Abstract
Organ fibrosis, including idiopathic pulmonary fibrosis, is associated with significant morbidity and mortality. Because currently available therapies have limited effect, there is a need to better understand the mechanisms by which organ fibrosis occurs. We have recently reported that transforming growth factor (TGF)-β, a key cytokine that promotes fibrogenesis, induces the expression of the enzymes of the de novo serine and glycine synthesis pathway in human lung fibroblasts, and that phosphoglycerate dehydrogenase (PHGDH; the first and rate-limiting enzyme of the pathway) is required to promote collagen protein synthesis downstream of TGF-β. In this study, we investigated whether inhibition of de novo serine and glycine synthesis attenuates lung fibrosis in vivo. We found that TGF-β induces mRNA and protein expression of PHGDH in murine fibroblasts. Similarly, intratracheal administration of bleomycin resulted in increased expression of PHGDH in mouse lungs, localized to fibrotic regions. Using a newly developed small molecule inhibitor of PHGDH (NCT-503), we tested whether pharmacologic inhibition of PHGDH could inhibit fibrogenesis both in vitro and in vivo. Treatment of murine and human lung fibroblasts with NCT-503 decreased TGF-β–induced collagen protein synthesis. Mice treated with the PHGDH inhibitor beginning 7 days after intratracheal instillation of bleomycin had attenuation of lung fibrosis. These results indicate that the de novo serine and glycine synthesis pathway is necessary for TGF-β–induced collagen synthesis and bleomycin-induced pulmonary fibrosis. PHGDH and other enzymes in the de novo serine and glycine synthesis pathway may be a therapeutic target for treatment of fibrotic diseases, including idiopathic pulmonary fibrosis.
Keywords: phosphoglycerate dehydrogenase, fibrosis, metabolism, serine, glycine
Clinical Relevance
Currently available therapies have limited efficacy in idiopathic pulmonary fibrosis, which is associated with significant morbidity and mortality. This study reports that the de novo serine synthesis pathway is necessary for transforming growth factor-β–induced collagen production and bleomycin-induced pulmonary fibrosis. Our findings suggest that phosphoglycerate dehydrogenase and other enzymes in the de novo serine synthesis pathway may be a therapeutic target for treatment of fibrotic diseases, including idiopathic pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal disease, which has a median survival of 3.5 years and affects roughly 89,000 people in the United States (1, 2). A defining feature of IPF is the differentiation of fibroblasts into myofibroblasts (3, 4). Fibroblasts under stimulation by transforming growth factor (TGF)-β, a key cytokine in the pathogenesis of IPF, alter their gene expression profile with de novo expression of cytoskeletal and contractile proteins normally found within smooth muscle cells, and components of the extracellular matrix, including collagen (5, 6).
Collagen, which is produced in excess in patients with IPF and those with other organ fibrosis, has a unique primary structure in which one-third of the amino acids are glycine (7). Glycine is a nonessential amino acid that is synthesized from serine through the action of two serine hydroxymethyltransferases (SHMT1 and SHMT2) (8). Serine can be produced de novo from the glycolytic intermediate, 3-phosphoglycerate, in a three-step process involving the enzymes, phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), and phosphoserine phosphatase (PSPH) (8). We recently demonstrated that treatment of human lung fibroblasts with TGF-β induces the expression of all of the enzymes required for de novo synthesis of glycine from 3-phosphoglycerate. Inhibition of PHGDH expression with siRNA inhibited collagen protein accumulation after TGF-β treatment (9). Whether de novo synthesis of serine and glycine is required for lung fibrosis in vivo has not been determined.
The serine/glycine synthesis pathway is part of a wider network that links glycolysis with one-carbon metabolism and nucleotide synthesis, and has thus been the subject of intense study for cancer therapy (10, 11). Small molecule inhibitors of PHGDH have recently been described, and have been shown to inhibit cancer cell proliferation both in vitro and in vivo (12). Here, we used one of these inhibitors, NCT-503, to determine the requirement of PHGDH for lung fibrosis in vivo. Our results show that, similar to PHGDH knockdown, pharmacologic inhibition of PHGDH inhibits TGF-β–induced collagen protein production in both human and mouse fibroblasts. Using the bleomycin model of lung fibrosis, we found that bleomycin-induced fibrosis leads to increased expression of PHGDH and SHMT2 in mouse lungs. Furthermore, mice treated with PHGDH inhibitors beginning 7 days after intratracheal instillation of bleomycin had attenuation of lung fibrosis. Our results suggest that PHGDH inhibition represents a novel therapeutic option for the treatment of organ fibrosis, such as IPF.
Methods
Fibroblast Culture
Normal human lung fibroblasts (Lonza) and NIH-3T3 fibroblasts (ATCC) were cultured as previously described (9). Cells were serum starved in Dulbecco’s modified Eagle medium containing 0.1% bovine serum albumin and 2 mM glutamine for 24 hours before treatment with TGF-β (1 ng/ml; Millipore-Calbiochem). NCT-503 (National Center for Advancing Translational Sciences) was dissolved in DMSO. siRNAs (250 pmol) were transfected into 1 × 106 cells using the NIH-3T3 program on a Lonza Nucleofector 2b. For a list of siRNAs used, see the data supplement.
Western Blotting
Cells were lysed, and electrophoresis was performed as previously described (9). For whole-lung homogenates, lungs were lysed in urea sample buffer (13). For a list of primary antibodies used, see the data supplement.
Quantitative PCR
RNA as isolated using Direct-zol RNA MiniPrep Plus (Zymo Research) and reverse transcribed using iScript Reverse Transcription Supermix (Bio-Rad). Quantitative mRNA expression was determined by real-time RT-PCR using ITaq Universal SYBR Green Supermix (Bio-Rad). For a list of primers used, see the data supplement.
Bleomycin-induced Pulmonary Fibrosis
The protocol for the use of animals was approved by the Institutional Animal Care and Use Committee at the University of Chicago. Male C57BL/6J mice (8–12 wk old; Jackson Laboratory) were intubated to intratracheally administer bleomycin (1.0 IU/kg; Fresenius Kabi). Mice were killed after 14 or 21 days as indicated, as we have previously reported (14–17). For NCT-503 experiments, mice were treated with NCT-503 (40 mg/kg intraperitoneally, daily) or vehicle (5% ethanol, 35% polyethylene glycol 300 [Sigma], and 60% aqueous 30% solution of hydroxypropyl-β-cyclodextrin [Sigma]), starting on Day 8 after bleomycin instillation. For serine/glycine dietary restriction experiments, mice were switched to control or serine/glycine-free diet (TestDiet 5CC7 or 5W53, respectively; TestDiet) on the day of bleomycin instillation.
For enhanced green fluorescent protein labeling of lung fibroblasts, 3-week-old ROSAmT/mG; Col1a2-CreERT2+/− mice were injected with tamoxifen (1 mg in corn oil) daily for 5 days, as described previously (18). At 8 weeks, mice were treated with bleomycin. For details on cell sorting and imaging, see the data supplement.
Histology
A 20-gauge angiocath was sutured into the trachea, the lungs and heart were removed en bloc, and the lungs inflated to 15 cm H2O with 4% paraformaldehyde. Lungs were fixed in paraffin and 5-μM sections were stained with hematoxylin and eosin and Masson’s trichrome stain. Images of the stained sections were obtained on a CRi Pannoramic whole-slide scanner (Perkin Elmer). For a list of primary antibodies used, see the data supplement.
Measurement of Lung Collagen
Lung collagen was measured using a modification of a previously described method for the precipitation of lung collagen using picrosirius red, as previously described (16, 17). Measurement of hydroxyproline was performed as described previously (19).
Statistical Analysis
Data were analyzed in Prism 7 (GraphPad Software, Inc.). All data are shown as mean (±SEM). Significance was determined by two-tailed Student’s t test (for comparisons between two samples), or by one-way ANOVA using Newman–Keuls correction for multiple comparisons (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
TGF-β Induces the Expression of Enzymes of the De Novo Serine and Glycine Synthesis Pathway in Mouse Fibroblasts
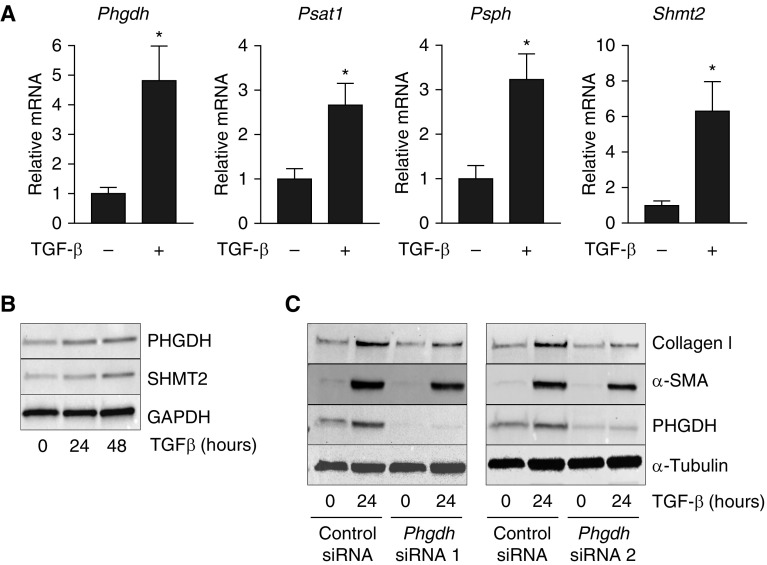
We have recently demonstrated that treatment of normal human lung fibroblasts (NHLFs) with TGF-β induces the expression of enzymes of the de novo serine and glycine synthesis pathway (9). To determine whether TGF-β has a similar effect in murine fibroblasts, we treated NIH-3T3 cells with TGF-β and measured the mRNA expression of the enzymes responsible for the synthesis of glycine from 3-phosphoglycerate (PHGDH, PSAT1, PSPH, and SHMT2) by qRT-PCR. Similar to what we observed in NHLFs (9), treatment of murine fibroblasts with TGF-β induced mRNA expression of all of these enzymes responsible for glycine synthesis (Figure 1A). Furthermore, TGF-β increased the protein levels of PHGDH and SHMT2 in NIH-3T3 cells (Figure 1B). To determine whether murine fibroblasts depend on the de novo serine and glycine synthesis pathway for collagen protein production, we knocked down Phgdh in NIH-3T3 cells using two independent siRNAs and induced myofibroblast differentiation with TGF-β. Knockdown of Phgdh inhibited the TGF-β–induced accumulation of collagen protein (Figure 1C). These results suggest that, similar to NHLFs, murine fibroblasts depend on de novo glycine synthesis to support collagen protein production.
Figure 1.
Phosphoglycerate dehydrogenase (PHGDH) is required for collagen protein production in transforming growth factor (TGF)-β–treated murine fibroblasts. (A) NIH-3T3 cells were treated with TGF-β for 24 hours or left untreated. mRNA expression of Phgdh, phosphoserine aminotransferase 1 (Psat1), phosphoserine phosphatase (Psph), and serine hydroxymethyltransferase 2 (Shmt2) were measured by qRT-PCR (mean ± SEM, n = 3). (B) NIH-3T3 cells were treated with TGF-β for 24 or 48 hours, or left untreated. Expression of PHGDH and SHMT2 proteins were determined by Western blot. (C) Phgdh was knocked down in NIH-3T3 cells using two independent siRNAs. Cells were treated with TGF-β for 24 hours or left untreated. Collagen I, α-smooth muscle actin (α-SMA), and PHGDH protein levels were determined by Western blot. *P < 0.05.
Pharmacologic Inhibition of PHGDH Reduces TGF-β–induced Collagen Protein Accumulation in Human and Mouse Fibroblasts
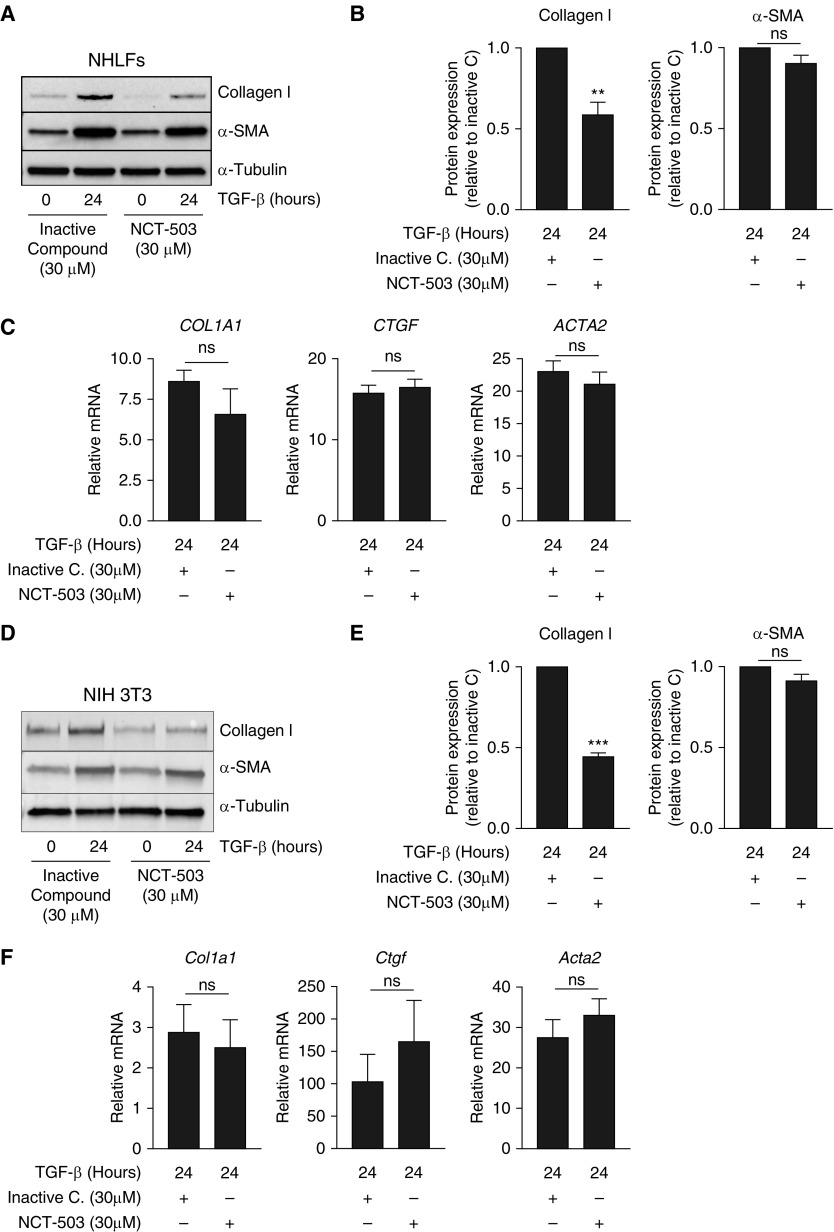
Pacold and colleagues (12) have recently developed pharmacologic inhibitors of PHGDH, which effectively inhibit cancer cell growth both in vitro and in vivo. To determine whether pharmacologic inhibition of PHGDH could abrogate TGF-β–induced collagen protein production, we treated NHLFs with TGF-β in the presence of increasing doses of the PHGDH inhibitor, NCT-503. Treatment with NCT-503 resulted in a dose-dependent decrease in the induction of collagen protein after TGF-β stimulation (see Figure E1A in the data supplement). Treatment of cells with a chemically similar, inactive control compound had no effect on the induction of collagen after TGF-β (Figure E1B). When compared with inactive control compound–treated cells, NHLFs treated with NCT-503 had significantly reduced collagen protein accumulation after TGF-β treatment, whereas induction of α-smooth muscle actin was minimally affected (Figures 2A and 2B). As we have previously shown with the inhibition of glucose metabolism using 2-deoxyglucose (9), pharmacologic inhibition of PHGDH with NCT-503 had no effect on the ability of NHLFs to induce TGF-β–induced target gene transcription, suggesting that the effect of PHGDH inhibition occurs post-transcriptionally (Figure 2C). Similar results were observed in NIH-3T3 cells (Figures 2D–2F).
Figure 2.
Pharmacologic inhibition of PHGDH reduces TGF-β–induced collagen protein expression in fibroblasts. (A) Normal human lung fibroblasts (NHLFs) were treated with TGF-β for 24 hours or left untreated in the presence of either inactive control compound or NCT-503. Collagen I and α-SMA protein induction was measured by Western blot. (B) Quantification of blots in A normalized to TGF-β–induced protein levels in the presence of inactive control compound (mean ± SEM, n = 4). (C) NHLFs were treated with TGF-β for 24 hours or left untreated in the presence of either inactive control compound or NCT-503. mRNA expression of collagen type I α 1 (COL1A1), connective tissue growth factor (CTGF), and α-SMA (ACTA2) was measured by qRT-PCR (mean ± SEM, n = 3). (D) NIH-3T3 cells were treated with TGF-β for 24 hours or left untreated in the presence of either inactive control compound or NCT-503. Collagen I and α-SMA protein induction was measured by Western blot. (E) Quantification of blots in D normalized to TGF-β–induced protein levels in the presence of inactive control compound (mean ± SEM, n = 4). (F) NIH-3T3 cells were treated with TGF-β for 24 hours or left untreated in the presence of either inactive control compound or NCT-503. mRNA expression of Col1a1, Ctgf, and Acta2 was measured by qRT-PCR (mean ± SEM, n = 3). ns = not significant. **P < 0.01, ***P < 0.001.
Enzymes of the de Novo Serine and Glycine Synthesis Pathway Are Upregulated in Mouse Lungs during Bleomycin-induced Fibrosis
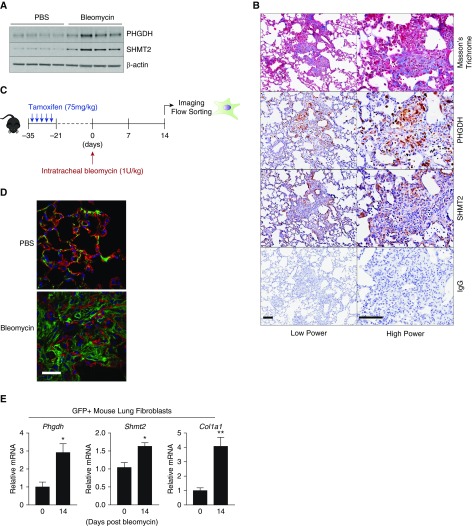
We have previously shown that PHGDH and SHMT2 are highly expressed in the fibrotic foci characteristic of human IPF (9). To determine whether the expression of these enzymes was increased in experimental lung fibrosis in mice, we induced fibrosis in mouse lungs by intratracheal instillation of bleomycin. At 14 days after bleomycin treatment, we found that PHGDH and SHMT2 proteins were significantly upregulated in whole-lung homogenates (Figure 3A). Although bleomycin-induced lung fibrosis does not completely recapitulate the fibrotic foci observed in human IPF, the expression of PHGDH and SHMT2 was localized to regions of fibrosis indicated by staining with Masson’s trichrome (Figure 3B).
Figure 3.
PHGDH and SHMT2 are induced in fibrotic mouse lungs after bleomycin instillation. (A) The protein expression of PHGDH and SHMT2 was determined by Western blot in whole-lung homogenates 14 days after intratracheal instillation of bleomycin or PBS (control). (B) Representative histological assessment of PHGDH and SHMT2 protein expression in the lungs of mice 14 days after intratracheal instillation of bleomycin. Scale bars: 100 μm. Low and high power ×200 and ×400, respectively. (C) Schematic of experimental timeline. ROSAmT/mG; Col1a2-CreERT2+/− were injected with tamoxifen five times before intratracheal instillation of bleomycin. At 14 days after bleomycin, mice were killed and lungs were either imaged or digested to sort enhanced GFP (eGFP)-positive cells. (D) Representative image of tdTomato and eGFP expression in lungs of ROSAmT/mG; Col1a2-CreERT2+/− mice 14 days after instillation of either bleomycin or PBS (control). Scale bar: 25 μm. Magnification ×400. (E) GFP-positive lung cells were collected from ROSAmT/mG; Col1a2-CreERT2+/− mice 14 days after instillation of bleomycin or PBS (control), and the expression of Phgdh, Shmt2, and Col1a1 mRNAs were measured by qRT-PCR (mean ± SEM, n = 4). *P < 0.05, **P < 0.01.
To determine whether bleomycin-induced fibrosis leads to increased expression of the enzymes of the de novo serine/glycine synthesis pathway specifically in fibroblasts, we generated mice that allow us to fluorescently label and sort fibroblasts from the lung. Cells of the ROSAmT/mG mouse expresses tdTomato before Cre-mediated recombination. Activation of Cre recombinase results in the loss of red fluorescence and activation of GFP expression (20). To conditionally activate reporter recombination in fibroblasts, we crossed these mice with mice expressing a tamoxifen-inducible Cre recombinase under the control of fibroblast-specific Col1a2 promoter (21). As shown in Figure 3D, after tamoxifen injection, GFP expression is restricted to lung fibroblasts, whereas epithelial cells retain tdTomato expression.
We isolated GFP-positive cells from the lungs of ROSAmT/mG; Col1a2-CreERT2+/− mice 14 days after instillation of either bleomycin or PBS (control). Concurrent with the induction of myofibroblastic differentiation and collagen mRNA expression, we observed induction of Phgdh and Shmt2 mRNA in GFP-positive cells from fibrotic lungs (Figure 3E). Together, these results reveal that induction of fibrosis in vivo leads to upregulation of the enzymes of the de novo serine and glycine synthesis pathway in lung fibroblasts.
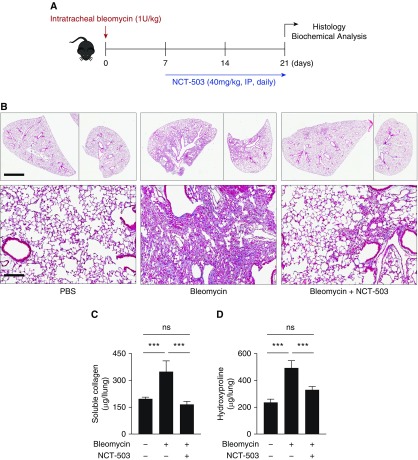
Pharmacologic Inhibition of PHGDH Reduces Bleomycin-induced Lung Fibrosis in Mice
NCT-503 has recently been shown to inhibit the growth of PHGDH-dependent xenografts in mice (12). To determine whether pharmacologic inhibition of PHGDH with NCT-503 would effectively inhibit lung fibrosis in vivo, we used the bleomycin-induced lung fibrosis model. We intratracheally instilled bleomycin into the lungs of mice, and began treatment with NCT-503 or vehicle 8 days later, a time point when the inflammatory response subsides and the fibrotic response is fully active (22) (Figure 4A). At 21 days after bleomycin treatment, mice were killed and lung fibrosis was analyzed by Masson’s trichrome staining. As shown in Figure 4B, mice treated with NCT-503 had greatly reduced lung fibrosis when compared with vehicle-treated mice. The results of our histological analysis were confirmed by the measurement of lung collagen using hydroxyproline and picrosirus red assay on whole-lung homogenates (Figures 4C and 4D). Lungs from mice treated with NCT-503 had much less collagen protein when compared with lungs from vehicle-treated mice.
Figure 4.
Pharmacologic inhibition of PHGDH reduces bleomycin-induced fibrosis in mice. (A) Schematic of experimental timeline. Bleomycin or PBS (control) was intratracheally instilled into the lungs of mice on Day 0; 7 days later, NCT-503 (40 mg/kg) or vehicle was administered daily by intraperitoneal injection. Mice were killed at Day 21. (B) Representative histological representation of mouse lungs at Day 21 after instillation of PBS or bleomycin. Mice were treated starting on Day 7 with either NCT-503 or vehicle. Scale bars: 2.5 mm (low magnification, ×1) and 150 μm (high magnification, ×200). (C and D) Measurement of collagen levels in whole-lung homogenates measured by (C) picrosirius red assay, and (D) hydroxyproline assay (mean ± SEM, n = 6). ***P < 0.001.
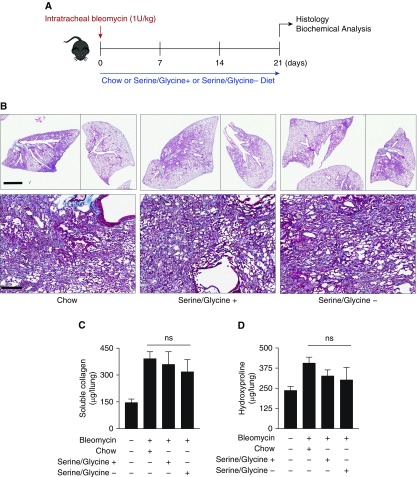
Dietary Serine and Glycine Restriction Does Not Reduce Bleomycin-induced Lung Fibrosis in Mice
Serine and glycine are nonessential amino acids and, as such, dietary restriction of serine and glycine is well tolerated in vivo. Removal of serine and glycine from mouse diet has been demonstrated to reduce circulating levels of these amino acids in serum and reduce tumor growth and T cell expansion in vivo (23–25). Although we have previously shown that NHLFs grown in serine- and glycine-free medium have no defect in induction of collagen protein in response to TGF-β (9), we sought to determine whether dietary restriction of serine and glycine might be effective at reducing fibrosis in vivo. Beginning on the day of bleomycin instillation, mice were either maintained on normal chow diet or switched to a defined diet either containing or lacking serine and glycine (Figure 5A). At 21 days after bleomycin treatment, mice were killed and the severity of lung fibrosis was analyzed by Masson’s trichrome staining. As shown in Figure 5B, serine and glycine restriction did not appear to inhibit bleomycin-induced fibrosis as NCT-503 treatment did. Total lung collagen protein content measured by hydroxyproline or picrosirius red assays was not significantly affected by serine and glycine restriction, suggesting that serine and glycine restriction did not protect mice from bleomycin-induced fibrosis.
Figure 5.
Dietary serine and glycine restriction does not inhibit bleomycin-induced fibrosis in mice. (A) Schematic of experimental timeline. Bleomycin or PBS (control) was intratracheally instilled into the lungs of mice on Day 0. On the day of instillation, mouse food was switched to either chow or defined food, either containing serine and glycine or lacking serine and glycine. Mice were killed at Day 21. (B) Representative histological representation of mouse lungs at Day 21 after instillation of PBS or bleomycin. Scale bars: 2.5 mm (low magnification, ×1) and 150 μm (high magnification, ×200). (C and D) Measurement of collagen levels in whole-lung homogenates measured by (C) picrosirius red assay and (D) hydroxyproline assay (mean ± SEM, n = 4).
Discussion
Although the etiology of IPF is unknown, it is believed to result from an abnormal wound-healing response after injury. The defining feature of IPF is the accumulation of myofibroblasts, the primary effector cell in fibrogenesis, responsible for deposition of extracellular matrix and progressive impairment of gas exchange due to replacement of alveoli with fibrotic tissue (1, 2). The impact of currently approved therapies remains limited, and thus a greater understanding of the cellular requirements for fibrosis is needed. Although most research has focused on specific signaling mediators of the fibrotic response, relatively little is understood about the metabolic requirements of myofibroblasts.
We have previously shown that stimulation of NHLFs with TGF-β induces expression of the enzymes of the de novo serine and glycine synthesis pathway, and that collagen protein production by NHLFs is dependent on the activity of this pathway (9). This is due to the contribution of glycolytic metabolites to the biosynthesis of glycine, an amino acid that constitutes one-third of the primary structure of collagen protein. The de novo serine and glycine synthesis pathway connects glycolysis with one-carbon metabolism and nucleotide synthesis, and has thus been the subject of intense study for cancer therapy (10, 11). Recently, pharmacologic inhibitors of PHGDH, the first enzyme of this pathway, have been developed and demonstrated to inhibit cancer cell growth, both in vitro and in vivo (12). Here, we show that pharmacologic inhibition of PHGDH attenuates collagen protein production in TGF-β–treated fibroblasts in vitro, and inhibits bleomycin-induced collagen production, and consequently lung fibrosis in vivo.
We find that, similar to NHLFs, murine fibroblasts induce expression of PHGDH, PSAT1, PSPH, and SHMT2 after exposure to TGF-β. As in NHLFs, siRNA-mediated inhibition of PHGDH expression resulted in reduced collagen protein accumulation after TGF-β stimulation, suggesting that the requirement of this pathway for fibrogenesis is conserved across species. Pharmacologic inhibition of PHGDH with NCT-503 has a similar effect as PHGDH knockdown on collagen protein production in both human and murine fibroblasts, suggesting that the effects of this drug are specific to its inhibition of PHGDH.
Using the bleomycin-induced model of lung fibrosis, we show that PHGDH and SHMT2 protein expression are induced in fibrotic lungs. Furthermore, using fibroblast-specific labeling, we show that PHGDH and SHMT2 are specifically induced in fibroblasts during the fibrotic response, concomitant with induction of collagen expression. These results bolster our previous findings that PHGDH and SHMT2 are highly expressed in the fibrotic foci of patients with IPF (9), and suggest that upregulation of the de novo serine and glycine synthesis pathway is an essential, conserved component of the fibrotic response.
Collagen is the main structural protein in the extracellular space, and is produced in excess in patients with IPF (26). The altered matrix environment of fibrotic lungs has bioactive and mechanical properties that potentiate profibrotic signaling and promotes further fibroblast activation in a feed-forward cycle (27–29). Indeed, lysyl oxidases, which cross-link collagen molecules and promote their accumulation and deposition, have been targeted in IPF clinical trials (30, 31). We thus hypothesized that inhibition of matrix protein synthesis would significantly inhibit fibrogenesis in vivo. Our results reveal a dramatic effect of PHGDH inhibition on bleomycin-induced fibrosis in vivo. Histological analysis as well as hydroxyproline and picrosirius red assay on whole-lung homogenates demonstrate that the collagen content of the lungs of NCT-503–treated mice is almost reduced to nonfibrotic levels. As our in vitro data demonstrate that NCT-503 does not affect the transcriptional response of fibroblasts to TGF-β, the effect of NCT-503 in vivo is likely due to its effects on matrix protein production and inhibition of the feed-forward cycle involving profibrotic signaling, which leads to fibroblast activation, development of an altered matrix environment, and further fibroblast activation.
Although we cannot rule out the possibility that off-target effects of NCT-503 played a role in the inhibition of the fibrotic response after bleomycin, Pacold and colleagues (12) demonstrated that a similar dosing schedule of NCT-503 led to inhibition of PHGDH-dependent tumor growth in mice over a time course similar to that in our experiments. No effect was seen in tumors that grow independently of PHGDH (12). Thus, we believe that our results suggest that PHGDH is a viable therapeutic target for IPF.
We previously demonstrated that cultured fibroblasts are able to produce collagen protein independent of extracellular serine and glycine, suggesting that myofibroblasts preferentially use glucose-derived glycine for the production of collagen protein (9). Our observation that dietary restriction of serine and glycine did not affect bleomycin-induced fibrosis suggests that myofibroblasts also produce collagen protein, independent of extracellular serine and glycine in vivo. Ma and colleagues (25) have recently demonstrated that dietary restriction of serine and glycine reduces the circulating levels of these amino acids over time courses similar to those in our experiment. Similar reductions in plasma serine and glycine have also been observed after several months of dietary restriction (23, 24), suggesting that the maximal reduction in the plasma levels of these amino acids occurs within the timeframe of our experiment. Although it is impossible to completely eliminate circulating serine and glycine, our results suggest that myofibroblast activation in vivo occurs independent of plasma serine and glycine content, and their de novo synthesis is sufficient to support collagen protein synthesis. Because bleomycin-induced fibrosis resolves after several weeks, we are precluded from analyzing the long-term effects of dietary serine and glycine restriction on progressive fibrosis as would be seen in humans. It remains possible that long-term dietary interventions over several years could slow the progression of IPF.
In summary, our results demonstrate that glucose-derived serine and glycine metabolism plays a key role in the fibrotic response both in vitro and in vivo. Recently, glucose metabolism has been shown to be upregulated in the lungs of patients with IPF; however, the contribution of glycolysis to disease pathogenesis has not been fully elucidated (32, 33). Cancer cells engage in high levels of glycolysis to provide the biosynthetic intermediates required for cellular proliferation (34, 35). This has led to intense interest in targeting glucose metabolism and biosynthetic pathways in an attempt to exploit metabolic “Achilles’ heels” for therapeutic purposes. Our findings suggest that the biosynthetic requirements of myofibroblasts are potential therapeutic targets for IPF and other fibrotic diseases.
Acknowledgments
Acknowledgment
The authors thank the University of Chicago Human Tissue Resource Center for its support.
Footnotes
This work was supported by National Institutes of Health (NIH) grants K01 AR066579 (R.B.H.), R56 HL127395 (N.O.D.), F32 HL134288 (D.W.), T32 HL007605 (P.S.W., L.J.W., and D.W.), and R01 ES015024 and R21 ES025644 (G.M.M.), and American Heart Association grants 15POST25590003 (R.N.) and 14FTF19840029 (R.D.G.).
Author Contributions: Conceptualization—R.B.H. and G.M.M.; methodology—R.B.H., A.Y.M., N.O.D., R.D.G., and G.M.M.; investigation—R.B.H., R.N., A.Y.M., Y.T., L.J.W., E.O’L., K.A.S., P.S.W., D.W., B.A., S.A., and R.D.G.; resources—J.M.R., N.O.D., and R.D.G.; formal analysis—R.B.H., R.N., A.Y.M., D.W., and G.M.M.; supervision—R.B.H. and G.M.M.; writing of original draft: R.B.H. and G.M.M.; review and editing of the writing—R.B.H., R.N., J.M.R., N.O.D., R.D.G., and G.M.M.; funding acquisition—R.B.H., R.N., D.W., N.O.D., R.D.G., and G.M.M.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0186OC on October 11, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 2.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. 2012;21:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheppard D. Transforming growth factor β: a central modulator of pulmonary and airway inflammation and fibrosis. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 7.Shoulders MD, Raines RT. Collagen structure and stability. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigdelioglu R, Hamanaka RB, Meliton AY, O’Leary E, Witt LJ, Cho T, et al. Transforming growth factor (TGF)-β promotes de novo serine synthesis for collagen production. 2016;291:27239–27251. doi: 10.1074/jbc.M116.756247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 12.Pacold ME, Brimacombe KR, Chan SH, Rohde JM, Lewis CA, Swier LJ, et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. 2016;12:452–458. doi: 10.1038/nchembio.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamanaka RB, Mutlu GM. Pfkfb3, a direct target of p63, is required for proliferation and inhibits differentiation in epidermal keratinocytes. 2017;137:1267–1276. doi: 10.1016/j.jid.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. 2007;175:587–594. doi: 10.1164/rccm.200603-312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, et al. Proapoptotic Bid is required for pulmonary fibrosis. 2006;103:4604–4609. doi: 10.1073/pnas.0507604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain M, Budinger GR, Lo A, Urich D, Rivera SE, Ghosh AK, et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. 2011;183:1490–1498. doi: 10.1164/rccm.201009-1409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutlu GM, Budinger GR, Wu M, Lam AP, Zirk A, Rivera S, et al. Proteasomal inhibition after injury prevents fibrosis by modulating TGF-β(1) signalling. 2012;67:139–146. doi: 10.1136/thoraxjnl-2011-200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzy RD, Li L, Smith C, Dorry SJ, Koo HY, Chen L, et al. Pulmonary fibrosis requires cell-autonomous mesenchymal fibroblast growth factor (FGF) signaling. 2017;292:10364–10378. doi: 10.1074/jbc.M117.791764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kach J, Sandbo N, La J, Denner D, Reed EB, Akimova O, et al. Antifibrotic effects of noscapine through activation of prostaglandin E2 receptors and protein kinase A. 2014;289:7505–7513. doi: 10.1074/jbc.M113.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts : a potentially powerful technique for investigating gene function in fibrosis. 2002;160:1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. 2017;544:372–376. doi: 10.1038/nature22056. [DOI] [PubMed] [Google Scholar]
- 25.Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, et al. Serine is an essential metabolite for effector T cell expansion. 2017;25:345–357. doi: 10.1016/j.cmet.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Fulmer JD, Bienkowski RS, Cowan MJ, Breul SD, Bradley KM, Ferrans VJ, et al. Collagen concentration and rates of synthesis in idiopathic pulmonary fibrosis. 1980;122:289–301. doi: 10.1164/arrd.1980.122.2.289. [DOI] [PubMed] [Google Scholar]
- 27.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinković A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. 2013;48:422–430. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. 2014;43:1430–1438. doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]
- 31.Woodcock HV, Maher TM. The treatment of idiopathic pulmonary fibrosis. 2014;6:16. doi: 10.12703/P6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. 2012;186:740–751. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, et al. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. 2015;192:1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 35.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]