Abstract
The FOSL1/AP-1 transcription factor regulates gene expression, thereby controlling various pathophysiological processes. It is a major effector of RAS-ERK1/2 signaling and is activated in human lung epithelia by tumorigenic stimuli. Recent evidence shows an inverse correlation between FOSL1 expression and the survival of patients with lung cancer and adenocarcinomas; however, its role in lung tumorigenesis remains elusive. In this work, we sought to determine the role of FOSL1 in Kras-induced lung adenocarcinoma in vivo and its downstream effector mechanisms. We used mice expressing the Kras oncogene in the lung with concomitant Fosl1 deletion, Kras-activated murine alveolar epithelial cells (mAECs) with Fosl1 deletion, and KRAS mutant human lung adenocarcinoma (HLAC) cells with FOSL1 deficiency, and performed cell proliferation and gene expression analyses. Mutant Kras induced Fosl1 expression in vitro (mAECs) and in vivo (lung tissue), and mice with Fosl1 deletion showed reduced levels of mutant Kras–induced lung tumorigenesis and survived longer than Fosl1-sufficient mice. Studies with mutant Kras–activated mAECs and KRAS-mutant HLAC cells revealed that FOSL1 regulates mutant KRAS–induced gene expression, thereby controlling cell proliferation and survival. In contrast, FOSL1 depletion in non–KRAS-mutant HLAC cells and nonmalignant human lung epithelia had no effect. Our data support the notion that FOSL1-mediated expression of amphiregulin and apoptotic and antioxidative genes plays a role in regulating HLAC cell proliferation and survival. FOSL1 is a determinant of lung cancer in vivo and regulates HLAC cell proliferation and survival, largely in the context of KRAS mutations. Activation of FOSL1 in adenocarcinomas may be a prognostic marker and potential target for human lung cancer with KRAS mutations.
Keywords: AP-1, apoptosis, cyclins, epidermal growth factors, tumor cell growth
Clinical Relevance
Mutant KRAS activation leading to lung tumor growth is a major cause of lung cancer deaths, but thus far its crucial downstream targets and mechanisms that control tumor cell growth are not well defined. Here, we show for the first time that FOSL1 is a determinant of lung cancer in vivo and regulates human lung adenocarcinoma cell proliferation and survival, largely in the context of KRAS mutations. Thus, activation of FOSL1 in adenocarcinomas may be a prognostic marker and potential target for human lung cancer with KRAS mutations.
Lung cancer is a prominent cause of cancer deaths in the United States and around the world. Alveolar epithelial cells (AECs) are major progenitors of lung cancer and are direct targets of procarcinogenic agents. Members of the AP-1 family regulate the expression of genes that are critical for various pathophysiological processes (1) that are also an integral part of various lung diseases and malignancy. Accumulating evidence suggests an important role for FOSL1 (aka FRA-1), a member of the FOS family, in cancer cell progression and maintenance of the transformed state in several cell types (2). Recent studies have shown that the FOSL1/AP-1 transcription factor regulates tumor heterogeneity, and especially tumor cell clonal evolution and epithelial and mesenchymal plasticity (3). Various procarcinogens, such as tobacco smoke (4), NNK (5), and tumor-promoting stimuli (6, 7), activate FOSL1 expression in lung epithelia. FOSL1 induction is required for asbestos-induced malignant transformation of rat pleural mesothelial cells, and FOSL1 silencing reverses the malignant phenotype of mesothelioma in vitro (5, 8). The transition from the small cell to non–small cell lung cancer phenotype is accompanied by specific induction of FOSL1, suggesting that this transcription factor maintains the differentiated state of lung cancer cell types (9). FOSL1 is also expressed in other cancer cell types, including breast cancer (10), colon cancer (11), and hepatocellular carcinoma (12), suggesting an important role for this AP-1 transcription factor in both Kras- and non-Kras–driven tumorigenesis and progression (see reviews in References 3 and 13).
We previously demonstrated an obligatory role for metalloproteinase-EGF receptor (EGFR)–mediated, RAS-activated MAP kinase signaling in controlling smoke-induced FOSL1 expression in lung epithelial cells (4). Ectopic FOSL1 enhances lung epithelial cell motility and invasion, and causes anchorage-independent growth (14). Although the above data suggest a role for FOSL1 in mediating tumor epithelial cell progression in vitro, we found that FOSL1 overexpression in nonmalignant lung epithelia alone is not sufficient to induce significant tumor growth in athymic mice (14). This suggests that additional potential modifications (such as phosphorylation) and/or the presence of some other activated proto-oncogene(s) are required to impart the full oncogenic potential of FOSL1 in vivo.
Consistent with the above experimental data, emerging evidence shows a correlation between elevated levels of FOSL1 expression and poor survival of patients with lung cancer and adenocarcinomas. However, whether FOSL1 is a critical determinant in driving the development and progression of lung cancer in vivo remains unclear. In the present study, we examined for the first time the role of FOSL1 in regulating KRAS-induced human lung tumorigenesis using an experimental model of lung cancer. Here, we report that FOSL1 is required for mutant Kras–induced lung tumorigenesis in vivo, and promotes human lung adenocarcinoma (HLAC) growth and survival by regulating multiple gene networks that control cell proliferation and survival in the setting of KRAS mutations.
Methods
Mice
Mice bearing “floxed” Fosl1 alleles (15) were bred with mice with LSL-KrasG12D (16) to generate bitransgenic Fosl1F/F:KrasG12D mice. Both Fosl1F/F:KrasG12D mice and KrasG12D mice (8–10 weeks old, male and female) were infected with adenoviral vectors encoding Cre to activate Kras and concurrently delete Fosl1. The animal protocols were approved by the Johns Hopkins Bloomberg School of Public Health and the University of Illinois at Chicago. More details are provided in the data supplement.
Cell Culture, Small Interfering RNA Transfection, Cell Proliferation, and Clonogenicity Assays
Details regarding cell culture, small interfering RNA (siRNA) transfection, cell proliferation, and clonogenicity assays are provided in the data supplement.
Histological, Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling, and Gene Expression Analyses
Details regarding histological, TUNEL, and gene expression analyses are provided in the data supplement.
Statistical Analysis
All histological and survival data were analyzed using GraphPad Prism4 software. Data are expressed as mean ± SD unless otherwise indicated. The survival data were also analyzed using a Kaplan–Meier survival plot. A two-tailed Student’s t test was used to calculate significance, and P = 0.05 or less was considered significant. Outliers identified by Grubbs’s test using the GraphPad calculator were removed from the data analysis.
Results
FOSL1 Overexpression Correlates Inversely with the Survival of Patients with Lung Cancer and Is Overexpressed in HLAC Cells
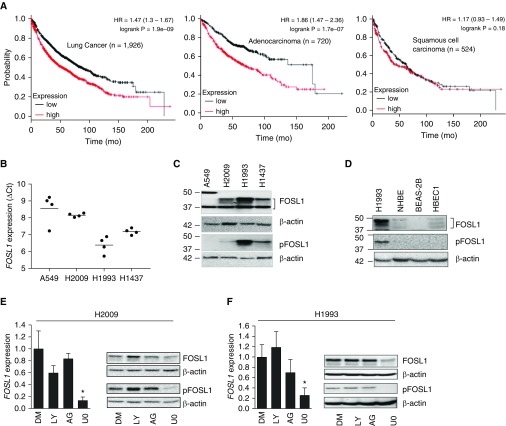
We assessed the relationship between FOSL1 expression (Affymetrix ID 204420_at) and the survival of patients with lung cancer using the Kaplan-Meier Plotter (http://kmplot.com/analysis/; for more details, see the data supplement). FOSL1 mRNA expression and patient survival curves were plotted for all patients with lung cancer (n = 1,926) (Figure 1A, left panel), and also stratified for adenocarcinoma samples (n = 720) (Figure 1A, middle panel). High-level FOSL1 mRNA expression was associated with poor overall survival rates in all patients with lung cancer. In contrast, relatively low-level FOSL1 mRNA expression was associated with increased survival in all patients with lung cancer (P = 1.9e-09). Interestingly, increased FOSL1 expression was associated with poor survival of patients with lung cancer and adenocarcinoma (P = 1.7e-07), but not patients with squamous cell carcinoma (n = 524, Figure 1A, right panel).
Figure 1.
FOSL1 expression in human lung cancer and its relationship with survival rates in patients with lung cancer. (A) FOSL1 (Affymetrix ID 204420_at) expression and Kaplan–Meier plot of cumulative survival rates for all patients with lung cancer (n = 1,926; left), patients with lung adenocarcinoma (n = 720; middle), and patients with squamous cell carcinoma (n = 524; right). HR = hazard ratio. (B) FOSL1 expression and regulation in human lung adenocarcinoma (HLAC) cells. FOSL1 mRNA levels were quantified by qRT-PCR using β-actin as a reference. Values shown are ΔCt. (C and D) Immunoblot analysis of the levels of native FOSL1 and the activated (phosphorylated) form of FOSL1 (pFOSL1) in (C) HLAC cells and (D) nonmalignant human lung epithelial cells. H1993 was used to compare the relative expression levels. (E and F) Effects of EGFR, PI3K-AKT1/2, and MEK1/2-ERK1/2 pathway inhibition on FOSL1 expression in HLAC cells. H2009 and H1993 cells were treated with U0126 (UO, 10 μM), LY294002 (LY, 10 μM), AG1478 (AG, 10 μM), or DMSO (DM, vehicle) for 6 hours, RNA was isolated, and FOSL1 mRNA expression was analyzed by qRT-PCR. Values are expressed relative to control (DMSO). *P ≤ 0.05, compared with DMSO. (E and F) Western blot showing FOSL1 and pFOSL1 levels in H2009 and H1993 cells treated with inhibitors as indicated. Readers may view the uncut gels and comments on the antibody probing protocol for Figure 1 in the data supplement. HBEC = human bronchial epithelial cell line; NHBE = normal human bronchial epithelial cells.
To verify the above findings, we examined FOSL1 mRNA expression in four HLAC cell lines: A549, H1993, H2009, and H1437. Consistent with the data from the patients with lung cancer, the analysis revealed elevated levels of FOSL1 mRNA expression in A549, H1993, H2009, and H1437 cells (Figure 1B). Corroborating the qRT-PCR results, immunoblot analysis showed an increased level of FOSL1 expression, which was associated with its activation (phosphorylation, pFOSL1) status (Figure 1C). We next analyzed FOSL1 expression in primary cultured normal human bronchial epithelial (NHBE) cells, and immortalized and nonmalignant human bronchial epithelial cell line (BEAS-2B) and human bronchial epithelial cell line (HBEC1). Immunoblot analysis revealed lower levels of FOSL1 and pFOSL1 in NHBE, BEAS-2B, and HBEC1 cells than in HLAC H1993 cells (Figure 1D).
Signaling by ERK1/2, not AKT1/2, Controls FOSL1 Expression in HLAC Cells
Previously, we have shown that signaling by both ERK1/2 and AKT1/2 regulates FOSL1 induction via tumor-promoting stimuli (e.g., phorbol ester and cigarette smoke) in human lung epithelial cells (4, 6, 7). To determine whether ERK1/2 and/or AKT1/2 signaling regulates FOSL1 expression in human lung cancer, we treated H2009 (Figure 1E) and H1993 (Figure 1F) cells with U0126 (MEK1/2-ERK1/2 inhibitor), LY294002 (PI3K/AKT inhibitor), and AG1478 (EGFR inhibitor) for 6 hours, and analyzed FOSL1 (mRNA and protein) expression. Both qRT-PCR and immunoblot analyses revealed decreased levels of FOSL1 expression in H2009 and H1993 cells treated with UO126 (Figures 1E and 1F), whereas LY294002 and AG1478 had no such effect, demonstrating that FOSL1 expression in HLAC cells is largely regulated by MEK1/2-ERK1/2 signaling.
Deletion of Fosl1 Decreases Mutant Kras–induced Lung Tumor Growth and Promotes Survival
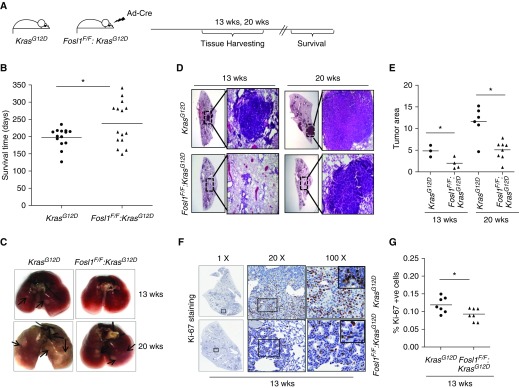
We first assessed Fosl1 activation by mutant Kras in vivo in the lung and alveolar epithelia. We found an increased expression of Fosl1 mRNA in the lungs of mice and in primary type 2 AECs (AEC2s) with mutant Kras activation (Figure E1 in the data supplement), demonstrating that mutant Kras induces Fosl1 expression in lung epithelia. To define the role of FOSL1 in human lung tumorigenesis in vivo, we generated a bitransgenic mouse model bearing Fosl1 “floxed” alleles and LSL-KrasG12D (notated as Fosl1F/F:KrasG12D). Fosl1F/F:KrasG12D mice were infected with adenoviral Cre to express oncogenic Kras and concomitantly delete Fosl1 in the lung (Figure 2A). Mice were killed at 13 weeks and 20 weeks after infection to monitor lung tumor development and progression. The survival rates of these mice after tumor initiation were also monitored. There was a significant increase in the median survival rate of Fosl1F/F:KrasG12D mice as compared with KrasG12D mice (Figure 2B). This result suggests an important role for Fosl1 in mediating oncogenic-Kras–induced lung tumorigenesis.
Figure 2.
Disruption of Fosl1 reduces mutant Kras–induced lung tumorigenesis in vivo. (A) Schema showing the timeline of tissue harvesting and survival monitoring of KrasG12D mice and Fosl1F/F:KrasG12D mice after adenoviral Cre vector infection. (B) Survival rates of KrasG12D mice and Fosl1F/F:KrasG12D mice after mutant Kras activation. *P ≤ 0.05, KrasG12D versus Fosl1F/F:KrasG12D mice. (C) Representative lungs harvested from KrasG12D mice and Fosl1F/F:KrasG12D mice at 13 weeks and 20 weeks after infection. Arrows indicate the position of adenomas. (D) Representative photomicrographs of hematoxylin and eosin–stained lung tumor sections at 13 weeks and 20 weeks after mutant Kras activation. Low (×1, left) and high (×20, right) magnifications are shown. (E) Quantification of tumor area (percent) in KrasG12D mice and Fosl1F/F:KrasG12D mice. Values are mean ± SD. *P ≤ 0.05, versus KrasG12D mice. (F) Fosl1 deletion affects Kras-induced tumor cell proliferation. Lung tumor sections at 13 weeks of Kras activation were immunostained with Ki-67 antibody. Shown are representative photomicrographs at low (×1, left) intermediate (×20, middle), and high (×100, right) resolution. (G) Quantification of Ki-67+ cells in lungs of KrasG12D mice and Fosl1F/F:KrasG12D mice. *P ≤ 0.05, versus KrasG12D mice.
Consistent with published reports, the KrasG12D mice displayed visibly distinct adenomas on both lobes of the lungs at 13 weeks after tumor initiation (Figure 2C, top panel). However, fewer or no adenomas were visible on the lungs of Fosl1F/F:KrasG12D mice. At 20 weeks after Kras activation, large adenocarcinomas were visible in KrasG12D mice, but there were fewer and smaller adenocarcinomas in Fosl1F/F:KrasG12D mice (Figure 2C, bottom panel). These differences in the rate of development and the size of adenomas/adenocarcinomas were clearly visible in hematoxylin and eosin–stained lung sections obtained from both groups of mice at the respective time points (Figure 2D). Consistent with these observations, the tumor area in KrasG12D mice at 13 weeks and 20 weeks was quantified as 4.9% and 12%, respectively. However, it was significantly smaller in their Fosl1F/F:KrasG12D counterparts, which had an average lung tumor area of 2% and 5.1% at 13 weeks and 20 weeks, respectively (Figure 2E). To determine whether the reduced tumor burden in Fosl1F/F:KrasG12D mice was accompanied by a reduction in tumor cell proliferation, we performed Ki-67 immunostaining of the lungs at 13 weeks after tumor initiation (Figure 2F). The number of proliferating (Ki-67+) cells in the transformed areas of lung sections from Fosl1F/F:KrasG12D mice was significantly lower than that observed for KrasG12D mice (Figure 2G). Collectively, these data suggest that the Fosl1/AP-1 transcription factor is important for mutant Kras–induced lung tumor cell proliferation and tumor growth in vivo.
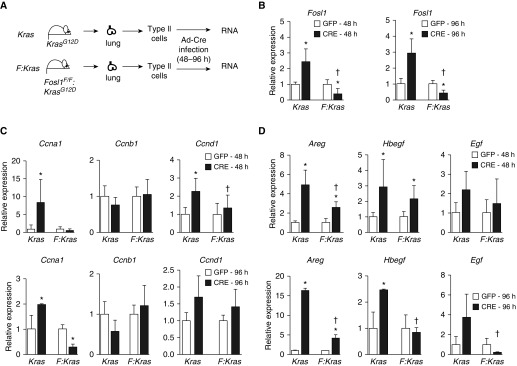
Fosl1 Selectively Upregulates Mutant Kras–Induced Cell-Cycle Gene Expression in Alveolar Epithelia
To examine the mechanisms underlying the Fosl1-mediated decrease in Kras-induced lung epithelial cell proliferation, AEC2s were isolated from lungs of KrasG12D mice and Fosl1F/F:KrasG12D mice, cultured for 4 days, and then infected with adenoviral vectors expressing Cre to activate mutant Kras (outlined in Figure 3A). Cells were infected with adenoviral vectors expressing green fluorescent protein to serve as a control. At 48 hours and 96 hours after infection, RNA was isolated from AEC2s for gene expression analysis. As anticipated, an ∼3-fold increased expression of Fosl1 mRNA was found in KrasG12D AEC2s with Kras activation. Fosl1 expression was markedly (∼80%) lower in Kras-activated Fosl1F/F:KrasG12D AEC2s at both 48 hours and 96 hours after infection, demonstrating adequate deletion of Fosl1 in the AEC2s (Figure 3B). To further determine the role of Fosl1 in regulating Kras-induced cell proliferation, we analyzed the expression levels of several genes encoding cyclins and growth factors involved in cell-cycle progression. Increased expression of Ccna1 and Ccnd1 was noted in KrasG12D AEC2s expressing mutant Kras at both 48 hours (Figure 3C, top) and 96 hours (Figure 3C, bottom) after infection, but their expression was not significantly induced in Fosl1F/F:KrasG12D AEC2s with Fosl1 deletion. Ccnb1 mRNA expression was not altered in AEC2s of either genotype with Kras activation, suggesting selective upregulation of cyclins by mutant Kras in lung epithelia. As increased expression of various growth factors that regulate EGFR activation is important for cancer cell growth and survival (17), we examined the expression of the EGFR ligands Areg, Hbegf, and Egf in Kras-activated AEC2s without and with Fosl1 deletion. Areg and Hbegf were significantly upregulated by mutant Kras in KrasG12D AEC2s, but they showed significantly lower induction in KrasG12D AEC2s lacking Fosl1 (Figure 3D). These data indicate that the Fosl1 transcription factor is required for upregulating mutant Kras–induced gene expression involved in AEC proliferation and survival.
Figure 3.
Mutant Kras–induced cell-cycle gene expression in primary alveolar epithelia with and without Fosl1 deletion. (A) Type 2 alveolar epithelial cells (AEC2s) were isolated from lungs of KrasG12D (Kras) mice and Fosl1F/F:KrasG12D (Fosl1:Kras) mice. Kras AEC2s and Fosl1:Kras AEC2s (abbreviated as Kras and F:Kras, respectively) were cultured for 4 days and infected with adenovirus vectors encoding Cre or GFP for 48 hours or 96 hours. RNA was isolated and gene expression was analyzed by qRT-PCR using β-actin as a reference. (B) Fosl1 mRNA expression in AEC2s of the indicated genotypes at 48 hours and 96 hours after adenoviral-Cre/GFP infection. (C) Analysis of cell-cycle progression and gene expression at 48 hours (top) and 96 hours (bottom) after infection. (D) Expression levels of EGFR ligands in AEC2s of the Kras and Fosl1:Kras genotypes at 48 hours (left panel) and 96 hours (bottom) after infection. Values are expressed relative to the respective genotypes infected with adenoviral GFP. *P ≤ 0.05, Cre versus GFP; †P ≤ 0.05, versus Kras.
FOSL1 Is Required for the Growth of HLAC Cells with Mutant KRAS
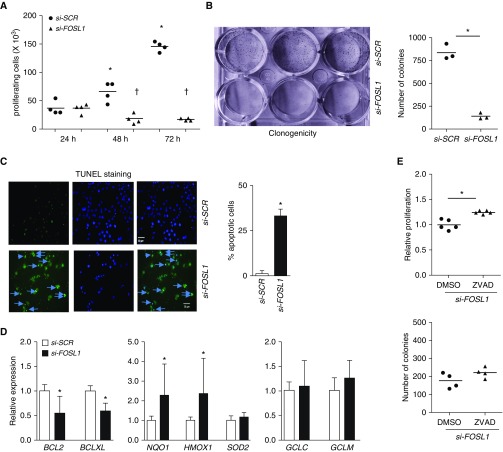
To determine whether FOSL1 is required for the promotion of KRAS mutant HLAC cell proliferation and survival, we used H2009 cells expressing elevated levels of FOSL1 (Figure 1C) and harboring the KRAS mutation. We used custom FOSL1 siRNA that was previously shown to be effective and specific for depleting FOSL1 (18, 19) (Figure E2). FOSL1 knockdown using the siRNA-mediated approach significantly reduced proliferation of H2009 cells (Figure 4A) and markedly impaired their survival, as assessed by long-term clonogenicity assays (Figure 4B). Similar results were obtained using smart pooled FOSL1-specific siRNAs (Figure E3). We used custom FOSL1 siRNA for the rest of studies. FOSL1 silencing also reduced KRAS mutant H2030 (Figure E4) and A549 (Figure E5A) cell proliferation, but its knockdown had no antiproliferative effect in nonmalignant immortalized bronchial epithelial cells (BEAS-2B; Figure E5B). Likewise, FOSL1 depletion had no antiproliferative effects in HLAC cells that harbored wild-type KRAS (H1993 and CL1–5), despite elevated expression of FOSL1 in these cells (unpublished data). These results suggest that FOSL1 is largely important for mutant KRAS–regulated HLAC cell growth and survival, but not for the survival of non–Kras-mutant cells (H1993 and CL1–5) in vitro.
Figure 4.
Effects of FOSL1 deficiency on KRAS mutant HLAC cell proliferation and survival. (A) To measure cell proliferation, H2009 cells (15,000/well) transfected with scramble (SCR) siRNA or custom FOSL1 siRNA (25 nM) for 24 hours were plated and harvested at the indicated time points and counted. Data are mean ± SD. *P ≤ 0.05 versus 24 hours si-SCR; †P ≤ 0.05, versus si-SCR. (B) To measure long-term survival, H2009 cells (1,500/well) transfected with SCR or FOSL1 siRNA were seeded on a 12-well plate and cultured for 7 days. Colonies were stained with crystal violet, dried, photographed, and quantified. Data are mean ± SD. *P ≤ 0.05, versus si-SCR. (C) FOSL1 deficiency promotes apoptosis in KRAS mutant HLAC cells. To examine whether FOSL1 deficiency affects cell proliferation by inducing apoptosis, H2009 cells (15,000/well) were transfected with si-SCR or custom si-FOSL1 siRNA (25 nM) for 72 hours and stained with TUNEL and DAPI. Images were then captured and TUNEL-positive cells (see arrows) were enumerated. Merged images stained with TUNEL and DAPI are shown. Data are mean ± SD (n = 3); *P ≤ 0.05 versus si-SCR. Scale bars: 50 μm. (D) H2009 cells were transfected with si-SCR or si-FOSL1 for 72 hours, RNA was isolated, and antiapoptotic and antioxidative gene expression (as indicated) was analyzed. Values are shown relative to si-SCR. Data are mean ± SD. *P ≤ 0.05, versus si-SCR. (E) To determine the role of apoptosis, H2009 cells transfected with FOSL1 siRNA were seeded on a 12-well plate and then cultured in the presence of ZVAD or vehicle (DMSO) for 3 days to measure their proliferation rate (top) or 7 days to measure their clonogenicity (bottom) as in Figure 6. Data are mean ± SD. *P ≤ 0.05, versus DMSO.
FOSL1 Maintains the Survival of KRAS Mutant HLAC Cells by Repressing Apoptosis through Modulation of Antioxidative and Apoptotic Gene Expression
To delineate the mechanisms by which FOSL1 promotes HLAC cell proliferation and survival, we assessed apoptosis in FOSL1 siRNA-transfected KRAS mutant H2009 cells. TUNEL staining revealed the induction of apoptosis, as shown by increased levels of TUNEL-positive cells, in FOSL1-depleted cells (Figure 4C). FOSL1 depletion resulted in reduced levels of antiapoptotic BCL2 and BCLXL expression (Figure 4D), suggesting the modulation of apoptotic gene expression by FOSL1 in KRAS mutant HLAC cells. As optimal antioxidative gene expression is important for mitigating oxidative stress and maintaining tumor cell survival, we examined the effect of FOSL1 silencing on their expression in H2009 cells (Figure 4D). NQO1 and HMOX1 expression was upregulated, whereas GCLC and GCLM expression was not altered due to FOSL1 deficiency, suggesting that this transcription factor selectively modulates antioxidative gene expression and maintains HLAC cell survival.
Apoptosis is a complex process that is regulated by multiple proteins and pathways. Thus, to verify that FOSL1 regulates KRAS mutant HLAC cell survival through the modulation of apoptosis, H2009 cells with FOSL1 knockdown were supplemented with ZVAD (a general inhibitor of apoptosis) and their proliferation and potential for clonogenicity were assessed (Figure 4E, top). ZVAD supplementation rescued cell growth defects observed in H2009 cells with the FOSL1 knockdown. ZVAD modestly improved H2009 cell survival (Figure 4E, bottom), as assessed by long-term clonogenicity assays, imparted by FOSL1 deficiency.
FOSL1 Promotes HLAC Cell Growth by Regulating AREG Expression
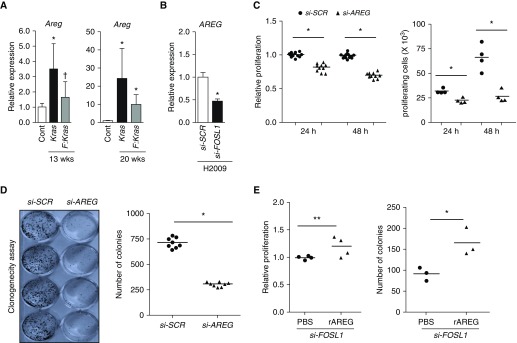
Our studies in primary cultured murine AEC2s suggest that FOSL1 upregulates the expression of growth factors that activate EGFR signaling induced by mutant Kras. Among EGFR ligands, Areg expression was markedly upregulated in alveolar epithelia by the Kras oncogene in a Fosl1-dependent manner (Figure 3D). As AREG has been implicated in human cancer (20), we analyzed Areg expression in Kras-induced lung tumors with and without Fosl1 deletion. Kras-induced lung tumors at 13 weeks showed increased levels of Areg expression in lungs of Kras mutant mice, but its expression was significantly lower in lung tumors obtained from mice with Fosl1 deletion (Figure 5A). Likewise, Areg expression in Kras-induced lung tumors at 20 weeks in mice lacking Fosl1 was lower than in mice without Fosl1 deletion. To verify that FOSL1 regulates AREG expression in human lung cancer, we measured the latter expression in H2009 cells with FOSL1 depletion. AREG mRNA expression in H2009 cells was reduced by FOSL1 deficiency (Figure 5B), suggesting a potential role for AREG in mediating KRAS-induced HLAC cell growth. Indeed, AREG silencing decreased H2009 cell growth, as assessed by acute cell proliferation (Figure 5C) and long-term clonogenicity assays (Figure 5D). Supplementation of AREG in FOSL1 siRNA-transfected H2009 cells improved their growth defects imparted by FOSL1 deficiency (Figure 5E).
Figure 5.
FOSL1 deficiency dampens mutant Kras–induced AREG expression in lung tumors, and FOSL1 promotes KRAS mutant HLAC cell proliferation by regulating AREG expression. (A) Areg mRNA expression in lung tumors of KrasG12D mice and Fosl1F/F:KrasG12D mice at 13 weeks and 20 weeks after Kras oncogene activation. Values are expressed relative to the lung tissue of KrasG12D mice (notated as Cont) without adenoviral Cre infection. Data are mean ± SD (n = 3–6). *P ≤ 0.05 versus Cont; †P ≤ 0.05 versus Kras. (B) AREG expression in H2009 cells transfected with si-SCR or si-FOSL1 for 72 hours. Data are mean ± SD (n = 3–4). *P ≤ 0.05 versus si-SCR. (C) H2009 cells (15,000/well) transfected with SCR siRNA or AREG siRNA (100 nM) for 24 hours were seeded onto a 96-well plate. Cells were harvested at 24 hours and 48 hours thereafter, and their proliferation was measured by CellTiter-Glo assay (left) or cell counting (right). Data are mean ± SD. *P ≤ 0.05, versus si-AREG. (D) To measure clonogenicity, H2009 cells (1,500/well) transfected with si-SCR or si-AREG for 24 hours were seeded onto a 12-well plate and cultured for 7 days. Colonies were stained with crystal violet, dried, and photographed (left), and the number of colonies was enumerated (right). Data are mean ± SD. *P ≤ 0.05 versus si-SCR. (E) Exogenous AREG improves HLAC cell survival imparted by FOSL1 deficiency. H2009 cells transfected with FOSL1 siRNA were seeded on a 96-well or 12-well plate and then cultured in the presence of recombinant AREG (rAREG; 50 ng/ml) or vehicle (PBS) for 3 days to measure their proliferation rate (left) or 7 days to measure clonogenicity (right) as in Figure 4. Data are mean ± SD. *P ≤ 0.05, versus PBS. **P = 0.06 versus PBS.
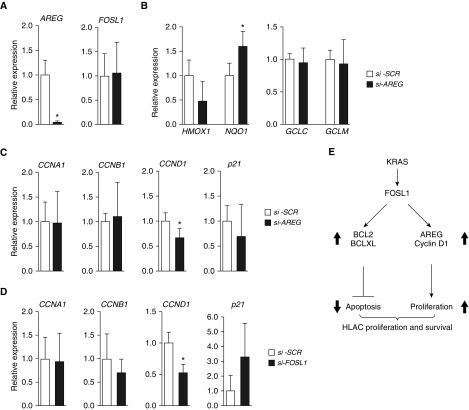
AREG silencing did not alter HMOX1, GCLC, or GCLM expression levels, although NQO1 expression was modestly increased (Figure 6A). In contrast, AREG silencing significantly reduced the expression levels of Cyclin D1, but not Cyclin A1 and Cyclin B1 (Figure 6B). Likewise, FOSL1 silencing significantly reduced the expression levels of Cyclin D1, but not other cyclins (Figure 6C). FOSL1 silencing resulted in a modest increase in p21, cyclin dependent-kinase inhibitor 1 expression. In contrast, AREG depletion had no such effect on p21 expression. Collectively, these results suggest that AREG acts as a downstream effector of FOSL1 and mediates HLAC cell growth by selectively regulating FOSL1-dependent Cyclin D1 expression, but not by modulating antioxidative gene expression.
Figure 6.
Effects of AREG deficiency on FOSL1 and FOSL1-regulated cell-cycle and antioxidative gene expression in HLAC cells. (A) AREG and FOSL1 expression in H2009 cells transfected with SCR siRNA or AREG siRNA (100 nM) for 72 hours. (B) Analysis of antioxidative and (C) cell-cycle gene expression in H2009 cells transfected with si-SCR or si-AREG. The indicated gene expression was quantified by qRT-PCR analysis. (D) Analysis of cell-cycle gene expression in H2009 cells with FOSL1 depletion. Data in A–D are mean ± SD (n =3–4). *P ≤ 0.05 versus si-SCR. (E) KRAS oncogene–induced FOSL1 activation promotes increased expression of AREG, cyclin D1, BCL2, and BCLXL. BCL2 and BCLXL are required for cell survival, whereas AREG maintains cell proliferation by regulating cyclin D1 expression. Decreased levels of AREG expression due to lack of the FOSL1 transcription factor result in impaired HLAC cell growth.
Discussion
High-level FOSL1 expression in HLAC is associated with poor survival of patients with lung cancer and adenocarcinoma. Using a mouse model of lung cancer, we show that Fosl1 deletion results in increased survival of mice with mutant Kras–induced lung tumorigenesis, demonstrating an important role for this transcription factor in promoting mutant KRAS–induced lung cancer in vivo. Furthermore, our data reveal that by upregulating AREG expression and modulating antiapoptotic and antioxidative gene expression, FOSL1 maintains oncogenic KRAS–induced lung cancer cell growth and survival.
The FOSL1/AP-1 transcription factor has been shown to regulate KRAS mutant HLAC cell proliferation and survival (21), and is important for oncogenic KRAS–induced non–lung cancer cell proliferation and progression in vitro (2, 22). Our studies provide a novel insight into the role of FOSL1-mediated mechanisms in regulating lung cancer cell proliferation. Loss-of-function (siRNA-mediated knockdown) and gain-of-function (exogenous supplementation) studies identified AREG as a downstream effector of FOSL1 that is required for promoting KRAS mutant HLAC cell proliferation (Figure 5). Consistent with this result, we observed decreased levels of AREG expression in the lung tumors of Fosl1F/F:KrasG12D mice as compared with their KrasG12D counterparts (Figure 5A), and in oncogenic Kras–activated mouse lung alveolar epithelia with Fosl1 deletion (Figure 3D). These data suggest that by upregulating AREG expression in alveolar epithelia, FOSL1 promotes Kras oncogene–induced lung tumor cell proliferation and growth in vivo. The EGFR ligands EGF, TGF-α, HBEGF, and AREG are differentially expressed during development and inflammatory and wound-healing responses, and are known to elicit distinct cellular responses (23). For example, EGF induces the epithelial–mesenchymal transition, whereas AREG promotes proliferative responses, suggesting that EGFR ligands have different effects on epithelial cell proliferation and differentiation. Several lines of evidence show that EGFR ligands provoke distinct cellular responses by differentially modulating EGFR degradation or recycling. For example, the binding of EGF and TGF-α largely promotes EGFR degradation. In contrast, AREG binding to EGFR facilitates its recycling, but not its degradation, resulting in an increased level of EGFR-activated signaling (reviewed in Ref. 24). Although AREG plays a key role in tissue development and morphogenesis, several studies have shown an important role for AREG in cancer cell survival and progression (20). Recently, it was reported that AREG expression correlates with epithelial cell hyperplasia and the development of cigarette smoke–induced lesions in the human lung (25). AEC2 stem cell renewal during alveolar epithelium repair after lung injury is mediated by EGFR ligand–activated signaling and by Kras-activated signaling during lung cancer development (26). Our results from Fosl1-sufficient and Fosl1-deficient Kras-induced lung tumors and lung alveolar epithelia, and KRAS mutant HLAC cells with FOSL1 depletion reveal that FOSL1 is important for the upregulation of AREG expression during Kras-induced lung tumorigenesis. AREG silencing results in decreased proliferation, and exogenous AREG rescues cell growth defects caused by FOSL1 deficiency, implying that AREG serves as a downstream effector of FOSL1 and mediates HLAC cell proliferation. Note that consistent with our results, Vallejo and colleagues (27) recently reported that Fosl1 regulates mutant Kras–induced lung tumorigenesis through the expression of genes involved in cell-cycle progression (e.g., aurora kinase A). Via a cross-species meta-analysis of gene expression, they also identified AREG, in addition to FOSL1, as a top candidate gene in lung cancer (27). Nonetheless, our studies demonstrate for the first time a causal link between FOSL1-regulated AREG expression and human lung cancer cell survival.
Increased proliferation and resistance to death are the essential hallmarks of the cancer cell (28). Here, we found that FOSL1 silencing attenuated HLAC cell proliferation rates and promoted cell death, but had no effect on nonmalignant human lung epithelia (Figure E5), suggesting that FOSL1 is important for KRAS mutant HLAC cell proliferation and survival. Dysregulation of apoptotic and antiapoptotic gene expression has been implicated in lung cancer cell survival. The findings that blocking of apoptosis reversed the effects of FOSL1 deficiency on HLAC cell proliferation, and that HLAC cells have high levels of FOSL1 expression (transcripts and protein) support our contention that FOSL1 maintains HLAC cell survival by inhibiting apoptosis. We have previously shown that mouse embryonic fibroblasts lacking Fosl1 are resistant to oxidant stress, and this phenotype is associated with increased expression of NFE2L2 (aka Nrf2)-regulated antioxidant genes (HMOX1, NQO1, GCLM, and GCLC) (29). However, our studies with HLAC cells showed that FOSL1 depletion increased expression of HMOX1 and NQO1, but did not alter GCLC or GCLM expression (Figure 4D), suggesting that FOSL1 differentially regulates antioxidant gene expression in HLAC cells as compared with normal cells. Studies with the apoptosis inhibitor ZVAD showed that inhibition of apoptosis prevented the effects of FOSL1 deficiency on HLAC cell survival, suggesting that the FOSL1 transcription factor maintains lung cancer cell survival by regulating apoptosis. The exact mechanisms by which FOSL1 modulates apoptosis in HLAC cells warrant a detailed investigation.
Our studies show that FOSL1 contributes to HLAC cell growth in an oncogenic mutation–dependent manner. Knockdown of FOSL1 expression inhibited the proliferation of KRAS mutant HLAC cells (H2009, H2030, and A549), but did not affect non–KRAS-mutant cells (CL1–5 with a p53 mutation, and H1993 with a p53 mutation and c-MET amplification; unpublished data). These findings suggest that FOSL1 promotes cancer cell proliferation and survival in a contextual manner (i.e., based on genetic and/or epigenetic status). Consistent with this notion, it was reported that the bromodomain and extra-terminal domain (BET) protein BRD4 promotes the proliferation and survival of certain HLAC cells (A549 and H23) by upregulating FOSL1 (21). FOSL1 knockdown phenocopied the inhibitory effect of BET inhibition on the growth of HLAC cells with KRAS mutations. However, depletion of high-level FOSL1 expression in BET inhibition–insensitive EGFR mutant HLAC cells (e.g., H3255 and H1650) had no effect. Likewise, we found that, despite the elevated levels of FOSL1 in H1993 and CL1–5 cells, siRNA-mediated depletion of this transcription factor had no inhibitory effect on their growth and survival (data not shown). Together, these results suggest that HLAC cells with mutant KRAS are largely dependent on FOSL1 for their survival and maintenance. The observation that cells that did not bear KRAS mutations also expressed elevated levels of FOSL1 expression, but its knockdown did not affect their growth and survival, indicates that FOSL1 may not be required to mediate cancer cell growth and survival functions in the setting of non-KRAS mutations. FOSL1 largely dimerizes with JUN, which can form complexes with other FOSL1 counterparts, such as FOS, FOSB, and FOSL2 (FRA-2). Thus, it is possible that other FOS family members may compensate FOSL1-deficiency in regulating non–KRAS-mutant HLAC cell growth. Further studies are needed to determine whether FOSL1 targeting is only applicable to HLAC cells with KRAS mutations, and whether a combined targeting of FOSL1 and its counterparts is required to mitigate lung cancer with KRAS and other genetic mutation(s). It is noteworthy that studies performed with cell cultures often do not mimic in vivo conditions due to the lack of circulating and infiltrated immune and inflammatory mediators, and multiple cell–cell interactions. Thus, the lack of effect of FOSL1 deficiency on non–KRAS-mutant human lung cancer cell growth remains to be verified in vivo using non–Kras-mutant experimental models of lung cancer.
In summary, our studies show the importance of FOSL1 for mediating KRAS-induced lung tumorigenesis in vivo, and suggest that the elevated expression of this transcription factor in patients with lung adenocarcinomas may be a prognostic marker for lung cancer deaths. Mechanistically, our data link AREG expression and regulation of antiapoptotic and antioxidative gene expression with FOSL1-mediated HLAC cell proliferation and survival, largely in the context of oncogenic KRAS mutations.
Footnotes
This work was supported by National Institutes of Health grants ES18998 and ES11863, and the University of Illinois at Chicago Cancer Center Pilot Project.
Author Contributions: Conception and design: I.M.E., M.V., and S.P.R.; analysis and interpretation: I.M.E., M.V., C.R.T., H.R.P., N.M.R., and S.P.R.; drafting the manuscript for important intellectual content: I.M.E., M.V., and S.P.R.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2017-0164OC on November 7, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 2.Tulchinsky E. Fos family members: regulation, structure and role in oncogenic transformation. 2000;15:921–928. doi: 10.14670/HH-15.921. [DOI] [PubMed] [Google Scholar]
- 3.Dhillon AS, Tulchinsky E. FRA-1 as a driver of tumour heterogeneity: a nexus between oncogenes and embryonic signalling pathways in cancer. 2015;34:4421–4428. doi: 10.1038/onc.2014.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Adiseshaiah P, Reddy SP. Matrix metalloproteinase/epidermal growth factor receptor/mitogen-activated protein kinase signaling regulate fra-1 induction by cigarette smoke in lung epithelial cells. 2005;32:72–81. doi: 10.1165/rcmb.2004-0198OC. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. 2002;62:6065–6069. [PubMed] [Google Scholar]
- 6.Adiseshaiah P, Papaiahgari SR, Vuong H, Kalvakolanu DV, Reddy SP. Multiple cis-elements mediate the transcriptional activation of human fra-1 by 12-O-tetradecanoylphorbol-13-acetate in bronchial epithelial cells. 2003;278:47423–47433. doi: 10.1074/jbc.M303505200. [DOI] [PubMed] [Google Scholar]
- 7.Adiseshaiah P, Peddakama S, Zhang Q, Kalvakolanu DV, Reddy SP. Mitogen regulated induction of FRA-1 proto-oncogene is controlled by the transcription factors binding to both serum and TPA response elements. 2005;24:4193–4205. doi: 10.1038/sj.onc.1208583. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Nino ME, Scapoli L, Martinelli M, Land S, Mossman BT. Microarray analysis and RNA silencing link fra-1 to cd44 and c-met expression in mesothelioma. 2003;63:3539–3545. [PubMed] [Google Scholar]
- 9.Risse-Hackl G, Adamkiewicz J, Wimmel A, Schuermann M. Transition from SCLC to NSCLC phenotype is accompanied by an increased TRE-binding activity and recruitment of specific AP-1 proteins. 1998;16:3057–3068. doi: 10.1038/sj.onc.1201845. [DOI] [PubMed] [Google Scholar]
- 10.Gallenne T, Ross KN, Visser NL, Salony, Desmet CJ, Wittner BS, Wessels LFA, Ramaswamy S, Peeper DS. Systematic functional perturbations uncover a prognostic genetic network driving human breast cancer. 2017;8:20572–20587. doi: 10.18632/oncotarget.16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iskit S, Schlicker A, Wessels L, Peeper DS. Fra-1 is a key driver of colon cancer metastasis and a Fra-1 classifier predicts disease-free survival. 2015;6:43146–43161. doi: 10.18632/oncotarget.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao XQ, Ge YS, Shu QH, Ma HX. Expression of Fra-1 in human hepatocellular carcinoma and its prognostic significance. 2017;39:1–8. doi: 10.1177/1010428317709635. [DOI] [PubMed] [Google Scholar]
- 13.Young MR, Colburn NH. Fra-1 a target for cancer prevention or intervention. 2006;379:1–11. doi: 10.1016/j.gene.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Adiseshaiah P, Lindner DJ, Kalvakolanu DV, Reddy SP. FRA-1 proto-oncogene induces lung epithelial cell invasion and anchorage-independent growth in vitro, but is insufficient to promote tumor growth in vivo. 2007;67:6204–6211. doi: 10.1158/0008-5472.CAN-06-4687. [DOI] [PubMed] [Google Scholar]
- 15.Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 16.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 18.Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. 2003;4:67–79. doi: 10.1016/s1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 19.Diesch J, Sanij E, Gilan O, Love C, Tran H, Fleming NI, Ellul J, Amalia M, Haviv I, Pearson RB, et al. Widespread FRA1-dependent control of mesenchymal transdifferentiation programs in colorectal cancer cells. 2014;9:e88950. doi: 10.1371/journal.pone.0088950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A. The multiple roles of amphiregulin in human cancer. 2011;1816:119–131. doi: 10.1016/j.bbcan.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Lockwood WW, Zejnullahu K, Bradner JE, Varmus H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. 2012;109:19408–19413. doi: 10.1073/pnas.1216363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. 2005;41:2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Singh B, Carpenter G, Coffey RJ. EGF receptor ligands: recent advances. 2016;5:pii: F1000. doi: 10.12688/f1000research.9025.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berasain C, Avila MA. Amphiregulin. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Zuo WL, Yang J, Gomi K, Chao I, Crystal RG, Shaykhiev R. EGF-amphiregulin interplay in airway stem/progenitor cells links the pathogenesis of smoking-induced lesions in the human airway epithelium. 2017;35:824–837. doi: 10.1002/stem.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallejo A, Perurena N, Guruceaga E, Mazur PK, Martinez-Canarias S, Zandueta C, Valencia K, Arricibita A, Gwinn D, Sayles LC, et al. An integrative approach unveils FOSL1 as an oncogene vulnerability in KRAS-driven lung and pancreatic cancer. 2017;8:14294. doi: 10.1038/ncomms14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaz M, Machireddy N, Irving A, Potteti HR, Chevalier K, Kalvakolanu D, Reddy SP. Oxidant-induced cell death and Nrf2-dependent antioxidative response are controlled by Fra-1/AP-1. 2012;32:1694–1709. doi: 10.1128/MCB.06390-11. [DOI] [PMC free article] [PubMed] [Google Scholar]