Abstract
Defining the mechanisms of cellular pathogenesis in rare lung diseases such as Hermansky-Pudlak syndrome (HPS) is often complicated by loss of the differentiated phenotype of cultured primary alveolar type 2 (AT2) cells, as well as by a lack of durable cell lines that are faithful to both AT2-cell and rare disease phenotypes. We used CRISPR/Cas9 gene editing to generate a series of HPS-specific mutations in the MLE-15 cell line. The resulting MLE-15/HPS cell lines exhibit preservation of AT2 cellular functions, including formation of lamellar body–like organelles, complete processing of surfactant protein B, and known features of HPS specific to each trafficking complex, including loss of protein targeting to lamellar bodies. MLE-15/HPS1 and MLE-15/HPS2 (with a mutation in Ap3β1) express increased macrophage chemotactic protein-1, a well-described mediator of alveolitis in patients with HPS and in mouse models. We show that MLE-15/HPS9 and pallid AT2 cells (with a mutation in Bloc1s6) also express increased macrophage chemotactic protein-1, suggesting that mice and humans with BLOC-1 mutations may also be susceptible to alveolitis. In addition to providing a flexible platform to examine the role of HPS-specific mutations in trafficking AT2 cells, MLE-15/HPS cell lines provide a durable resource for high-throughput screening and studies of cellular pathophysiology that are likely to accelerate progress toward developing novel therapies for this rare lung disease.
Keywords: CRISPR/Cas9, gene editing, MLE-15 cell line, Hermansky-Pudlak syndrome
The Hermansky-Pudlak syndromes (HPSs) constitute a family of genetic diseases in which the affected genes encode subunits of multimeric complexes that are used in protein and membrane targeting to lysosome-related organelles, specifically the biogenesis of lysosome-related organelles complex-1, -2, and -3 (BLOC-1–BLOC-3, respectively) and the adaptor protein 3 complex (AP-3) (1). Whereas albinism and bleeding diathesis are common to all patients with HPS, significant morbidity and mortality result from pulmonary fibrosis in selected genotypes, specifically those affecting BLOC-3 and AP-3. Advances in the mechanistic understanding of HPS lung disease have been possible with the use of mouse models (2), and researchers have demonstrated the central role of the alveolar type 2 (AT2) cell in the pathogenesis of fibrotic lung disease in BLOC-3 and AP-3 subtypes (3). However, progress in identifying how the underlying cell biology of HPS causes AT2 cell injury has been limited by the lack of robust cellular models that can easily be manipulated in culture.
CRISPR/Cas9 gene editing has been used to generate small mutations in a site-directed manner for permanent gene inactivation in a variety of cell types through a process of nonhomologous end joining that creates microinsertions or microdeletions (indels) of the targeted sequence (4). We have developed MLE-15 cell lines in which a subunit of each of the four HPS-related trafficking complexes has been inactivated using CRISPR/Cas9 gene editing. The MLE-15 cell line was chosen for its close similarity to primary AT2 cells. The MLE-15 cell line was established from tumors in transgenic mice expressing the simian virus 40 early region, including the large T antigen from a well-characterized 3.7 kD region of the human SFTPC promoter (5, 6). The initial characterization of MLE-15 cells demonstrated expression of RNA for most surfactant proteins, expression of surfactant protein B proprotein (SFTPB), and multivesicular bodies and lamellar body–like structures (6). Further characterization by others has also demonstrated expression of surfactant protein A and surfactant protein C proproteins, processing of SFTPB proprotein to its mature 8 kD form (SP-B), and secretion of SP-B into culture media (6, 7).
We targeted mutations in MLE-15 cells that would inactivate representative HPS genes associated with fibrotic lung disease in humans (Hps1 of the BLOC-3 complex associated with HPS type 1 [8–10] and Ap3β1 of the AP-3 complex associated with HPS type 2 [11]), a subtype of HPS not associated with fibrotic lung disease (Hps3 of the BLOC-2 complex associated with HPS type 3 [12, 13]), and one of the very rare BLOC-1 mutations (Bloc1s6 also known as pallidin) (14). We demonstrate that gene editing disrupted the targeted genes, with the expected additional effect of reduced expression of nontargeted subunits of the respective BLOC or AP-3 complexes (13, 15–17). Gene editing resulted in misrouting of known cargo proteins and increased macrophage chemotactic protein-1 (MCP-1) expression, as expected from prior work (2, 3, 18–20). Together, these experiments demonstrate that the MLE-15/HPS cell lines faithfully replicate several known characteristics of primary AT2 cells from HPS mouse models, providing a robust platform from which to elucidate cellular mechanisms of AT2 cell injury in this family of rare lung diseases.
Methods
Additional methodologic details available in the data supplement.
Plasmid Construction for Generating Mutations in Cell Lines
Table E1 in the data supplement lists the single-guidance RNA sequences used to generate plasmids used for developing each MLE-15/HPS cell line as well as the flanking genomic PCR primers that facilitated screening and sequencing of genomic PCR and RT-PCR products.
MLE-15 Cell Culture
MLE-15 cells were originally obtained from Dr. Jeffrey Whitsett (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) and cultured as previously described (6), as detailed in the data supplement.
Transfection and Puromycin Selection
MLE-15 cells were transfected by performing nucleofection using the Amaxa Cell Line Nucleofector Kit T (VCA-1002) and the Nucleofector II device with the X005 cycle (all from Lonza). Media supplemented with 5 μg/ml puromycin were added 24 hours after transfection to select for positive clones, as detailed in the data supplement.
Animals
Wild-type (WT) C57BL/6J, Hps1ep/Hps1ep (pale ear), Ap3β1pe/Ap3β1pe (pearl), Hps3coa/Hps3coa (cocoa), and Bloc1s6pa/Bloc1s6pa (pallid) mice were bred and maintained at the Laboratory Animal Facility at Vanderbilt University Medical Center as previously described (18). All animal protocols were reviewed and approved by the institutional animal care and use committee of Vanderbilt University Medical Center and adhered to the principles of the NIH Guide for the Care and Use of Laboratory Animals. Both male and female mice were used between 8 and 10 weeks of age. Lung homogenates for Western immunoblotting were generated from flash-frozen lung tissue after lavage as previously described (21).
AT2 cell isolation
Preparation of AT2 cells was performed as previously described (21), with the following modification. Epithelial cells were separated from macrophages and other blood cells by negative selection on antibody-coated plates (42 μg of CD45 and 16 μg of CD32 antibodies; BD Pharmingen) before plating.
Quantitative Real-Time PCR Analysis
Total RNA was isolated and reverse transcribed by using standard methods with the RNeasy kit (Qiagen) and the SuperScript VILO cDNA Synthesis Kit (Life Technologies), with additional details provided in the data supplement.
Immunoblotting
Immunoblotting was performed as previously described (18) using 80–100 μg of proteins in each lane of precast 4–12% NuPAGE gels (Thermo Fisher Scientific). Blots were incubated with the primary antibodies as described previously (18). Antibodies used and procedural details are listed in the data supplement.
Immunocytochemistry and Fluorescence Microscopy
Primary AT2 and MLE-15 cells were transfected by nucleofection as described above using plasmids expressing ATP-binding cassette transporter A3/enhanced GFP (ABCA3-EGFP) or ABCA3-mCherry and EGFP-RAB38, and cells were plated onto coverslips for immunostaining and fluorescence microscopy as described in the data supplement.
Measurement of MCP-1
MCP-1 concentrations in cell culture media were measured by ELISA according to the manufacturer’s recommendations (MJE00; R&D Systems). RNA was prepared from the cells for quantitative PCR (qPCR) analysis of Ccl2 RNA expression as described above.
Statistical Methods
Differences in amplification efficiencies between the sample groups in qPCR experiments were assessed using one-way ANOVA with post hoc testing using the Kruskal-Wallis test for differences in normalized expression between groups. Comparison of MCP-1 concentrations between two groups was conducted using the Mann-Whitney U test. Prism software (version 6.0c; GraphPad Software) was used for all statistical analyses, and values of P < 0.05 were considered significant.
Results
Generation of MLE-15/HPS Clones and Validation of Gene Expression
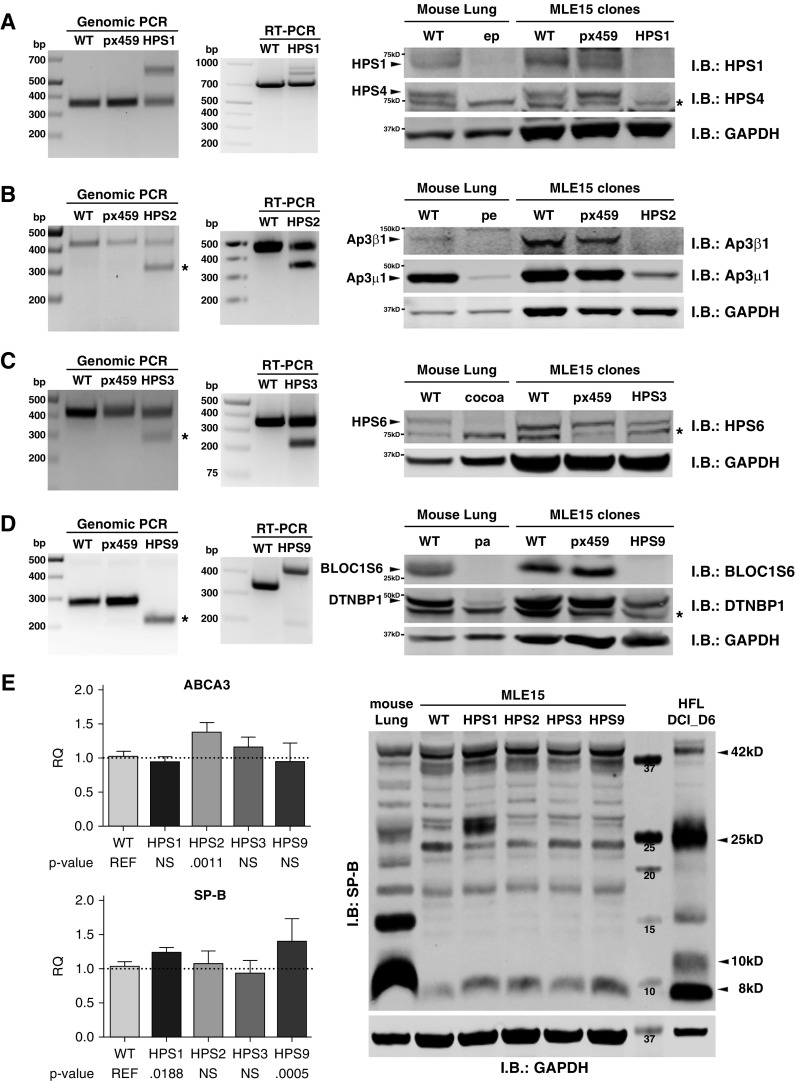
Using two guidance RNAs, we targeted larger genomic segments for gene editing such that two Cas9-mediated double-stranded DNA breaks would result in a deletion easily detected by gel electrophoresis of genomic PCR products. This strategy is best illustrated in Figure 1, in which a single PCR product resulted from a large deletion on both alleles of the MLE-15/HPS9 clone shown in Figure 1D. In practice, we found that most clones demonstrated the expected large genomic deletion only on one allele, accompanied by a small indel on the other allele. This is best illustrated in Figure 1B for the MLE-15/HPS2 clone and in Figure 1C for the MLE-15/HPS3 clone. The MLE-15/HPS1 clone shown in Figure 1A demonstrated a small indel on one allele (lower band) and a large insertion of nonspecific sequence on the other allele (upper band). Sequencing of genomic PCR products (Table 1) enabled us to distinguish small indel products from WT sequence, eliminating heterozygous clones (0.1% of clones screened). In parallel experiments, we transfected MLE-15 cells with an empty pX459 vector (no single-guidance RNA) to control for any potential effects of puromycin selection. These parent cell lines (labeled pX459 in Figure 1) behaved similarly to WT MLE-15 cells.
Figure 1.
Confirmation of Hermansky-Pudlak syndrome (HPS) gene editing in MLE-15 cells. (A–D) Genomic PCR (bp = base pairs), RNA RT-PCR (bp), and composite immunoblotting (I.B.) are combined using lysates from wild-type MLE-15 cells (WT), a representative parent cell line created with an empty vector (pX459), a representative gene-edited clone, and lung tissue from WT and HPS mice for (A) MLE-15/HPS1 cells targeting Hps1, (B) MLE-15/HPS2 cells targeting Ap3β1, (C) MLE-15/HPS3 cells targeting Hps3, and (D) MLE-15/HPS9 cells targeting Bloc1s6. In the panels representing genomic PCR, an asterisk identifies the band resulting from DNA cleavage using both single-guidance RNAs. Genomic and RT-PCR sequencing results are provided in Table 1. Lung homogenates were prepared for immunoblotting from adult lung tissue of C57BL6/J (WT) mice and the HPS strains pale ear (ep)/HPS1, pearl (pe)/HPS2, cocoa/HPS3, and pallid (pa)/HPS9. Each lane contains 80 μg of total protein, and GAPDH immunoblotting is used as a loading control. Nonspecific bands are designated with an asterisk to the right of the immunoblot; bands representing the detected protein are denoted with an arrowhead; and size markers appear to the left of the blot. (E) Validation of alveolar type 2 (AT2) cell characteristics after MLE-15/HPS clone selection. Quantitative PCR for Abca3 (ABCA3) and Sftpb (SP-B) from triplicate samples of MLE-15 cells and MLE-15/HPS clones (n = 4–5 experiments; normalized to Rn18s and Gapdh RNA and reported as relative quantity (RQ); mean ± SD with P values listed below; NS = not significant), together with immunoblotting of WT mouse lung homogenate, WT MLE-15 cell lysate, and MLE-15/HPS cell lysate, using 100 μg of protein per lane, in addition to 25 μg of lysate from cultured human fetal lung explants (HFL DCI D6) as described previously (38). Immunoblotting is shown for surfactant protein B proprotein (SFTPB) with GAPDH as a loading control. Arrowheads to the right of the image denote the positions of the SFTPB proprotein at 42 kD, the major 25 kD intermediate, a 10 kD intermediate common to human AT2 cells, and the mature 8 kD SP-B. ABCA3 = ATP-binding cassette transporter A3.
Table 1.
Genomic and RT-PCR Sequencing Results from MLE-15/Hermansky-Pudlak Syndrome Clones
| Gene Target | Allele | Genomic Sequence Variant* | Protein Mutation† |
|---|---|---|---|
| Hps1 | Allele 1 (upper band) | C.103_104ins229 (net gain: 230) | —‡ |
| Allele 2 (lower band) | C.14_96delins76 (net loss: 7) | p.Leu5Trpfs*51 | |
| Ap3b1 | Allele 1 (upper band) | g.825_830del (net loss: 6) | p.Met1Ser |
| Allele 2 (lower band) | g.825_951delinsA (net loss: 126) | p.Met1Leu | |
| Hps3 | Allele 1 (upper band) | g.182_192del (net loss: 11) | p.Val16fs*20 |
| Allele 2 (lower band) | g.189_316delinsTC (net loss: 127) | p.Glu19Serfs*15 | |
| Bloc1s6 | Allele 1 | g.40_111del (net loss: 72) | p.Pro8Leufs*11 |
Genomic sequence variant: deletion mutation and insertion mutation by Human Genome Variation Society (HGVS) nomenclature (http://varnomen.hgvs.org/).
Protein mutation: translational consequence of deletion by HGVS nomenclature.
Large genomic insertion resulted in a faint band containing a nonspecific sequence.
Validation studies were performed using clones grown for fewer than 10 passages after confirmation by genomic PCR sequencing. Validation of a representative MLE-15/HPS1 clone appears in Figure 1A. Sequencing of RT-PCR products from MLE-15/HPS1 RNA demonstrated the small deletion found by genomic PCR sequencing (Table 1); the faint bands of larger size did not yield an interpretable sequence. BLOC-3 is a heterodimer of the HPS1 and HPS4 proteins (9, 10). The pale ear mouse contains a 7-bp duplication flanking a large insertion within exon 19 of Hps1, resulting in a premature stop codon within the inserted element (22). Immunoblotting for HPS1 and HPS4 confirmed both proteins are expressed in lung homogenates from WT mice, as well as in WT and empty vector (px459) MLE-15 cells, but not in lung homogenates from pale ear mice or the MLE-15/HPS1 gene-edited cells.
Validation of the MLE-15/HPS2 clone with a mutation in Ap3β1 appears in Figure 1B. Sequencing of RT-PCR products from MLE-15/HPS2 RNA demonstrated the same small deletions (larger product) and large deletions (smaller product) predicted from genomic PCR sequencing. AP-3 is a heterotetrameric complex consisting of two large subunits (δ- and β-subunits) and two smaller subunits (μ- and σ-subunits) (23). The pearl mouse has a mutation of the Ap3β1 gene involving a 793-bp tandem duplication that results in a reading frame shift and premature stop codon, truncating the protein 130 amino acids from the amino-terminus (11). Immunoblotting showed the β1-subunit of AP-3 in lung homogenates from WT mice, as well as in WT and empty vector MLE-15 cell lysates, but not in pearl mouse lung homogenates or MLE-15/HPS2 cell lysates. In addition, immunoblotting for the μ1-subunit of AP-3 was significantly reduced in both lung homogenates from pearl mice and MLE-15/HPS2 cells, reflecting a prior observation that loss of one AP-3 subunit results in degradation of other AP-3 subunits (24).
The MLE-15/HPS3 clone (Figure 1C) presented a technical challenge because of a paucity of suitable antibody reagents to confirm loss of the murine HPS3 protein. Sequencing of RT-PCR products from the MLE-15/HPS3 clone confirmed the deletions found in genomic PCR sequencing, similarly predicting a frameshift mutation and a shortened HPS3 protein. BLOC-2 is a heterotrimeric complex of HPS3, HPS5, and HPS6 proteins (13). The cocoa mouse carries a splice site mutation resulting in a frameshift and loss of expression of the Hps3 mRNA (25). We performed immunoblotting for HPS6 because deletion of one subunit of BLOC-2 has been shown to promote degradation of the other subunits (13). Lung homogenate from cocoa mice and from MLE-15/HPS3 cells indeed demonstrated reduced HPS6 protein compared with WT lung homogenate and lysates from WT and empty vector MLE-15 cells.
The largest HPS-related complex is BLOC-1, consisting of eight different protein subunits (26). A substitution at nucleotide 787 of Bloc1s6 (also known as pallidin or HPS9) leads to a premature stop codon and no protein expression in pallid mice (27). RT-PCR using the MLE-15/HPS9 clone demonstrated a single product larger than the WT product owing to elimination of an mRNA splice site as predicted from genomic PCR screening (Figure 1D). We found no evidence of BLOC1s6 protein expression in lung homogenate from pallid mice or in MLE-15/HPS9 cells when compared with WT lung homogenate or with lysates from empty vector cells (pX459) and WT MLE-15 cells. Moreover, DTNBP1 (also known as dysbindin or dystrobrevin-binding protein-1), another BLOC-1 subunit, was significantly reduced in both pallid lung homogenate and MLE-15/HPS9 cells compared with WT lung homogenate or MLE-15 cells.
We next assessed for preservation of the AT2 cell phenotype, specifically related to the lamellar body–like organelles of MLE-15 cells, in the gene-edited MLE-15/HPS cells (Figure 1E). Prior studies have shown that BLOC-3 and AP-3 disruption does not impair trafficking of ABCA3 to lamellar bodies, nor does it impair processing of SFTPB to mature 8 kD SP-B or trafficking of SP-B to lamellar bodies (18, 21, 28). Given the presence of small lamellar body–like organelles in MLE-15 cells and the role of ABCA3 in phospholipid transfer into lamellar bodies (29–31), we first assessed the expression of Abca3 in WT MLE-15 cells and MLE-15/HPS clones. Compared with the original MLE-15 cells, qPCR demonstrated a modest increase in Abca3 RNA only in the MLE-15/HPS2 clone, but qPCR cycle thresholds that ranged from 25.6 to 26.3 suggested low Abca3 RNA expression. We were unable to detect any endogenous ABCA3 protein by immunoblotting or by immunostaining using a reliable monoclonal antibody (3C9; ab24751; Abcam) (data not shown), likely owing to low ABCA3 abundance. By comparison, Sftpb RNA expression was robust in WT MLE-15 and MLE-15/HPS cells, with qPCR cycle thresholds ranging from 17.7 to 18.2, and MLE-15/HPS1 and /HPS9 clones specifically showed a significant but modest increase in Sftpb RNA. Immunoblotting demonstrated SFTPB proprotein and the major 25 kD intermediate, as well as fully processed 8 kD SP-B (32), in all MLE-15/HPS clones, although overall expression in MLE-15 cells was much lower than in WT mouse lung lysate.
Immunostaining for SFTPB in WT MLE-15 cells has been shown previously to localize to small organelles of approximately 500 nm (7). Because we were unable to immunostain successfully for endogenous ABCA3, we transfected MLE-15 cells to express human ABCA3-EGFP. In WT MLE-15 cells and MLE-15/HPS clones (Figure E1), ABCA3-EGFP surrounded individual SP-B-positive puncta in the perinuclear region. These data demonstrate that gene editing preserves important aspects of the AT2 cell phenotype in MLE-15/HPS cells.
MLE-15/HPS2 and MLE-15/HPS1 Cell Lines Exhibit Disrupted Trafficking of Known Lamellar Body Cargo Proteins
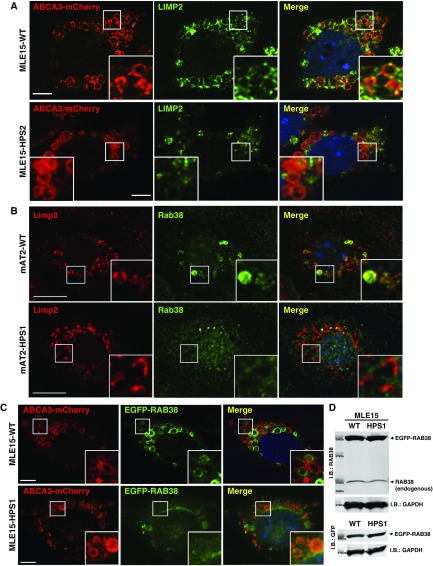
AP-3 and BLOC complexes facilitate the targeting of proteins relevant to lysosome-related organelles. Lamellar bodies are lysosome-related organelles containing many transmembrane proteins within their limiting membrane (33, 34), but lamellar body trafficking has been incompletely characterized. To date, there are no BLOC-1– or BLOC-2–dependent cargo proteins that could be used to test disruption of lamellar body targeting in gene-edited MLE-15/HPS3 or MLE-15/HPS9 cells. We recently showed that AP-3 was necessary for lamellar body targeting of lysosome integral membrane protein-2 (LIMP2) in primary AT2 cells (18). In keeping with our prior report, MLE-15/HPS2 cells demonstrated disruption of LIMP2 targeting to ABCA3-mCherry–positive organelles (Figure 2A).
Figure 2.
Disruption of protein trafficking to lamellar bodies in MLE-15/HPS cells. (A) Representative images of MLE-15/WT and MLE-15/HPS2 cells transfected with ABCA3-mCherry and subsequently immunostained for the presence of endogenous lysosome membrane protein-2 (LIMP2); nuclei are identified by DAPI staining. Scale bars: 10 μm. (B) Representative images of primary AT2 cells from WT and pale ear mice immunostained for endogenous LIMP2 and RAB38. Scale bars: 10 μm; nuclei are identified by DAPI staining. (C) Representative images of MLE-15/WT and MLE-15/HPS1 cells 24 hours after transfection with ABCA3-mCherry and enhanced GFP (EGFP)-RAB38 plasmids. Scale bars: 10 μm; nuclei are identified by DAPI staining. (D) I.B. showing cell lysates from MLE-15/WT and MLE-15/HPS1 cells (80 μg of total protein each; molecular mass markers denoted with size in Da = kilodaltons) using either RAB38 or GFP antisera to detect RAB38-GFP, with GAPDH as a loading control.
RAB38 decorates lamellar bodies of AT2 cells in a GTP-dependent manner (19). Using LIMP2 as an endogenous marker of lamellar bodies, endogenous RAB38 was localized to the lamellar body, limiting membranes of WT mouse AT2 cells but not in pale ear mouse AT2 cells that are defective in BLOC-3 (Figure 2B). We transfected MLE-15 and MLE-15/HPS1 cells to express human RAB38-EGFP and ABCA3-mCherry and similarly found a loss of RAB38 targeting to ABCA3-mCherry–positive organelles in the MLE-15/HPS1 cells (Figure 2C). Disruption of BLOC-3 function was not associated with loss of endogenous RAB38, nor did it alter the effectiveness of RAB38-EGFP expression (Figure 2D). Because BLOC-3 has been validated as a guanine nucleotide exchange factor (GEF) for RAB32 and RAB38 (35), these experiments indicate that BLOC-3 provides the GEF function that is necessary for targeting RAB38-EGFP specifically to lamellar bodies in AT2 cells.
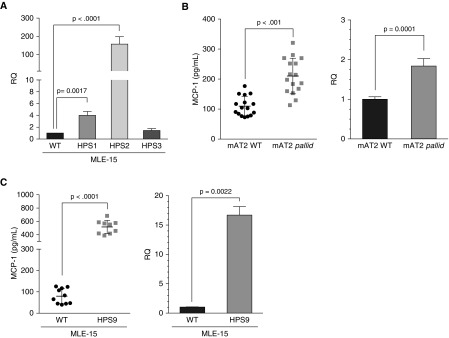
MLE-15/HPS1, MLE-15/HPS2, and MLE-15/HPS9 Cells Secrete Increased MCP-1 into Culture Media
Recent studies have provided strong evidence that injury to primary AT2 cells is central to the development of pulmonary fibrosis in HPS (2, 20, 21). MCP-1, also known as CCL2, is an important driver of macrophage alveolitis in patients with HPS1 (36) and in mice (2). By qPCR, WT MLE-15 and MLE-15/HPS3 cells exhibited low amounts of Ccl2 RNA, whereas Ccl2 RNA was significantly elevated in MLE-15/HPS1 and MLE-15/HPS2 cells (Figure 3A), consistent with prior reports of primary AT2 cells from cocoa, pale ear, and pearl mice (2, 20). Pallid mice carrying a mutation in Bloc1s6 have not been examined previously for MCP-1 production. Primary AT2 cells from pallid mice (Figure 3B) and MLE-15/HPS9 cells (Figure 3C) both exhibited increased MCP-1 in culture media, as well as increased Ccl2 RNA by qPCR, when compared with WT primary AT2 and MLE-15 cells.
Figure 3.
Macrophage chemotactic protein-1 (MCP-1)/Ccl2 expression by MLE-15/HPS cell lines. (A) Ccl2 qRT-PCR using RNA from WT MLE-15 cells and MLE-15/HPS1, MLE-15/HPS2, and MLE-15/HPS3 cells cultured for 24 hours (n = 7 experiments/triplicate samples; normalized to Rn18s and Gapdh and reported as relative quantity compared to WT/RQ; mean ± SD). (B and C) MCP-1 expression in cell culture media evaluated by ELISA and Ccl2 qPCR using (B) primary mouse AT2 cells from WT/C57BL/6 (mAT2 WT) and pallid (mAT2 pallid) mice (n = 2 experiments/triplicate samples; qPCR normalized to Rn18s and Gapdh and reported as RQ; mean ± SD) and (C) WT MLE-15 and MLE-15/HPS9 cells. (n = 3 experiments/triplicate samples; qPCR normalized to Rn18s and Gapdh and reported as RQ; mean ± SD).
Discussion
The efficient gene editing of MLE-15 cells afforded by CRISPR/Cas9 provides a valuable tool for cell modeling of rare genetic lung diseases. Our long-term goal is to examine BLOC and AP-3 functions in lamellar body trafficking and to understand the role of disrupted trafficking on AT2 cell injury, so it was critical to choose a cell line demonstrating a stable AT2 cell phenotype for gene editing. Expression of fully processed SP-B protein and presence of lamellar organelles that undergo regulated secretion of surfactant proteins in MLE-15 cells (7) imply sufficient proteolytic machinery, intact trafficking pathways, and functional exocytosis that would contribute to robust MLE-15 CRISPR cell models of HPS. Although WT MLE-15 cells have low endogenous Abca3 expression, we found that ABCA3-EGFP trafficked appropriately to small organelles containing SP-B and that SFTPB proprotein was appropriately processed to the mature 8 kD product, suggesting that MLE-15 cells exhibit essential pathways for lamellar body homeostasis. Importantly, the trafficking of exogenous ABCA3 and endogenous SFTPB was not disrupted in MLE-15/HPS clones, in keeping with prior reports based on mouse models of HPS and patients with HPS (18, 21, 28).
HPSs comprise an ideal family of rare lung diseases to demonstrate the usefulness of gene editing of cell lines, because comparisons of MLE-15/HPS cells could be made to lung tissue from HPS mice. As predicted (13, 15–17), targeting a single subunit (Bloc1s6, Hps3, Hps1, and, Ap3β1) from each trafficking complex (BLOC-1, -2, and -3 and AP-3, respectively) for gene disruption also resulted in loss of other subunits of the complex in each MLE-15/HPS clone. The definitive test of successfully gene-edited MLE-15/HPS clones would be disruption of protein trafficking, but there is a general lack of understanding of suitable AT 2 cell cargo proteins using BLOC and AP-3 trafficking. We were limited to demonstrating disrupted AP-3 trafficking in MLE-15/HPS2 cells and BLOC-3 disruption in MLE-15/HPS1 cells. LIMP2 is the only known lamellar body protein that has been shown to be trafficked by an HPS-related complex, specifically AP-3 (18). Loss of AP-3 in the MLE-15/HPS2 clone disrupted LIMP2 trafficking to lamellar body–like organelles, consistent with our prior studies using primary AT2 cells (18). The MLE-15/HPS1 cell line has enabled us to advance the understanding of the role of BLOC-3 in lamellar body homeostasis by demonstrating that BLOC-3 provides the GEF function that enables RAB38 targeting to lamellar bodies.
MLE-15/HPS cells would be valuable reagents in the investigation of mechanisms of HPS lung disease. MCP-1 production and a macrophage-predominant alveolitis are well-known features of patients with HPS1 (36). Primary AT2 cells from pale ear and pearl mice, but not cocoa mice, adapt to HPS subunit loss and trafficking complex dysfunction, in part by increasing production of MCP-1 (20, 21), and the MLE-15/HPS1, MLE-15/HPS2, and MLE-15/HPS3 clones behaved predictably in this respect. We extended these observations to the rare BLOC-1 mutations, showing increased MCP-1 production from primary AT2 cells from both pallid mice and MLE-15/HPS9 cells. These data predict that patients with HPS9 and other BLOC-1 mutations are likely to exhibit increased alveolar MCP-1 and may be at risk for pulmonary fibrosis similarly to patients with HPS1. In summary, we have demonstrated the strength and potential value of gene editing of MLE-15/HPS cell lines by validating some of the known effects of HPS mutations on AT2 cell physiology through comparisons between primary AT2 cells from HPS mouse models and MLE-15/HPS cells. MLE-15/HPS clones provide a robust alternative to primary AT2 cell culture as we begin to examine the impact of HPS mutations on AT2 cell physiology.
The limitations of the MLE-15/HPS gene-edited cells as a model also deserve comment. Some mutations of HPS genes in humans, including HPS1 and AP3B1 (15, 16), have been associated with detectable amounts of residual protein subunits that may be important in the pathogenesis of HPS types 1 and 2. It is not clear whether the endoplasmic reticulum stress responses described in some studies of HPS reflect residual HPS subunit expression or are part of a cellular response to disrupted protein trafficking (37). The MLE-15/HPS1, MLE-15/HPS2, and MLE-15/HPS9 clones clearly demonstrate loss of the target protein by immunoblotting and thus may represent a more extreme phenotype than the natural human diseases, affording an opportunity to understand the impact of residual subunit expression on cell stress responses. Another limitation is that we were unable to fully validate the MLE-15/HPS3 clone, owing to a lack of reagents to detect murine HPS3 by immunoblotting and a lack of suitable cargo proteins to test for disruption of BLOC-2 trafficking. The Hps3 genomic and mRNA sequences from MLE-15/HPS3 cells demonstrate a reading frameshift that predicts HPS3 loss, but the presence of reduced but not absent HPS6 leaves open the possibility that we did not achieve sufficient loss of the HPS3 protein. We anticipate fully validating the MLE-15/HPS3 cells in the future as antibody reagents become available and BLOC-2 cargo proteins are identified. Finally, the MLE-15 cells are a transformed cell line and exhibit unrestrained proliferation that is dependent on the continuous expression of the simian virus 40 large T antigen (5). There are circumstances where this may be a liability, so confirmation of findings in primary AT2 cells is always recommended. The MLE-15/HPS clones reduce the reliance on primary AT2 cells for detailed mechanistic studies of protein trafficking, cellular function, and disease pathogenesis that are challenging to do with primary AT2 cells that lose their differentiated phenotype in cell culture or achieve inadequate protein inactivation by transient experimental strategies, such as siRNA. Moreover, the MLE-15/HPS clones may foster enhanced drug discovery through high-throughput screening that would be difficult with primary AT2 cells.
Acknowledgments
Acknowledgment
The authors thank Dr. Daniel Swarr (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) for advice in developing the original strategy for gene editing using CRISPR/Cas9. Experiments, image analysis, and presentation using the Nikon Spinning Disk MCN T-2216 were performed in the Vanderbilt Cell Imaging Shared Resource and Nikon Center of Excellence (supported by NIH grants CA68485, DK20593, DK58404, DK59637, and EY08126). Finally, the authors acknowledge the continued encouragement and support of the patients and families in the HPS community who inspire our work.
Footnotes
Supported by the American Heart Association (GRA 12050265 [S.H.G.]) and the NIH (grants HL119503 [L.R.Y.] and GM108807 [S.H.G.]).
Author Contributions: S.K.: developed reagents, guided the experiments, analyzed data, created figures, and wrote the manuscript; A.Q.: developed reagents, guided the experiments, and analyzed data; P.W., S.M., and P.G.: executed experiments and analyzed data; L.R.Y.: guided experiments, analyzed data, and edited the manuscript; and S.H.G.: conceived of the project, guided the experiments, analyzed data, created figures, and wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0324MA on November 30, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vicary GW, Vergne Y, Santiago-Cornier A, Young LR, Roman J. Pulmonary fibrosis in Hermansky–Pudlak syndrome. 2016;13:1839–1846. doi: 10.1513/AnnalsATS.201603-186FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young LR, Gulleman PM, Short CW, Tanjore H, Sherrill T, Qi A, et al. Epithelial-macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky-Pudlak syndrome. 2016;1:e88947. doi: 10.1172/jci.insight.88947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, et al. The alveolar epithelium determines susceptibility to lung fibrosis in Hermansky-Pudlak syndrome. 2012;186:1014–1024. doi: 10.1164/rccm.201207-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 5.Wikenheiser KA, Clark JC, Linnoila RI, Stahlman MT, Whitsett JA. Simian virus 40 large T antigen directed by transcriptional elements of the human surfactant protein C gene produces pulmonary adenocarcinomas in transgenic mice. 1992;52:5342–5352. [PubMed] [Google Scholar]
- 6.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, et al. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grek CL, Newton DA, Qiu Y, Wen X, Spyropoulos DD, Baatz JE. Characterization of alveolar epithelial cells cultured in semipermeable hollow fibers. 2009;35:155–174. doi: 10.1080/01902140802495870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh J, Bailin T, Fukai K, Feng GH, Ho L, Mao JI, et al. Positional cloning of a gene for Hermansky-Pudlak syndrome, a disorder of cytoplasmic organelles. 1996;14:300–306. doi: 10.1038/ng1196-300. [DOI] [PubMed] [Google Scholar]
- 9.Martina JA, Moriyama K, Bonifacino JS. BLOC-3, a protein complex containing the Hermansky-Pudlak syndrome gene products HPS1 and HPS4. 2003;278:29376–29384. doi: 10.1074/jbc.M301294200. [DOI] [PubMed] [Google Scholar]
- 10.Nazarian R, Falcón-Pérez JM, Dell’Angelica EC. Biogenesis of lysosome-related organelles complex 3 (BLOC-3): a complex containing the Hermansky-Pudlak syndrome (HPS) proteins HPS1 and HPS4. 2003;100:8770–8775. doi: 10.1073/pnas.1532040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L, Seymour AB, Jiang S, To A, Peden AA, Novak EK, et al. The β3A subunit gene (Ap3B1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- 12.Anikster Y, Huizing M, White J, Shevchenko YO, Fitzpatrick DL, Touchman JW, et al. Mutation of a new gene causes a unique form of Hermansky-Pudlak syndrome in a genetic isolate of central Puerto Rico. 2001;28:376–380. doi: 10.1038/ng576. [DOI] [PubMed] [Google Scholar]
- 13.Gautam R, Chintala S, Li W, Zhang Q, Tan J, Novak EK, et al. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2) 2004;279:12935–12942. doi: 10.1074/jbc.M311311200. [DOI] [PubMed] [Google Scholar]
- 14.McGarry MP, Reddington M, Novak EK, Swank RT. Survival and lung pathology of mouse models of Hermansky-Pudlak syndrome and Chediak-Higashi syndrome. 1999;220:162–168. doi: 10.1046/j.1525-1373.1999.d01-24.x. [DOI] [PubMed] [Google Scholar]
- 15.Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 16.Chiang P-W, Oiso N, Gautam R, Suzuki T, Swank RT, Spritz RA. The Hermansky-Pudlak syndrome 1 (HPS1) and HPS4 proteins are components of two complexes, BLOC-3 and BLOC-4, involved in the biogenesis of lysosome-related organelles. 2003;278:20332–20337. doi: 10.1074/jbc.M300090200. [DOI] [PubMed] [Google Scholar]
- 17.Starcevic M, Dell’Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- 18.Kook S, Wang P, Young LR, Schwake M, Saftig P, Weng X, et al. Impaired lysosomal integral membrane protein 2-dependent peroxiredoxin 6 delivery to lamellar bodies accounts for altered alveolar phospholipid content in adaptor protein-3-deficient pearl mice. 2016;291:8414–8427. doi: 10.1074/jbc.M116.720201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Yu K, Robert KW, DeBolt KM, Hong N, Tao J-Q, et al. Rab38 targets to lamellar bodies and normalizes their sizes in lung alveolar type II epithelial cells. 2011;301:L461–L477. doi: 10.1152/ajplung.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young LR, Pasula R, Gulleman PM, Deutsch GH, McCormack FX. Susceptibility of Hermansky-Pudlak mice to bleomycin-induced type II cell apoptosis and fibrosis. 2007;37:67–74. doi: 10.1165/rcmb.2006-0469OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atochina-Vasserman EN, Bates SR, Zhang P, Abramova H, Zhang Z, Gonzales L, et al. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. 2011;184:449–458. doi: 10.1164/rccm.201011-1882OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng GH, Bailin T, Oh J, Spritz RA. Mouse Pale Ear (ep) is homologous to human Hermansky-Pudlak syndrome and contains a rare ‘AT-AC’ intron. 1997;6:793–797. doi: 10.1093/hmg/6.5.793. [DOI] [PubMed] [Google Scholar]
- 23.Dell’Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peden AA, Rudge RE, Lui WWY, Robinson MS. Assembly and function of AP-3 complexes in cells expressing mutant subunits. 2002;156:327–336. doi: 10.1083/jcb.200107140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T, Li W, Zhang Q, Novak EK, Sviderskaya EV, Wilson A, et al. The gene mutated in cocoa mice, carrying a defect of organelle biogenesis, is a homologue of the human Hermansky-Pudlak syndrome-3 gene. 2001;78:30–37. doi: 10.1006/geno.2001.6644. [DOI] [PubMed] [Google Scholar]
- 26.Dell’Angelica EC. The building BLOC(k)s of lysosomes and related organelles. 2004;16:458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Huang L, Kuo YM, Gitschier J. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. 1999;23:329–332. doi: 10.1038/15507. [DOI] [PubMed] [Google Scholar]
- 28.Guttentag SH, Akhtar A, Tao JQ, Atochina E, Rusiniak ME, Swank RT, et al. Defective surfactant secretion in a mouse model of Hermansky-Pudlak syndrome. 2005;33:14–21. doi: 10.1165/rcmb.2004-0293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ban N, Matsumura Y, Sakai H, Takanezawa Y, Sasaki M, Arai H, et al. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. 2007;282:9628–9634. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, et al. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. 2007;48:621–632. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, et al. ABCA3 is critical for lamellar body biogenesis in vivo. 2007;282:23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- 32.Guttentag SH, Beers MF, Bieler BM, Ballard PL. Surfactant protein B processing in human fetal lung. 1998;275:L559–L566. doi: 10.1152/ajplung.1998.275.3.L559. [DOI] [PubMed] [Google Scholar]
- 33.Ridsdale R, Na CL, Xu Y, Greis KD, Weaver T. Comparative proteomic analysis of lung lamellar bodies and lysosome-related organelles. 2011;6:e16482. doi: 10.1371/journal.pone.0016482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Chintagari NR, Narayanaperumal J, Ayalew S, Hartson S, Liu L. Proteomic analysis of lamellar bodies isolated from rat lungs. 2008;9:34. doi: 10.1186/1471-2121-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouhani FN, Brantly ML, Markello TC, Helip-Wooley A, O’Brien K, Hess R, et al. Alveolar macrophage dysregulation in Hermansky-Pudlak syndrome type 1. 2009;180:1114–1121. doi: 10.1164/rccm.200901-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahavadi P, Korfei M, Henneke I, Liebisch G, Schmitz G, Gochuico BR, et al. Epithelial stress and apoptosis underlie Hermansky-Pudlak syndrome-associated interstitial pneumonia. 2010;182:207–219. doi: 10.1164/rccm.200909-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korimilli A, Gonzales LW, Guttentag SH. Intracellular localization of processing events in human surfactant protein B biosynthesis. 2000;275:8672–8679. doi: 10.1074/jbc.275.12.8672. [DOI] [PubMed] [Google Scholar]