Abstract
Objective: The goal of this study was to estimate the prevalence of mild cognitive impairment (MCI) in Georgia. Method: A population-based study was conducted using Georgian version of the Montreal Cognitive Assessment (MoCA) and its cognitive domain index score. Results: Of the initial cohort of 1,000 subjects, 851 met inclusion criteria. The prevalence of MCI was 13.3%, and it was associated with age >65 years (odds ratio [OR] = 4.51, 95% confidence interval [CI] = [3.00, 6.75]), urban residence (OR = 0.53, 95% CI = [0.33, 0.88]), lower education (OR = 3.99, 95% CI = [2.66, 5.93]), and hypertension (OR = 2.51, 95% CI = [1.68, 3.76]), while amnestic MCI was documented in 9.3%, with higher risk in older subjects (OR = 2.69, 95% CI = [1.66, 4.20]), and diabetics (OR = 2.69, 95% CI = [1.25, 5.98]). Conclusion: In this first population-based study of MCI in Georgia, prevalence was comparable with those reported from the United States and Europe. Observed association of MCI with cardiovascular risk factors has important clinical implication for dementia prevention in Georgia.
Keywords: mild cognitive impairment, dementia, epidemiology, Georgia
Introduction
Mild cognitive impairment (MCI) is a known risk factor for dementia (Petersen at al., 1999). Despite its current pronounced heterogeneity, the concept of MCI permits timely identification of patients at high risk of developing dementia, thus opening a potential therapeutic window and increasing the significance of controlling modifiable risk factors (Winblad et al., 2004).
Published prevalence rates for MCI vary from as low as 2% to 4% to greater than 20%. A number of prospective population-based studies in the United States, France, and Germany estimate the prevalence among older adults to be between 14% and 18% (Busse, Bischkopf, Riedel-Heller, & Angermeyer, 2003; Ganguli, Dodge, Shen, & DeKosky, 2004; Larrieu et al., 2002; Luck et al., 2007). However, epidemiological data on MCI in low and median income countries are sparse. Similar to Western countries, a population-based study in Kolkata, India, showed an overall prevalence rate of MCI at 14.9%, while a study in Brazil was half as common at 7.1% (Das et al., 2007; Herrera, Caramelli, Silveira, & Nitrini, 2002).
Results of the Delphi consensus study revealed a huge gap in data on cognitive impairment epidemiology, and therefore there is an urgent need for epidemiological research in Eastern Europe including Georgia (Ferri et al., 2005).
Given the lack of epidemiological data on MCI in Georgia, we sought to estimate the prevalence of MCI and to characterize its demographics and risk factors in a population-based study.
Method
A cross-sectional one-phase study was conducted to identify subjects with MCI among an urban and rural population of Georgia in individuals aged 40 years or older. The study was conducted during a 1 year period from March 1, 2014, to March 1, 2015. Institutional ethical approval was obtained from the Tbilisi State Medical University (Tbilisi, Georgia) before initiation of the study. Individual consent was obtained before enrollment in the study.
Georgia is a country in the South Caucasian region, bordering the Black Sea, with an area of 69,700 km2 and a population of 3.8 million according to 2014 census data (Results of General Population Census of Georgia, 2014). Georgian is a Kartvelian language spoken by Georgians and is the most pervasive of the family of Kartvelian languages. Georgian is written in its own Georgian scripts that is unique in their appearance and consists of a 33-letter alphabet. The predominant ethnic group are Georgians whom form about 86.8% of Georgia’s current population (Results of General Population Census of Georgia, 2014). The overall literacy rate in Georgia is 98.8%, while 53% of population resides in an urban area (Results of General Population Census of Georgia, 2014). Tbilisi is the capital of Georgia, is a major urban center, and almost 28.9% of the 1.1 million persons of Georgia reside in Tbilisi (Results of General Population Census of Georgia, 2014).
Results of General Population Census of Georgia a stratified survey method was used for the study. Georgia is divided into 12 territories (nine regions, one city, and two autonomous republics), and comprised the sampling frame for the study. Primary survey regions were stratified according to population and region type (urban vs. rural). Based on this strategy, one urban area, Tbilisi (central location, six administrative districts, population 1.1 million), and two rural areas—Kakheti (eastern location, eight administrative districts, population 318,000) and Imereti (western location, 12 administrative districts, population 534,000)—were selected. Survey subregions included all administrative districts of Tbilisi (six administrative districts, population 1.1 million) and one administrative district in both the Kakheti region (Sagarejo municipality rural settlement with population 681), and the Imereti region (Sachkhere municipality rural settlement with population 815; Results of General Population Census of Georgia, 2014). Both these settlements in Kakheti and Imereti regions are listed as rural settlements in 2014 Results of General Population Census of Georgia. We randomly selected these two villages: one in eastern and one in western Georgia because of cultural differences.
Study investigators contacted random households within each survey region. Where there was no response, the household was replaced by the next in order.
Sample size calculation was made based on previously reported MCI prevalence that was in a range of 2% to 20% (Busse et al., 2003; Das et al., 2007; Ganguli et al., 2004; Herrera et al., 2002; Larrieu et al., 2002; Luck et al., 2007). Averaging these data, 10% was used as an expected prevalence, allowable margin of error 2%, and 95% confidence interval (CI). Using formula n = (z2) P(1 – P) / d2 where n = sample size, z = z statistic for the level of confidence, P = expected prevalence, and d = allowable margin of error (Arya, Antonisamy, & Kumar, 2012), calculation yielded sample size of n = 864 subjects. Given about 15% possibility of incomplete data, we targeted total of N = 1,000 subjects. To have rural population representation in the study, we decided to recruit 20% (n = 200) of aforementioned sample of N = 1,000 from two rural regions of Georgia described above. No information was collected on households that either refused to be enrolled or were unavailable to participate.
Cognitive Testing
All individuals in these households were evaluated using the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005). The MoCA was previously translated into Georgian language and was validated showing its reliability and accuracy for evaluation of MCI (Janelidze et al., 2017).
MoCA cognitive domain index score (CDIS) was used to evaluate memory, executive function, visuospatial, language, attention, and orientation abnormalities (Julayanont, Brousseau, Chertkow, Phillips, & Nasreddine, 2014). CDIS was calculated as follows:
The memory index score (MIS) was calculated by adding the number of words remembered in free delayed recall with a score ranging from 0 to 5.
The executive index score (EIS) was calculated by adding raw scores for the modified Trail-Making Test Part B, clock drawing, digit span forward and backward, letter A tapping, serial-7 subtraction, letter fluency, and abstraction, with a score ranging from 0 to 13.
The visuospatial index score (VIS) was determined by adding the raw scores of the cube copy, clock drawing, and naming, with a score ranging from 0 to 7.
The language index score (LIS) was obtained by adding the raw scores for naming, sentence repetition, and letter fluency, with a score ranging from of 0 to 6.
The attention index score (AIS) was obtained by adding the raw scores for digit span forward and backward, letter A tapping, serial-7 subtraction, sentence repetition, and the words recalled in both immediate recall trials, with a score ranging from 0 to 18.
The orientation index score (OIS) was calculated as a sum of points for the orientation section of the MoCA, with a score ranging from 0 to 6 (Julayanont et al., 2014).
MIS, VIS, EIS, and LIS were used to categorize the MCI subtypes into single domain amnestic and nonamnestic, as well as multidomain amnestic (memory impairment plus one other impaired domain) and nonamnestic (Julayanont et al., 2014). Participants who scored less than 1.5 SD below the age- and education-adjusted mean value in MIS, VIS, EIS, and LIS were considered as being impaired in that cognitive domain.
Definition of MCI
We used International Working Group on MCI consensus criteria which defines MCI as follows: (a) the individual is neither normal nor demented (see the Diagnostic and Statistical Manual of Mental Disorders [4th ed.; DSM-IV; American Psychiatric Association, 1994] criteria); (b) there is evidence of cognitive deterioration, shown by objectively measured (>1.5 SD below mean value) decline; and (c) activities of daily life are preserved and complex instrumental functions are either intact or minimally impaired (Gauthier et al., 2006; Winblad et al., 2004).
For the multiple domain MCI type, we defined as deficits evidenced by scores >1.5 SD below mean values in more than one areas of cognitive functioning with or without memory impairment. Based on these criteria, MCI was divided in five categories: any MCI (MoCA >1.5 SD below mean value), amnestic, nonamnestic, multidomain amnestic, and multidomain nonamnestic.
We excluded individuals with MoCA < 16 from analysis classifying them moderately to severely impaired similar to Ganguli, Chang, Snitz, Saxton, Vanderbilt, & Lee (2010) study that excluded subjects with Mini Mental Status Examination (MMSE) < 21. This MoCA score threshold was chosen based on the evidence from Alzheimer’s Disease (AD) Imaging Initiative study, where MoCA cut-off score of 16 corresponded to MMSE score of 21 (Trzepacz, Hochstetler, Wang, Walker, & Saykin, 2015).
Data Collection
Demographic information was collected on each participant including: age, gender, level of education, and region (urban vs. rural). We also obtained information on the following medical conditions: smoking, hypertension (HTN), diabetes mellitus (DM), and hyperlipidemia (HLD). These medical conditions were reported by subjects and no medical record review or testing was performed. Only current smoking was documented.
Because of the fact that until recently, full school education in Georgia lasted 11 years, subjects were classified into two groups: (a) General (school graduation) with <12 years (11 years) of education (11 years); (b) Higher-with ≥12 years of education. Based on these, study subjects were asked simple question if they have general education (11 years) or higher education which means >12 years.
Statistical Analysis
Values were reported as mean (±SD), percentage, and the two-tailed p or Fisher’s exact two-sided tests were performed to compare the means or the distributions of variables as appropriate. One-way ANOVA was used for comparison of multiple groups. Spearman r correlation was used for correlations; p < .05 was considered statistically significant. SPSS version 22 and GraphPad Prism7 were used for statistical analysis.
Results
In all, 1,000 subjects were evaluated for the study, 149 were excluded for following reasons: n = 46 with MoCA < 16, n = 31 with incomplete data, n = 25 could not complete the test due to unknown reasons and n = 47 due to neurologic or psychiatric problems. In all, 851 subjects were enrolled in the study. Participants had a mean age of 56.5 ± 11.8 years, 63% were women, and 71% had ≥12 years of education. The rates were similar to the Georgian population >40 years of age, except for a higher rate of women (63.3% vs. 56%; Table 1). The prevalence of medical disorders among subjects included the following: HTN 26%, diabetes 4.6%, HLD 8.4%, and current smoking 23.6%.
Table 1.
Demographic Characteristics of Studied Versus Georgian Population >40 Years.
| Variable | All (n = 851) | Tbilisi (n = 719) | Rural (n = 132) | Georgian populationa |
|---|---|---|---|---|
| Age (M ± SD) | 56.5 ± 11.8 | 56.2 ± 11.8 | 57.9 ± 11.8 | 58.6 ± 12.5 |
| 40-49 years (%) (n = 298) |
35.1 | 35.4 | 32.5 | 27.7 |
| 50-59 years (%) (n = 222) |
26.1 | 24.3 | 35.6 | 29.6 |
| 60-69 years (%) (n = 188) |
22.1 | 22.2 | 21.2 | 21.1 |
| 70-79 years (%) (n = 120) |
14.1 | 13.2 | 18.9 | 14.9 |
| ≥80 years (%) (n = 23) |
3.0 | 2.7 | 2.3 | 2.7 |
| Female (%) (n = 539) |
63.3 | 65.8 | 50.0** | 56.0 |
| Higher education (%) (n = 608) |
71.4 | 80.0 | 25.0* | 78b |
Georgian population >40 years old per Results of General Population Census of Georgia (2014).
Urban rate per census, rural reported as 22% (Results of General Population Census of Georgia, 2014).
p < .0001 in rural versus Tbilisi. **p < .001 in rural versus Tbilisi.
The prevalence of any MCI was 13.3%. There was significantly higher prevalence of any MCI in males (odds ratio [OR] = 1.54, 95% CI = [1.03, 2.27], p < .05), older subjects with age >65 years (OR = 4.51, 95% CI = [3.00, 6.75], p < .0001) and general versus higher education group (OR = 3.99, 95% CI = [2.66, 5.93], p < .0001; Table 2), as well as among those with HTN (OR = 2.51, 95% CI = [1.68, 3.76], p < .0001; Table 3), while residents of Tbilisi compared with rural population had lower (OR = 0.53, 95% CI = [0.33, 0.88], p < .05) prevalence of any MCI (Table 2).
Table 2.
Prevalence of Different Types of MCI According to Demographics.
| All | aMCI n (%) |
naMCI n (%) |
mdaMCI n (%) |
namdMCIn (%) | Any MCI n (%) |
|---|---|---|---|---|---|
| n = 851 | 79 (9.3) | 90 (10.5) | 23 (2.7) | 31 (3.6) | 114 (13.3) |
| Tbilisi (n = 719) | 64 (8.9) | 74 (10.2) | 18 (2.5) | 22 (3.0) | 87 (12.1) |
| Rural (n = 132) | 15 (11.3) | 16 (12.1) | 5 (3.8) | 9 (6.8) | 27 (20.4) |
| OR (95% CI) | 0.78 [0.41, 1.32] | 0.83 [0.47, 1.49] | 0.83 [0.25, 1.63] | 0.43 [0.19; 0.98]§ | 0.53 [0.33, 0.88]§ |
| Age ≥65 (n = 238) | 38 (16.0) | 29 (9.9) | 8 (3.3) | 12 (5.1) | 66 (27.7) |
| Age <65 (n = 613) | 41 (6.7) | 61 (12.1) | 15 (2.4) | 19 (3.1) | 48 (7.8) |
| OR (95% CI) | 2.65 [1.66, 4.20]* | 1.25 [0.79, 1.99] | 1.39 [0.61, 3.18] | 1.67 [0.81, 3.56] | 4.51 [3.00, 6.75]* |
| Male (n = 312) | 34 (10.9) | 29 (9.3) | 11 (3.5) | 10 (3.2) | 52 (16.6) |
| Female (n = 539) | 45 (8.3) | 61 (11.3) | 12 (2.2) | 21 (3.8) | 62 (11.5) |
| OR (95% CI) | 1.34 [0.83, 2.13] | 0.80 [0.51, 1.26] | 1.60 [0.73, 3.74] | 0.82 [0.40, 1.76] | 1.54 [1.03, 2.27]§ |
| General education (n = 243) | 22 (9.0) | 40 (16.4) | 11 (4.5) | 17 (6.7) | 64 (26.3) |
| Higher education (n = 608) | 57 (9.3) | 50 (8.2) | 12 (2.0) | 14 (2.3) | 50 (8.2) |
| OR (95% CI) | 0.96 [0.58, 1.60] | 2.20 [1.40, 3.40]† | 2.35 [1.07, 5.51]‡ | 3.19 [1.60, 6.68]† | 3.99 [2.66, 5.93]* |
Note. Any MCI = MoCA < 22. MCI = mild cognitive impairment; aMCI = amnestic MCI; naMCI = nonamnestic MCI; mdaMCI = multidomain MCI; namdMCI = nonamnestic multidomain MCI; OR = odds ratio; CI = confidence interval; MoCA = Montreal Cognitive Assessment.
nonsignificant p < .1. *p < .0001. ‡p < .001. †p < .01. §p < .05.
Table 3.
Prevalence of Different Types of MCI According Risk Factors.
| aMCI n (%) |
naMCI n (%) |
mdaMCI n (%) |
namdMCI n (%) |
Any MCI n (%) |
|
|---|---|---|---|---|---|
| HTN+ (n = 225) | 28 (12.4) | 29 (12.8) | 9 (4.0) | 10 (4.4) | 50 (22.2) |
| HTN– (n = 626) | 51 (8.1) | 61 (9.7) | 14 (2.2) | 21 (3.3) | 64 (10.2) |
| OR (95% CI) | 1.60 [0.97, 2.58] | 1.37 [0.86, 2.18] | 1.84 [0.75, 4.19] | 1.34 [0.65, 2.91] | 2.51 [1.68, 3.76]* |
| DM+ (n = 39) | 8 (20.5) | 2 (5.1) | 2 (5.1) | 3 (7.6) | 9 (23.1) |
| DM– (n = 812) | 71 (8.7) | 88 (10.8) | 21 (2.6) | 28 (3.4) | 105 (12.9) |
| OR (95% CI) | 2.69 [1.25, 5.98]§ | 0.44 [0.10, 1.71] | 2.04 [0.46, 8.31] | 2.33 [0.72, 7.11] | 2.02 [0.93, 4.22] |
| HLD+ (n = 65) | 10 (15.4) | 3 (4.6) | 2 (3.1) | 0 (0.0) | 10 (15.3) |
| HLD– (n = 786) | 69 (8.8) | 87 (11.1) | 21 (2.7) | 31 (4.0) | 104 (13.2) |
| OR (95% CI) | 1.89 [0.93, 3.72] | 0.39 [0.13, 1.19] | 1.16 [0.26, 4.52] | 0.00 [0.00, 1.28] | 1.35 [0.66, 2.70] |
| Smoking+ (n = 201) | 8 (4.0) | 18 (8.9) | 4 (2.0) | 4 (2.0) | 22 (10.9) |
| Smoking– (n = 650) | 71 (10.9) | 72 (11.1) | 19 (2.9) | 27 (4.1) | 92 (14.1) |
| OR (95% CI) | 0.34 [0.16, 0.70]† | 0.79 [0.46, 1.35] | 0.67 [0.24, 1.92] | 0.47 [0.17, 1.32] | 0.74 [0.45, 1.21] |
Note. Any MCI = MoCA < 22; MCI = mild cognitive impairment; aMCI = amnestic MCI; naMCI = nonamnestic MCI; mdaMCI = multidomain MCI; namdMCI = nonamnestic multidomain MCI; HTN = hypertension; OR = odds ratio; CI = confidence interval; DM = diabetes mellitus; HLD = hyperlipidemia; MoCA = Montreal Cognitive Assessment.
nonsignificant p < .1. *p < .0001. ‡p < .001. †p < .01. §p < .05.
The mean MoCA score of the study population was 25.2 ± 3.1. There was a statistically significant negative correlation between age and MoCA score (r = −.252, p < .0001), as well as age and CDISs including MIS (r = −.225, p < .0001), EIS (r = −.091, p = .008), LIS (r = −.215, p < .0001), VIS (r = −.089, p = .009), AIS (r = −.268, p < .0001), and a negative trend with OIS, although not significant (r = −.065, p = .058).
The prevalence of amnestic MCI (aMCI) was 9.3% (Table 2), and it was significantly higher with subjects older than 65 years, as well as those with diabetes (Table 3). On the contrary, smokers had significantly lower prevalence of aMCI (Table 3). Smokers were significantly younger (54.6 ± 11.1 vs. 57.1 ± 11.9, p = .008), but there was no difference in proportion of subjects with degree between smokers and nonsmokers (66.3% vs. 72.0%, p = .77).
Nonamnestic MCI (naMCI) was documented in 10.5% of subjects with significantly higher prevalence in high school nondegree graduates (Table 2). Multidomain amnestic MCI (mdaMCI) was observed in 2.7% of subjects with significantly higher prevalence among high school nondegree graduates (Table 2). Finally, nonamnestic multidomain MCI (namdMCI) prevalence was 3.4% with significant increase in high school nondegree graduates and significant decrease among Tbilisi residents (Table 2).
Figure 1 shows prevalence of MCI types according different age groups. There was significant increase in prevalence of all types of MCIs according age groups except naMCIs including both single and multidomain (Figure 1).
Figure 1.
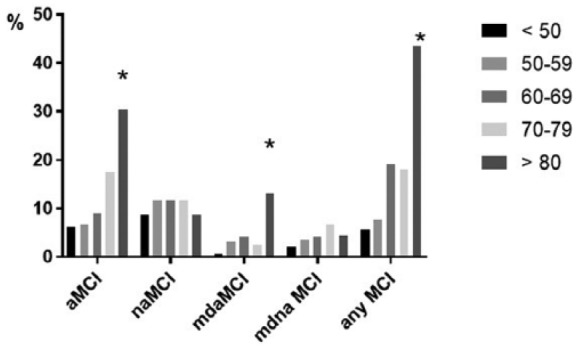
Prevalence of different MCI subtypes according age.
Note. GraphPad Prism7 software was used to create Figure 1. Any MCI = MoCA score < 22. MCI = mild cognitive impairment; aMCI = amnestic MCI; naMCI = nonamnestic MCI; mdaMCI = multidomain MCI; MoCA = Montreal Cognitive Assessment.
Discussion
In this population-based, cross-sectional study of MCI in Republic of Georgia the prevalence of MCI was 13.3%. This is similar to 16.0% reported by Petersen et al. (2010), 17.7% observed by Ganguli et al. (2010), 18.8% by Lopez et al. (2003; all in U.S. cohorts), and 19.4% by Busse, Hensel, Gühne, Angermeyer, & Riedel-Heller (2006) in Germany. However, 13.3% reported here is much lower compared with 42.0% documented in France (Artero et al., 2008), 28.3% in Northern Manhattan (Manly et al., 2005), and 39.1% in Australia (Sachdev et al., 2012). On the contrary, our finding is almost twice higher compared with 6.3% found in some U.S. studies, 6.5% in Finland, and 5.1% in Germany (Busse et al., 2003; Ganguli et al., 2004; Hänninen, Hallikainen, Tuomainen, Vanhanen, & Soininen, 2002) There are several explanations for varying estimates of MCI prevalence reported in these studies including age, MCI criteria used, and cut-points for abnormality for neuropsychological test scores (Rosebud & Knopman, 2013). In the current study, among older subjects >65 years the overall prevalence of MCI was 27.7%, which is similar to studies from U.S. reporting 22.2% and 23.4% (Fisk, Merry, & Rockwood, 2003; Plassman et al., 2008; Unverzagt et al., 2001).
We documented aMCI in 9.9% of studied subjects, which is comparable with the prevalence of 14.9% reported in Kolkata, India (Das et al., 2007). Higher prevalence of aMCI in India most likely reflects older age, as mean age of their cohort was 10 years older compared with the current study. Lower prevalence of aMCI ranging from 2.4% to 5.3% was reported in population-based cohorts from Canada and Finland (Fisk et al., 2003; Hänninen et al., 2002). In Finnish study, it is possible that exclusion of subjects older than 76 years resulted an underestimation of the prevalence, while Canadian study used more strict aMCI criteria that might yield relatively lower population prevalence estimate (Fisk et al., 2003; Hänninen et al., 2002).
In this study, multiple domain MCIs including amnestic and nonamnestic ranged from 3.3% to 5.3%. Similar to aMCI, these rates were comparable with 8.8% reported from Kolkata, India (Das et al., 2007). As in aMCI, slightly lower prevalence of multiple domain MCIs in the current study most likely is a reflection of younger age.
Age is the strongest associated factor with MCI (Busse et al., 2006; Das et al., 2007; Ganguli et al., 2010, 2011; Hänninen et al., 2002; Trzepacz et al., 2015; Unverzagt et al., 2001). In the current study, age >65 years was associated with 4.5-fold increase in any MCI prevalence (Table 2), and each 10-year increase in age was associated with statistically significant increase in prevalence of any and aMCIs including single and multidomain (Figure 1). In addition, age negatively correlated with CDIS scores including memory, language, executive, visual, and attention scores, while orientation was nearly significant.
This study found statistically significant 1.5-fold increase in prevalence of any MCI among males compared with female subjects. This is similar to studies from India, Finland, and the United States (Das et al., 2007; Hanninen et al., 2002; Petersen et al., 2010; Plassman et al., 2008) but opposite to report of Luck et al. (2007) showing 1.4-fold increase of MCI among women in a cohort recruited from primary care clinic in Germany. Although men may have a higher risk of MCI, women are disproportionally affected with AD (Mielke, Vemuri, & Rocca, 2014).
We found 47% lower prevalence of MCI and 57% lower risk of namdMCI in Tbilisi compared with rural residents (Table 2). Most likely explanation of this finding is the fact that proportion of subjects with higher education in Tbilisi was more than three-fold higher compared with rural regions (Table 1). However, those with general education compared with higher education cohort had significantly higher prevalence of all types of MCI except aMCI (Table 2). A protective effect of education on cognitive function has been noted in other studies as well (Fisk et al., 2003; Hänninen et al., 2002).
In this study, smoking was associated with 66% lower prevalence of aMCI. Earlier case–control studies also reported reduced risk of AD among smokers (Lee, 1994). The most likely explanation of smoking paradox reported in current study is the fact that smokers were significantly younger. Subsequent cohort study examining smoking in midlife, have found that smoking is a risk factor for AD (Ott et al., 1998).
We found statistically significant more than two-fold higher prevalence of any MCI in subjects with HTN and diabetes (Table 3). In addition, aMCI was strongly associated with diabetes, but not with HTN (Table 3). Several studies have identified association of vascular risk factors with MCI and their role in progression of MCI to dementia (DeCarli et al., 2001; Di Carlo et al., 2000; Kivipelto et al., 2001; Solfrizzi et al., 2004). In a recent positron emission tomography study of individuals without dementia from three U.S. communities, a cumulative number of midlife vascular risk factors was associated with elevated brain amyloid deposition suggesting a role of vascular disease in the development of AD (Gottesman et al., 2017).
In the current study, mean age, proportion of subjects in age subgroups (10-year increments), as well as proportion of individuals with general and/or higher education was comparable with the Georgian population older than 40 years (Table 1). The female prevalence of 63% reported in this study was higher than 56% documented among Georgian population of corresponding age older than 40 years in Georgian Census (2014). However, female prevalence of 50.0% in rural community of studied participants was compatible to 47.7% in rural regions of Georgian population reported in Results of General Population Census of Georgia (2014).
Although 23.6% prevalence of smoking in this study was compatible to previously reported 27.7% (Grim et al., 1999), prevalences of other vascular risk factors were much lower in this study including HTN (26.0% vs. 56%), DM (4.6% vs. 10.0%), and HLD (31.0% vs. 7.6%). However, Grim et al. (1999) study was conducted almost 20 years ago, and recent meta-analysis of HTN in low- and middle-income countries shows 31.5% prevalence in Europe and Central Asia, which is comparable with 26.0% reported here (Sarki, Nduka, Stranges, Kandala, & Uthman, 2015). In addition, prevalence of DM in Georgia recently was reported to be 2.2%, which is close to observed 4.6% in this study, while HLD prevalence of 8.7% is exactly the same as documented here (Wilkins et al., 2017).
As for the large-scale epidemiological study of the MCI complete neuropsychological testing in the field is not feasible, we used MoCA with its CDIS (Julayanont et al., 2014; Nasreddine et al., 2005). The Georgian version of MoCA was previously validated showing reliability and accuracy of this test for evaluation of MCI (Janelidze et al., 2017). In that study, MoCA < 22 was optimal to detect MCI with 100% sensitivity and 69% specificity (Janelidze et al., 2017). In the current study, based on 1.5 SD below normative values, the same cut-off MoCA < 22 was found to be an optimal threshold for MCI. Lam et al. (2013) have shown that MoCA is a valid tool for assessment of cognition that shows good agreement with existing neuropsychological screening tests and global measures. In addition, MoCA subscores for different cognitive domains also demonstrated validity when compared with neuropsychological testing–derived measures (Lam et al., 2013). In that study, in the case of memory, executive, and visuospatial dysfunction, the MoCA’s subscores were reasonable screens for domain-specific impairment (Lam et al., 2013). In another study, individuals with MCI with a low MoCA MIS score were at high risk of conversion to AD (Julayanont et al., 2014).
The study has some limitations. One possible limitation is inclusion of subjects with relatively younger age >40 years. We considered this age cut-off because of previously reported high prevalence of vascular risk factors in a similar age group (mean age of 55 years) of Georgian population with a prevalence of HTN 56%, DM 10%, and HLD 31% (Grim et al., 1999). Another potential shortcoming of this study which is a common problem with cross-sectional studies is nonresponse and lack of the data on nonresponse rate. We cannot completely rule out possibility that those who refuse to participate are more impaired than those who agree to participate. The lack of information on nonresponders and those that refused to participate might create possible bias. However, given the fact that demographic profile of the studied population is comparable with Georgian population of the same age, sampling biases are less likely and the results are generalizable to whole Georgian population. Representability of rural community in the study can be potential methodological shortcoming. As 20% rate of rural residence is lower than 42% reported in recent 2014 census, this might be potential limitation of the study. However, this sample size allowed appropriate subgroups analysis. Another potential limitation is the selection of rural sites for the study. The rate of MCI was lower in Tbilisi as compared with rural regions. The two rural regions were selected for the study were based on study investigators, geographic region (east and west), and population. Each region was defined as a rural settlement based on a 2014 census (Results of General Population Census of Georgia, 2014). We registered cardiovascular comorbidities based on survey without confirmation in medical or pharmacy records. However, in a recent study, self-reported diagnosis sensitivity for HTN was 83%, for diabetes 73%, and for hypercholesterolemia 59% while specificity was >80% for all three conditions indicating that self-reports are reasonably accurate for certain chronic conditions and can provide a useful estimate for broad measures of population prevalence (Martin, Leff, Calonge, Garrett, & Nelson, 2000).
In conclusion, in this population-based cross-sectional study, prevalence of MCI in Georgia was 13.3%, and it was associated with advanced age, male gender, rural residence, lower education, HTN, and diabetes. These findings have significant implications not only for better understanding of MCI profile in Georgia, but also for public health planning in this country as aggressive vascular risk factor control intervention potentially may prevent progression of MCI to dementia.
Footnotes
Author Contributions: All authors made substantial contributions to the conception and design of the study and analysis and interpretation of data. Marina Janelidze: study concept and design, contributed to drafting the paper and revising it critically for intellectual content; Nino Mikeladze: data acquisition, analysis and interpretation of data, contributed to drafting the paper and revising it critically for intellectual content; Nazibrola Bochorishvili: data acquisition, contributed to drafting the paper and revising it critically for intellectual content; Ann Dzagnidze: data acquisition, contributed to drafting the paper and revising it critically for intellectual content; Mariam Kapianidze: data acquisition, contributed to drafting the paper and revising it critically for intellectual content; Nino Mikava: data acquisition, contributed to drafting the paper and revising it critically for intellectual content; Irene Khatiashvili; data acquisition, contributed to drafting the paper and revising it critically for intellectual content; Ekaterina Mirvelashvili: study design, statistical analysis of the data; Nino Shiukashvili: data collection, statistical analysis of the data; John K. Lynch: design, analysis and interpretation of data and revising the manuscript critically for intellectual content; Zurab Nadareishvili: study concept and design, analysis and interpretation of data, drafting the paper and revising it critically for intellectual content, preparation the manuscript, and final approval.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Rustaveli Georgian National Scientific Foundation, Grant N DI/16/8-313/13, Tbilisi, Georgia.
ORCID iD: Zurab Nadareishvili  https://orcid.org/0000-0002-5526-1342
https://orcid.org/0000-0002-5526-1342
References
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Artero S., Ancelin M. L., Portet F., Dupuy A., Berr C., Dartigues J. F., . . . Ritchie K. (2008). Risk profiles for mild cognitive impairment and progression to dementia are gender specific. Journal of Neurology, Neurosurgery, & Psychiatry, 79, 979-984. [DOI] [PubMed] [Google Scholar]
- Arya R., Antonisamy B., Kumar S. (2012). Sample size estimation in prevalence studies. Indian Journal of Pediatrics, 79, 1482-1488. [DOI] [PubMed] [Google Scholar]
- Busse A., Bischkopf J., Riedel-Heller S. G., Angermeyer M. C. (2003). Mild cognitive impairment: Prevalence and incidence according to different diagnostic criteria. Results of the Leipzig Longitudinal Study of the Aged (LEILA75+). The British Journal of Psychiatry, 182, 449-454. [PubMed] [Google Scholar]
- Busse A., Hensel A., Gühne U., Angermeyer M. C., Riedel-Heller S. G. (2006). Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology, 67, 2176-2185. [DOI] [PubMed] [Google Scholar]
- Das S. K., Bose P., Biswas A., Dutt A., Banerjee T. K., Hazra A., Roy T. (2007). An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology, 68, 2019-2026. [DOI] [PubMed] [Google Scholar]
- DeCarli C., Miller B. L., Swan G. E., Reed T., Wolf P. A., Carmelli D. (2001). Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Archives of Neurology, 58, 643-647. [DOI] [PubMed] [Google Scholar]
- Di Carlo A., Baldereschi M., Amaducci L., Maggi S., Grigoletto F., Scarlato G., Inzitari D. (2000). Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. Journal of the American Geriatrics Society, 48, 775-782. [DOI] [PubMed] [Google Scholar]
- Ferri C. P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M. . . . Alzheimer’s Disease International. (2005). Global prevalence of dementia: A Delphi consensus study. The Lancet, 366, 2112-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk J. D., Merry H. R., Rockwood K. (2003). Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology, 61, 1179-1184. [DOI] [PubMed] [Google Scholar]
- Ganguli M., Chang C. C., Snitz B. E., Saxton J. A., Vanderbilt J., Lee C. W. (2010). Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. The American Journal of Geriatric Psychiatry, 18, 674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M., Dodge H. H., Shen C., DeKosky S. T. (2004). Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology, 63, 115-121. [DOI] [PubMed] [Google Scholar]
- Ganguli M., Snitz B. E., Saxton J. A., Chang C.-C. H., Lee C. W., Bilt J. V., . . . Petersen R. S. (2011). Outcomes of mild cognitive impairment by definition: A population study. Archives of Neurology, 68, 761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S., Reisberg B., Zaudig M., Petersen R. C., Ritchie K., Broich K., . . . Winblad B. (2006). Mild cognitive impairment. The Lancet, 367, 1262-1270. [DOI] [PubMed] [Google Scholar]
- Gottesman R. F., Schneider A. L., Zhou Y., Coresh J., Green E., Gupta Knopman D. S., . . . Mosley T. H. (2017). Association between midlife vascular risk factors and estimated brain amyloid deposition. The Journal of the American Medical Association, 317, 1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim C. E., Grim C. M., Petersen J. R., Li J., Tavill F., Kipshidze N. N., . . . Kipshidze N. (1999). Prevalence of cardiovascular risk factors in the Republic of Georgia. Journal of Human Hypertension, 13, 243-247. [DOI] [PubMed] [Google Scholar]
- Hänninen T., Hallikainen M., Tuomainen S., Vanhanen M., Soininen H. (2002). Prevalence of mild cognitive impairment: A population-based study in elderly subjects. Acta Neurologica Scandinavica, 106, 148-154. [DOI] [PubMed] [Google Scholar]
- Herrera E., Jr., Caramelli P., Silveira A. S., Nitrini R. (2002). Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Disease and Associated Disorders, 16, 103-108. [DOI] [PubMed] [Google Scholar]
- Janelidze M., Mikeladze N., Bochorishvili N., Dzagnidze A., Kapianidze M., Mikava N., . . . Nadareishvili Z. (2017). Validity of the Georgian Montreal Cognitive Assessment for the Screening of Mild Cognitive Impairment and Dementia. American Journal of Alzheimer’s Disease & Other Dementias®, 31, 36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julayanont P., Brousseau M., Chertkow H., Phillips N., Nasreddine Z. (2014). Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive Impairment to Alzheimer’s disease. Journal of the American Geriatrics Society, 62, 679-684. [DOI] [PubMed] [Google Scholar]
- Kivipelto M., Helkala E. L., Hänninen T., Laakso M. P., Hallikainen M., Alhainen K., . . . Nissinen A. (2001). Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology, 56, 1683-1689. [DOI] [PubMed] [Google Scholar]
- Lam B., Middleton L. E., Masellis M., Stuss D. T., Harry R. D., Kiss A., Black S. E. (2013). Criterion and convergent validity of the Montreal Cognitive Assessment with screening and standardized neuropsychological testing. Journal of the American Geriatrics Society, 61, 2181-2185. [DOI] [PubMed] [Google Scholar]
- Larrieu S., Letenneur L., Orgogozo J. M., Fabrigoule C., Amieva H., Le Carret N., . . . Dartigues J. F. (2002). Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology, 59, 1594-1599. [DOI] [PubMed] [Google Scholar]
- Lee P. N. (1994). Smoking and Alzheimer’s disease: A review of the epidemiological evidence. Neuroepidemiology, 13, 131-144. [DOI] [PubMed] [Google Scholar]
- Lopez O. L., Jagust W. J., DeKosky S. T., Becker J. T., Fitzpatrick A., Dulberg C., Kuller L. H. (2003). Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Archives of Neurology, 60, 1385-1389. [DOI] [PubMed] [Google Scholar]
- Luck T., Riedel-Heller S. G., Kaduszkiewicz H., Bickel H., Jessen F., Pentzek M., . . . Weyerer S. (2007). Mild cognitive impairment in general practice: age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe). Dementia and Geriatric Cognitive Disorders, 24, 307-312. [DOI] [PubMed] [Google Scholar]
- Manly J. J., Bell-McGinty S., Tang M. X., Schupf N., Stern Y., Mayeux R. (2005). Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Archives of Neurology, 62, 1739-1746. [DOI] [PubMed] [Google Scholar]
- Martin L. M., Leff M., Calonge N., Garrett C., Nelson D. E. (2000). Validation of self-reported chronic conditions and health services in a managed care population. American Journal of Preventive Medicine, 18, 215-218. [DOI] [PubMed] [Google Scholar]
- Mielke M. M., Vemuri P., Rocca W. A. (2014). Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clinical Epidemiology, 6, 37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., . . . Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695-699. [DOI] [PubMed] [Google Scholar]
- Ott A., Slooter A. J., Hofman A., van Harskamp F., Witteman J. C., Van Broeckhoven C., . . . Breteler M. M. (1998). Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: The Rotterdam Study. The Lancet, 351, 1840-1843. [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Roberts R. O., Knopman D. S., Geda Y. E., Cha R. H., Pankratz V. S., . . . Rocca W. A. (2010). Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology, 75, 889-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., Kokmen E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56, 303-308. [DOI] [PubMed] [Google Scholar]
- Plassman B. L., Langa K. M., Fisher G. G., Heeringa S. G., Weir D. R., Ofstedal M. B., . . . Wallace R. B. (2008). Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine, 148, 427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results of general population census of Georgia. (2014). Retrieved from http://www.geostat.ge
- Rosebud R., Knopman D. S. (2013). Classification epidemiology of MCI. Clinics in Geriatric Medicine, 29, 753-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P. S., Lipnicki D. M., Crawford J., Reppermund S., Kochan N. A., Trollor J. N., . . . Brodaty H. (2012). Risk profiles for mild cognitive impairment vary by age and sex: The Sydney memory and ageing study. The American Journal of Geriatric Psychiatry, 20, 854-20865. [DOI] [PubMed] [Google Scholar]
- Sarki A. M., Nduka C. U., Stranges S., Kandala N. B., Uthman O. A. (2015). Prevalence of hypertension in low- and middle-income countries: A systematic review and meta-analysis. Medicine (Baltimore), 94(50), e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V., Panza F., Colacicco A. M., D’Introno A., Capurso C., Torres F. . . . Italian Longitudinal Study on Aging Working Group. (2004). Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology, 63, 1882-1891. [DOI] [PubMed] [Google Scholar]
- Trzepacz P. T., Hochstetler H., Wang S., Walker B., Saykin A. J. (2015). Relationship between the Montreal Cognitive Assessment and Mini-Mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatrics, 15, 107-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt F. W., Gao S., Baiyewu O., Ogunniyi A. O., Gureje O., Perkins A., . . . Hendrie H. C. (2001). Prevalence of cognitive impairment: Data from the Indianapolis Study of Health and Aging. Neurology, 57, 1655-1662. [DOI] [PubMed] [Google Scholar]
- Wilkins E., Wilson L., Wickramasinghe K., Bhatnagar P., Leal J., Luengo-Fernandez R., . . . Townsend N. (2017). European Cardiovascular Disease Statistics 2017. Brussels, Belgium: European Heart Network. [Google Scholar]
- Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L. O., Petersen R. C. (2004). Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256, 240-246. [DOI] [PubMed] [Google Scholar]


