Abstract
Objectives: To determine whether a dual-task gait and aerobic exercise intervention differentially impacted older adults with normal blood pressure (BP) dipping status (dippers) compared to those with nondipping status (nondippers). Methods: This study was a secondary analysis involving participants (mean age = 70.3 years, 61% women) who attended a laboratory-based exercise intervention over a 6-month period (40 min/day and 3 days/week). Participants were assessed in measures of cognition, mobility, and cardiovascular health at baseline, 3, 6, and 12 months (after a 6-month no-contact follow-up). Results: We observed improvements in cognition in both groups at 6 and 12 months, although no between-group differences were seen. Nondippers demonstrated superior improvements in usual gait velocity and step length after the exercise intervention compared to dippers. Dippers reduced daytime systolic BP at 6 and 12 months to a greater extent than nondippers. Discussion: BP dipping status at baseline did not influence exercise benefits to cognition but did mediate changes in mobility and cardiovascular health.
Keywords: exercise, aging, blood pressure, cognition, mobility
Introduction
Blood pressure (BP) dipping characterizes the diurnal BP pattern and is expressed as the percentage drop in mean systolic BP from day to night or the systolic day-to-night ratio (O’Brien et al., 2013). Several BP dipping patterns are commonly observed, including normal dipping status (dippers, i.e., those who experience a 10%-20% drop in mean systolic BP from day to night), extreme dipping status (i.e., those who experience a greater than or equal to 20% drop in mean systolic BP from day to night), nondipping status (nondippers, i.e., those who experience a drop of less than 10% in mean systolic BP from day to night), and reverse dipping status (i.e., those who experience higher mean systolic BP levels at night compared to day, expressed as a negative BP dipping percentage; O’Brien et al., 2013; Salles et al., 2016). Nondipping status is considered an independent cardiovascular disease (CVD) risk factor (Salles et al., 2016) and has been associated with an increased risk of severe cardiovascular and cerebrovascular events in older adults (Salles et al., 2016; Verdecchia, 2000) and all-cause mortality in older hypertensive adults (Fagard et al., 2008). It is assumed that because of the exposure to higher BP levels during nighttime hours when individuals lie supine while sleeping, the brain is less protected from hydrostatic forces and the cerebral vasculature is exposed to pathologically higher pulsatile flow (Fagard et al., 2008). The sustained elevation in pulsatile flow subsequently damages the cerebral microvasculature and contributes to the development of vascular-related brain injury, including microbleeds, lacunar infarcts, and white matter (WM) hyperintensities (O’Rourke & Safar, 2005).
Previous observations have also identified a negative relationship between nondipping status and cognition. In older hypertensive adults, nondipping status has been associated with smaller total brain volumes (Nagai, Hoshide, Ishikawa, Shimada, & Kario, 2008), poorer global cognitive functioning (GCF; Bellelli et al., 2004), and worse memory and information processing speed (PS; van Boxtel et al., 2006). Abnormal BP dipping may also be associated with the development of mild cognitive impairment (MCI), as the prevalence of MCI is greatest among community-dwelling older adults who are extreme dippers (32%), nondippers (30%), and reverse-dippers (50%) when compared to those with normal dipping status (13.2%; Guo et al., 2010).
Healthy lifestyle choices, such as the habitual participation in aerobic exercise (AE), consistently reduces cardiovascular risk factor burden, and evidence suggests that exercise may also be an important strategy to reduce the risk of cognitive impairment and slow the progression of dementia (Barnes, Yaffe, Satariano, & Tager, 2003). Previous meta-analyses suggest that AE can improve cognitive function within a number of cognitive domains, including PS, memory, and executive function (EF) in healthy older adults (Colcombe et al., 2004; Smith et al., 2010) and can improve verbal fluency (VF) in those with indications of underlying cognitive impairment (Gates et al., 2013). Aerobic-based exercise programs incorporating some form of cognitive training (e.g., dual-task [DT] training) might impart a significantly larger global cognitive benefit than those focusing on single strategies (Gregory, Gill, & Petrella, 2013). Nevertheless, the influence of combined exercise interventions on cognition, mobility, and cardiovascular health in older adults with nondipping status has yet to be explored.
Thus, the primary objective of this study was to determine whether a 6-month DT gait and AE intervention differentially impacted older adults with normal systolic BP dipping status (dippers) compared to those with nondipping status (nondippers) on measures of global and domain-specific cognitive functioning, usual and DT gait performance and cardiovascular health (carotid intima-media thickness [cIMT] and carotid arterial compliance [cAC]; ambulatory systolic and diastolic BP). As a secondary objective, we investigated whether the exercise intervention would increase the magnitude of BP dipping in nondippers.
Methods
Study Design and Participants
This study was a secondary subgroup analysis of a 6-month, intervention-based case-series study. Study design, participant recruitment, as well as study eligibility have been published previously (Gregory et al., 2017). Briefly, we invited community-dwelling older adults aged 60 years or above to take part in this case-series study. Participants were recruited from London, Ontario through the use of town hall announcements, calls to past research participants, and the distribution of advertisements to various locations throughout the community (i.e., Boys & Girls Clubs, Kiwanis Clubs, media outlets). Participants without dementia (i.e., no self-reported diagnosis and/or Mini-Mental State Examination [MMSE] score > 24; Folstein, Folstein, & McHugh, 1975) and preserved instrumental activities of daily living (i.e., scoring ≥ 6/8 on the Lawton–Brody Instrumental Activities of Daily Living scale; Lawton & Brody, 1969) were included in this study. Significant neurological (i.e., Parkinson’s) or orthopedic (i.e., severe osteoarthritis) conditions, clinical depression (i.e., >16 on Center for Epidemiologic Studies–Depression [CES-D] Scale; Radloff, 1977), or at the discretion of the study physician, or BP unsafe for exercise (i.e., 180/100 mmHg or <100/60 mmHg; American College of Sports Medicine, 2014), and those who reported a recent severe cardiovascular complication (i.e., congestive heart failure, stroke) or could not comprehend the questionnaire material were excluded from participation. The Western University Health Sciences Research Ethics Board approved this study and all participants provided written informed consent. The study was registered under ClinicalTrials.gov Identifier: NCT01572311.
Exercise Intervention
Exercise training utilized a Biodex GaitTrainer2 treadmill (providing visuospatial feedback related to the user’s step length on a screen fixed atop of the treadmill) under the supervision of research personnel. During each session, participants underwent a 5-min warm-up, 15 min of DT gait training, 15 min of moderate-intensity AE (i.e., 75%-85% maximal heart rate; Stuckey, Knight, & Petrella, 2012) and a 5-min cool down. During the DT gait training, participants walked at a self-selected pace while receiving visuospatial step-length feedback and answering cognitively challenging questions (i.e., VF and arithmetic). For the first 7 min, participants prioritized providing correct responses to the VF and arithmetic tasks, and after a 1-min break from the cognitive tasks, participants prioritized modifying their step length to achieve or surpass an individualized step length goal. Following the DT gait training, the visuospatial step length feedback was removed and participants underwent 15 min of moderate-intensity AE. The incline and speed of the treadmill was increased until training heart rates were achieved, and the training intensity was monitored every 5 min. A 10-point modified Borg Rating of Perceived Exertion scale and a built-in handgrip heart rate monitor on the treadmill were used to monitor exercise intensity. In total, the DT gait and AE intervention took place 3 days/week for 6 months, with each session lasting 40 minutes.
Data Collection
Participants were assessed at baseline and 3, 6, and 12 months (i.e., after the 6-month no-contact follow-up period). Medical history, current health status, and demographics were collected at baseline and included age, sex, ethnicity, years of education, body mass index, cognitive status (i.e., Montreal Cognitive Assessment [MoCA] and the MMSE; Nasreddine et al., 2005), systolic BP dipping, and cardiorespiratory fitness (i.e., predicted maximal oxygen consumption [pVO2max]) estimated using the Step Test and Exercise Prescription (STEP) tool (Stuckey et al., 2012).
Study Outcomes
Cognition outcomes
To investigate changes in cognition outcomes, a composite score was derived from a neuropsychological battery that included four cognitive domains (EF, PS, memory, and VF). The selected battery included reliable and well-validated (Hachinski et al., 2006) measures of EF (Trail-Making Tests [TMT] A and B), PS (Digital Symbol Substitution Test [DSST]), memory (Auditory Verbal Learning Test [AVLT]), as well as VF (category: semantic [animal naming] and phonemic [letter: Controlled Oral Word Association Test]). The composite scores were derived by first converting individual outcomes from the neuropsychological tests to standardized z scores ([raw score–baseline group mean]/baseline group standard deviation). Next, following previous methods by our group (Gill et al., 2016) and others (Barnes et al., 2013), standardized scores were averaged within each cognitive domain, and then, domain-specific composite scores were averaged to create a GCF score, thereby ensuring that all four cognitive domains were equally weighted.
Mobility outcomes
Gait characteristics were collected using a valid and reliable (Brach, Perera, Studenski, & Newman, 2008) electronic walkway system (GAITRite® System, Peekskill, NY, USA). Participants were required to complete two usual and DT walking trials at a comfortable pace. The serial sevens task (Dorfman et al., 2014) was used as the DT condition and there was no instruction provided to prioritize either gait or responses to the cognitive tasks during the DT trials. In each trial, participants were instructed to start 1 m before and continue to walk until 1 m beyond the electronic walkway, to measure steady-state walking. Gait performance over two walking trials were averaged and used for analysis. Usual and DT gait velocity, step length, and stride time variability (gait variability) were used as outcomes.
BP dipping status and cardiovascular health outcomes
The 24-hr ambulatory BP and carotid ultrasonography were used to evaluate cardiovascular health and to determine BP dipping status among participants. The 24-hr BP was recorded using an appropriately sized, valid, and reliable (Iqbal, Fotherby, & Potter, 1996) ambulatory BP cuff and monitor (SpaceLabsTM 90207, SpaceLabs Healthcare Ltd, Hertford, UK). Measurements occurred two times an hour during the daytime (i.e., 06:00 to 22:00) and once an hour during the nighttime (i.e., 22:00 to 06:00).
The percent drop in daytime to nighttime mean systolic BP was used to calculate BP dipping status for all participants at baseline. Participants were identified as dippers if they presented ≥10% to 20% drop in systolic BP from daytime to nighttime, whereas those who demonstrated <10% to 1% reduction in systolic BP from daytime to nighttime were classified as nondippers (O’Brien et al., 2013). Extreme (>20% BP reduction) or reverse (<0% BP reduction) dippers were excluded from analysis. Daytime and nighttime systolic and diastolic BP were also considered as outcomes for this study and we investigated changes in the magnitude of BP dipping following the exercise intervention among nondippers.
Carotid arterial diameters were measured following previous techniques (Gregory et al., 2016). Briefly, a 10 MHz linear array B-mode ultrasonography transducer (Vingmed, GE Ultrasound A/S, Horten, Norway) was used to collect a longitudinal two-dimensional image of the cephalic portion of the right common carotid artery. Arterial diameters were measured leading-edge-to-leading-edge at peak systole and end diastole and averaged across three cardiac cycles. cIMT was determined by subtracting the carotid arterial lumen diameter from the outer arterial diameter at end diastole. cAC was determined using the following equation: cAC = (π[Dmax/2]2 – π[Dmin/2]2)/ΔP, where Dmax was the systolic carotid arterial diameter, Dmin was the diastolic carotid arterial diameter, and ΔP was resting brachial pulse pressure (Gregory et al., 2016). To control for external factors, cardiovascular assessments were conducted in a quiet and temperature-controlled room (~23 °C) and participants were instructed not to engage in vigorous physical activity or drink alcohol for 24 hr, to avoid caffeine intake and smoking for 12 hr, and to fast for 4 hr before assessments.
Analysis
We conducted linear mixed models for repeated measurements (Fitzmaurice, Laird, & Ware, 2011) to assess differences between groups in mean change from baseline to 6 months while adjusting for age, education, gender, presence of hypertension (self-reported diagnosis and/or prescribed medication) and type 2 diabetes (self-reported diagnosis and/or prescribed medication). Within the models, we also examined differences between groups from baseline to 3 and 12 months. The terms included in the model were group (dippers vs. nondippers), time, group × time, age, gender, years of education, presence of hypertension, and presence of type 2 diabetes. Time was modeled categorically using three indicator variables representing each time point (with baseline as the reference category). Data from all participants were analyzed regardless of compliance with the exercise intervention and attendance at follow-up assessments (Fitzmaurice et al., 2011).
Finally, to address participant dropout, we compared baseline characteristics of those who dropped out versus those who completed the 6-month assessment, using the independent-samples t test for normally distributed data, the Mann–Whitney U test for nonnormal data, and the chi-square test of proportions for categorical variables. Interpretation of study results were primarily based on mean estimation and associated 95% confidence intervals. Two-sided p values less than .05 were considered as statistically significant. All analyses were performed using SPSS® Statistics for Mac, Version 21 (Armonk, NY, USA).
Results
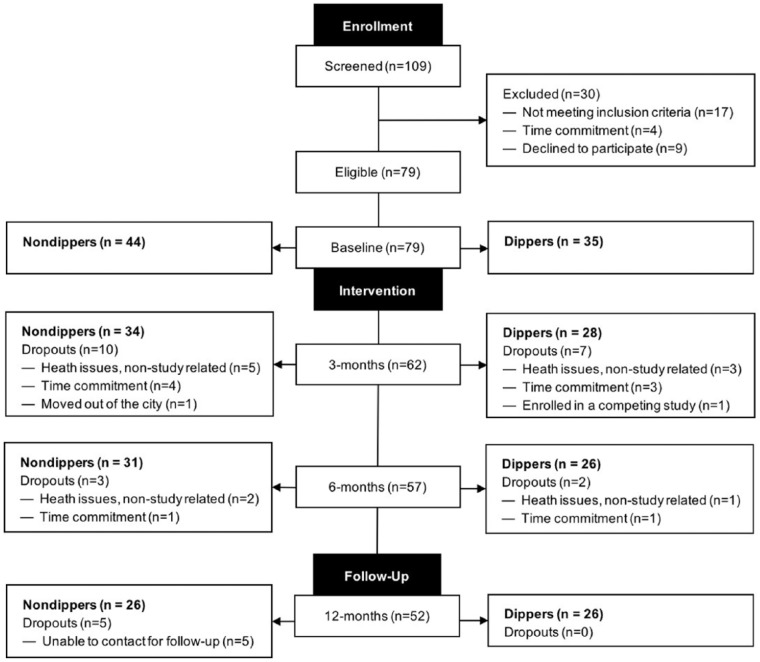
The flow of participants throughout the study is presented in Figure 1. Of 109 participants assessed for eligibility, 79 were enrolled at baseline, 57 successfully completed the exercise intervention, and 52 completed the study. At baseline, 35 participants met the criteria for normal dipping status (i.e., dippers), whereas 44 participants were classified as having nondipping status (i.e., nondippers). Participants were mostly women, primarily Caucasian, and highly educated (see Table 1). Differences in baseline characteristics between those who attended 6-month assessments and those who dropped out of the study are not statistically significant. In addition, participants classified as dippers attended 79.4% (approximately 62 of 78 sessions) of the exercise sessions and nondippers attended 79.8% (approximately 62 of 78 sessions).
Figure 1.
Participant flow for the 6-month case-series study with a 6-month no-contact follow-up.
Table 1.
Baseline Characteristics.
| Characteristicsa | Dippers (n = 35) | Nondippers (n = 44) |
|---|---|---|
| Age (years) | 68.7 (5.7) | 71.6 (7.1) |
| Women | 23 (65.7%) | 25 (56.8%) |
| Education (years) | 14.2 (2.8) | 15.4 (3.8) |
| Caucasian | 32 (91.4%) | 43 (97.7%) |
| Body mass index (kg/m2) | 29.8 (4.7) | 29.4 (4.7) |
| pVO2max (mL·min−1·kg−1) | 29 (7.6) | 27.8 (7) |
| MMSE scoreb | 28.4 (1.5) | 28.5 (1.2) |
| MoCA scoreb | 25.2 (2.5) | 24.8 (2.3) |
| CES-D scorec | 6.6 (5) | 6.5 (6.3) |
| Systolic BP dipping (%) | 13.5 (2.7) | 4.2 (4.4) |
| Medical history | ||
| Hypertension | 17 (48.6%) | 28 (63.6%) |
| Hypercholesterolemia | 8 (22.9%) | 17 (38.6%) |
| Type 2 diabetes | 4 (11.4%) | 8 (18.2%) |
| Previous cardiovascular event | 2 (5.7%) | 4 (9.1%) |
| Previous stroke | 3 (8.6%) | 2 (4.5%) |
| Osteoarthritis | 3 (8.6%) | 2 (4.5%) |
Note. pVO2max = predicted maximal oxygen consumption; MMSE = mini-mental status examination; MoCA = Montreal cognitive assessment; CES-D = center for epidemiological studies depression scale; BP = blood pressure.
Data presented as either mean (standard deviation) or no. (%) where applicable.
Range from 0 to 30, lower scores indicate greater cognitive impairment.
Range from 0 to 60, scores above 16 indicate clinical depression.
Cognition Outcomes
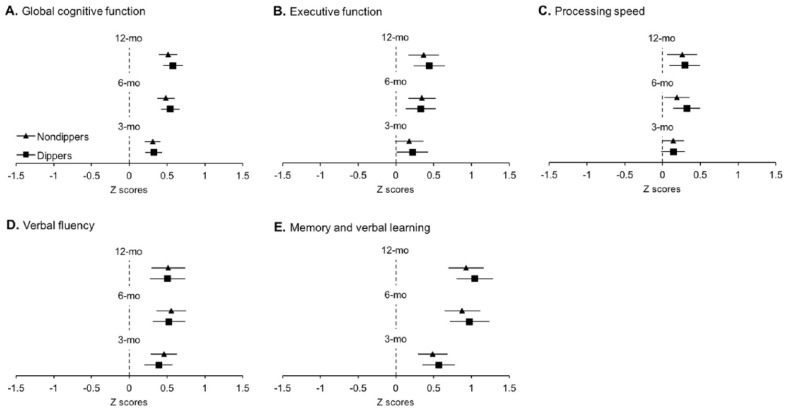
At baseline, both groups demonstrated similar performance on the cognitive tests (see Table 2). No differences between groups were seen in GCF at 6 months, and similar results were seen at both 3 and 12 months. Accordingly, no differences between groups were found for any of the domain-specific cognitive outcomes across all time points (see Table 3). Despite the finding of no differences between groups, there were numerous within-group changes over time in standardized GCF and domain-specific cognition (see Figure 2). Both groups demonstrated equal improvements in standardized GCF, memory, and VF at 3, 6, and 12 months. Regarding EF, while dippers demonstrated improvements as early as 3 months and retained these improvements to 6 and 12 months, nondippers demonstrated improvements only at 6 and 12 months. In contrast, nondippers improved in PS at 3 months, which was retained at 6 and 12 months, whereas dippers demonstrated improvements in PS at 6 and 12 months only.
Table 2.
Baseline Study Outcomes.
| Outcomesa | Dippers (n = 35) | Nondippers (n = 44) | p |
|---|---|---|---|
| Cognition, z scores | |||
| Global cognitive function | .22 (.7) | −.17 (.7) | .075 |
| Executive function | .17 (.8) | −.13 (.9) | .42 |
| Processing speed | .23 (1) | −.19 (.9) | .12 |
| Memory | .28 (.8) | −.22 (.9) | .12 |
| Verbal fluency | .18 (.8) | −.15 (.8) | .18 |
| Mobility | |||
| Usual gait velocity (cm/s) | 117.7 (18) | 106.7 (18.5) | .01a |
| Usual step length (cm) | 63.7 (6.1) | 61.3 (7.8) | .15 |
| Usual gait variability (%) | 2.6 (4.5) | 2.3 (1.1) | .69 |
| DT gait velocity (cm/s) | 96.8 (23.4) | 85.8 (9.4) | .22 |
| DT step length (cm) | 59.5 (6.8) | 56.2 (9.4) | .38 |
| DT gait variability (%) | 7.4 (8.5) | 6.2 (4.8) | .91 |
| Cardiovascular | |||
| cIMT (mm) | .64 (.12) | .683 (.137) | .34 |
| cAC (mm/mmHg2 × 10−1) | .709 (.269) | .827 (.385) | .15 |
| Daytime SBP (mmHg) | 130.2 (11.5) | 131.9 (12.8) | .80 |
| Daytime DBP (mmHg) | 73.2 (7.4) | 74.1 (10.1) | .52 |
| Nighttime SBP (mmHg) | 112.5 (9.9) | 126.5 (14.9) | <.001b |
| Nighttime DBP (mmHg) | 61.2 (6.6) | 67.9 (11.1) | <.001b |
Note. DT = dual-task gait; cIMT = carotid intima-media thickness; cAC = carotid arterial compliance; SBP = systolic blood pressure; DBP = diastolic blood pressure. Boldface values indicate statistically significant results.
Data presented as mean (standard deviation).
Differences between groups at baseline adjusting for age, education, gender, presence of hypertension, and type 2 diabetes.
Table 3.
Estimated Changes Between Groups From Baseline.
| Outcomes | Difference between groups in
estimated mean change from baseline (95% CI) |
|||||
|---|---|---|---|---|---|---|
| 3 months | p | 6 months | p | 12 months | p | |
| Cognition, z scores | ||||||
| Global cognitive function | −0.014 [–0.162, 0.134] | .85 | −0.055 [–0.238, 0.11] | .51 | −0.064 [–0.238, 0.11] | .46 |
| Executive function | −0.043 [–0.319, 0.234] | .75 | 0.019 [–0.243, 0.282] | .88 | −0.075 [–0.356, 0.207] | .59 |
| Processing speed | 0.002 [–0.204, 0.208] | .98 | −0.133 [–0.37, 0.104] | .26 | −0.032 [–0.311, 0.248] | .82 |
| Memory | −0.076 [–0.36, 0.208] | .59 | −0.096 [–0.443, 0.251] | .58 | −0.116 [–0.445, 0.214] | .48 |
| Verbal fluency | 0.07 [–0.179, 0.319] | .57 | 0.032 [–0.251, 0.315] | .82 | 0.01 [–0.304, 0.325] | .94 |
| Mobility | ||||||
| Usual gait velocity (cm/s) | 4.82 [–1.38, 11.03] | .12 | 7.58 [1.03, 14.13] | .024a | 6.97 [–0.17, 14.12] | .055 |
| Usual step length, cm | 2.02 [–0.04, 4.08] | .054 | 3.01 [0.92, 5.11] | .006a | 2.12 [–0.17, 4.42] | .06 |
| Usual gait variability, % | 0.49 [–0.97, 1.95] | .51 | 0.43 [–1.08, 1.93] | .57 | 0.48 [–0.94, 1.9] | .51 |
| DT gait velocity, cm/s | 2.93 [–6.87, 12.73] | .55 | 3.39 [–6.53, 13.31] | .51 | −8.04 [–18.8, 2.72] | .14 |
| DT step length, cm | 1.87 [–1.01, 4.75] | .21 | 1.75 [–1.77, 5.27] | .32 | −.61 [–4.43, 3.21] | .75 |
| DT gait variability, % | −1.85 [–5.83, 2.13] | .36 | −2.04 [–7.14, 3.06] | .43 | 1.84 [–1.1, 4.78] | .22 |
| Cardiovascular health | ||||||
| cIMT, mm | −0.025 [–0.089, 0.04] | .44 | 0.034 [–0.038, 0.106] | .35 | −0.018 [–0.089, 0.054] | .62 |
| cAC, mm/mmHg2 × 10−1 | 0.061 [–0.14, 0.263] | .54 | −0.019 [–0.206, 0.168] | .83 | 0.07 [–0.129, 0.268] | .48 |
| Daytime SBP, mmHg | 4.57 [–0.62, 9.77] | .08 | 6.93 [1.12, 12.74] | .020a | 7.49 [2.32, 12.66] | .005a |
| Daytime DBP, mmHg | 0.26 [–2.8, 3.31] | .87 | 2.73 [–0.59, 6.1] | .11 | 3.2 (–0.45, 6.85 | .08 |
| Nighttime SBP, mmHg | −3.08 [–8.46, 2.29] | .26 | −5.59 [–0.11, 0.1] | .054 | −0.22 [–5.24, 4.79] | .93 |
| Nighttime DBP, mmHg | −2.67 [6.1, 0.73] | .12 | −3.5 [–6.95, 0.05] | .057 | −1.27 [–4.41, 1.86] | .42 |
Note. CI = confidence interval; DT = dual-task gait; IMT = carotid intima-media thickness; cAC = carotid arterial compliance; SBP = systolic blood pressure; DBP = diastolic blood pressure. Boldface values indicate statistically significant results.
Significant differences between groups in estimated mean change from baseline (reference category = dippers) adjusting for age, education, gender, presence of hypertension and type 2 diabetes.
Figure 2.
Estimated mean changes in cognition.
Note. Solid triangles (nondippers) and squares (dippers) represent estimated group mean changes from baseline; bars represent associated 95% confidence intervals. Confidence intervals not including zero (i.e., not crossing the vertical dotted line) indicate significant differences from baseline (see Supplemental Table 1). All analyses were adjusted for age, education, gender, presence of hypertension, and type 2 diabetes.
Mobility Outcomes
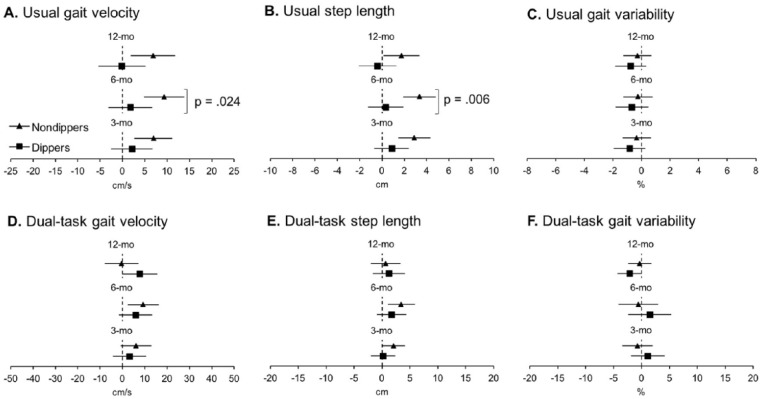
Nondippers performed worse than dippers in usual gait velocity at baseline (see Table 2). Despite this baseline difference, the effects of the exercise intervention yielded greater improvements in usual gait velocity and usual step length at 6 months in nondippers compared to dippers (see Table 3). No differences between groups were observed in usual gait variability at any time points. With regard to DT gait velocity, step length, and gait variability, there were no differences between groups at any time points. Within-group changes in mobility over time are presented in Figure 3. In summary, nondippers demonstrated increased usual gait velocity and step length at all time points and increased DT gait velocity at 6 months along with increased DT step length at 3 and 6 months, while dippers reduced DT gait variability at 12 months.
Figure 3.
Estimated mean changes in mobility.
Note. Solid triangles (nondippers) and squares (dippers) represent estimated group mean changes from baseline; bars represent associated 95% confidence intervals. Confidence intervals not including zero (i.e., not crossing the vertical dotted line) indicate significant differences from baseline (see Supplemental Table 1). The p value indicates significant differences between groups in estimated mean change from baseline adjusting for age, education, gender, presence of hypertension, and type 2 diabetes.
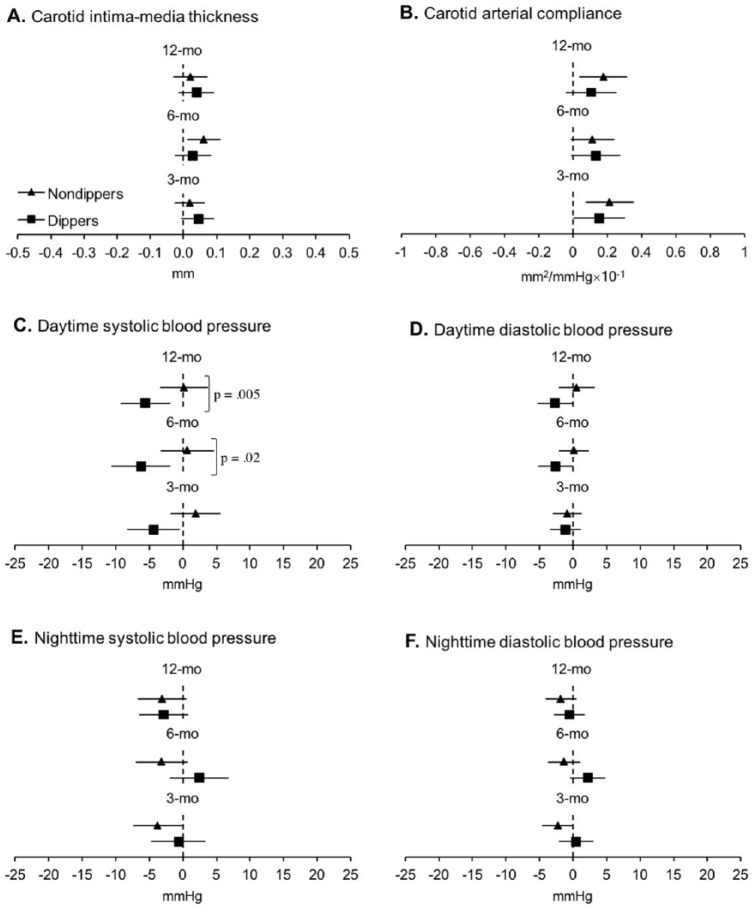
Cardiovascular Outcomes
At baseline, groups only differed in nighttime systolic and diastolic BP (see Table 2), where nondippers had higher mean values, which was expected due to the BP dipping grouping factor. At 6 and 12 months, dippers decreased daytime systolic BP to a greater extent than nondippers, with no differences in nighttime systolic BP. No differences between groups in cIMT and cAC were seen at any time points (see Table 3). Within-group analyses demonstrated that dippers reduced daytime systolic BP from baseline to 3, 6, and 12 months and daytime diastolic BP at 6 and 12 months and improved cAC at 3 months, while nondippers reduced nighttime systolic BP at 3 months, increased cIMT at 6 months, and improved cAC at 3 and 12 months (see Figure 4). In addition, we noted that regardless of unchanged systolic BP after the exercise intervention, nondippers successfully increased the magnitude of BP dipping at 6 months, mean change: 2.97%, 95% confidence interval (CI) = [0.81% to 4.14%], p < .001. Furthermore, 25% (n = 11) of the individuals who met the criteria for nondipping BP status at baseline were reclassified as dippers after the exercise intervention.
Figure 4.
Estimated mean changes in cardiovascular health from baseline.
Note. Solid triangles (nondippers) and squares (dippers) represent estimated group mean changes from baseline; bars represent associated 95% confidence intervals. Confidence intervals not including zero (i.e., not crossing the vertical dotted line) indicate significant differences from baseline (see Supplemental Table 1). The p value indicate significant differences between groups in estimated mean change from baseline adjusting for age, education, gender, presence of hypertension, and type 2 diabetes.
Discussion
The results of the current study demonstrate that 6 months of DT gait and AE training impart similar benefits to GCF and domain-specific cognitive function in community-dwelling older adults without dementia, regardless of BP dipping status. Furthermore, in those with BP nondipping status, the exercise program yielded greater improvements in mobility, an important functional aspect of daily life, although it minimally impacted cardiovascular health.
Based on the current available literature, this is the first study to report that dippers and nondippers respond similarly to a combined DT gait and AE exercise intervention on measures of cognition. In older adults without known cognitive impairment, habitual participation in aerobic-based exercise alone (Erickson & Kramer, 2009), or combined with DT interventions (Gill et al., 2016), appears to stimulate general benefits to cognition; similar benefits have also been reported in individuals with cognitive impairment (ten Brinke et al., 2014). In this study, both dippers and nondippers retained the gains in GCF and domain-specific cognitive functioning 6 months following the intervention endpoint, suggesting a long-lasting effect of the intervention on cognitive function. Nonetheless, it is also plausible that extraneous factors, such as continuation in exercise practice or engagement in cognitive training following the end of the intervention, could explain this finding.
With regard to mobility, nondippers presented, on average, 9% (–11 cm/s) slower gait velocity at baseline compared to dippers. Due to the lack of differences between groups in GCF and domain-specific cognitive function at baseline, these findings suggest that nondipping status alone may be a risk factor for mobility impairment and the progression of cognitive difficulties, even prior to the manifestation of objective cognitive impairment. These results are further strengthened when considering that several confounders were controlled for in our analysis (e.g., age, the presence of hypertension and diabetes). Given that hypertension (Filomena et al., 2015) and nondipping status (Sander, Winbeck, Klingelhofer, & Conrad, 2000) are primary risk factors for CVD (Filomena et al., 2015), it is plausible that individuals with nondipping status may present higher vascular-induced neuropathological changes in brain regions responsible for the control of gait (Kim et al., 2016), which, in turn, may significantly contribute to poorer gait performance. Previous research has shown that gait abnormalities are associated with decreased WM integrity in older adults with cerebrovascular small vessel disease (de Laat et al., 2011), and there have been reports suggesting higher prevalence of WM hyperintensities in individuals with nondipping status (Sander et al., 2000).
A surprising find of the current study is that the 6-month DT gait and AE exercise intervention yielded greater improvements in usual and DT gait speed and step length in nondippers. Although no differences between groups in DT gait outcomes were observed. Increased gait velocity and step length following exercise interventions have been well documented in the literature and is the primary mechanism by which exercise enhances gait performance (Plummer, Zukowski, Giuliani, Hall, & Zurakowski, 2015). To our knowledge, this is the first study to report nondippers, those at increased CVD risk and potentially with greater CVD morbidity and benefited more from the exercise intervention compared to dippers. Thus, our results indicate exercise may reverse mobility impairment associated with nondipping status and contribute to reduction in the risk of future mobility impairment. Regarding the lack of differences between groups for DT gait outcomes, we believe that under DT conditions, the degree to which nondipping impairment affect neurocognitive control of gait is less evident that for usual task, and therefore, there would be less room for improvement and differences between groups. This could be related to the stages of cerebrovascular burden in the brain of those with nondipping status. Perhaps if our population were either older or demonstrated greater CVD risk factor burden compared to dippers, the differences in DT gait between groups would have been more pronounced. As well, it could be that our sample size was too small to detect differences in these outcomes
With regard to cardiovascular health, we observed that dippers significantly improved in daytime systolic BP to a greater extent at 6 and 12 months, compared to nondippers. Even though previous investigations suggest that the impact of aerobic-based exercise on measures of ambulatory BP is limited (Cornelissen, Buys, & Smart, 2013), our findings indicate that, at least in dippers, a 6-month DT gait and AE intervention is an effective strategy to improve daytime systolic BP. In those with nondipping status, it is plausible that the exercise intervention was not intense enough or long enough to improve cardiovascular health in a vascular-impaired population, corroborating previous investigations (Ling et al., 2014). Along with unchanged BP at 6 months in nondippers, we observed that these individuals presented negative changes in cIMT, despite improved cAC at 3 months. Increased cIMT may be a result of age-related alterations in vascular wall structure that occur to maintain intra-arterial pressure and blood flow homeostasis (Engelen, Ferreira, Stehouwer, Boutouyrie, & Laurent, 2012), which is seemingly more severe in those with nondipping status (Cuspidi et al., 2016). Improvements in cAC were seen in dippers and nondippers; these findings are not surprising as aerobic-based exercise interventions have successfully increased cAC in older adults, even when changes in cIMT are not apparent (Seals, Desouza, Donato, & Tanaka, 2008). Finally, with regard to changes in the magnitude of BP dipping in nondippers, we observed these individuals increased BP dipping after the intervention. This finding aligns with previous studies and demonstrates that exercise may improve BP dipping profile, even when daytime and nighttime systolic BP remain unchanged (Ling et al., 2014; Sherwood, Smith, Hinderliter, Georgiades, & Blumenthal, 2016).
The current study presents limitations. Participants were predominantly Caucasian, well educated, and relatively healthy; thus, results must be generalized only to similar populations. This investigation used data from a case-series study where the grouping variable was derived as part of a post hoc investigation; although several possible confounders were controlled for in the statistical analysis, there could be unknown and/or unmeasured confounders that could have impacted the study results. For instance, the BP measurements that were used to determine the subgroups in this study (24-hr ambulatory BP) could have been affected by participant’s individual routine, as some participants may live different lifestyles and may have different sleeping patterns. As well, the exploratory/secondary analysis nature of this study is a limitation, and more randomized controlled trials originally designed to investigate this population are warranted. Moreover, cognition was assessed using traditional neuropsychological outcomes, which could lead to the occurrence of practice effects; however, practice effects can be significantly diminished when assessments are performed at least 3 months apart (Bartels, Wegrzyn, Wiedl, Ackermann, & Ehrenreich, 2010), as was done in this study. Finally, compliance to the recommendations for ideal cardiovascular assessments provided 24 hr prior to participant visits was not monitored or enforced.
Conclusion
In a sample of community-dwelling older adults without dementia, 6 months of DT gait and AE training improved cognition in dippers and nondippers, yielded measurable improvements in mobility in nondippers, and significantly improved daytime systolic BP in dippers. Our results may indicate that CVD risk factor burden that is associated with cognitive deterioration and mobility impairment may be reversed with an exercise intervention. Future studies should focus on determining whether patients with more advanced stages of cognitive impairment (early dementia or MCI) who also show BP nondipping patterns can experience improvements in cognition and mobility following exercise.
Supplemental Material
Supplemental material, Supplemental_Table_1 for The Impact of Blood Pressure Dipping Status on Cognition, Mobility, and Cardiovascular Health in Older Adults Following an Exercise Program by Narlon C. Boa Sorte Silva, Michael A. Gregory, Dawn P. Gill, Cheri L. McGowan and Robert J. Petrella in Gerontology and Geriatric Medicine
Footnotes
Authors’ Note: Michael A. Gregory is now affiliated with McMaster University, Canada.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Canadian Institutes of Health Research (CIHR) Open Operating Grant (No. 130474) and the Mitacs Globalink Graduate Fellowship Award.
ORCID iDs: NC Boa Sorte Silva  https://orcid.org/0000-0002-4847-3742
https://orcid.org/0000-0002-4847-3742
References
- American College of Sports Medicine. (2014). ACSM’s guidelines for exercise testing and prescription (9th ed.). Baltimore, MD: Wolters Kluwer/Lippincott Williams & Wilkins Health. [Google Scholar]
- Barnes D. E., Santos-Modesitt W., Poelke G., Kramer A. F., Castro C., Middleton L. E., Yaffe K. (2013). The Mental Activity and eXercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. Journal of the American Medical Association Internal Medicine, 173, 797-804. doi: 10.1001/jamainternmed.2013.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. E., Yaffe K., Satariano W. A., Tager I. B. (2003). A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatrics Society, 51, 459-465. doi: 10.1046/j.1532-5415.2003.51153.x [DOI] [PubMed] [Google Scholar]
- Bartels C., Wegrzyn M., Wiedl A., Ackermann V., Ehrenreich H. (2010). Practice effects in healthy adults: A longitudinal study on frequent repetitive cognitive testing. BMC Neuroscience, 11(1), Article 118. doi:1471-2202-11-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellelli G., Frisoni G. B., Lucchi E., Guerini F., Geroldi C., Magnifico F., . . . Trabucchi M. (2004). Blunted reduction in night-time blood pressure is associated with cognitive deterioration in subjects with long-standing hypertension. Blood Pressure Monitoring, 9, 71-76. doi: 10.1097/00126097-200404000-00003 [DOI] [PubMed] [Google Scholar]
- Brach J. S., Perera S., Studenski S., Newman A. B. (2008). The reliability and validity of measures of gait variability in community-dwelling older adults. Archives of Physical Medicine and Rehabilitation, 89, 2293-2296. doi: S0003-9993(08)01481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S. J., Kramer A. F., Erickson K. I., Scalf P., McAuley E., Cohen N. J., . . . Elavsky S. (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences, 101, 3316-3321. doi: 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen V. A., Buys R., Smart N. A. (2013). Endurance exercise beneficially affects ambulatory blood pressure: A systematic review and meta-analysis. Journal of Hypertension, 31, 639-648. doi: 10.1097/HJH.0b013e32835ca964 [DOI] [PubMed] [Google Scholar]
- Cuspidi C., Sala C., Tadic M., Gherbesi E., Grassi G., Mancia G. (2016). Nondipping pattern and carotid atherosclerosis. Journal of Hypertension, 34, 385-392. doi: 10.1097/HJH.0000000000000812 [DOI] [PubMed] [Google Scholar]
- de Laat K. F., Tuladhar A. M., van Norden A. G., Norris D. G., Zwiers M. P., de Leeuw F. E. (2011). Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain, 134(1), 73-83. doi: 10.1093/brain/awq343 [DOI] [PubMed] [Google Scholar]
- Dorfman M., Herman T., Brozgol M., Shema S., Weiss A., Hausdorff J., Mirelman A. (2014). Dual-task training on a treadmill to improve gait and cognitive function in elderly idiopathic fallers. Journal of Neurologic Physical Therapy, 38, 246-253. doi: 10.1097/NPT.0000000000000057 [DOI] [PubMed] [Google Scholar]
- Engelen L., Ferreira I., Stehouwer C. D., Boutouyrie P., Laurent S. (2012). Reference intervals for common carotid intima-media thickness measured with echotracking: Relation with risk factors. European Heart Journal, 34, 2368-2380. [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Kramer A. F. (2009). Aerobic exercise effects on cognitive and neural plasticity in older adults. British Journal of Sports Medicine, 43, 22-24. doi: 10.1136/bjsm.2008.052498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard R. H., Celis H., Thijs L., Staessen J. A., Clement D. L., De Buyzere M. L., De Bacquer D. A. (2008). Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension, 51, 55-61. doi: 10.1161/HYPERTENSIONAHA.107.100727 [DOI] [PubMed] [Google Scholar]
- Filomena J., Riba-Llena I., Vinyoles E., Tovar J. L., Mundet X., Castañé X., . . . Delgado P. (2015). Short-term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension, 66(3), 634-640; discussion 445. doi: 10.1161/HYPERTENSIONAHA.115.05440 [DOI] [PubMed] [Google Scholar]
- Fitzmaurice G. M., Laird N. M., Ware J. H. (2011). Wiley Series in Probability and Statistics. Applied longitudinal analysis (2nd ed.). Hoboken, NJ: John Wiley. doi: 10.1198/jasa.2005.s24 [DOI] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gates N., Singh M. A. F., Sachdev P. S., Valenzuela M. (2013). The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. American Journal of Geriatric Psychiatry, 21, 1086-1097. doi: 10.1016/j.jagp.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Gill D. P., Gregory M. A., Zou G., Liu-Ambrose T., Shigematsu R., Hachinski V., . . . Petrella R. J. (2016). The healthy mind, healthy mobility trial: A novel exercise program for older adults. Medicine & Science in Sports & Exercise, 48, 297-306. doi: 10.1249/MSS.0000000000000758 [DOI] [PubMed] [Google Scholar]
- Gregory M. A., Boa Sorte Silva N. C., Gill D. P., McGowan C. L., Liu-Ambrose T., Shoemaker J. K., . . . Petrella R. J. (2017). Combined dual-task gait training and aerobic exercise to improve cognition, mobility, and vascular health in community-dwelling older adults at risk for future cognitive decline. Journal of Alzheimer’s Disease, 57, 747-763. doi: 10.3233/JAD-161240 [DOI] [PubMed] [Google Scholar]
- Gregory M. A., Gill D. P., Petrella R. J. (2013). Brain health and exercise in older adults. Current Sports Medicine Reports, 12, 256-271. doi: 10.1249/JSR.0b013e31829a74fd [DOI] [PubMed] [Google Scholar]
- Gregory M. A., Gill D. P., Zou G., Liu-Ambrose T., Shigematsu R., Fitzgerald C., . . . Petrella R. J. (2016). Group-based exercise combined with dual-task training improves gait but not vascular health in active older adults without dementia. Archives of Gerontology and Geriatrics, 63, 18-27. doi: 10.1016/j.archger.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Guo H., Tabara Y., Igase M., Yamamoto M., Ochi N., Kido T., . . . Kohara K. (2010). Abnormal nocturnal blood pressure profile is associated with mild cognitive impairment in the elderly: The J-SHIPP study. Hypertension Research, 33(1), 32-36. doi: 10.1038/hr.2009.172 [DOI] [PubMed] [Google Scholar]
- Hachinski V., Iadecola C., Petersen R. C., Breteler M. M., Nyenhuis D. L., Black S. E., . . . Leblanc G. G. (2006). National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke, 37, 2220-2241. doi: 10.1161/01.STR.0000237236.88823.47 [DOI] [PubMed] [Google Scholar]
- Iqbal P., Fotherby M. D., Potter J. F. (1996). Validation of the SpaceLabs 90207 automatic non-invasive blood pressure monitor in elderly subjects. Blood Pressure Monitoring, 1, 367-373. [PubMed] [Google Scholar]
- Kim Y. J., Kwon H. K., Lee J. M., Cho H., Kim H. J., Park H. K., . . . Seo S. W. (2016). Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology, 86, 1199-1207. doi: 10.1212/WNL.0000000000002516 [DOI] [PubMed] [Google Scholar]
- Lawton M. P., Brody E. M. (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9, 179-186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- Ling C., Diaz K. M., Kretzschmar J., Feairheller D. L., Sturgeon K. M., Perkins A., . . . Brown M. D. (2014). Chronic aerobic exercise improves blood pressure dipping status in African American non-dippers. Blood Pressure Monitoring, 19, 353-358. doi: 10.1097/MBP.0000000000000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M., Hoshide S., Ishikawa J., Shimada K., Kario K. (2008). Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. Journal of Hypertension, 26, 1636-1641. doi: 10.1097/HJH.0b013e3283018333 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., . . . Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- O’Brien E., Parati G., Stergiou G., Asmar R., Beilin L., Bilo G., . . . Zhang Y. (2013). European Society of Hypertension position paper on ambulatory blood pressure monitoring. Journal of Hypertension, 31, 1731-1768. doi: 10.1097/HJH.0b013e328363e964 [DOI] [PubMed] [Google Scholar]
- O’Rourke M. F., Safar M. E. (2005). Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension, 46, 200-204. doi: 10.1161/01.HYP.0000168052.00426.65 [DOI] [PubMed] [Google Scholar]
- Plummer P., Zukowski L. A., Giuliani C., Hall A. M., Zurakowski D. (2015). Effects of physical exercise interventions on gait-related dual-task interference in older adults: A systematic review and meta-analysis. Gerontology, 62(1), 94-117. doi: 10.1159/000371577 [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385-401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Salles G. F., Reboldi G., Fagard R. H., Cardoso C. R., Pierdomenico S. D., Verdecchia P., . . . Roush G. C. (2016). Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: The Ambulatory Blood Pressure Collaboration in Patients with Hypertension (ABC-H) meta-analysis. Hypertension, 67, 693-700. doi: 10.1161/HYPERTENSIONAHA.115.06981 [DOI] [PubMed] [Google Scholar]
- Sander D., Winbeck K., Klingelhofer J., Conrad B. (2000). Extent of cerebral white matter lesions is related to changes of circadian blood pressure rhythmicity. Archives of Neurology, 57, 1302-1307. [DOI] [PubMed] [Google Scholar]
- Seals D. R., Desouza C. A., Donato A. J., Tanaka H. (2008). Habitual exercise and arterial aging. Journal of Applied Physiology, 105, 1323-1332. doi: 10.1152/japplphysiol.90553.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A., Smith P. J., Hinderliter A. L., Georgiades A., Blumenthal J. A. (2016). Effects of exercise and stress management training on nighttime blood pressure dipping in patients with coronary heart disease: A randomized controlled trial. American Heart Journal, 183, 85-90. doi: 10.1016/j.ahj.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., Blumenthal J. A., Hoffman B. M., Cooper H., Strauman T. A., Welsh-Bohmer K., . . . Sherwood A. (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72, 239-252. doi: 10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey M. I., Knight E., Petrella R. J. (2012). The Step Test and Exercise Prescription Tool in Primary Care : A Critical Review. Critical ReviewsTM in Physical and Rehabilitation Medicine, 24(1), 109-123. [Google Scholar]
- ten Brinke L. F., Bolandzadeh N., Nagamatsu L. S., Hsu C. L., Davis J. C., Miran-Khan K., Liu-Ambrose T. (2014). Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. British Journal of Sports Medicine, 49, 248-254. doi: 10.1136/bjsports-2013-093184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel M. P., Henskens L. H., Kroon A. A., Hofman P. A., Gronenschild E. H., Jolles J., de Leeuw P. W. (2006). Ambulatory blood pressure, asymptomatic cerebrovascular damage and cognitive function in essential hypertension. Journal of Human Hypertension, 20(1), 5-13. doi: 10.1038/sj.jhh.1001934 [DOI] [PubMed] [Google Scholar]
- Verdecchia P. (2000). Prognostic value of ambulatory blood pressure : Current evidence and clinical implications. Hypertension, 35, 844-851. doi: 10.1161/01.HYP.35.3.844 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Table_1 for The Impact of Blood Pressure Dipping Status on Cognition, Mobility, and Cardiovascular Health in Older Adults Following an Exercise Program by Narlon C. Boa Sorte Silva, Michael A. Gregory, Dawn P. Gill, Cheri L. McGowan and Robert J. Petrella in Gerontology and Geriatric Medicine