulating immune cells on PH pathogenesis must also be considered (39–41). For example, previous work demonstrated depleted PPARγ levels in pulmonary artery endothelial cells (10) in PH. In addition, loss of bone morphogenetic protein receptor type 2 led to reductions in PGC1α, mitochondrial dysfunction, and a propensity to undergo apoptosis after HYP reoxygenation (42), suggesting that these derangements are not confined to a single cell compartment. Third, although the present study does not specifically address the mechanisms by which HYP decreases PPARγ in vitro or in vivo, our published studies have defined both transcriptional (21, 22) and post-translational (10) mechanisms that are activated by HYP to decrease PPARγ levels. Finally, the length of time in which pulmonary vascular SMCs display proliferative responses to HYP in vivo is not well defined and may be limited (43–45). Therefore, translating our in vitro findings to the pathobiology of PH in vivo will require more rigorous studies linking temporal and cell compartment–specific alterations in PPARγ and PGC1α to mitochondrial, metabolic, and proliferative derangements that cause pulmonary vascular remodeling.
The current study extends previous reports to demonstrate a novel role for PPARγ, through modulation of PGC1α, in regulating mitochondrial structure, function, and cellular metabolism in pulmonary vascular wall cells. Because PPARγ or PGC1α knockdown stimulated HPASMC proliferation (Figure 1) and comparable derangements in mitochondrial protein expression and bioenergetics (Figures 2, 3, and E2), and because manipulation of PPARγ regulated PGC1α, whereas manipulation of PGC1α had no effect on PPARγ (Figures E1 and E4), our findings collectively support a model in which PPARγ regulates mitochondrial bioenergetic function through PGC1α. Although a metabolomic analysis of late-stage human PAH lung samples suggested that derangements in metabolism may differ according to the stage and severity of disease (46), our studies along with previous reports provide evidence that depletion of PPARγ causes metabolic derangements in pulmonary vascular wall cells and proliferative cellular phenotypes. Because loss of PPARγ function has been implicated in diverse disorders (5), discovering common pathways that link PPARγ downregulation to mitochondrial dysfunction may shed light on other disorders that share similar pathobiological mechanisms. Our findings identify additional mechanistic pathways whereby PPARγ activation could potentially decrease metabolic derangements. Pharmacological activation of PPARγ using rosiglitazone was previously shown to decrease chronic HYP-induced PH, right ventricular hypertrophy, and vessel thickness (24). Taken together, these considerations suggest that therapeutic strategies that target PPARγ have the potential to mitigate the fundamental metabolic derangements that cause vascular cell hyperproliferation and vascular remodeling during PH pathogenesis.
Acknowledgments
Acknowledgments
The authors thank Sherry Adesina, Ph.D., for her contributions to Figure 2, and Dr. Russ Price (Emory University) for providing the adenovirus overexpressing PGC1α and GFP. They also thank Brian C. Brockway, M.S. (medical illustrator at Medical Media Services, Atlanta VA Medical Center), and Brandy E. Wade, Ph.D., for their contributions to the illustration depicted in Figure 7.
Note Added in Proof: While the current paper was under review, Calvier and colleagues (47) reported that PPARγ also regulates PASMC proliferation through modulation of TGFβ-induced alterations in signaling and cellular metabolism.
Footnotes
This work was supported in part by grants from the National Institute on Alcohol Abuse and Alcoholism (1K99AA021803 and 4R00AA021803 to S.M.Y.), the American Heart Association (13SDG14150004 to B.-Y.K. and 11SDG5440016 to K.M.B.), the National Heart, Lung, and Blood Institute (2P01HL095070-06A1 to A.S.M. and HL133053-01A1 to B.-Y.K., and HL102167 to R.L.S. and C.M.H.), and the Veterans Affairs Basic Laboratory Research and Development Merit Review Award Program (1I01BX001910 to C.M.H.). The contents of this report do not represent the views of the Department of Veterans Affairs or the United States government.
Author Contributions: S.M.Y., B.-Y.K., and K.M.B. designed and analyzed experiments, and prepared the manuscript; J.M.K. and T.C.M. designed and analyzed experiments; G.T. and A.S.M. designed and analyzed mitochondria structure experiments; R.L.S. and C.M.H. designed experiments and prepared the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0293OC on November 28, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 2.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. 2009;54(1) Suppl:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARγ signaling and metabolism: the good, the bad and the future. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green DE, Sutliff RL, Hart CM. Is peroxisome proliferator-activated receptor gamma (PPARγ) a therapeutic target for the treatment of pulmonary hypertension? 2011;1:33–47. doi: 10.4103/2045-8932.78101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, et al. Peroxisome proliferator-activated receptor gamma (PPARγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 7.Tian J, Smith A, Nechtman J, Podolsky R, Aggarwal S, Snead C, et al. Effect of PPARγ inhibition on pulmonary endothelial cell gene expression: gene profiling in pulmonary hypertension. 2009;40:48–60. doi: 10.1152/physiolgenomics.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijli KM, Kleinhenz JM, Murphy TC, Kang BY, Adesina SE, Sutliff RL, et al. Peroxisome proliferator-activated receptor gamma depletion stimulates Nox4 expression and human pulmonary artery smooth muscle cell proliferation. 2015;80:111–120. doi: 10.1016/j.freeradbiomed.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green DE, Murphy TC, Kang BY, Searles CD, Hart CM. PPARγ ligands attenuate hypoxia-induced proliferation in human pulmonary artery smooth muscle cells through modulation of microRNA-21. 2015;10:e0133391. doi: 10.1371/journal.pone.0133391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang BY, Park KK, Kleinhenz JM, Murphy TC, Green DE, Bijli KM, et al. Peroxisome proliferator-activated receptor γ and microRNA 98 in hypoxia-induced endothelin-1 signaling. 2016;54:136–146. doi: 10.1165/rcmb.2014-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Wang G, Han D, Zhang Y, Xu J, Lu J, et al. Activation of PPAR-γ ameliorates pulmonary arterial hypertension via inducing heme oxygenase-1 and p21(WAF1): an in vivo study in rats. 2014;98:39–43. doi: 10.1016/j.lfs.2013.12.208. [DOI] [PubMed] [Google Scholar]
- 12.Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, et al. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. 2007;292:L885–L897. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 14.Hondares E, Mora O, Yubero P, Rodriguez de la Concepción M, Iglesias R, Giralt M, et al. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1alpha gene transcription: an autoregulatory loop controls PGC-1alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation. 2006;147:2829–2838. doi: 10.1210/en.2006-0070. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 16.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. 2010;176:1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 19.Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, et al. PGC1α-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. 2013;187:865–878. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao J, Li J, Liu Y, Lu P, Sun X, Sugumaran PK, et al. The key role of PGC-1α in mitochondrial biogenesis and the proliferation of pulmonary artery vascular smooth muscle cells at an early stage of hypoxic exposure. 2012;367:9–18. doi: 10.1007/s11010-012-1313-z. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Bijli KM, Ramirez A, Murphy TC, Kleinhenz J, Hart CM. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. 2013;63:151–160. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Murphy TC, Nanes MS, Hart CM. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. 2010;299:L559–L566. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Janocha AJ, Leahy RA, Klatte R, Dudzinski D, Mavrakis LA, et al. A novel method for pulmonary research: assessment of bioenergetic function at the air-liquid interface. 2014;2:513–519. doi: 10.1016/j.redox.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, et al. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. 2010;42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 26.Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel W, Fang HB, et al. PGC1α promotes tumor growth by inducing gene expression programs supporting lipogenesis. 2011;71:6888–6898. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casanova E, Baselga-Escudero L, Ribas-Latre A, Arola-Arnal A, Blade C, Arola L, et al. Epigallocatechin gallate counteracts oxidative stress in docosahexaenoxic acid-treated myocytes. 2014;1837:783–791. doi: 10.1016/j.bbabio.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Simpson RL, Bennett RG. Relaxin activates peroxisome proliferator-activated receptor γ (PPARγ) through a pathway involving PPARγ coactivator 1α (PGC1α) 2015;290:950–959. doi: 10.1074/jbc.M114.589325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 30.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. 2010;15:659–668. doi: 10.1111/j.1440-1843.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 31.Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. 2013;2:e005157. doi: 10.1161/JAHA.112.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shertzer HG, Krishan M, Genter MB. Dietary whey protein stimulates mitochondrial activity and decreases oxidative stress in mouse female brain. 2013;548:159–164. doi: 10.1016/j.neulet.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 34.Green DE, Murphy TC, Kang BY, Bedi B, Yuan Z, Sadikot RT, et al. Peroxisome proliferator-activated receptor-γ enhances human pulmonary artery smooth muscle cell apoptosis through microRNA-21 and programmed cell death 4. 2017;313:L371–L383. doi: 10.1152/ajplung.00532.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampson MJ, Lovell RS, Craigen WJ. Isolation, characterization, and mapping of two mouse mitochondrial voltage-dependent anion channel isoforms. 1996;33:283–288. doi: 10.1006/geno.1996.0193. [DOI] [PubMed] [Google Scholar]
- 36.Isarangkul D, Wiyakrutta S, Kengkoom K, Reamtong O, Ampawong S. Mitochondrial and cytoskeletal alterations are involved in the pathogenesis of hydronephrosis in ICR/Mlac-hydro mice. 2015;8:9192–9204. [PMC free article] [PubMed] [Google Scholar]
- 37.Rafikov R, Sun X, Rafikova O, Meadows ML, Desai AA, Khalpey Z, et al. Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells. 2015;6:278–286. doi: 10.1016/j.redox.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabinovitch M. PPARγ and the pathobiology of pulmonary arterial hypertension. 2010;661:447–458. doi: 10.1007/978-1-60761-500-2_29. [DOI] [PubMed] [Google Scholar]
- 39.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. 2012;55:218–228. doi: 10.1016/j.pcad.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmüller P, et al. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. 2014;129:1332–1340. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. 2015;21:596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paddenberg R, Stieger P, von Lilien AL, Faulhammer P, Goldenberg A, Tillmanns HH, et al. Rapamycin attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice. 2007;8:15. doi: 10.1186/1465-9921-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinlan TR, Li D, Laubach VE, Shesely EG, Zhou N, Johns RA. eNOS-deficient mice show reduced pulmonary vascular proliferation and remodeling to chronic hypoxia. 2000;279:L641–L650. doi: 10.1152/ajplung.2000.279.4.L641. [DOI] [PubMed] [Google Scholar]
- 45.Sheikh AQ, Lighthouse JK, Greif DM. Recapitulation of developing artery muscularization in pulmonary hypertension. 2014;6:809–817. doi: 10.1016/j.celrep.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Peng J, Lu C, Hsin M, Mura M, Wu L, et al. Metabolomic heterogeneity of pulmonary arterial hypertension. 2014;9:e88727. doi: 10.1371/journal.pone.0088727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calvier L, Chouvarine P, Legchenko E, Hoffmann N, Geldner J, Borchert P, et al. PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. 2017;25:1118–1134. doi: 10.1016/j.cmet.2017.03.011. [DOI] [PubMed] [Google Scholar]