Abstract
Transforming growth factor β1 (TGF-β1), a cytokine whose levels are elevated in the airways of patients with asthma, perpetuates airway inflammation and modulates airway structural cell remodeling. However, the role of TGF-β1 in excessive airway narrowing in asthma, or airway hyperresponsiveness (AHR), remains unclear. In this study, we set out to investigate the direct effects of TGF-β1 on human airway smooth muscle (HASM) cell shortening and hyperresponsiveness. The dynamics of AHR and single-cell excitation-contraction coupling were measured in human precision-cut lung slices and in isolated HASM cells using supravital microscopy and magnetic twisting cytometry, respectively. In human precision-cut lung slices, overnight treatment with TGF-β1 significantly augmented basal and carbachol-induced bronchoconstriction. In isolated HASM cells, TGF-β1 increased basal and methacholine-induced cytoskeletal stiffness in a dose- and time-dependent manner. TGF-β1–induced single-cell contraction was corroborated by concomitant increases in myosin light chain and myosin phosphatase target subunit 1 phosphorylation levels, which were attenuated by small interfering RNA–mediated knockdown of Smad3 and pharmacological inhibition of Rho kinase. Strikingly, these physiological effects of TGF-β1 occurred through a RhoA-independent mechanism, with little effect on HASM cell [Ca2+]i levels. Together, our data suggest that TGF-β1 enhances HASM excitation-contraction coupling pathways to induce HASM cell shortening and hyperresponsiveness. These findings reveal a potential link between airway injury–repair responses and bronchial hyperreactivity in asthma, and define TGF-β1 signaling as a potential target to reduce AHR in asthma.
Keywords: asthma, remodeling, bronchoconstriction, contraction, cytokines
Clinical Relevance
This article provides new insights into the mechanism by which airway injury–repair responses promote airway hyperresponsiveness and irreversible airway obstruction, thereby illuminating potential therapeutic targets to abrogate airway hyperresponsiveness in asthma.
Asthma, a chronic obstructive airway disease, affects ∼300 million individuals worldwide (1). Asthma is characterized by airway inflammation and airway hyperresponsiveness (AHR) to endogenous or exogenous stimuli (2). As ∼5–10% of individuals with asthma are refractory to inhaled corticosteroid therapy (3, 4), there is an urgent need to identify new therapeutic targets that modulate AHR to reduce asthma-related morbidity.
Transforming growth factor β1 (TGF-β1), a profibrotic cytokine that is elevated in the airways of patients with asthma, is released after airway injury–repair responses and may contribute to AHR (5, 6). Canonical TGF-β1 signaling begins when activated TGF-β1 binds to the serine-threonine kinase TGF-β type II receptor (TβR-II). After TGF-β1 binding, TβR-II recruits and phosphorylates TβR-I at serine/threonine residues. Activated TβR-I then recruits and phosphorylates Smad2 and Smad3 (Smad2/3), downstream TGF-β1 transcriptional mediators that translocate to the nucleus after association with Smad4 (7, 8). TGF-β1 can also signal through a variety of Smad-independent pathways in various cell types (9).
TGF-β1 enhances α-smooth muscle actin, myosin light-chain kinase (MLCK), and smooth muscle myosin heavy-chain contractile protein expression in human airway smooth muscle (HASM) cells, and promotes the differentiation of airway structural cells to a more contractile phenotype (6, 10, 11). However, the role of TGF-β1 in HASM cell excitation-contraction (E-C) coupling and AHR has yet to be defined.
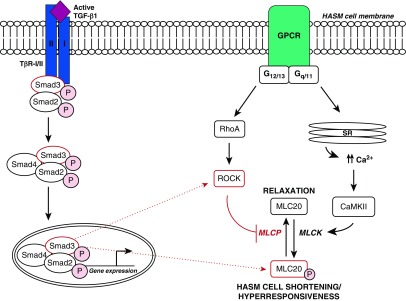
Classic HASM E-C coupling occurs when an agonist binds to a G protein–coupled receptor (GPCR) on the HASM cell membrane (see Figure 7). Agonist binding induces an influx of Ca2+ from the sarcoplasmic reticulum into the intracellular space ([Ca2+]i), activating Ca2+/calmodulin-dependent kinase II (CamKII). Upon phosphorylation by CamKII, MLCK evokes phosphorylation of the 20 kD myosin light chain (MLC20) and subsequent cell shortening (12, 13). HASM E-C coupling is also mediated by the sensitivity of the contractile apparatus to [Ca2+]i. MLC phosphatase (MLCP) regulates the relaxation of HASM through the dephosphorylation of MLC20. In addition, Rho-associated kinase (ROCK) suppresses MLCP activity in a process known as Ca2+ sensitization. Upon agonist binding to HASM cells, the small GTPase RhoA activates ROCK. ROCK suppresses MLCP activity by phosphorylating myosin phosphatase target subunit 1 (MYPT1), the myosin-binding subunit of MLCP (14). As inactive MLCP can no longer dephosphorylate MLC20, a given level of Ca2+ induces higher levels of phosphorylated MLC20 and a subsequent increase in HASM contractility.
Figure 7.
Proposed role of TGF-β1 signaling in HASM cell shortening and hyperresponsiveness. After activated TGF-β1 binding to TβR-I/II, TβR-I kinase activity induces the phosphorylation of Smad2/3. After association with Smad4, the phosphorylated Smad2/3 complex translocates to the nucleus to mediate gene expression. Through a Smad3-dependent pathway, TGF-β1 augments HASM cell MLC phosphorylation and hyperresponsiveness to multiple agonists. Additionally, TGF-β1 exerts these effects through a ROCK-dependent, RhoA-independent pathway. However, further details regarding the mechanism by which Smad3 may induce ROCK, MLC phosphorylation, and hyperresponsiveness in HASM cells remain unclear. The dotted lines represent potential mechanisms regulating TGF-β1–induced HASM cell shortening and hyperresponsiveness. The figure is modified from a previously published image (6). Ca2+ = intracellular calcium; Ca2+/CaMKII = calcium/calmodulin-dependent kinase II; GPCR = G protein–coupled receptor; MLCK = myosin light light-chain kinase; MLCP = myosin light-chain phosphatase; SR = sarcoplasmic reticulum.
TGF-β1 modulates [Ca2+]i levels and ROCK activation in some cell types, suggesting a potential role for TGF-β1 in HASM cell E-C coupling. TGF-β1 augments [Ca2+]i release in human pulmonary fibroblasts and human osteoblasts (15, 16). Furthermore, TGF-β1 activates RhoA/ROCK signaling in human periodontal ligament cells, rodent fibroblasts, and mammary epithelial cells (6, 17, 18). Using primary HASM cells and human precision-cut lung slices (hPCLS), we aimed to elucidate the role of TGF-β1 signaling in HASM cell E-C coupling and AHR.
Methods
HASM Cell Culture
Primary HASM cell lines were derived from the trachea of otherwise healthy, aborted-transplant human lung donors from the National Disease Research Interchange (Philadelphia, PA) and International Institute for the Advancement of Medicine (Edison, NJ). HASM cells were isolated and cultured as previously described (19).
hPCLS Preparation and Assays
hPCLS were prepared from human lung donors from the National Disease Research Interchange and the International Institute for the Advancement of Medicine (20), and bronchoconstriction assays were conducted as previously described (21). The log of the half-maximal (EC50) and 25% maximal (EC25) effective concentrations, as well as the maximal agonist-induced contraction (Emax), was calculated from generated dose-response curves.
Immunoblot Analysis
HASM cells were serum starved for 24 hours before treatment and collected as previously described (22). Immunoblots represent findings from individual experiments and are representative of at least three biological replicates.
Magnetic Twisting Cytometry
Magnetic twisting cytometry (MTC) measures dynamic changes in the cytoskeletal stiffness as a surrogate for agonist-induced force generation at the single-cell level (23). MTC was performed as described in previous studies (24).
Small Interfering RNA Transfection
RNA knockdown was performed using a reverse transfection procedure. HASM cells were trypsinized and resuspended in plain media, and then incubated with Hi-Perfect Transfection Reagent (#301705; Qiagen) and small interfering RNA (siRNA) before plating for a final siRNA concentration of 10 μM.
[Ca2+]i Studies
[Ca2+]i was measured using Fluo-8 dye as described previously (22). After Fluo-8 incubation, images were taken every second for 90 seconds. Carbachol (10 μM) or histamine (1 μM) was manually added to the chamber after 10 frames were recorded. HASM cell fluorescence was normalized to baseline and the area under the curve (AUC) was examined.
TGF-β Ligand and Receptor Transcript Expression
Baseline transcript expression levels were obtained from previously published data (25). Briefly, primary HASM cells were isolated from five white donors who died of fatal asthma or from 10 donors with no history of chronic illness or medication use. Construction, sequencing, and data analysis of the total RNA-sequencing library were conducted as previously described (25). For each sample, kallisto (v0.42.3) was used to quantify hg38 transcripts and Sleuth was used for differential expression analysis (26, 27).
Statistical Analysis
GraphPad Prism software (GraphPad) was used to determine significance evaluated at a P value of <0.05 using a Student’s unpaired t test or multiple t tests with Holm-Sidak correction unless otherwise noted. Data are represented as mean ± SEM, with a minimum of three biological replicates per condition.
Materials
Compounds were purchased from Sigma Aldrich (carbachol and perchloric acid), Enzo Life Sciences (Y-27632), and R&D Systems (TGF-β1 and SB-431542). Antibodies were purchased from Cell Signaling Technologies (pMLC [3674S], pMYPT1-s507 [3040S], GAPDH [2118S], and RhoA [2117S]), Abcam (Smad3 [ab28379], Smad2 [ab71109], and pSmad3 [ab52903]), EMT Millipore (MLC [MABT180]), BD Biosciences (total MYPT1 [612165]), and Santa Cruz Biotechnology (Smad4 [sc-7966]). siRNA was purchased from ThermoFisher Scientific (Smad3 [VHS41114]), Dharmacon (Smad2 [L-003561-00]), and Qiagen (RhoA [S102654211]).
Results
TGF-β1 Augments Basal and Agonist-mediated hPCLS Bronchoconstriction
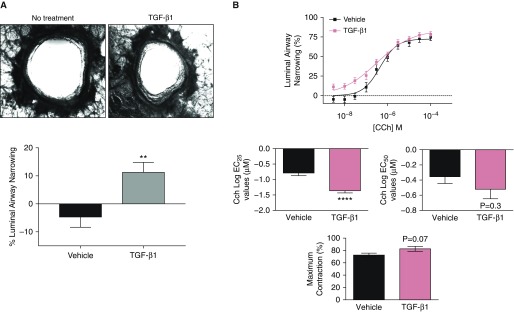
To determine the physiological effects of TGF-β1 on the airway, hPCLS airway lumen area were measured before and after treatment with TGF-β1 as shown in Figure 1. Overnight TGF-β1 treatment significantly decreased basal hPCLS luminal airway area (Figure 1A). To investigate the role of TGF-β1 in mediating AHR, hPCLS were treated with TGF-β1 or vehicle overnight and then stimulated with carbachol in a dose-dependent manner (Figure 1B). Because these slices were derived from donors from a heterogeneous population, as expected, we observed variations in the degree of response to contractile agonists. To determine the effect of TGF-β1 on carbachol-induced bronchoconstriction most accurately, we subjected hPCLS to a carbachol contractile test before conducting contractile studies. After washes and incubation to return the slice to the baseline luminal diameter, only hPCLS that exhibited bronchoconstriction of >40% were used for subsequent assays. As shown in Figure 1B, TGF-β1–pretreated hPCLS exhibited a 25% airway narrowing at a lower concentration of carbachol as compared with control (log EC25 = −0.7867 ± 0.088 versus log EC25 = −1.406 ± 0.085), along with a significant increase in the maximal contraction (Emax).
Figure 1.
Airway narrowing in transforming growth factor β1 (TGF-β1)–treated human precision-cut lung slices (hPCLS). (A) Top: images were taken both before and after 18 hours of TGF-β1 exposure in one lung slice. Bottom: luminal airway narrowing in TGF-β1 (100 ng/mL, 18 h) and vehicle-treated hPCLS (n = 9 donors). (B) Carbachol (Cch)-induced luminal airway narrowing in TGF-β1 (100 ng/mL, 18 h) and vehicle-treated hPCLS (n = 9 donors). **P ≤ 0.01; ****P ≤ 0.0001. EC25 = 25% maximal effective concentration; EC50 = half-maximal effective concentration.
TGF-β1 Augments Basal and Agonist-induced Cell Stiffening and [Ca2+]i in HASM cells
The direct effect of TGF-β1 on isolated HASM cell shortening was determined using MTC. TGF-β1 significantly increased basal HASM cell stiffness in a dose- and time-dependent manner, showing significantly increased contractile responses as early as 1 hour after exposure (Figure 2A). To further characterize the effects of TGF-β1 on the HASM cell contractile apparatus, methacholine- and histamine-induced cell stiffness was measured in HASM cells pretreated with TGF-β1 overnight (Figure 2C). TGF-β1 significantly enhanced HASM cell stiffness in response to both agonists. Furthermore, inhibition of TβR-I kinase activity with SB-431542, a TβR-I antagonist, significantly decreased the enhancement of basal and agonist-mediated HASM cell stiffness by TGF-β1 (Figures 2B and 2C).
Figure 2.
Agonist-induced human airway smooth muscle (HASM) cell [Ca2+]i and contractile responses of isolated HASM cells. (A) TGF-β1 (10–100 ng/mL) treatment increases the basal stiffness of isolated HASM cells (n = 221–477 individual cells). (B) SB-431542 (10 μM, 1 h pretreatment) decreases TGF-β1 (10 ng/mL, 18 h)-induced augmentation of basal HASM cell stiffness (n = 187–292). (C) SB-431542 (10 μM, 1 h pretreatment) decreases TGF-β1 (10 ng/mL, 18 h)-induced augmentation of Cch (n = 552–818) and histamine (Hist; n = 205–292 individual cells)–induced HASM cell stiffness. All magnetic twisting cytometry (MTC) data are represented as geometric mean (95% confidence interval). MTC statistical analysis was performed using one-way ANOVA with Tukey’s post hoc test. (D) Overnight TGF-β1 (10 ng/mL, 18 h) pretreatment augments [Ca2+]i in Cch-stimulated (10 μM) HASM cells. Three donors, two replicates per condition. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. AUC = area under the curve; [Ca2+]i = intracellular calcium; ns = not significant; Pa = pascal.
To determine the role of TGF-β1 in mediating HASM cell [Ca2+]i, Fluo-8 was used to measure [Ca2+]i after treatment of HASM cells with TGF-β1 (Figure 2D). Acute stimulation with TGF-β1 alone showed little augmentation of [Ca2+]i transients over that of vehicle control (Figure E1 in the data supplement). TGF-β1–pretreated HASM cells, however, showed a significantly increased AUC after carbachol stimulation compared with cells treated with carbachol alone (Figure 2D).
TGF-β1 Augments Basal and Agonist-induced HASM MLC20 Phosphorylation
We next treated HASM cells with TGF-β1 in the presence or absence of contractile agonists and investigated the phosphorylation of MLC20, a necessary signaling event in agonist-induced HASM contraction (Figure 3). TGF-β1 increased MLC20 phosphorylation in a time-dependent manner, significantly sustaining MLC20 phosphorylation for up to 72 hours after exposure (Figure 3A). Additionally, TGF-β1 increased basal MLC20 phosphorylation and significantly augmented MLC20 phosphorylation in response to both carbachol and histamine (Figure 3B). Inhibition of TβR-I kinase activity significantly reduced TGF-β1–induced MLC phosphorylation and HASM hyperresponsiveness to both agonists (Figure 3C).
Figure 3.
MLC20 phosphorylation in TGF-β1–treated HASM cells. (A) TGF-β1 (10 ng/mL) induces time-dependent MLC20 phosphorylation (pMLC) in HASM cells (n = 3 ± SEM). (B) TGF-β1 (10 ng/mL) pretreatment for 18 hours augments Cch (10 μM, 10 min) and Hist–induced (1 μM, 10 min) MLC20 phosphorylation (n = 5; *P ≤ 0.05, ****P ≤ 0.0001). (C) Inhibition of TGF-β receptor I (TβR-I) signaling decreases TGF-β1–induced pMLC. For these studies, HASM cells were stimulated with TGF-β1 for a total of 18 hours. Prior to collection, HASM cells were treated with the ALK5 inhibitor SB-431542 (5 μM) for 1 hour, stimulated acutely with vehicle, Cch (10 μM), or Hist (1 μM), then lysed and subjected to immunoblot (n = 5 ± SEM). *P ≤ 0.05; **P < 0.01; ***P < 0.001; ****P ≤ 0.0001. MLC20 = 20 kD myosin light chain 20.
Rho-associated Kinase Inhibition Prevents MLC20 Phosphorylation by TGF-β1
In addition to increases in [Ca2+]i, HASM E-C coupling is also mediated by the sensitivity of the contractile apparatus to Ca2+. To determine the role of ROCK activation in TGF-β1–mediated HASM cell shortening, we investigated the phosphorylation of MYPT1 as a surrogate for ROCK activation in TGF-β1–treated HASM cells (Figure 4). TGF-β1 significantly enhanced basal and carbachol-induced MYPT1 phosphorylation in treated HASM cells (Figure 4A).
Figure 4.
Effect of Rho kinase (ROCK) inhibition on TGF-β1–induced MLC20 phosphorylation and hyperresponsiveness in HASM cells. (A) HASM cells were treated with TGF-β1 (10 ng/mL) for 18 hours and then stimulated with Cch (10 μM) or Hist (1 μM) for 10 minutes (n = 5 ± SEM). (B) HASM cells were pretreated with the ROCK inhibitor Y-27632 for 15 minutes. HASM cells were then treated with TGF-β1 (10 ng/mL) for 18 hours and stimulated with Cch or Hist (n = 8 ± SEM, n = 4 ± SEM). pMYPT1/MYPT1 vehicle versus Y-27632 immunoblots show two different experiments representative of multiple donor observations. pMLC/MLC vehicle versus Y-27632 immunoblots show one representative immunoblot from multiple donor observations. (C) HASM cells were transfected with siRNA targeted against RhoA or a nontargeting (NT) siRNA pool, and then treated with TGF-β1 (10 ng/mL, 18 h) and stimulated with Cch (20 μM, 10 min; n = 3 ± SEM). *P < 0.05; **P < 0.01; ***P < 0.001. MYPT1 = myosin phosphatase target subunit 1; pMYTP1 = phosphorylated myosin phosphatase target subunit 1.
To determine the necessity of ROCK for TGF-β1–induced MLC20 phosphorylation, we investigated TGF-β1–induced MLC20 phosphorylation in HASM cells pretreated with the ROCK1/2 inhibitor Y-27632 (Figure 4B). ROCK1/2 inhibition significantly decreased basal and agonist-induced MLC20 phosphorylation by TGF-β1 as compared with vehicle control. Furthermore, pretreatment with Y-27632 decreased augmentation of basal and agonist-induced MYPT1 phosphorylation by TGF-β1.
RhoA activation acts upstream from ROCK activation in the HASM cell Ca2+-sensitization pathway (28, 29). To determine the contribution of RhoA to TGF-β1–induced MLC phosphorylation, HASM cells were transfected with siRNA targeting the small GTPase RhoA and treated with TGF-β1 overnight in the presence or absence of carbachol (Figure 4C). RhoA knockdown had little effect on the augmentation of basal and agonist-induced MLC20 phosphorylation by TGF-β1 in HASM cells.
Smad3 Knockdown Decreases TGF-β1–induced MLC20 Phosphorylation and ROCK Activation
Smad2/3 activation and phosphorylation play a major role in the canonical TGF-β1 signaling pathway. To determine the role of canonical TGF-β1 signaling in TGF-β1–induced HASM cell shortening, we transfected HASM cells with siRNA against Smad3 or Smad2 before TGF-β1 treatment and agonist stimulation (Figure 5). Smad3 knockdown significantly decreased the augmentation of basal and agonist-induced MLC20 and MYPT1 phosphorylation by TGF-β1, as well as carbachol-induced MLC phosphorylation, in HASM cells (Figure 5A). Conversely, Smad2 knockdown had little effect on TGF-β1–induced MLC20 or MYPT1 phosphorylation (Figure 5B). Although Smad4 knockdown also tended to decrease TGF-β1–induced MLC phosphorylation, the results were not significant relative to the diluent control (Figure E2).
Figure 5.
Effect of Smad knockdown on TGF-β1–induced MLC20 phosphorylation and hyperresponsiveness in HASM cells. HASM cells were transfected with siRNA against (A) Smad3 (n = 4–6 ± SEM) or (B) Smad2 (n = 3 ± SEM), pretreated with TGF-β1 (10 ng/mL) or vehicle control for 18 hours, and then stimulated with Cch (20 μM, 10 min). After treatment, HASM cells were lysed and subjected to immunoblot. **P < 0.01; ***P < 0.001.
No Significant Differences in TGF-β Family Ligand and Receptor Gene Expression between HASM Cells Derived from Donors with Fatal Asthma or No Asthma
Other investigators and we have demonstrated that HASM cells derived from donors with fatal asthma display distinct phenotypic differences compared with those derived from donors with no asthma (30–32). To determine whether the observed differences could be due to differential expression of TGF-β signaling pathways, we used RNA sequencing to measure TGF-β family ligand and receptor transcript expression in HASM cells derived from donors with fatal asthma or no asthma (Figure 6; Table E1). We observed no statistically significant differences between the two groups in terms of TGF-β1, TGF-β2, or TGF-β3 ligand or receptor transcript expression.
Figure 6.
TGF-β ligand and receptor basal gene expression in HASM cells derived from donors with no asthma (NA) or fatal asthma (FA). The mRNA expression of TGF-β (A) family ligands and (B) receptors was assessed by RNA sequencing in NA (n = 10) and FA (n = 5) donor–derived HASM cells. Shown are the transcripts with the highest expression levels, expressed as transcripts per million, for each gene.
Discussion
Severe and persistent asthma is a disease characterized by a repetitive cycle of airway injury and repair. Studies suggest that repeated airway-injury repairs lead to increased airway resistance and AHR (6, 33). However, the mechanisms that mediate AHR are not fully understood. Our findings suggest a unique role for TGF-β1 in mediating airway narrowing and augmenting agonist-induced shortening. To our knowledge, this is the first report to demonstrate that TGF-β1 directly modulates HASM cell shortening and hyperresponsiveness in asthma.
Several cytokines have been previously established as mediators of AHR in asthma (34). The role for TGF-β1 in AHR, however, has yet to be defined. In this study, we demonstrate that TGF-β1 augments basal and agonist-induced hPCLS bronchoconstriction, HASM cell stiffness, and MLC20 phosphorylation, suggesting a novel role for TGF-β1 in asthmatic airway obstruction through the mediation of HASM contraction (Figures 1, 2A–2C, and 3B). Additionally, TGF-β1–induced HASM cell contractility was both time and dose dependent (Figures 2A and 3A), peaking after overnight treatment for up to 72 hours after exposure (Figure 3A). Together, these results suggest a role for TGF-β1 in mediating HASM contraction and AHR in asthma.
HASM contraction is mediated by E-C coupling, a process in which downstream signaling mediates [Ca2+]i levels and ROCK activation. Interestingly, we found that TGF-β1 augmented HASM cell shortening induced by two agonists that signal through different GPCRs (Figures 2C and 3B). Thus, we hypothesized that TGF-β1’s effects on HASM shortening and hyperresponsiveness occur downstream of the receptor level through the modulation of [Ca2+]i and/or ROCK activation.
As a contractile agonist, TGF-β1 showed little augmentation of HASM cell [Ca2+]i transients over that of vehicle control (Figure E1). Overnight TGF-β1 treatment, however, enhanced carbachol-induced [Ca2+]i levels (Figure 2D). Thus, our data suggest that TGF-β1–induced [Ca2+]i augmentation may play a modest role in mediating TGF-β1–induced HASM cell shortening and hyperresponsiveness.
In addition to the elevation of HASM cell [Ca2+]i, HASM E-C coupling is mediated by the activation of RhoA/ROCK signaling. Through the phosphorylation of MYPT1, we demonstrate that overnight TGF-β1 treatment augments basal ROCK activation in HASM cells (Figure 4A). Additionally, we demonstrate for the first time that TGF-β1 augments agonist-induced HASM cell MLC phosphorylation through ROCK activation (Figure 4B), suggesting the necessity of ROCK for TGF-β1–induced HASM cell hyperresponsiveness. ROCK is a known downstream effector of RhoA, and our lab has previously established a role for RhoA in the agonist-induced Ca2+-sensitization pathway (29, 35). Surprisingly, RhoA knockdown had little effect on TGF-β1–induced MYPT1 and MLC20 phosphorylation (Figure 4C). Although the pathways by which TGF-β1 bypasses RhoA to activate ROCK in HASM cells remain unclear (Figure 7), it is plausible that TGF-β1 augments HASM cell contraction by enhancing other Rho-family small GTPases that modulate ROCK activation and MLC20 phosphorylation upstream. Rac1 and Cdc42 are two such small GTPases that have been shown to have a role in mediating both HASM cell actin polymerization and the contraction of airway smooth muscle in various species (36, 37). TGF-β1 may also enhance the expression of CPI-17, ROCK, or other downstream proteins in the HASM E-C coupling pathway, serving to prime the cell for augmented contractile responses to agonists (38).
Activation of Smad2/3 is the canonical TGF-β1 signaling pathway in multiple cell types. Although Smad2 and Smad3 are similar in structure and function, literature suggests that the two proteins serve different functions in the cell (39, 40). In our study, Smad3 knockdown significantly reduced TGF-β1–induced HASM cell shortening and hyperresponsiveness (Figure 5A). Interestingly, Smad3 knockdown also significantly decreased carbachol-induced HASM cell shortening. As the cooperation between TGF-β1 and muscarinic pathways has been previously described, it is plausible that Smad3 inhibition may negatively regulate muscarinic responses (41). Notably, Smad2 knockdown alone exhibited little effect on TGF-β1 or carbachol-induced HASM cell shortening (Figure 5B). Additionally, knockdown of Smad4—an important component in Smad2/3 nuclear translocation—showed little reduction in TGF-β1–induced HASM cell shortening (Figure E2). These data are in accordance with previous findings in fibroblasts demonstrating that Smad3 is a pivotal player in TGF-β1–induced contraction (42, 43). However, our study is the first to suggest the necessity of Smad3 for TGF-β1–induced contraction and hyperresponsiveness in HASM cells (Figure 5A). Furthermore, as TGF-β1’s effects occur through a pathway that is ROCK dependent but RhoA independent, the precise mechanisms linking TGF-β1 and Smad3 to ROCK activation remain an enigma (Figure 7).
In this study, we did not examine the effects of TGF-β1 on the expression of α-smooth muscle actin, smooth muscle myosin heavy chain, MLCK, or other contractile proteins. It is plausible that enhanced expression of these proteins may modulate TGF-β1’s effects on HASM cell hyperresponsiveness. However, given the significant enhancement of MLC phosphorylation and HASM cell stiffness at time points as short as 1 hour (Figures 2A and 3A), we posit that changes in contractile protein expression cannot fully explain the effects of TGF-β1 and Smad3 on basal and agonist-induced HASM cell shortening. Taken together, these data suggest a novel role for TGF-β1 and Smad3 in HASM cell E-C coupling and hyperresponsiveness.
Infiltrating eosinophils are a major source of TGF-β1 in the asthmatic airway (44, 45). Additionally, most airway structural cells produce some levels of TGF-β1 (46, 47). TGF-β1 protein levels are elevated in the bronchial lavage and epithelial lining fluid of patients with asthma, and TGF-β1 gene expression is increased in bronchial biopsies isolated from subjects with severe and moderate asthma as compared with biopsies from subjects with no asthma (44, 47–49). In HASM cells, we observed little difference in TGF-β ligand and receptor gene expression levels between donors with fatal asthma and those with no asthma (Figure 6). However, it is plausible that HASM cell TGF-β1 signaling in asthma increases after increased production of TGF-β1 from alternative airway cell types, modulation of HASM cell TGF-β1 protein production or degradation, increased HASM cell TβR-I/II activation, or increased activation of TGF-β1 on the HASM cell surface. Airway injury, increased reactive oxygen species generation, and HASM contraction alone have been demonstrated to induce TGF-β1 activation, further reinforcing the notion that TGF-β1 plays an important role in modulating airway function (6, 50). Thus, AHR may be closely linked to the airway injury–repair response that follows repeated asthma exacerbations. TGF-β1’s prolonged effects on HASM shortening also indicate that elevation of TGF-β1 after airway injury may increase a patient’s susceptibility to sustained airway obstruction. Therefore, our study establishes TGF-β1 as a potential link between airway injury and hyperresponsiveness in asthma. Furthermore, our data suggest that TGF-β1 or its signaling pathways may serve as an important therapeutic target for reducing AHR in asthma patients who are not sensitive to current therapies.
Footnotes
This work was supported by National Institutes of Health grants 3P01 HL114471-04S1 and R01 HL107361, and training grant T32GM008076.
Author Contributions: C.A.O., G.C., W.Z., E.J.Y., B.E.H., S.S.A., and R.A.P. contributed to the experimental conception and design. C.A.O., G.C., and W.Z. performed the experiments. C.A.O., G.C., W.Z., E.J.Y., M.S., B.E.H., S.S.A., and R.A.P. contributed to the analysis and interpretation of the data. C.A.O. wrote the manuscript. C.A.O., W.Z., E.J.Y., M.S., B.E.H., S.S.A., and R.A.P. edited and reviewed the manuscript for important intellectual content.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0247OC on October 6, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McDonald VM, Maltby S, Reddel HK, King GG, Wark PAB, Smith L, et al. Severe asthma: current management, targeted therapies and future directions—a roundtable report. 2017;22:53–60. doi: 10.1111/resp.12957. [DOI] [PubMed] [Google Scholar]
- 2.Carr TF, Bleecker E. Asthma heterogeneity and severity. 2016;9:41. doi: 10.1186/s40413-016-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hekking P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. 2015;135:896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Sheehan WJ, Phipatanakul W. Difficult-to-control asthma: epidemiology and its link with environmental factors. 2015;15:397–401. doi: 10.1097/ACI.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Zhang N, Lan F, Van Crombruggen K, Fang L, Hu G, et al. Transforming growth factor-β1 pathways in inflammatory airway diseases. 2014;69:699–707. doi: 10.1111/all.12403. [DOI] [PubMed] [Google Scholar]
- 6.Ojiaku CA, Yoo EJ, Panettieri RA., Jr Transforming growth factor β1 function in airway remodeling and hyperresponsiveness. The missing link? 2017;56:432–442. doi: 10.1165/rcmb.2016-0307TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata A, Chen Y-G. TGF-β signaling from receptors to Smads. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budi EH, Duan D, Derynck R. Transforming growth factor-β receptors and Smads: regulatory complexity and functional versatility. 2017;27:658–672. doi: 10.1016/j.tcb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Yeganeh B, Mukherjee S, Moir LM, Kumawat K, Kashani HH, Bagchi RA, et al. Novel non-canonical TGF-β signaling networks: emerging roles in airway smooth muscle phenotype and function. 2013;26:50–63. doi: 10.1016/j.pupt.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima T, Yamasaki A, Harada T, Chikumi H, Watanabe M, Okazaki R, et al. γ-Tocotrienol inhibits TGF-β1-induced contractile phenotype expression of human airway smooth muscle cells. 2017;60:16–23. [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Dong Y, Duan Y, Jiang X, Chen C, Deng L. Substrate stiffness influences TGF-β1-induced differentiation of bronchial fibroblasts into myofibroblasts in airway remodeling. 2013;7:419–424. doi: 10.3892/mmr.2012.1213. [DOI] [PubMed] [Google Scholar]
- 12.Nazinigouba O, Etienne R. Physiology of airway smooth muscle contraction: an overview. 2014;4:221. [Google Scholar]
- 13.Dixon RE, Santana LFA. A Ca2+- and PKC-driven regulatory network in airway smooth muscle. 2013;141:161–164. doi: 10.1085/jgp.201210953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koopmans T, Anaparti V, Castro-Piedras I, Yarova P, Irechukwu N, Nelson C, et al. Ca2+ handling and sensitivity in airway smooth muscle: emerging concepts for mechanistic understanding and therapeutic targeting. 2014;29:108–120. doi: 10.1016/j.pupt.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Nesti LJ, Caterson EJ, Li W-JJ, Chang R, McCann TD, Hoek JB, et al. TGF-β1 calcium signaling in osteoblasts. 2007;101:348–359. doi: 10.1002/jcb.21180. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S, Kolb MR, Duan F, Janssen LJ. Transforming growth factor-β evokes Ca2+ waves and enhances gene expression in human pulmonary fibroblasts. 2012;46:757–764. doi: 10.1165/rcmb.2011-0223OC. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wang T, Song M, Pan J. Rho plays a key role in TGF-β1-induced proliferation and cytoskeleton rearrangement of human periodontal ligament cells. 2014;59:149–157. doi: 10.1016/j.archoralbio.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Manickam N, Patel M, Griendling KK, Gorin Y, Barnes JL. RhoA/Rho kinase mediates TGF-β1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. 2014;307:F159–F171. doi: 10.1152/ajprenal.00546.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panettieri RA., Jr Isolation and culture of human airway smooth muscle cells. 2001;56:155–160. doi: 10.1385/1-59259-151-5:155. [DOI] [PubMed] [Google Scholar]
- 20.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced β(2)-adrenergic receptor tolerance in human small airways. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, et al. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. 2009;297:L530–L537. doi: 10.1152/ajplung.00133.2009. [DOI] [PubMed] [Google Scholar]
- 22.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon A-R, Stasinopoulos I, Kim JH, Yong HM, Kilic O, Wirtz D, et al. COX-2 dependent regulation of mechanotransduction in human breast cancer cells. 2015;16:430–437. doi: 10.1080/15384047.2014.1003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Himes BE, Koziol-White C, Johnson M, Nikolos C, Jester W, Klanderman B, et al. Modulates expression of the airway smooth muscle transcriptome in fatal asthma. 2015;10:e0134057. doi: 10.1371/journal.pone.0134057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pimentel H, Bray NL, Puente S, Melsted P, Pachter L. Differential analysis of RNA-seq incorporating quantification uncertainty. 2017;14:687–690. doi: 10.1038/nmeth.4324. [DOI] [PubMed] [Google Scholar]
- 27.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 28.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, et al. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 29.Chiba Y, Matsusue K, Misawa M. RhoA, a possible target for treatment of airway hyperresponsiveness in bronchial asthma. 2010;114:239–247. doi: 10.1254/jphs.10r03cr. [DOI] [PubMed] [Google Scholar]
- 30.Koziol-White CJ, Yoo EJ, Cao G, Zhang J, Papanikolaou E, Pushkarsky I, et al. Inhibition of PI3K promotes dilation of human small airways in a rho kinase-dependent manner. 2016;173:2726–2738. doi: 10.1111/bph.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trian T, Burgess JK, Niimi K, Moir LM, Ge Q, Berger P, et al. β2-Agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. 2011;6:e20000. doi: 10.1371/journal.pone.0020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berair R, Hollins F, Brightling C. Airway smooth muscle hypercontractility in asthma. 2013;2013:185971. doi: 10.1155/2013/185971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? 2013;305:L912–L933. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. 2002;29:859–866. doi: 10.1046/j.1440-1681.2002.03756.x. [DOI] [PubMed] [Google Scholar]
- 35.Jude J, Koziol-White C, Scala J, Yoo E, Jester W, Maute C, et al. Formaldehyde induces Rho-associated kinase activity to evoke airway hyperresponsiveness. 2016;55:542–553. doi: 10.1165/rcmb.2015-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang DD. Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. 2015;16:134. doi: 10.1186/s12931-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata K, Sakai H, Huang Q, Kamata H, Chiba Y, Misawa M, et al. Rac1 regulates myosin II phosphorylation through regulation of myosin light chain phosphatase. 2015;230:1352–1364. doi: 10.1002/jcp.24878. [DOI] [PubMed] [Google Scholar]
- 38.Sakai H, Suto W, Kai Y, Chiba Y. Mechanisms underlying the pathogenesis of hyper-contractility of bronchial smooth muscle in allergic asthma. 2017;53:37–47. doi: 10.1540/jsmr.53.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L, Liu X, Ren X, Tian Y, Chen Z, Xu X, et al. Smad2 and Smad3 have differential sensitivity in relaying TGFβ signaling and inversely regulate early lineage specification. 2016;6:21602. doi: 10.1038/srep21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown KA, Pietenpol JA, Moses HL. A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-β signaling. 2007;101:9–33. doi: 10.1002/jcb.21255. [DOI] [PubMed] [Google Scholar]
- 41.Oenema TA, Smit M, Smedinga L, Racké K, Halayko AJ, Meurs H, et al. Muscarinic receptor stimulation augments TGF-β1-induced contractile protein expression by airway smooth muscle cells. 2012;303:L589–L597. doi: 10.1152/ajplung.00400.2011. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi T, Liu X, Wen F-QQ, Kohyama T, Shen L, Wang XQ, et al. Smad3 mediates TGF-β1-induced collagen gel contraction by human lung fibroblasts. 2006;339:290–295. doi: 10.1016/j.bbrc.2005.10.209. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Wen FQ, Kobayashi T, Abe S, Fang Q, Piek E, et al. Smad3 mediates the TGF-β-induced contraction of type I collagen gels by mouse embryo fibroblasts. 2003;54:248–253. doi: 10.1002/cm.10098. [DOI] [PubMed] [Google Scholar]
- 44.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, et al. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 45.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, et al. Transforming growth factor β1 (TGFβ1) gene expression by eosinophils in asthmatic airway inflammation. 1996;15:404–409. doi: 10.1165/ajrcmb.15.3.8810646. [DOI] [PubMed] [Google Scholar]
- 46.Aubert JD, Dalal BI, Bai TR, Roberts CR, Hayashi S, Hogg JC. Transforming growth factor β1 gene expression in human airways. 1994;49:225–232. doi: 10.1136/thx.49.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redington AE, Roche WR, Holgate ST, Howarth PH. Co-localization of immunoreactive transforming growth factor-β1 and decorin in bronchial biopsies from asthmatic and normal subjects. 1998;186:410–415. doi: 10.1002/(SICI)1096-9896(199812)186:4<410::AID-PATH198>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, et al. Transforming growth factor-β1 in asthma. Measurement in bronchoalveolar lavage fluid. 1997;156:642–647. doi: 10.1164/ajrccm.156.2.9605065. [DOI] [PubMed] [Google Scholar]
- 49.Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LAS, Fitzpatrick AM. Airway TGF-β1 and oxidant stress in children with severe asthma: association with airflow limitation. 2012;129:388–396, 396.e1–396.e8. doi: 10.1016/j.jaci.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oenema TA, Maarsingh H, Smit M, Groothuis GMM, Meurs H, Gosens R. Bronchoconstriction induces TGF-β release and airway remodelling in guinea pig lung slices. 2013;8:e65580. doi: 10.1371/journal.pone.0065580. [DOI] [PMC free article] [PubMed] [Google Scholar]