Abstract
Background and Study Aims:
The prevalence of gallbladder cancer (GBC) varies between different parts of the world. This study is a review of literature and an update of a previously published study conducted in our university and aims to reassess the incidence of GBC over the past 2 decades.
Patients and Methods:
We conducted a retrospective study between 2002 and 2016. Data regarding demographics, clinical presentation, risk factors, histopathology, investigations, and treatments were obtained. A diagnosis of GBC established during surgery or primarily detected in the surgical specimen was classified as incidental.
Results:
Of 11 391 cholecystectomies performed, 31 cases (0.27%) of GBC were found. The mean age of patients with GBC was 68 years (43-103 years), 74% were women. The annual incidence of GBC was 0.2/100 000 (men: 0.1/100 000; women: 0.3/100 000). Biliary colic and acute cholecystitis were the main presentations. Diagnosis of GBC was “incidental” in 67% of cases. About 75% of patients with GBC had gallstones, 13% had polyps, and 3% had porcelain gallbladder. Adenocarcinoma was the dominant (87%) histologic type.
Conclusions:
The GBC rate in our region, similar to others parts of the world, is still low and has not changed over the past 2 decades. This study consolidates the previously published recommendations regarding the high index of suspicion of GBC in elderly with cholelithiasis.
Keywords: Gallbladder cancer, Jordan, jaundice
Introduction
Gallbladder cancer (GBC) is the most common malignant biliary tumor and the most aggressive touting the shortest median survival of all biliary tract cancers.1 First described by Stoll in 1777, it is still considered a highly malignant disease with poor outcomes.2 Only 10% of patients diagnosed with GBC are candidates for potentially curative surgical resection.3
According to the International Agency for Research on Cancer,4 the estimated number of new GBC cases worldwide in 2012 was 178 000 (age standardized rate: 2.2 per 100 000). The prevalence of GBC varies vastly between different parts of the world. The variable geographic incidence of GBC may reflect a risk factor distribution for this tumor.
In the period 1992-2002, declining overall GBC mortality trends were observed in 50 studied countries. Only Iceland, Costa Rica, and Korea were found to have an increase in mortality in men. The authors attribute this decline to the increased awareness of this disease and better diagnostic modalities resulting in appropriate staging of gallbladder and biliary cancers.5 Another recent study that analyzed data from the Danish Cancer Registry reported a minor improvement observed in the 5-year relative survival from 6% to 9% to 13% to 16% in the past 15 years for all age groups except those aged 90+ years.6
A previous published study conducted in our university revealed 33 GBC out of 4502 cholecystectomies performed over a period of 7 years.7 This study is a continuation and an update of the previous one and aims to assess whether the occurrence rate of GBC among patients with cholecystectomy has changed over the past 2 decades, compared with other parts of the world.
Patients and Methods
This is a retrospective study conducted between January 2002 and March 2016. Patients included were managed at King Abdullah University Hospital and affiliated hospitals in the north of Jordan. The histopathology department’s database was searched for GBC during this period. Medical records of affected patients were reviewed. Data regarding demographic features, clinical presentation, potential risk factors, investigations, and treatment modalities were obtained. If the diagnosis of GBC was established during surgery or first detected in the surgical specimen by a dedicated pathologist, it was classified as “incidental.” Patients’ confidentiality was protected in accordance with declaration of Helsinki provisions. This study was approved by the ethics committee at our institution (reference number: 278-2016). Analysis was performed with the IBM SPSS v.20.0 (Chicago, IL, USA) software.
Results
Over the 15-year study period, 11 391 patients underwent cholecystectomy. About 31/11 391 (0.27%) patients had GBC confirmed by histopathology. In the patients with GBC, the male:female ratio was 1:2.9. The mean age was 68 years (range: 43-103 years). The patients with GBC presented mainly with biliary colic or acute cholecystitis. Table 1 summarizes the characteristics and risk factors in these patients with cancer.
Table 1.
Patients’ characteristics and risk factors.
| Patients’ characteristics and risk factors | No. of patients (31) | % |
|---|---|---|
| Gender: male:female | 8:23 | |
| Age: range (mean), y | 43-103 (68) | |
| Advanced age, y | ||
| >70 | 11 | 34 |
| >60 | 22 | 69 |
| Incidental (not diagnosed pre-op) | 21 | 68 |
| Other risk factors | ||
| Gallstones | 23 | 74 |
| Porcelain gallbladder | 1 | 3 |
| Gallbladder polyps | 4 (1 with stones) | 13 |
| None | 3 | 10 |
The population in the catchment area of our university hospital and affiliated hospitals is estimated 0.75 to 1.5 million during the study period (Jordan population census 2004 and 2015).8 It worth mentioning that the population in Jordan has doubled mainly due to refugees from wars in the nearby countries. This makes the annual incidence rate in this area about 0.2 per 100 000 (men: 0.1/100 000; women: 0.3/100 000).
Diagnosis of GBC was “incidental” in about two-thirds of the study population. Three quarters of patients had gallstones, 4 had polyps, and 1 had porcelain gallbladder (GB). Cancer was suspected in a third of patients on the basis of ultrasound (US)/computed tomography scan findings or alarming clinical presentation (eg, mainly advanced age, weight loss with or without jaundice) prior to operation (Figures 1 and 2). Nonincidental GBC cases were mostly detected by US preoperatively. Thickened irregular gallbladder mucosa with intraluminal heterogeneous polypoid lesion was the most common finding. These patients presented frequently with jaundice, weight loss, and chronic epigastric discomfort.
Figure 1.
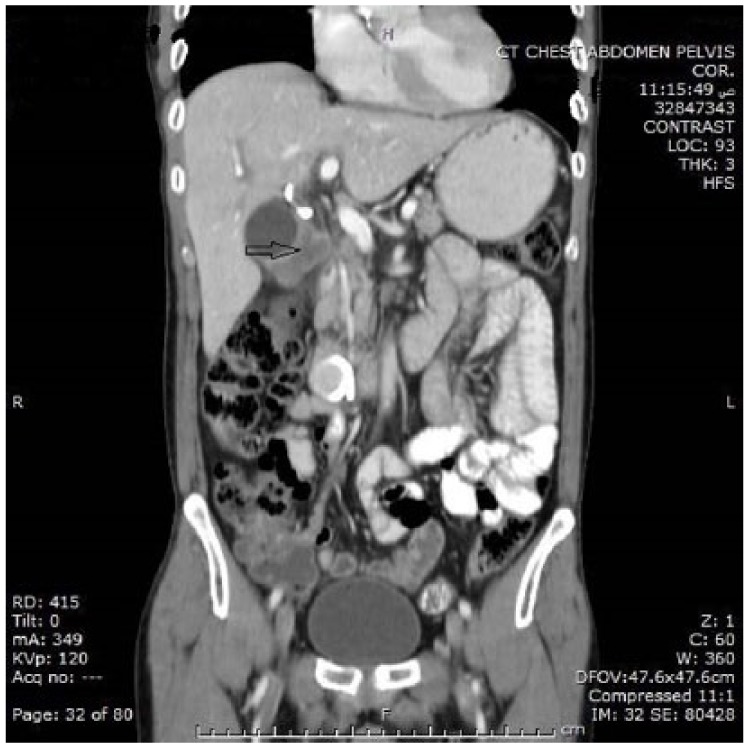
Thickened irregular gallbladder wall (CT—coronal section). CT indicates computed tomography.
Figure 2.
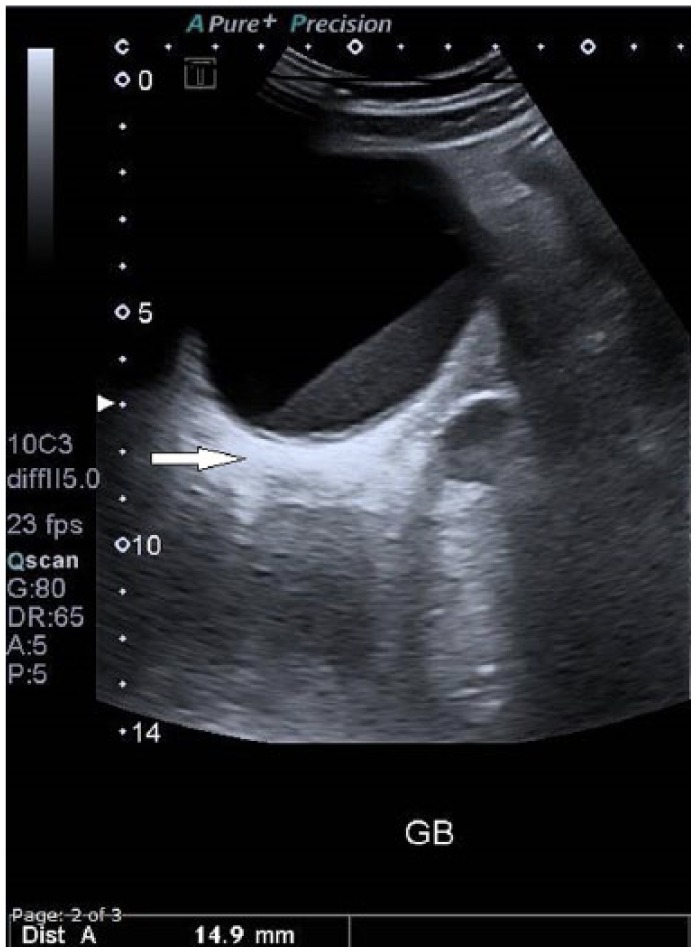
Thickened irregular gallbladder wall (ultrasound).
Table 2 lists the different histologic types for all patients (Figure 3 demonstrates high-grade primary adenocarcinoma of gallbladder [×40 power]). Based on histopathologic analysis, 2/31 GBCs were stage TIb, one had Tis (carcinoma in situ), and the rest of patients had pathologic stage of TII or more (TII:15, TIII:8, T4:5).9 Simple cholecystectomy was performed in 21 patients. The remaining 10 patients underwent diagnostic ± biopsy, hepatectomy, or extrahepatic biliary resection. There were no perioperative deaths.
Table 2.
Histology type.
| Histology type | No. of patients (31) |
|---|---|
| Mixed adenocarcinoma—small-cell carcinoma | 1 |
| Small-cell carcinoma | 1 |
| Squamous cell carcinoma | 1 |
| Poorly differentiated carcinoma | 1 |
| Adenocarcinoma | 27 |
| Papillary | 2 |
| In situ | 1 |
| Mucinous | 1 |
| Signet ring | 1 |
| With neuroendocrine differentiation | 1 |
| Not otherwise specified | 21 |
Figure 3.
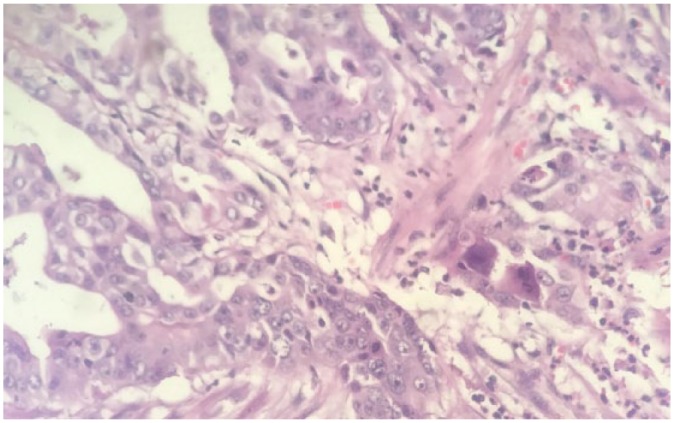
High-grade adenocarcinoma of gallbladder (×40 power).
Discussion
In Jordan, cancer is considered the leading cause of death after heart disease. According to the latest mortality report issued by Ministry of Health for 2012, more than one-third of deaths are attributed to cardiovascular diseases (36.6%) and 16.2% to cancer.10
Compared with a report published by Bani-Hani et al,7 almost 15 years ago, GBC rate and histologic patterns among patients with cholecystectomy in northern Jordan has not significantly changed (0.007 in the previous study and 0.003 in this study). Adenocarcinoma remains the dominant type in both studies (82% in the previous study and 87% in this study).
Our annual incidence of 0.2 per 100 000 (men: 0.1/100 000; women: 0.3/100 000) is lower than other countries in the Middle East, such as Turkey (men: 1.5/100 000; women: 2/100 000) and Iran (men: 0.4-1.2/100 000; women: 0.7-1.7/100 000).11 Our rate is considered very low compared with the rest of the world. The GBC incidence in Western world countries (United States, United Kingdom, Canada, Australia, and New Zealand) ranges between 0.4 and 0.8/100 000 in men and 0.6 and1.4/100 000 in women.12,13 High incidence rates have been reported in Pakistan (13.8/100 000), Bolivia (15.5/100 000), Poland (14/100 000), and Japan (7/100 000) with the highest incidence rate reported in women in Chile (27/100 000).
Although most of patients diagnosed with biliary cancers have no well-defined risk factors,14 several factors have been linked to the increased risk of GBC, including gallstones, gallbladder polyps, advanced age, female sex, porcelain GB, and chronic biliary tract infections.
Although the correlation between GBC and gallstones is not well established, in our study, gallstones seemed to be a predominant risk factor and the leading cause for the incidental diagnosis of GBC. Gallstones have been shown in other studies to be present in up to 90% of patients with GBC. However, the incidence of GBC in patients with gallstones varies from 0.3% to 3%.15 The relative risk of developing GBC in patients with gallstones was found to be 8.3 compared with general population.16
The exact mechanism by which gallstones can cause GBC is not well-understood. Carcinogenesis in this case is thought to be due to stone-induced chronic inflammation which may predispose to dysplasia and malignancy. Both gallstones and biliary duct stones have been implicated as risk factors for GBC and cholangiocarcinoma.14
Multiple reports have studied the correlation between gallstone size and the risk of developing GBC. These studies have shown an increasing relative risk of GBC with stone size ≥3 cm in diameter, compared with subjects with stones smaller than 1 cm.17,18 We were unable to find sufficient data regarding the diameter of the stones in our series due to a number of factors: (1) the stones are not always measured and mentioned in the US report and (2) most of the cholecystectomies were performed laparoscopically and the stones were usually crushed inside the gallbladder during extraction to facilitate delivery via the paraumbilical port wound.
Compared with the previous study of Bani-Hani et al,7 a minor shift of mean age at diagnosis (from 61.4 to 68 years) was noticed. This could be attributed to the increased life expectancy over this period. It is evident in the literature that GBC rate increases steadily with advanced age. The US data from 2010 revealed that GBC incidence rate was highest for individuals over the age of 75 years.19
Having 23 (74%) of our patients with GBC as women is not an unusual finding. Women are affected 2 to 6 times more often than men. The coexpression of both estrogen and progesterone receptors is increased in women with GBC as compared with men and compared with chronic cholecystitis.19,20 Female hormones are thought to play an important role in gallbladder carcinogenesis.21
Incidental GBC (IGBC), which is diagnosed during or after cholecystectomy, has better prognosis than non-IGBC. A study from Korea compared the clinical outcomes of IGBC and non-IGBC and suggests significant differences in disease stage and overall survival (survival rates at 1 year: 90% vs 23%) between both groups.22
Until now, most GBCs are still found incidentally in patients undergoing cholecystectomy for cholelithiasis or gallbladder polyps. This corresponds to what we found in our study, namely, that 68% of patients were diagnosed incidentally. However, a cancer is found in less than 1% of gallbladders removed for common reasons, ie, stones or polyps,14 thus there is no sufficient evidence to support the performance of prophylactic cholecystectomy for asymptomatic gallstone disease to prevent GBC.22
Non-IGBC cases in this study were mostly detected by US. Proper preoperative US maximizes the opportunity for appropriate surgery (radical vs simple cholecystectomy) at the start and saves the patient from undergoing more than one surgery. Further preoperative diagnostic attempts for GBC, mainly in high-risk group (elderly people), may improve outcomes, especially in non-IGBCs. A supportive evidence for this suggestion is still lacking because prospective study designs to investigate this disease are difficult to apply.
Only 13% (4 patients) of GBCs in our group evolved from gallbladder polyps. The literature estimates that almost 5% of population harbor gallbladder polyps. This frequency is likely increasing due to improved imaging technology and to more patients undergoing US scans.23
An imaging overlap of polyps with gallstones is not uncommon. Similarly, differentiating nonneoplastic from malignant polyps is an important major preoperative diagnostic challenge.18 Classically, polyp features that predict malignancy include rapidly growing polyps, polyps >10 mm in size, and solitary/sessile polyps in patients with gallstones of the age of 50 to 60 years. However, based on the systemic review of evidence of Wiles et al, a true correlation between growth of polyp and development of malignancy in gallbladder could not be established using the currently available evidence in literature. In addition, the presence of malignant neoplasms in a small percentage of gallbladder polyps measuring below 10 mm challenges the current practice of only removing gallbladders containing polyps measuring >10 mm.23 Therefore, cholecystectomy was proposed for all symptomatic polyps and asymptomatic polyps measuring >10 mm, with no US follow-up schedule on polyps <10 mm.
Findings in this study consolidate the previously published recommendations regarding the importance of a high index of suspicion preoperatively when dealing with cholelithiasis in elderly patients, particularly with large-sized stones, and the necessity of senior radiologist’s supervision over routine gallbladder US requested for stones. Intraoperatively, a proper gross inspection should be performed routinely by all surgeons. This inspection would help in the staging of GBC regardless of the surgical option chosen (radical vs simple cholecystectomy) at IGBC cases.
Our study has some limitations; first, it is a retrospective, nonrandomized study. Second, the sample size of patients with GBC was small to evaluate risk factors with statistical significance; in addition, people who do not undergo gallbladder surgery but likely have the disease—based on clinical or radiologic findings—were not included in the study as histologic diagnosis was essential. This may result in lower expectation of the actual number of cases. Despite these limitations, findings appeared to confirm the stable trend of GBC in our region over 2 decades and contributed to global research tracking cancer burden.
In conclusion, GBC average annual incidence rate in northern Jordan is low and has not changed over the past 2 decades. The GBC histopathology has also not changed and adenocarcinoma remained the most common subtype. This study reinforces the previously published recommendations regarding the high index of suspicion of GBC in elderly with cholelithiasis and the importance of proper gross inspection during routine cholecystectomy.
Acknowledgments
This abstract has been accepted for an oral presentation at the 52nd International Meeting of the European Society for Surgical Research (ESSR).
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conception and design: ARAM, MBH. Acquisition of data: ARAM, SAA, NA-M, SH, MA. Analysis and interpretation of data: ARAM, MBH, HQ. Participated in drafting the article or revising it: ARAM, MBH, HQ, SN, NA-Z. All Authors gave final approval of the version to be submitted and the revised version.
References
- 1. Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder cancer in the 21st century. J Oncol. 2015;2015:967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nevin JE, Moran TJ, Kay S, King R. Carcinoma of the gallbladder: staging, treatment, and prognosis. Cancer. 1976;37:141-148. [DOI] [PubMed] [Google Scholar]
- 3. Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 5. Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for gallbladder cancer across the world. HPB (Oxford). 2008;10:327-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjerregaard JK, Mortensen MB, Pfeiffer P. Trends in cancer of the liver, gall bladder, bile duct, and pancreas in elderly in Denmark, 1980-2012. Acta Oncol. 2016;55:40-45. [DOI] [PubMed] [Google Scholar]
- 7. Bani-Hani KE, Yaghan RJ, Matalka II, Shatnawi NJ. Gallbladder cancer in northern Jordan. J Gastroenterol Hepatol. 2003;18:954-959. [DOI] [PubMed] [Google Scholar]
- 8.Population and housing census report 2015, Jordan. http://www.dos.gov.jo/dos_home_a/main/population/census2015/Persons/Persons_3.1.pdf. Accessed April 10, 2018.
- 9. American Joint Committee on Cancer. Gallbladder. In: Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017:303-309. [Google Scholar]
- 10. Al-Sayaideh A, Nimri O, Al-Zaghal M, Arqoup K, Halasa W. Cancer incidence in Jordan 2012, Non-Communicable Diseases Directorate, Jordan Cancer Registry, Ministry of Health, Jordan. www.moh.gov.jo. Accessed October 25, 2016.
- 11. Moore MA. Overview of cancer registration research in the Asian Pacific from 2008-2013. Asian Pac J Cancer Prev. 2013;14:4461-4484. [DOI] [PubMed] [Google Scholar]
- 12. Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34:306-312. [DOI] [PubMed] [Google Scholar]
- 13. Vijayakumar A, Vijayakumar A, Patil V, Mallikarjuna MN, Shivaswamy BS. Early diagnosis of gallbladder carcinoma: an algorithm approach. ISRN Radiol. 2012;2013:239424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43:137-147. [DOI] [PubMed] [Google Scholar]
- 15. Lai CH, Lau WY. Gallbladder cancer, a comprehensive review. Surgeon. 2008;6:101-110. [DOI] [PubMed] [Google Scholar]
- 16. Maringhini A, Moreau JA, Melton LJ, 3rd, Hench VS, Zinsmeister AR, DiMagno EP. Gallstones, gallbladder cancer, and other gastrointestinal malignancies. An epidemiologic study in Rochester, Minnesota. Ann Intern Med. 1987;107:30-35. [DOI] [PubMed] [Google Scholar]
- 17. Lowenfels AB, Lindström CG, Conway MJ, Hastings PR. Gallstones and risk of gallbladder cancer. J Natl Cancer Inst. 1985;75:77-80. [PubMed] [Google Scholar]
- 18. Diehl AK. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250:2323-2326. [PubMed] [Google Scholar]
- 19. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factor. Int J Cancer. 2006;118:1591-1602. [DOI] [PubMed] [Google Scholar]
- 21. Gupta P, Agarwal A, Gupta V, Singh PK, Pantola C, Amit S. Expression and clinicopathological significance of estrogen and progesterone receptors in gallbladder cancer. Gastrointest Cancer Res. 2012;5:41-47. [PMC free article] [PubMed] [Google Scholar]
- 22. Cha BH, Bae JM. Comparison of clinical outcomes of incidental and non-incidental gallbladder cancers: a single-center cross-sectional study. Asian Pac J Cancer Prev. 2014;15:1281-1283. [DOI] [PubMed] [Google Scholar]
- 23. Wiles R, Varadpande M, Muly S, Webb J. Growth rate and malignant potential of small gallbladder polyps—systematic review of evidence. Surgeon. 2014;12:221-226. [DOI] [PubMed] [Google Scholar]


